Electrospun Polyimide Nanofibers Modified with Metal Oxide Nanowires and MXene for Photocatalytic Water Purification
Abstract
1. Introduction
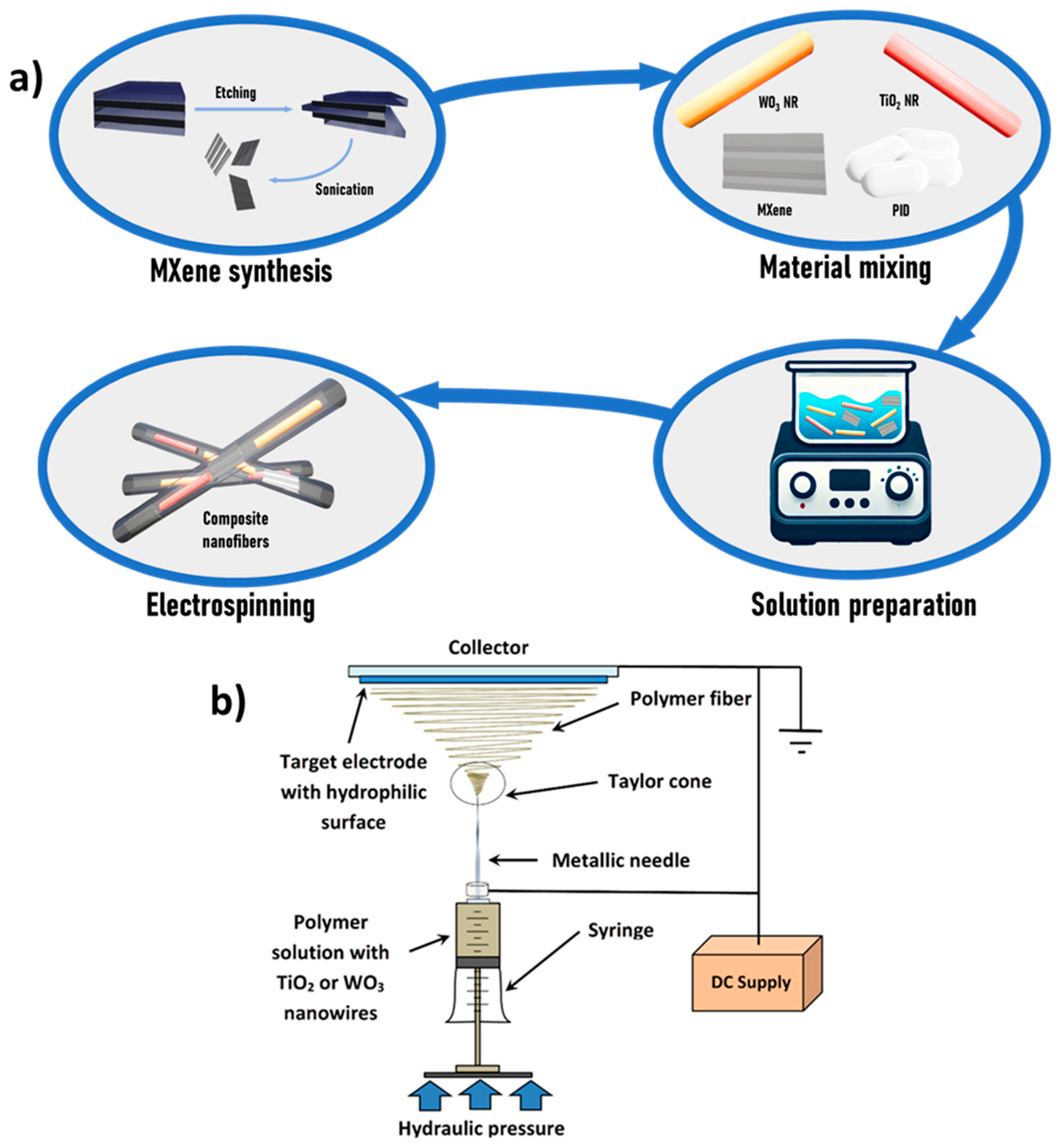
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis of MXenes
2.3. Solutions Preparation and Electrospinning
2.4. Characterization
2.5. Photodegradation Tests
3. Results and Discussion
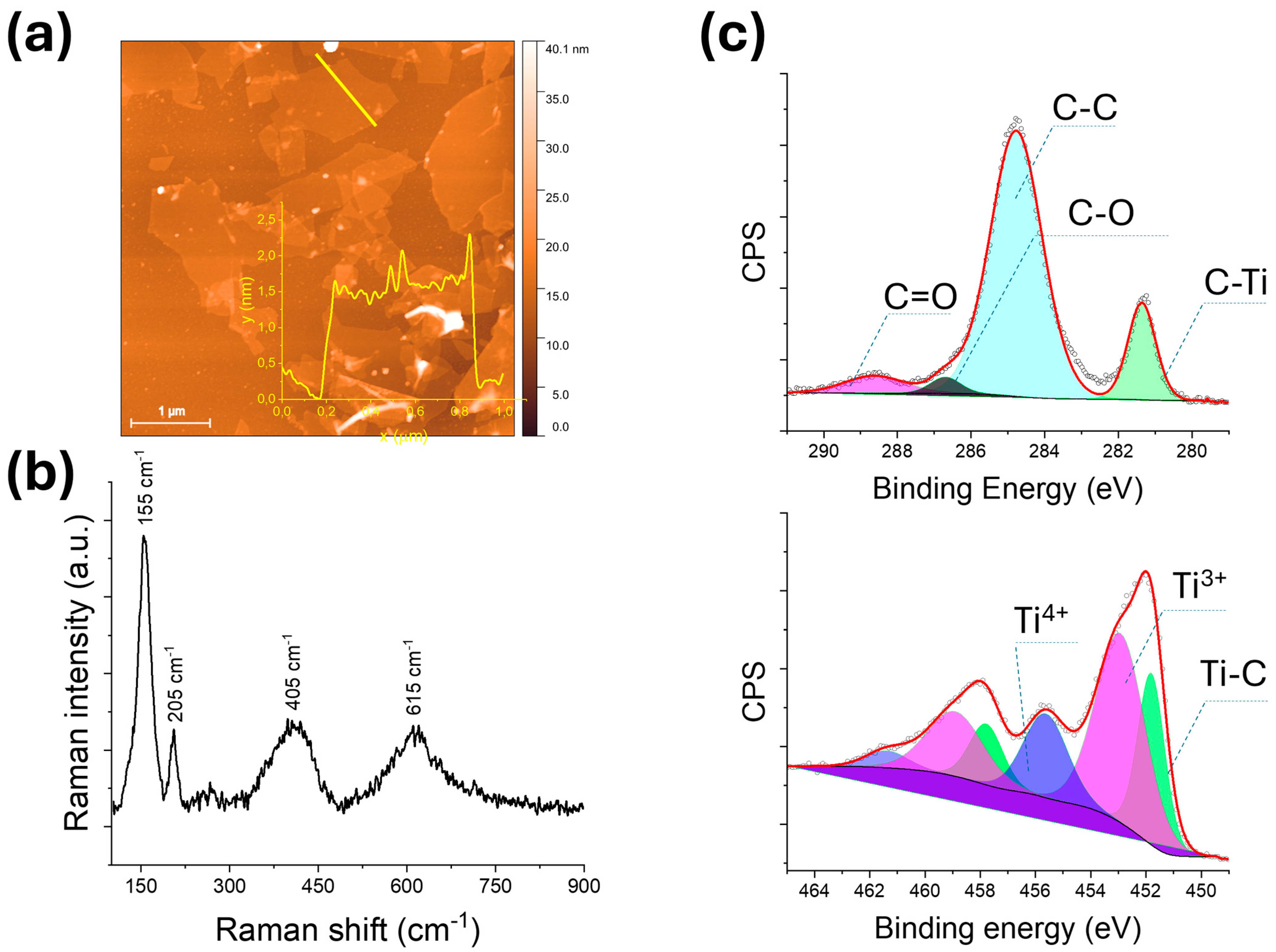
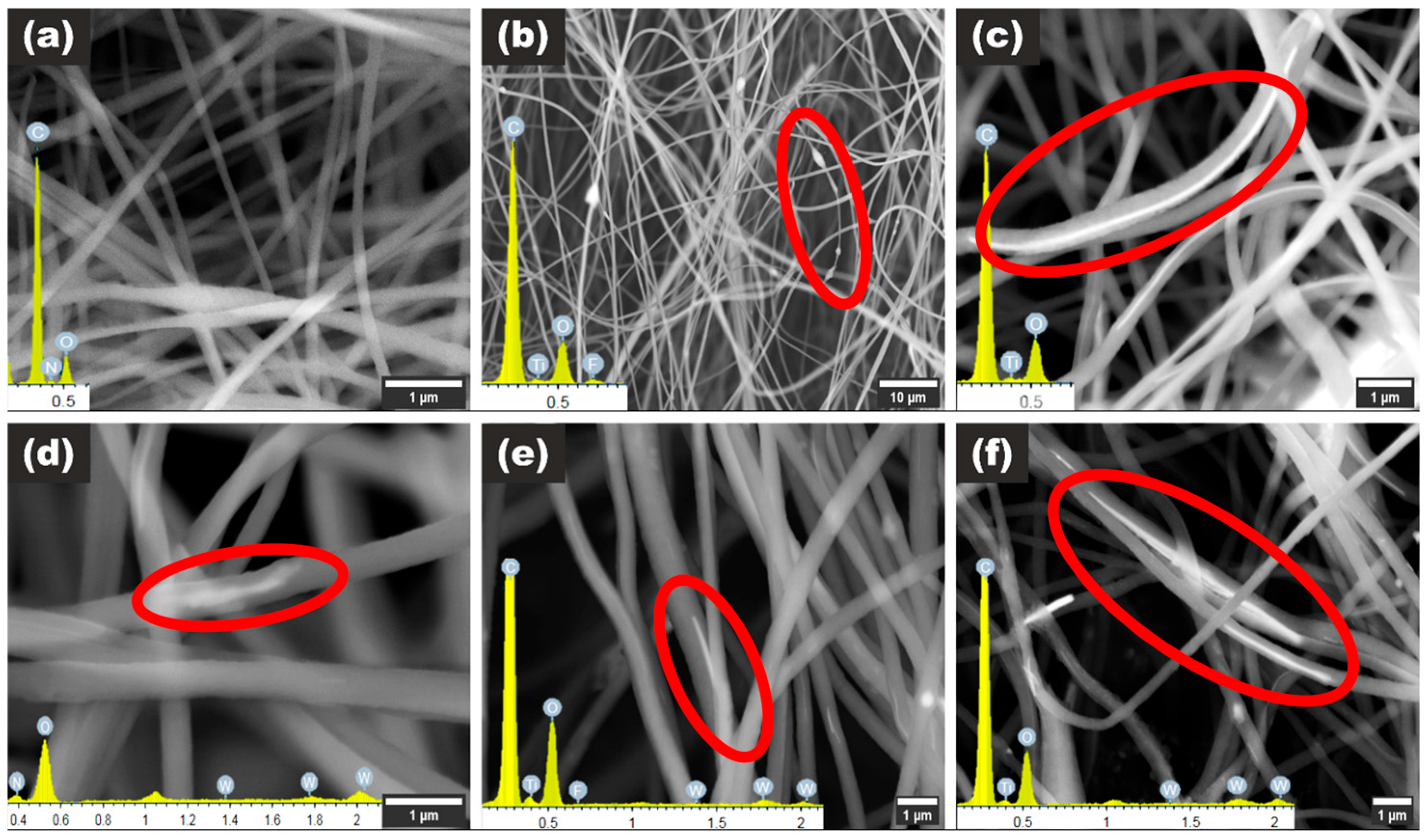
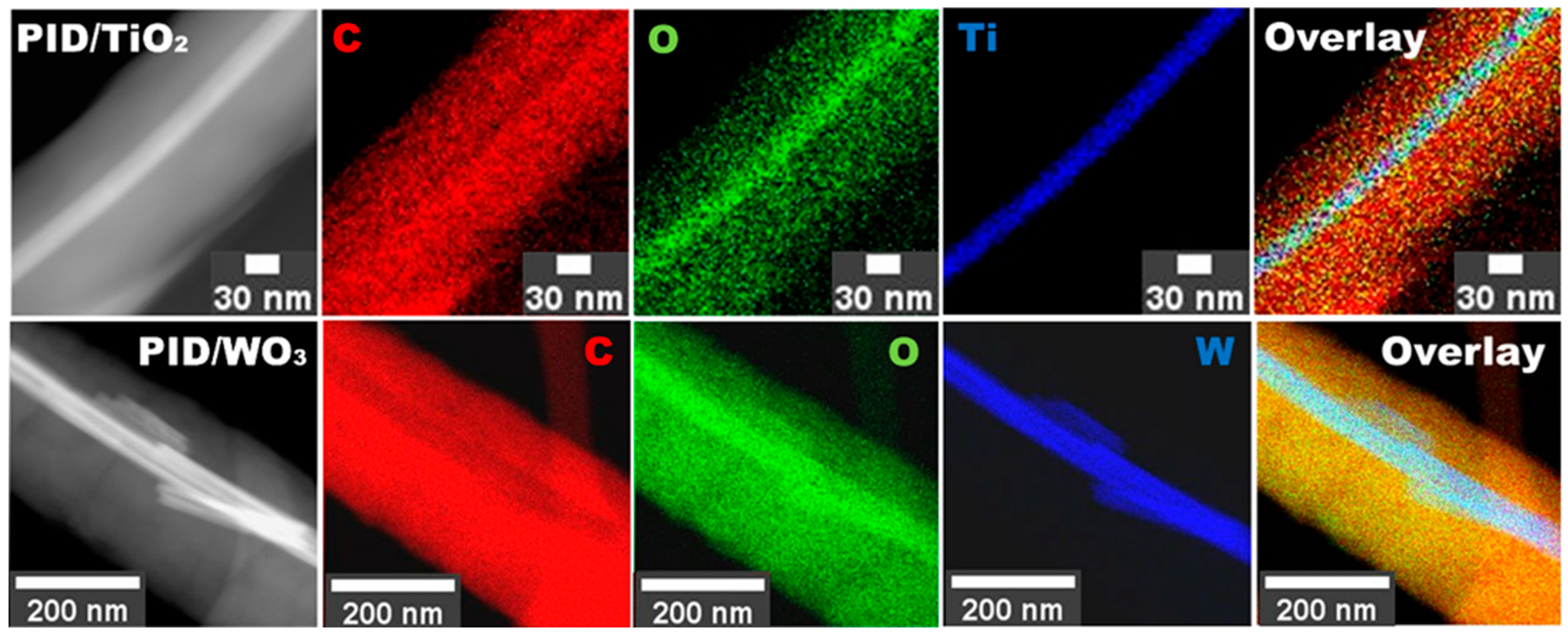
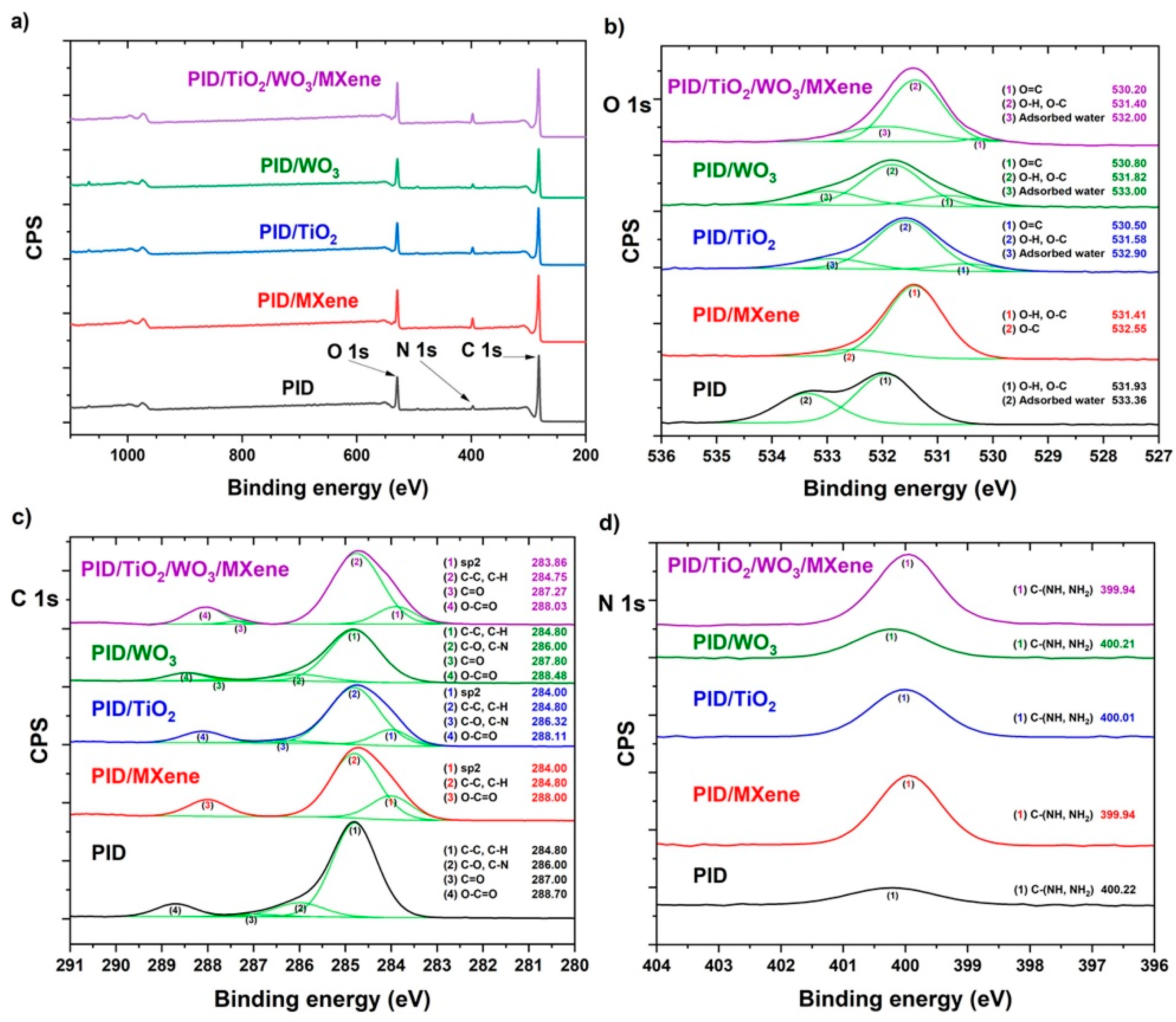
3.1. Structure Characterization
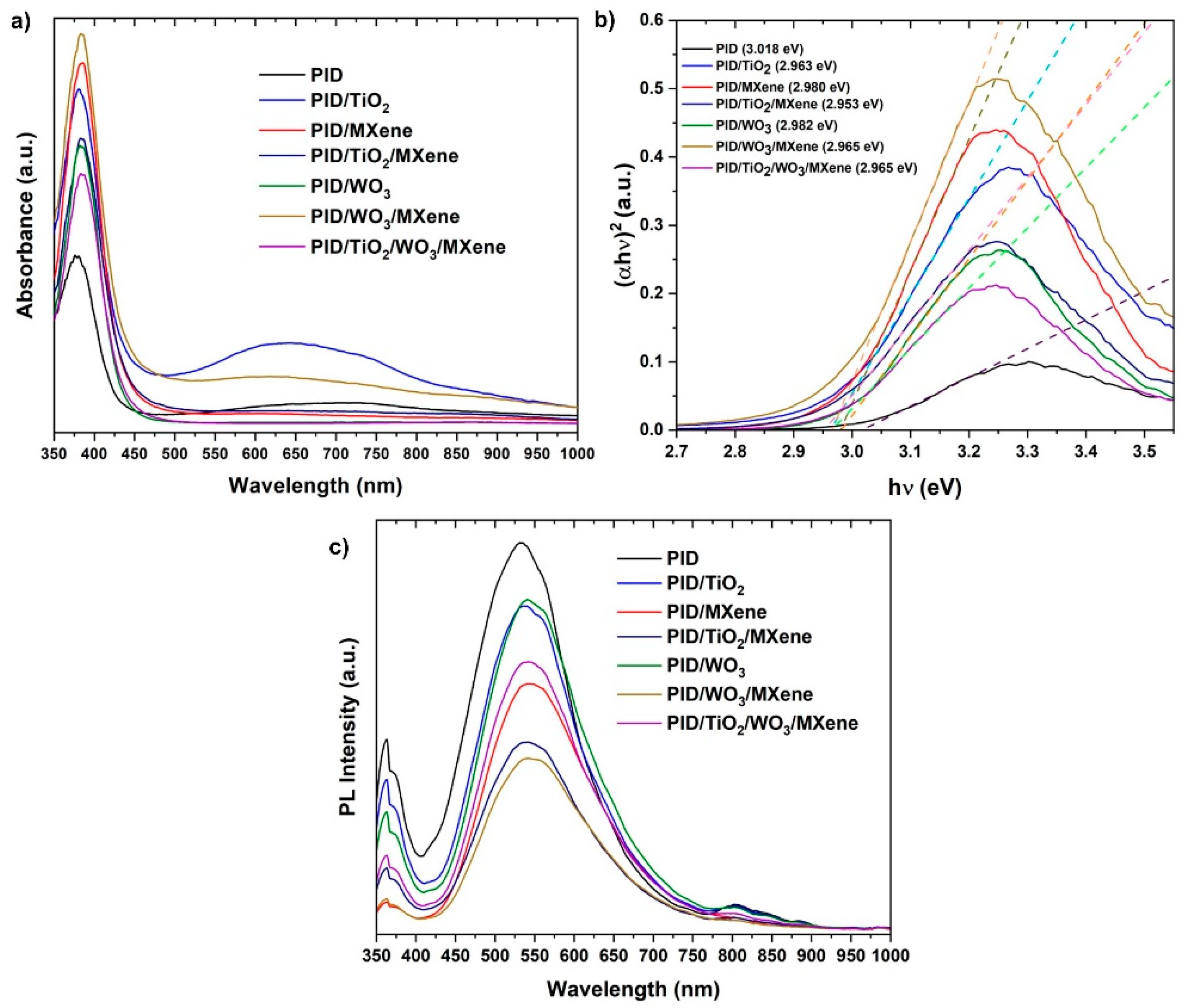
3.2. Chemical and Optical Properties of Produced Nanocaomposites
3.3. Dye Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PID | Polyimide |
| R6G | Rhodamine 6G |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| PL | Photoluminescence |
| EDX | Energy-dispersive X-ray spectroscopy |
| HR-TEM | High-resolution transmission electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| AFM | Atomic force microscopy |
References
- Hafeez, A.; Shamair, Z.; Shezad, N.; Javed, F.; Fazal, T.; Rehman, S.U.; Bazmi, A.A.; Rehman, F. Solar Powered Decentralized Water Systems: A Cleaner Solution of the Industrial Wastewater Treatment and Clean Drinking Water Supply Challenges. J. Clean. Prod. 2021, 289, 125717. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Zhu, W.; Liu, G.; Xian, T.; Yang, H. Design of CdZnS/BiOCl Heterostructure as a Highly-Efficient Piezo-Photocatalyst for Removal of Antibiotic. J. Environ. Chem. Eng. 2024, 12, 114405. [Google Scholar] [CrossRef]
- Mu, W.; Xu, M.; Sun, X.; Liu, G.; Yang, H. Oxygen-Vacancy-Tunable Mesocrystalline ZnO Twin “Cakes” Heterostructured with CdS and Cu Nanoparticles for Efficiently Photodegrading Sulfamethoxazole. J. Environ. Chem. Eng. 2024, 12, 112367. [Google Scholar] [CrossRef]
- Thanh, P.N.; Phung, V.-D.; Nguyen, T.B.H. Recent Advances and Future Trends in Metal Oxide Photocatalysts for Removal of Pharmaceutical Pollutants from Wastewater: A Comprehensive Review. Environ. Geochem. Health 2024, 46, 364. [Google Scholar] [CrossRef]
- Fareza, A.R.; Nugroho, F.A.A.; Abdi, F.F.; Fauzia, V. Nanoscale Metal Oxides–2D Materials Heterostructures for Photoelectrochemical Water Splitting—A Review. J. Mater. Chem. A 2022, 10, 8656–8686. [Google Scholar] [CrossRef]
- Okpara, E.C.; Olatunde, O.C.; Wojuola, O.B.; Onwudiwe, D.C. Applications of Transition Metal Oxides and Chalcogenides and Their Composites in Water Treatment: A Review. Environ. Adv. 2023, 11, 100341. [Google Scholar] [CrossRef]
- Sedghi, R.; Moazzami, H.R.; Hosseiny Davarani, S.S.; Nabid, M.R.; Keshtkar, A.R. A One Step Electrospinning Process for the Preparation of Polyaniline Modified TiO2/Polyacrylonitile Nanocomposite with Enhanced Photocatalytic Activity. J. Alloys Compd. 2017, 695, 1073–1079. [Google Scholar] [CrossRef]
- Chang, Z.; Sun, X.; Liao, Z.; Liu, Q.; Han, J. Design and Preparation of Polyimide/TiO2@MoS2 Nanofibers by Hydrothermal Synthesis and Their Photocatalytic Performance. Polymers 2022, 14, 3230. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Hou, K.; Chen, J.; Cheng, W.; Wu, Q.; Han, J.; Jiang, J. Ag/AgBr/AgVO3 Photocatalyst-Embedded Polyacrylonitrile/Polyamide/Chitosan Nanofiltration Membrane for Integrated Filtration and Degradation of RhB. ACS Appl. Mater. Interfaces 2022, 14, 24708–24719. [Google Scholar] [CrossRef]
- Araújo, E.S.; Da Costa, B.P.; Oliveira, R.A.P.; Libardi, J.; Faia, P.M.; De Oliveira, H.P. TiO2/ZnO Hierarchical Heteronanostructures: Synthesis, Characterization and Application as Photocatalysts. J. Environ. Chem. Eng. 2016, 4, 2820–2829. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Zheng, H.; Meng, X.; Chen, J.; Que, M.; Wang, W.; Liu, X.; Yang, L.; Zhao, Y. In Situ Phase Evolution of TiO2/Ti3C2T Heterojunction for Enhancing Adsorption and Photocatalytic Degradation. Appl. Surf. Sci. 2021, 545, 149031. [Google Scholar] [CrossRef]
- Konieva, A.; Deineka, V.; Diedkova, K.; Aguilar-Ferrer, D.; Lyndin, M.; Wennemuth, G.; Korniienko, V.; Kyrylenko, S.; Lihachev, A.; Zahorodna, V.; et al. MXene-Polydopamine-antiCEACAM1 Antibody Complex as a Strategy for Targeted Ablation of Melanoma. ACS Appl. Mater. Interfaces 2024, 16, 43302–43316. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Wang, M.; Zhang, B.; Feng, S.; Liu, W.; Liu, Y.; Liu, T.; Bi, Y.; Yang, Q.; et al. Surface Plasmon Effect of Ti3C2 Mxene and Degradation of Antibiotics Under Full Spectrum. SSRN J. 2022. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Peng, Q.; Zheng, H.; Xue, F.; Li, P.; Xu, Z.; He, X. Flame-Retardant MXene/Polyimide Film with Outstanding Thermal and Mechanical Properties Based on the Secondary Orientation Strategy. Nanoscale Adv. 2021, 3, 5683–5693. [Google Scholar] [CrossRef] [PubMed]
- Kalambate, P.K.; Dhanjai; Sinha, A.; Li, Y.; Shen, Y.; Huang, Y. An Electrochemical Sensor for Ifosfamide, Acetaminophen, Domperidone, and Sumatriptan Based on Self-Assembled MXene/MWCNT/Chitosan Nanocomposite Thin Film. Microchim. Acta 2020, 187, 402. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene Nanoflakes Decorating ZnO Tetrapods for Enhanced Performance of Skin-Attachable Stretchable Enzymatic Electrochemical Glucose Sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef] [PubMed]
- Prabu, G.T.V.; Dhurai, B. A Novel Profiled Multi-Pin Electrospinning System for Nanofiber Production and Encapsulation of Nanoparticles into Nanofibers. Sci. Rep. 2020, 10, 4302. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, D.; Liu, F. Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials 2022, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Näslund, L.-Å.; Kokkonen, E.; Magnuson, M. Interaction and Kinetics of H2, CO2, and H2O on Ti3C2Tx MXene Probed by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2025, 684, 161926. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, L.; Yao, N.; Bi, Z.; Zhang, B.; Hu, H. Field-Electron Emission from Polyimide-Ablated Films. Appl. Phys. A 2000, 71, 281–284. [Google Scholar] [CrossRef]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Célérier, S.; Barsoum, M.W. A Critical Analysis of the X-Ray Photoelectron Spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, H.-T.; Park, J.-K. Effects of Thermal Curing on the Structure of Polyimide Film. Polym. J. 1998, 30, 229–233. [Google Scholar] [CrossRef][Green Version]
- Sheng, W.; Shi, J.-L.; Hao, H.; Li, X.; Lang, X. Polyimide-TiO2 Hybrid Photocatalysis: Visible Light-Promoted Selective Aerobic Oxidation of Amines. Chem. Eng. J. 2020, 379, 122399. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Marzorati, S.; Longhi, M.; Coduri, M.; Artiglia, L.; Selli, E. Photocatalytic Activity of TiO2-WO3 Mixed Oxides in Relation to Electron Transfer Efficiency. Appl. Catal. B Environ. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Luo, S.; Luo, Z.; Zhang, X.; Huang, R.; Luo, M. In Situ Modification of Cobalt on MXene/TiO2 as Composite Photocatalyst for Efficient Nitrogen Fixation. J. Colloid Interface Sci. 2021, 585, 20–29. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Nirmala, R.; Jeong, J.W.; Navamathavan, R.; Kim, H.Y. Synthesis and Electrical Properties of TiO2 Nanoparticles Embedded in Polyamide-6 Nanofibers Via Electrospinning. Nano-Micro Lett. 2011, 3, 56–61. [Google Scholar] [CrossRef]
- Li, B.; He, T.; Ding, M. A Comparative Study of Insoluble and Soluble Polyimide Thin Films. Polymer 1999, 40, 789–794. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Patel, M.Y.; Mortelliti, M.J.; Dempsey, J.L. A Compendium and Meta-Analysis of Flatband Potentials for TiO2, ZnO, and SnO2 Semiconductors in Aqueous Media. Chem. Phys. Rev. 2022, 3, 011303. [Google Scholar] [CrossRef]
- Kalanur, S.S. Structural, Optical, Band Edge and Enhanced Photoelectrochemical Water Splitting Properties of Tin-Doped WO3. Catalysts 2019, 9, 456. [Google Scholar] [CrossRef]
- Ye, F.; Qian, J.; Xia, J.; Li, L.; Wang, S.; Zeng, Z.; Mao, J.; Ahamad, M.; Xiao, Z.; Zhang, Q. Efficient Photoelectrocatalytic Degradation of Pollutants over Hydrophobic Carbon Felt Loaded with Fe-Doped Porous Carbon Nitride via Direct Activation of Molecular Oxygen. Environ. Res. 2024, 249, 118497. [Google Scholar] [CrossRef]
- Othman, Z.; Sinopoli, A.; Mackey, H.R.; Mahmoud, K.A. Efficient Photocatalytic Degradation of Organic Dyes by AgNPs/TiO2/Ti3C2Tx MXene Composites under UV and Solar Light. ACS Omega 2021, 6, 33325–33338. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.A.; Kim, H. Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials 2022, 12, 2464. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, S.; Chen, J.; Sun, X.; Zhao, S.; Chang, J.; He, Z. Ti3C2@g-C3N4/TiO2 Ternary Heterogeneous Photocatalyst for Promoted Photocatalytic Degradation Activities. Coatings 2023, 13, 655. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Liu, Y.; Zhao, Q.; Li, X.; Zhou, Q.; Chen, Y.; Wang, S. Construction of Bronze TiO2/Ti3C2 MXene/Ag3PO4 Ternary Composite Photocatalyst toward High Photocatalytic Performance. Catalysts 2022, 12, 599. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on G-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Praus, P. 2D/2D Composites Based on Graphitic Carbon Nitride and MXenes for Photocatalytic Reactions: A Critical Review. Carbon Lett. 2024, 34, 227–245. [Google Scholar] [CrossRef]
- Pino, E.; Calderón, C.; Herrera, F.; Cifuentes, G.; Arteaga, G. Photocatalytic Degradation of Aqueous Rhodamine 6G Using Supported TiO2 Catalysts. A Model for the Removal of Organic Contaminants from Aqueous Samples. Front. Chem. 2020, 8, 365. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3C2 -MXene/Bismuth Ferrite Nanohybrids for Efficient Degradation of Organic Dyes and Colorless Pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef]
- Li, J.-Y.; Jiang, X.; Lin, L.; Zhou, J.-J.; Xu, G.-S.; Yuan, Y.-P. Improving the Photocatalytic Performance of Polyimide by Constructing an Inorganic-Organic Hybrid ZnO-Polyimide Core–Shell Structure. J. Mol. Catal. A Chem. 2015, 406, 46–50. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, X.; Yu, H.; Wang, Z.; Ren, C.; Zhou, L.; Lin, Y.-W.; Dou, L. WO3/Ag2CO3 Mixed Photocatalyst with Enhanced Photocatalytic Activity for Organic Dye Degradation. ACS Omega 2021, 6, 26439–26453. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. MXene-Based Photocatalysts in Degradation of Organic and Pharmaceutical Pollutants. Molecules 2022, 27, 6939. [Google Scholar] [CrossRef]
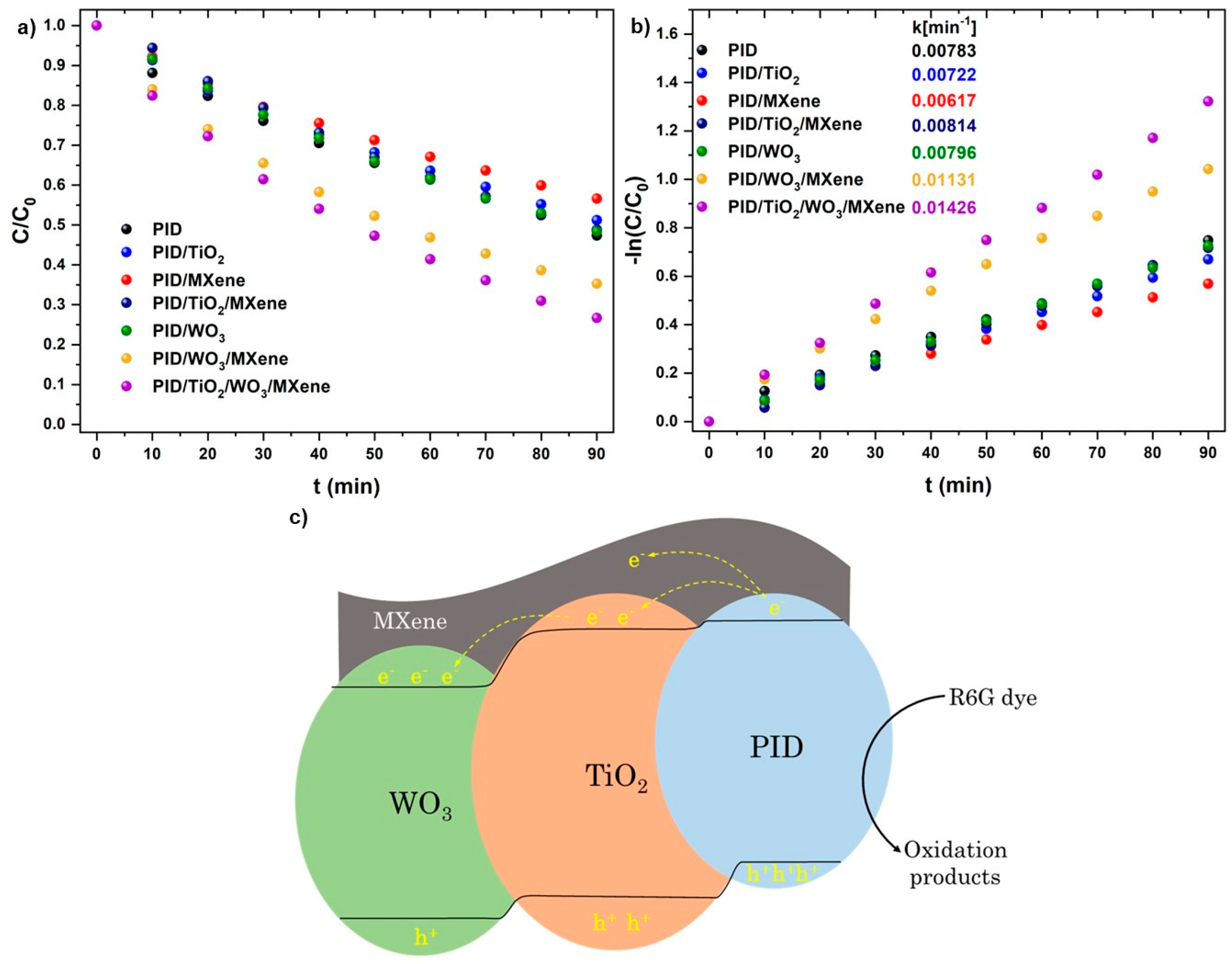
| Photocatalyst | Pollutant (C0, mg/L) | Lamp, Power (W) | Spectrum | Time (min) | k (min−1) | Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|
| PID/TiO2/WO3/MXene | R6G (10) | Xenon, 300 | UV–Vis | 90 | 0.01426 | 74 | this work |
| AgNPs/TiO2/Ti3C2Tx | MB (10) | Mercury, 400 | UV | 30 | 0.162 | 99 | [38] |
| RhB (10) | Mercury, 400 | UV | 40 | 0.143 | 99 | ||
| MB (10) | Solar simulator | Sunlight | 120 | 0.028 | 96 | ||
| RhB (10) | Solar simulator | Sunlight | 120 | 0.020 | 88 | ||
| Ag3PO4/TiO2@Ti3C2 (Petals) | MB (10) | Xenon, 300 | Visible | 40 | 0.289 | 94–100 | [39] |
| Ti3C2@TiO2/g-C3N4 (ternary) | RhB (10) | Xenon, 300 | Visible | 120 | 0.01575 | - | [40] |
| g-C3N4 | RhB (10) | Xenon, 300 | Visible | 120 | 0.01575 | - | [40] |
| TiO2(B)/Ti3C2/Ag3PO4 | RhB (10) | Visible lamp | Visible | 60 | 0.182–0.345 | 90–100 | [41] |
| TiO2(B) | RhB (10) | Visible lamp | Visible | 60 | 0.008 | - | [41] |
| TiO2(B)/20% Ti3C2 | RhB (10) | Visible lamp | Visible | 60 | 0.011 | - | [41] |
| MXene/g-C3N4 (1 wt% Ti3C2) | MB (10) | Halogen, 500 | Visible | 180 | ~0.0051 | ~60 | [42,43] |
| TiO2@Ti3C2 (baseline in Petals work) | RhB (9) | Solar simulator | Sunlight | 20 | 0.0093 | - | [39] |
| Supported TiO2 (Raschig rings) | R6G (5) | White light | Visible | - | 0.025 | 66–77 | [44] |
| Pristine Ti3C2 | Congo Red (10) | Visible lamp | Visible | 120 | - | ~12 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lys, A.; Myndrul, V.; Pavlenko, M.; Anastaziak, B.; Holec, P.; Vodseďálková, K.; Coy, E.; Bechelany, M.; Iatsunskyi, I. Electrospun Polyimide Nanofibers Modified with Metal Oxide Nanowires and MXene for Photocatalytic Water Purification. Nanomaterials 2025, 15, 1371. https://doi.org/10.3390/nano15171371
Lys A, Myndrul V, Pavlenko M, Anastaziak B, Holec P, Vodseďálková K, Coy E, Bechelany M, Iatsunskyi I. Electrospun Polyimide Nanofibers Modified with Metal Oxide Nanowires and MXene for Photocatalytic Water Purification. Nanomaterials. 2025; 15(17):1371. https://doi.org/10.3390/nano15171371
Chicago/Turabian StyleLys, Andrii, Valerii Myndrul, Mykola Pavlenko, Błażej Anastaziak, Pavel Holec, Kateřina Vodseďálková, Emerson Coy, Mikhael Bechelany, and Igor Iatsunskyi. 2025. "Electrospun Polyimide Nanofibers Modified with Metal Oxide Nanowires and MXene for Photocatalytic Water Purification" Nanomaterials 15, no. 17: 1371. https://doi.org/10.3390/nano15171371
APA StyleLys, A., Myndrul, V., Pavlenko, M., Anastaziak, B., Holec, P., Vodseďálková, K., Coy, E., Bechelany, M., & Iatsunskyi, I. (2025). Electrospun Polyimide Nanofibers Modified with Metal Oxide Nanowires and MXene for Photocatalytic Water Purification. Nanomaterials, 15(17), 1371. https://doi.org/10.3390/nano15171371










