In Situ Synthesis of RMB6-TMB2 Composite Nanopowders via One-Step Solid-State Reduction
Abstract
1. Introduction
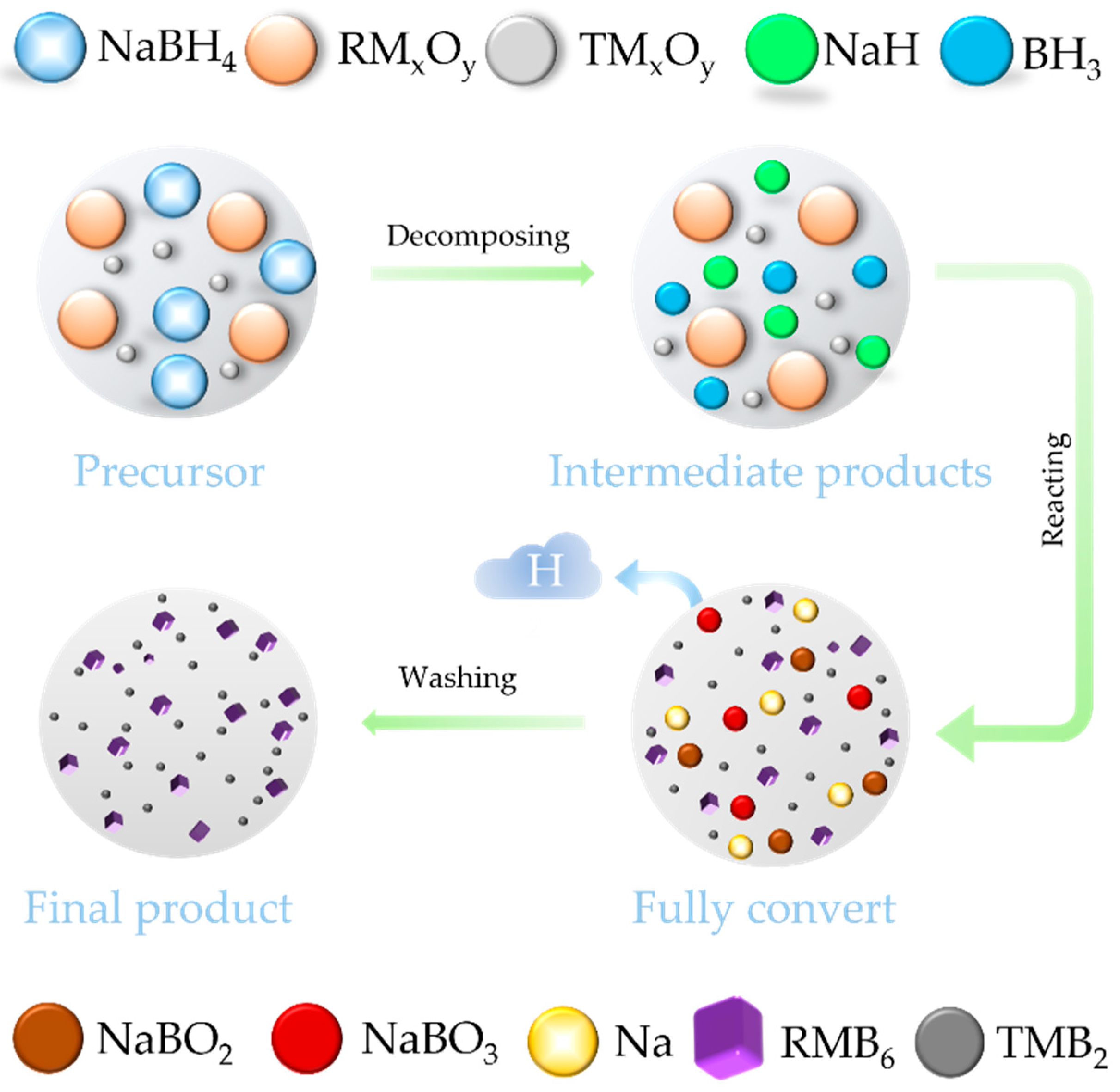
2. Experiment
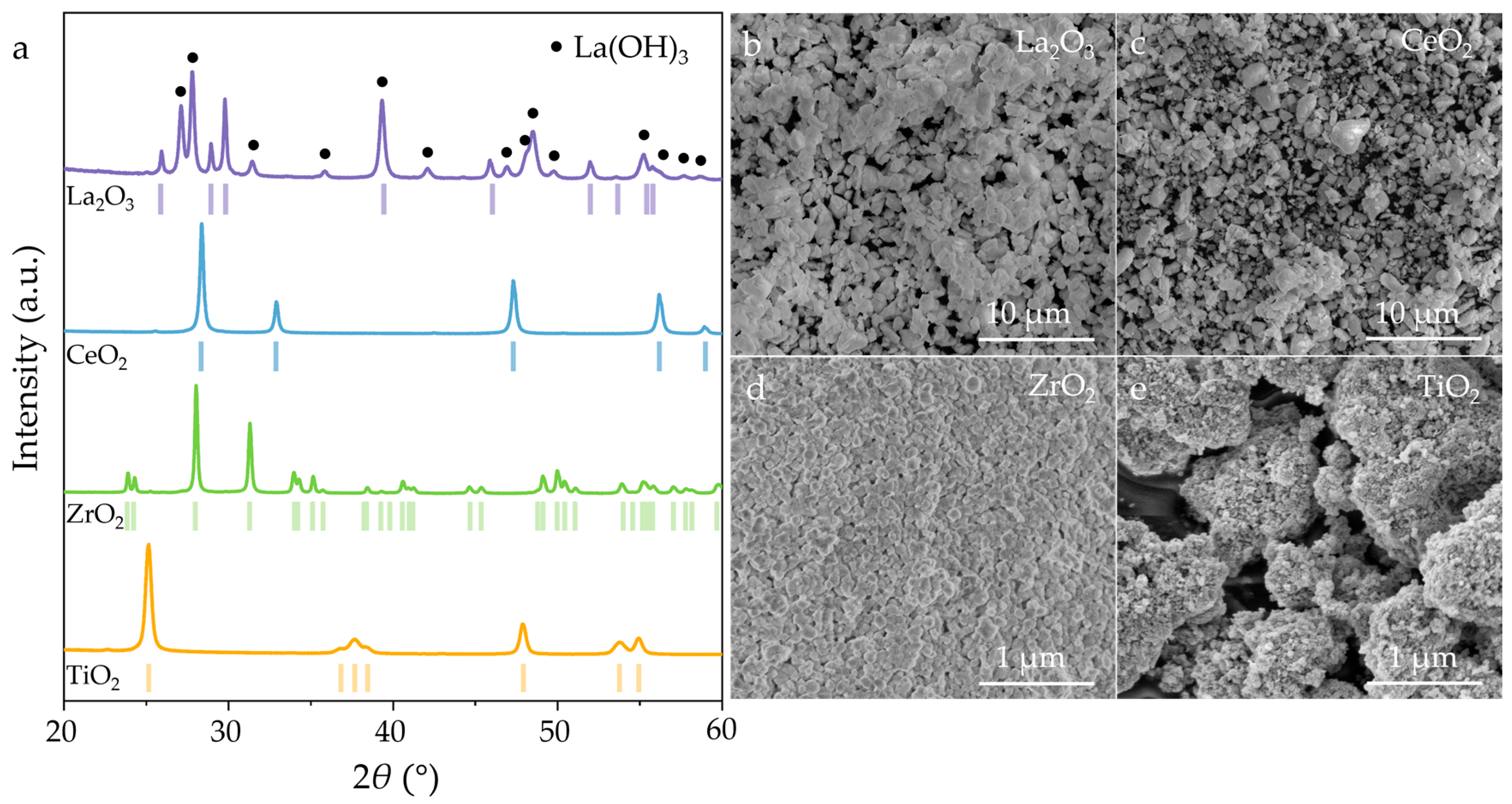
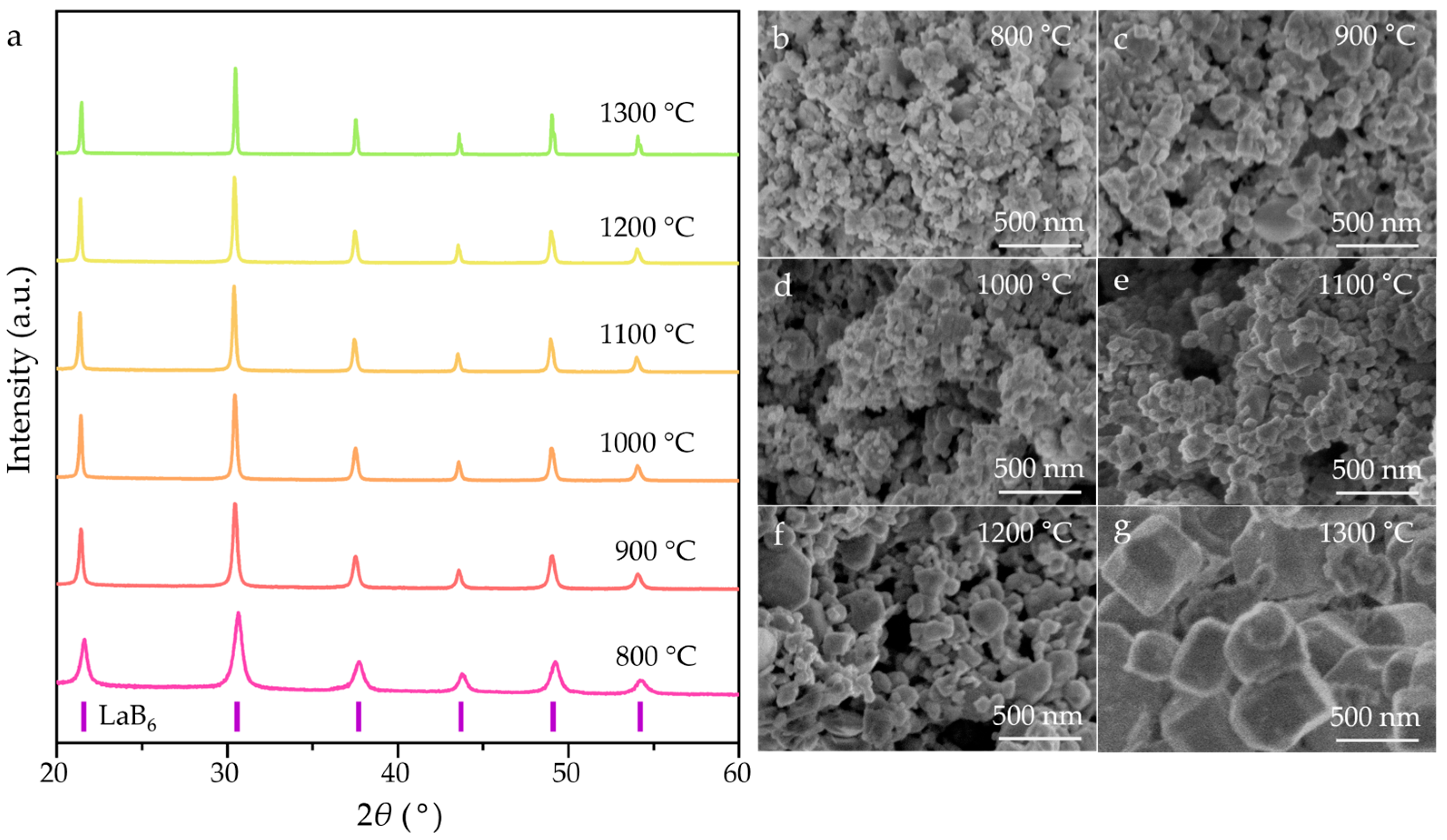
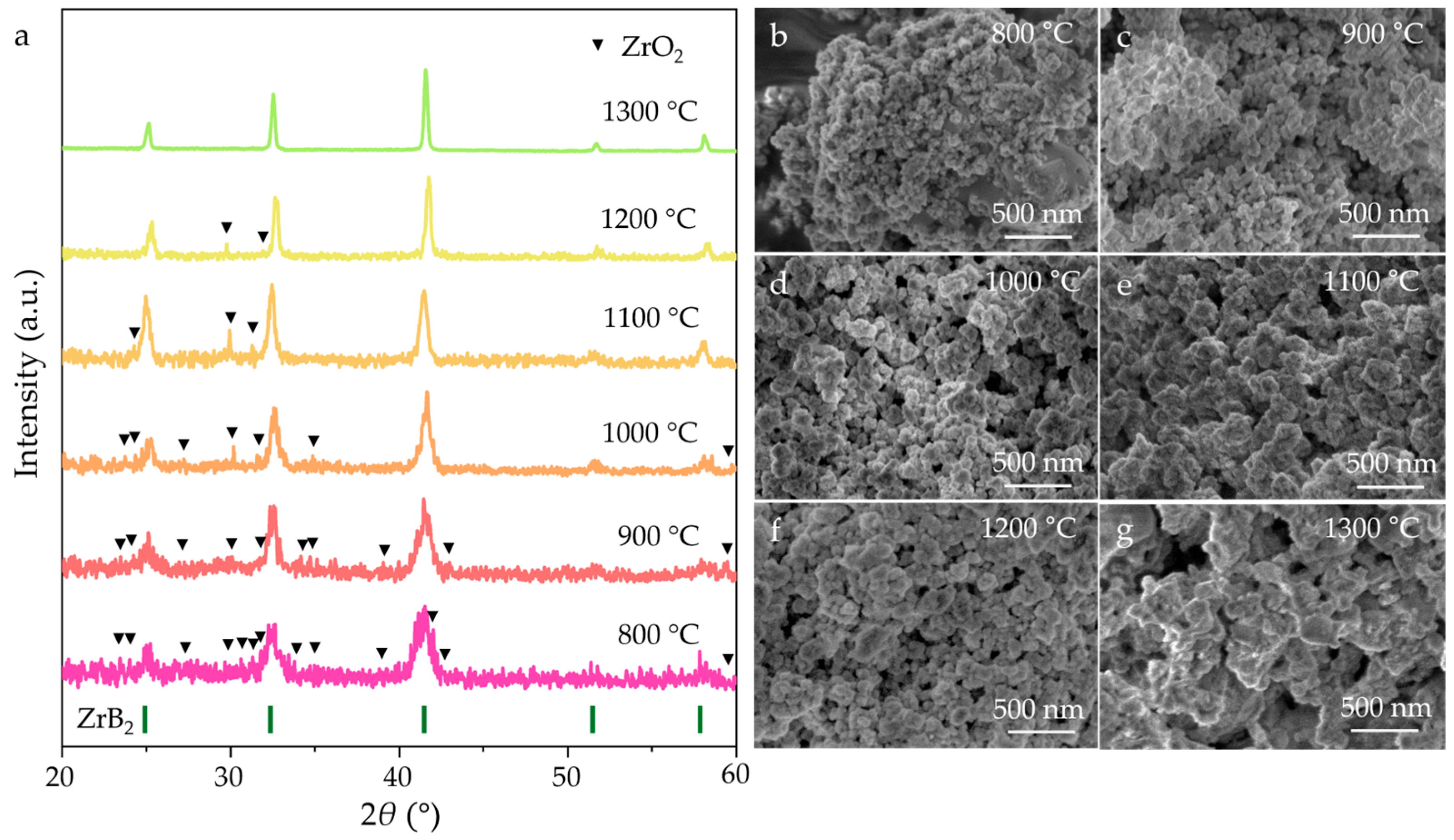
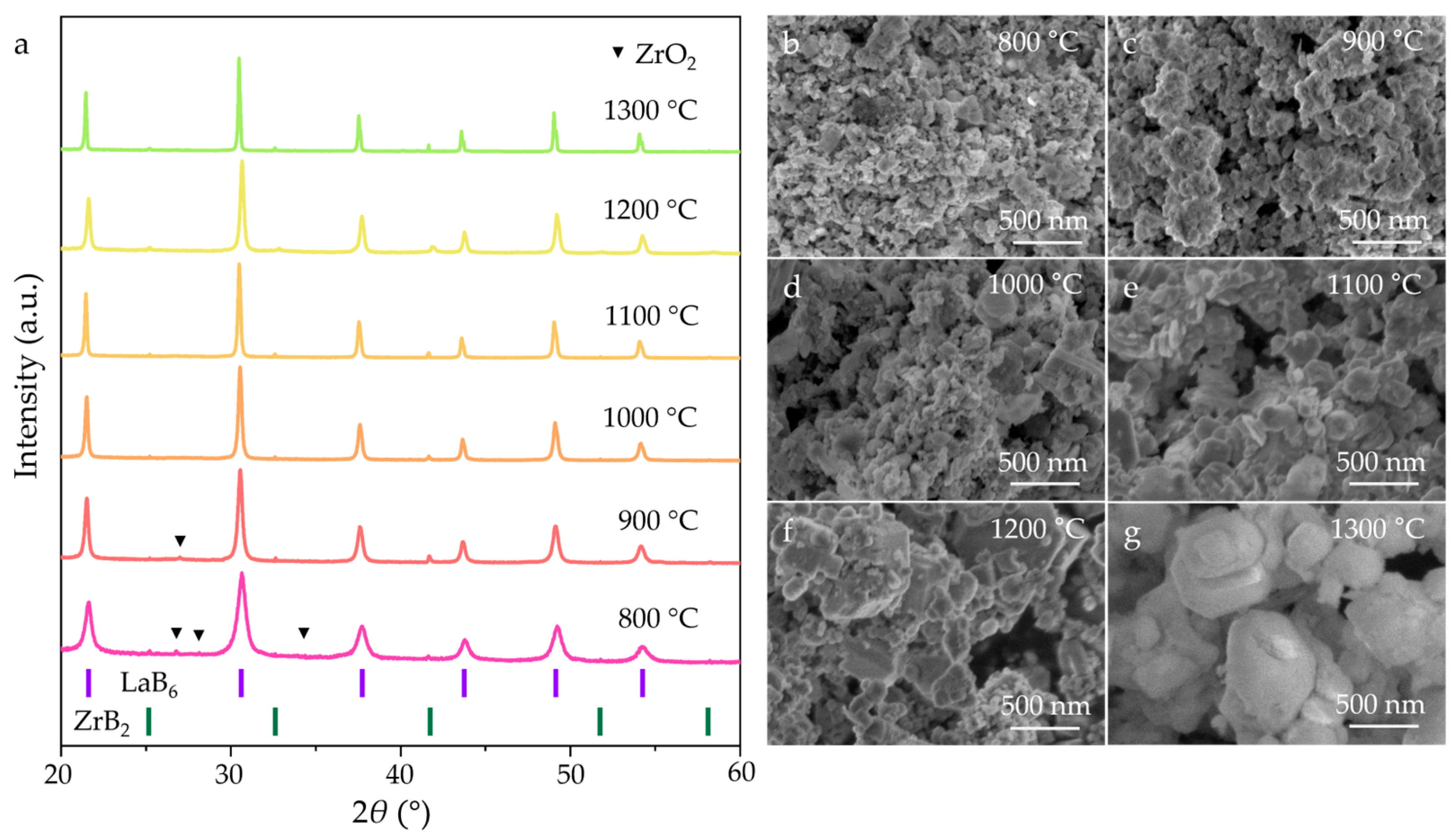
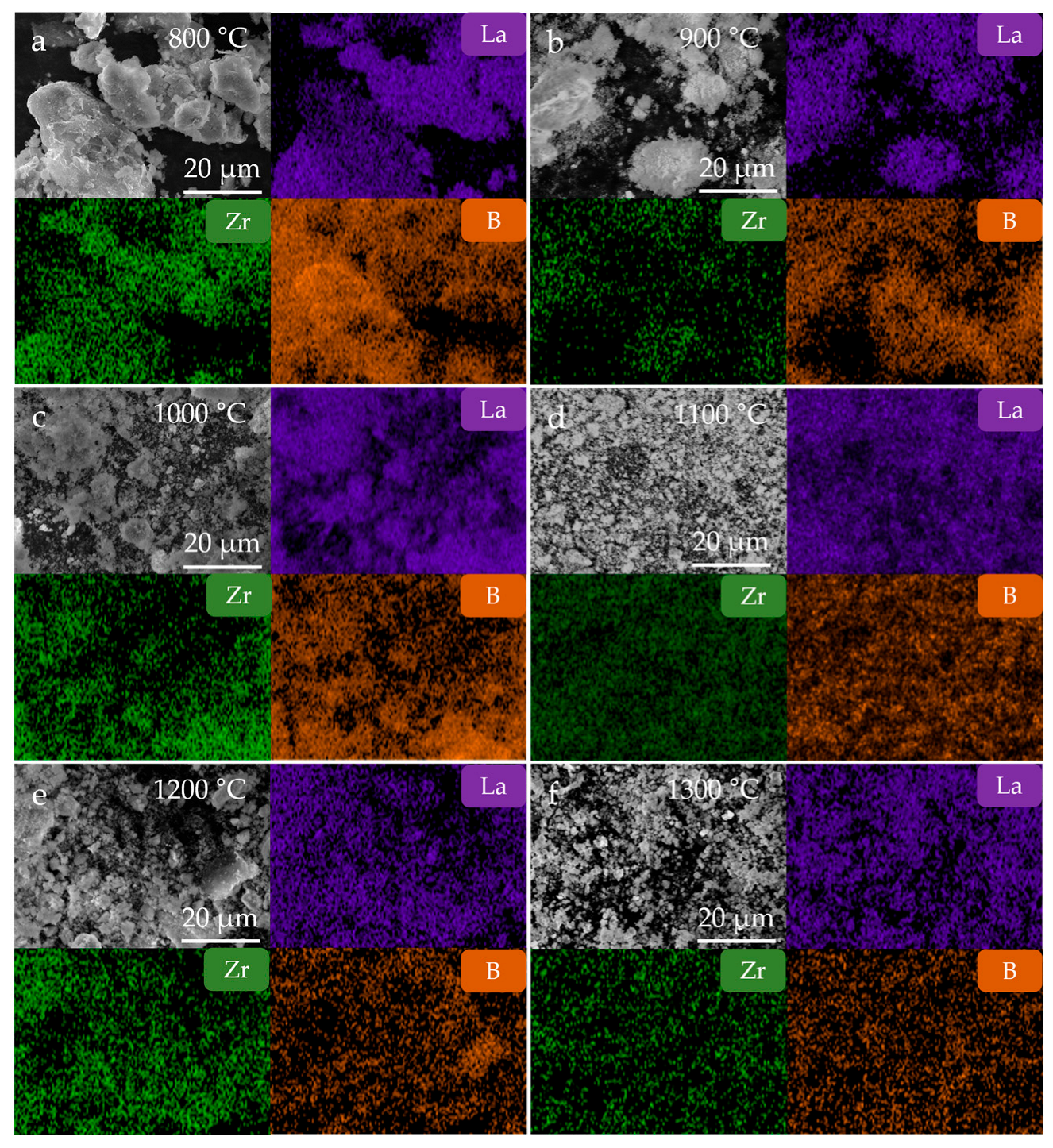
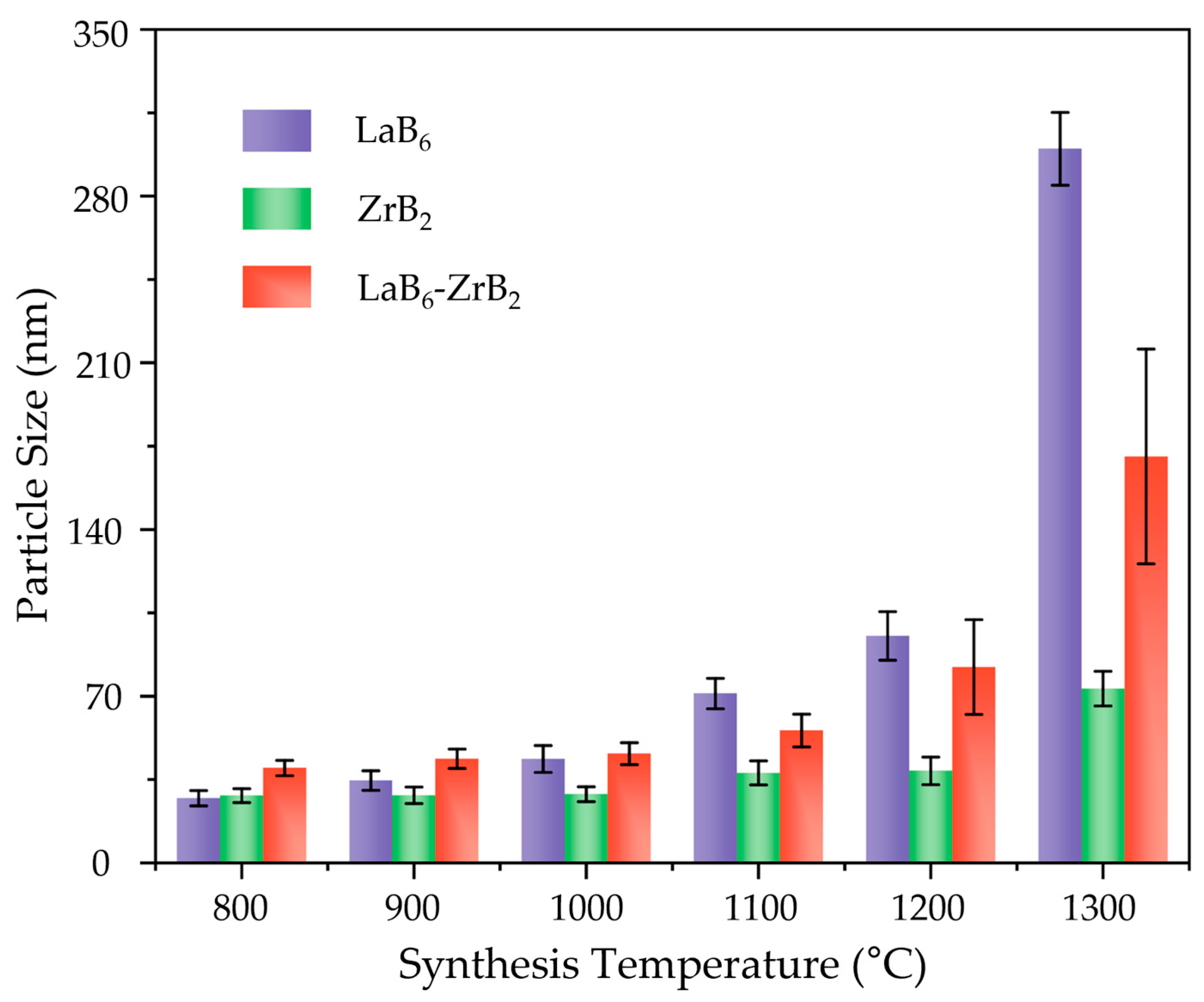
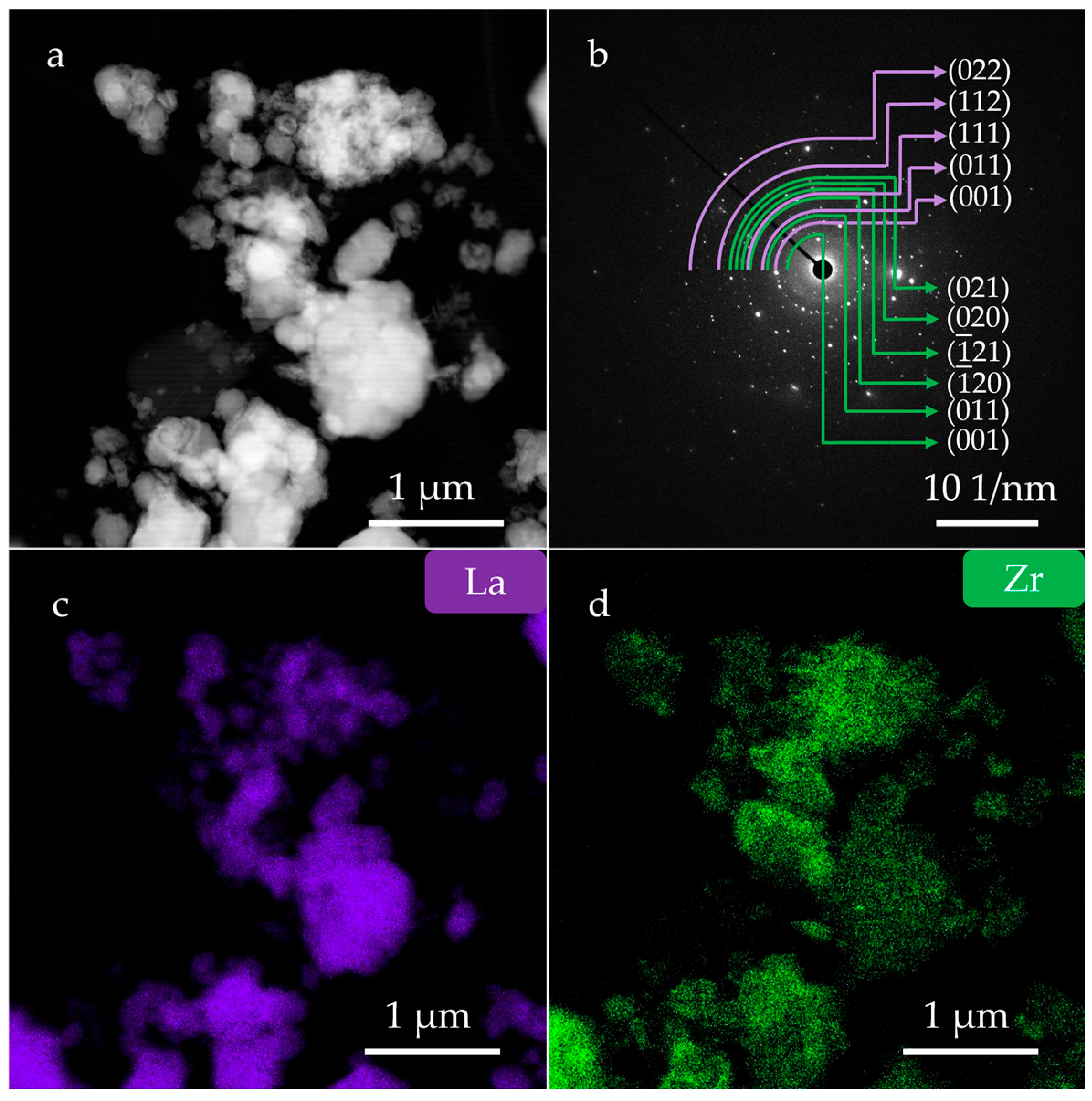
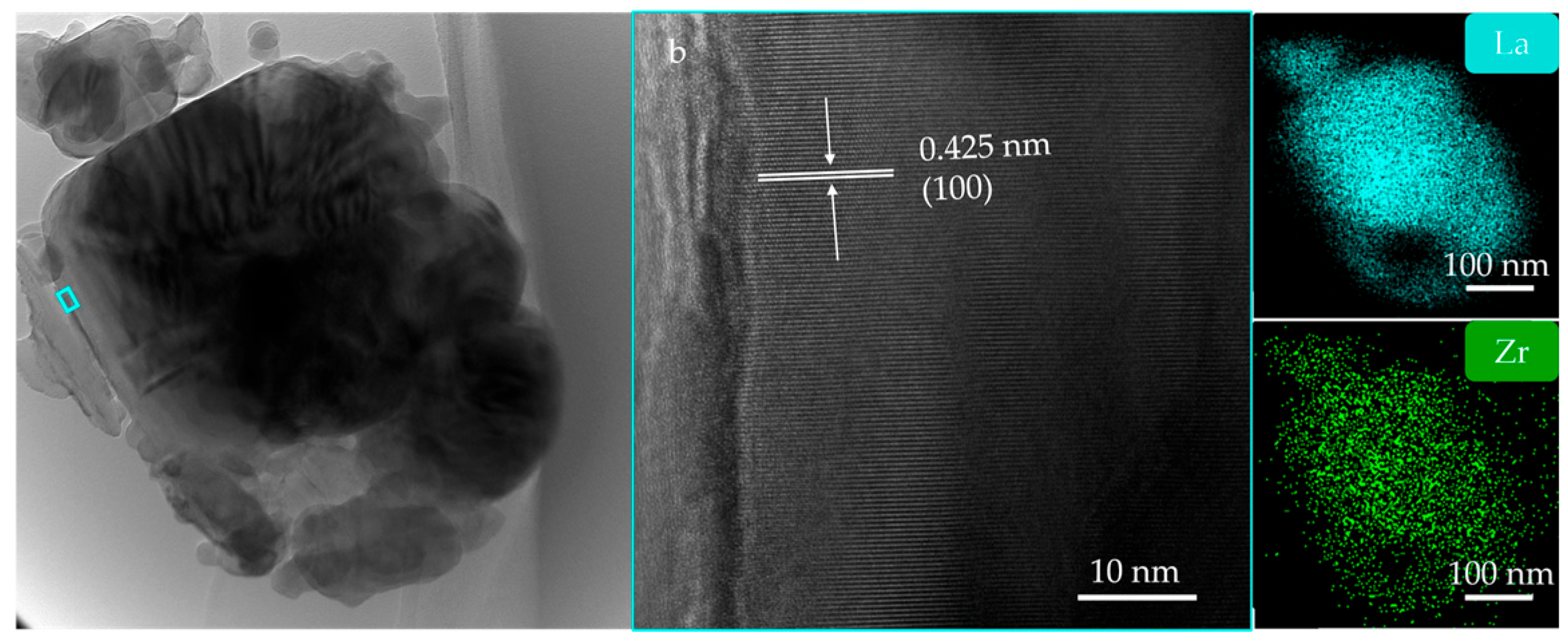
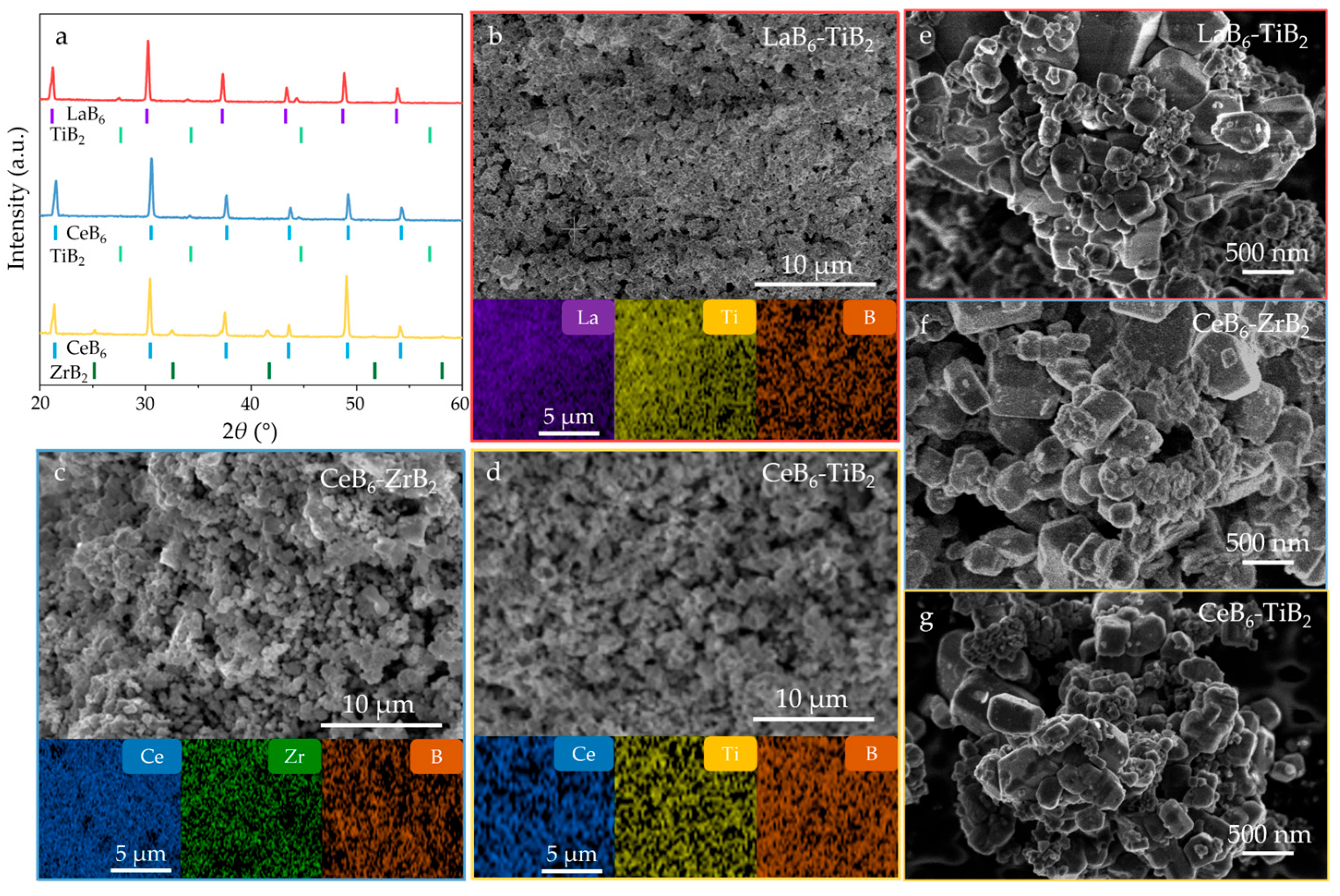
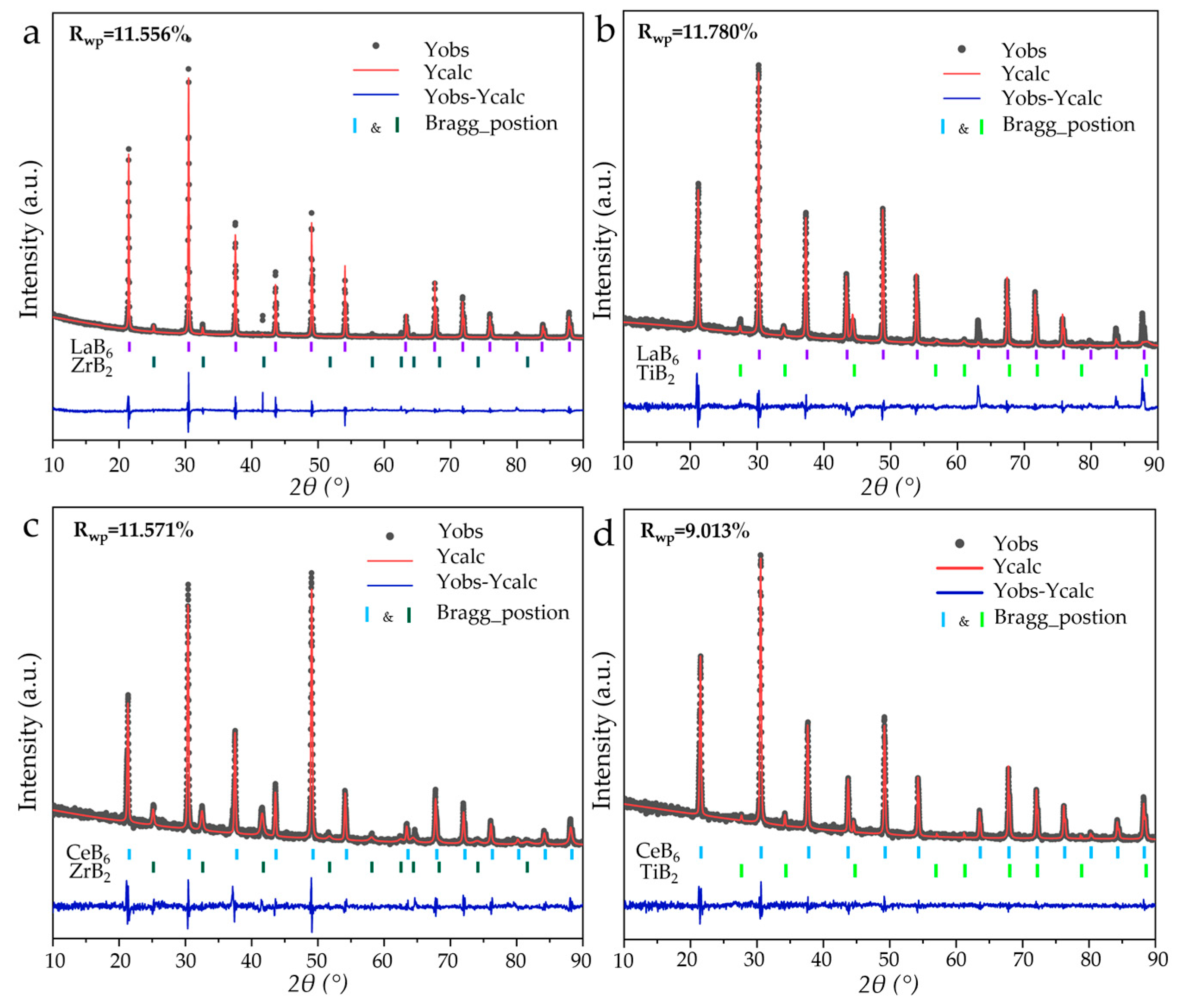
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reich, S.; Suhr, H.; Hankó, K.; Szepes, L. Deposition of Thin Films of Zirconium and Hafnium Boride by Plasma Enhanced Chemical Vapor Deposition. Adv. Mater. 1992, 4, 650–653. [Google Scholar] [CrossRef]
- Çamurlu, H.E.; Maglia, F. Preparation of Nano-Size ZrB2 Powder by Self-Propagating High-Temperature Synthesis. J. Eur. Ceram. Soc. 2009, 29, 1501–1506. [Google Scholar] [CrossRef]
- Kelly, J.P.; Kanakala, R.; Graeve, O.A. A Solvothermal Approach for the Preparation of Nanostructured Carbide and Boride Ultra-High-Temperature Ceramics. J. Am. Ceram. Soc. 2010, 93, 3035–3038. [Google Scholar] [CrossRef]
- Barış, M.; Şimşek, T.; Gökmeşe, H.; Akkurt, A. Characterization of W2B Nanocrystals Synthesized by Mechanochemical Method. J. Boron 2016, 1, 45–51. [Google Scholar]
- Hwang, Y.; Lee, J.K. Preparation of TiB2 Powders by Mechanical Alloying. Mater. Lett. 2002, 54, 1–7. [Google Scholar] [CrossRef]
- Qiu, H.Y.; Guo, W.M.; Zou, J.; Zhang, G.J. ZrB2 Powders Prepared by Boro/Carbothermal Reduction of ZrO2: The Effects of Carbon Source and Reaction Atmosphere. Powder Technol. 2012, 217, 462–466. [Google Scholar] [CrossRef]
- Aksu, M.; Aydln, E. Nano and Microsized EuB6 via Magnesiothermic Reduction. J. Ceram. Process Res. 2013, 14, 4–7. [Google Scholar] [CrossRef]
- Liu, B.; Chen, S.L.; Ma, M.D.; Pan, Y.L.; Zhao, Z.S.; Zhou, H.; Sun, L.; Chen, S.; Guo, X.G.; Tong, K.; et al. Producing High Performance TiB2 Ceramics by High-Pressure Sintering Forging. Ceram. Int. 2025, 51, 20116–20123. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.Y.; Ning, S.Y.; Wang, Z.H.; Xu, B.; Zhang, J.X. Preparation, Characterization and Properties of La0.6Ce0.3Pr0.1B6-ZrB2 Directionally Solidified Eutectic Grown via the Optical Floating Zone Technique. J. Alloys Compd. 2020, 818, 152924. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Y.; Luo, S.F.; Han, C.L.; Yang, X.Y.; Zhang, J.X. Spark Plasma Sintering of Polycrystalline La0.6Ce0.3Pr0.1B6-ZrB2 Composites. J. Am. Ceram. Soc. 2023, 106, 2118–2129. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zhao, G.; Tang, J.; Zhou, O.; Qin, L.C. Single-Crystalline GdB6 Nanowire Field Emitters. J. Am. Chem. Soc. 2005, 127, 13120–13121. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Meucci, M.; Zoli, L.; Sciti, D. Lanthanum Hexaboride for Solar Energy Applications. Sci. Rep. 2017, 7, 718. [Google Scholar] [CrossRef]
- Cahill, J.T.; Graeve, O.A. Hexaborides: A Review of Structure, Synthesis and Processing. J. Mater. Res. Technol. 2019, 8, 6321–6335. [Google Scholar] [CrossRef]
- Plankovsky, S. Review of LaB6, Re-W Dispenser and BaHfO3-W Cathode Development. IEEE Trans. Electron. Devices 2009, 56, 760–765. [Google Scholar] [CrossRef]
- Lafferty, J. Boride Cathodes. J. Appl. Phys. 1951, 22, 299–309. [Google Scholar] [CrossRef]
- Bellucci, A.; Mastellone, M.; Girolami, M.; Serpente, V.; Generosi, A.; Paci, B.; Mezzi, A.; Kaciulis, S.; Carducci, R.; Polini, R.; et al. Nanocrystalline Lanthanum Boride Thin Films by Femtosecond Pulsed Laser Deposition as Efficient Emitters in Hybrid Thermionic-Photovoltaic Energy Converters. Appl. Surf. Sci. 2020, 513, 145829. [Google Scholar] [CrossRef]
- Bellucci, A.; Mastellone, M.; Girolami, M.; Serpente, V.; Sani, E.; Sciti, D.; Trucchi, D. Thermionic Emission Measurement of Sintered Lanthanum Hexaboride Discs and Modelling of Their Solar Energy Conversion Performance. Ceram. Int. 2021, 47, 20736–20739. [Google Scholar] [CrossRef]
- Aono, M.; Nishitani, R.; Oshima, C.; Tanaka, T.; Bannai, E.; Kawai, S. Direct Observation of LaB6 (001) Surface at High Temperatures by x-Ray and Ultraviolet Photoelectron Spectroscopy, Low-Energy Electron Diffraction, Auger Electron Spectroscopy, and Work-Function Measurements. J. Appl. Phys. 1979, 50, 4802–4807. [Google Scholar] [CrossRef]
- Ning, S.; Iitaka, T.; Yang, X.; Wang, Y.; Zhao, J.; Li, Z.; Zhang, J. Enhanced Thermionic Emission Performance of LaB6 by Ce Doping. J. Alloys Compd. 2018, 760, 1–5. [Google Scholar] [CrossRef]
- Ağaoğulları, D.; Duman, İ.; Öveçoğlu, M.L. Synthesis of LaB6 Powders from La2O3, B2O3 and Mg Blends via a Mechanochemical Route. Ceram. Int. 2012, 38, 6203–6214. [Google Scholar] [CrossRef]
- Fan, Q.; Zhao, Y.; Li, D. Synthesis of Single-Crystalline Lanthanum Hexaboride Nanowires by Au Catalyst. Ceram. Int. 2013, 39, 6271–6275. [Google Scholar] [CrossRef]
- Loboda, P. Structure and Properties of Reinforced Ceramic Materials Produced by Directional Solidification. Powder Metall. Met. Ceram. 2018, 57, 13–26. [Google Scholar] [CrossRef]
- Kusunoki, T.; Hashizume, T.; Kasuya, K.; Arai, N. Stabilization of Cold-Field-Emission Current from a CeB6 Single-Crystal Emitter by Using a Faceted (100) Plane. J. Vac. Sci. Technol. B 2020, 39, 013202. [Google Scholar] [CrossRef]
- Dub, S.; Kislaya, G.; Loboda, P. Study of Mechanical Properties of LaB6 Single Crystal by Nanoindentation. J. Superhard Mater. 2013, 35, 158–165. [Google Scholar] [CrossRef]
- Demirskyi, D.; Suzuki, T.S.; Yoshimi, K.; Vasylkiv, O.O. Consolidation and High-temperature Strength of Monolithic Lanthanum Hexaboride. J. Am. Ceram. Soc. 2022, 105, 4277–4290. [Google Scholar] [CrossRef]
- Sonber, J.K.; Sairam, K.; Murthy, T.S.R.C.; Nagaraj, A.; Subramanian, C.; Hubli, R.C. Synthesis, Densification and Oxidation Study of Lanthanum Hexaboride. J. Eur. Ceram. Soc. 2014, 34, 1155–1160. [Google Scholar] [CrossRef]
- Xu, B.; Yang, X.Y.; Cheng, H.F.; Zhao, J.J.; Wang, Y.; Zhu, E.; Zhang, J.X. Preparation, Characterization and Property of High-Quality LaB6 Single Crystal Grown by the Optical Floating Zone Melting Technique. Vacuum 2019, 168, 108845. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zhang, J.X.; Liu, D.M.; Lin, Z.L.; Huang, Q.; Bao, L.H.; Ma, R.; Wei, Y. Synthesis and Properties of Nanostructured Dense LaB6 Cathodes by Arc Plasma and Reactive Spark Plasma Sintering. Acta Mate 2010, 58, 4978–4985. [Google Scholar] [CrossRef]
- Bogomol, I.; Nishimura, T.; Vasylkiv, O.; Sakka, Y.; Loboda, P. High-Temperature Strength of Directionally Reinforced LaB6-TiB2 Composite. J. Alloys Compd. 2010, 505, 130–134. [Google Scholar] [CrossRef]
- Guo, X.G.; Liu, A.; Hao, L.J.; Zhou, H.; Chen, S.; Ying, P.; Liu, B.; Li, B.Z.; Gao, Y.F.; Zhao, Z.S.; et al. Ultrafine-Grained LaB6–ZrB2 Composite Ceramics with Superior Mechanical and Thermionic Emission Properties Prepared by High-Pressure Sintering. J. Adv. Ceram. 2024, 13, 2021–2029. [Google Scholar] [CrossRef]
- Paderno, Y.; Paderno, V.; Filippov, V. Some Peculiarities of Structure Formation in Eutectic D-and f-Transition Metals Boride Alloys. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 1991; Volume 231, pp. 561–569. [Google Scholar]
- Yang, X.Y.; Wang, P.; Wang, Z.H.; Hu, K.; Cheng, H.F.; Li, Z.; Zhang, J.X. Microstructure, Mechanical and Thermionic Emission Properties of a Directionally Solidified LaB6-VB2 Eutectic Composite. Mater. Des. 2017, 133, 299–306. [Google Scholar] [CrossRef]
- Paderno, Y.; Paderno, V.; Filippov, V. Some Peculiarities of Eutectic Crystallization of LaB6-(Ti, Zr)B2 Alloys. J. Solid. State Chem. 2000, 154, 165–167. [Google Scholar] [CrossRef]
- Min, G.H.; Gao, R.L.; Yu, H.S.; Han, J.D. Mechanical Properties of LaB6-ZrB2 Composites. Key Eng. Mater. 2005, 297–300, 1630–1638. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, J.; Gong, H.; Zou, F.; Guo, Y.; Luo, H. Synthesis, Characterization and Formation Mechanism of Ta-W Nanocrystalline Composite Powders Fabricated by Nano-In Situ Composite Method. RSC Adv. 2016, 6, 106011–106018. [Google Scholar] [CrossRef]
- Deng, X.C.; Zhang, G.H.; Zhu, Z.G. In-Situ Preparation of WC-Co Composite Powders. Int. J. Refract. Met. Hard Mater. 2025, 128, 106995. [Google Scholar] [CrossRef]
- Naren, G.; Ren, T.Y.; Bao, L.H.; Chao, L.M.; Tegus, O. Preparation and Optical Absorption Properties of Ternary Rare Earth Boride LaxPr1-xB6 Submicron Powders. J. Nanosci. Nanotechnol. 2020, 20, 5064–5069. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Y.; Yang, Z.; Shi, L.; Ma, J.; Qian, Y. Preparation and Some Properties of Nanocrystalline ZrB2 Powders. Scr. Mater. 2004, 50, 959–961. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Qian, Y.; Shi, L.; Yang, Z.; Ma, J. A Facile One-Step Route to Nanocrystalline TiB2 Powders. Mater. Res. Bull. 2004, 39, 609–613. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Shi, L.; Yang, Z.; Ma, J.; Qian, Y. Synthesis and Oxidation of Nanocrystalline HfB2. J. Alloys Compd. 2004, 368, 353–356. [Google Scholar] [CrossRef]
- Bates, S.E.; Buhro, W.E.; Frey, C.A.; Sastry, S.M.; Kelton, K. Synthesis of Titanium Boride (TiB)2 Nanocrystallites by Solution-Phase Processing. J. Mater. Res. 1995, 10, 2599–2612. [Google Scholar] [CrossRef]
- Ma, M.D.; Yang, X.Y.; Meng, H.; Zhao, Z.S.; He, J.L.; Chu, Y.H. Nanocrystalline High-Entropy Hexaboride Ceramics Enable Remarkable Performance as Thermionic Emission Cathodes. Fundam. Res. 2023, 3, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, A.; Sanderson, R. Oxidation and Thermal Decomposition of Sodium and Potassium Borohydrides. J. Inorg. Nucl. Chem. 1957, 4, 230–231. [Google Scholar] [CrossRef]
- Zoli, L.; Galizia, P.; Silvestroni, L.; Sciti, D. Synthesis of Group IV and V Metal Diboride Nanocrystals via Borothermal Reduction with Sodium Borohydride. J. Am. Ceram. Soc. 2018, 101, 2627–2637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, L.; Zhou, H.; Xu, J.; Liu, A.; Ma, M.; Sun, R.; Qin, W.; Gao, Y.; Liu, B.; et al. In Situ Synthesis of RMB6-TMB2 Composite Nanopowders via One-Step Solid-State Reduction. Nanomaterials 2025, 15, 1341. https://doi.org/10.3390/nano15171341
Guo X, Wang L, Zhou H, Xu J, Liu A, Ma M, Sun R, Qin W, Gao Y, Liu B, et al. In Situ Synthesis of RMB6-TMB2 Composite Nanopowders via One-Step Solid-State Reduction. Nanomaterials. 2025; 15(17):1341. https://doi.org/10.3390/nano15171341
Chicago/Turabian StyleGuo, Xiaogang, Linyan Wang, Hang Zhou, Jun Xu, An Liu, Mengdong Ma, Rongxin Sun, Weidong Qin, Yufei Gao, Bing Liu, and et al. 2025. "In Situ Synthesis of RMB6-TMB2 Composite Nanopowders via One-Step Solid-State Reduction" Nanomaterials 15, no. 17: 1341. https://doi.org/10.3390/nano15171341
APA StyleGuo, X., Wang, L., Zhou, H., Xu, J., Liu, A., Ma, M., Sun, R., Qin, W., Gao, Y., Liu, B., Li, B., Sun, L., & Yu, D. (2025). In Situ Synthesis of RMB6-TMB2 Composite Nanopowders via One-Step Solid-State Reduction. Nanomaterials, 15(17), 1341. https://doi.org/10.3390/nano15171341






