A Mean Field Poisson–Boltzmann Theory Assessment of Copper Oxide Nanosheets Interaction Potential in Physiological Fluids
Abstract
1. Introduction
2. Backgrounds
3. Theoretical Foundation
3.1. Mean Field Electrostatic Theory
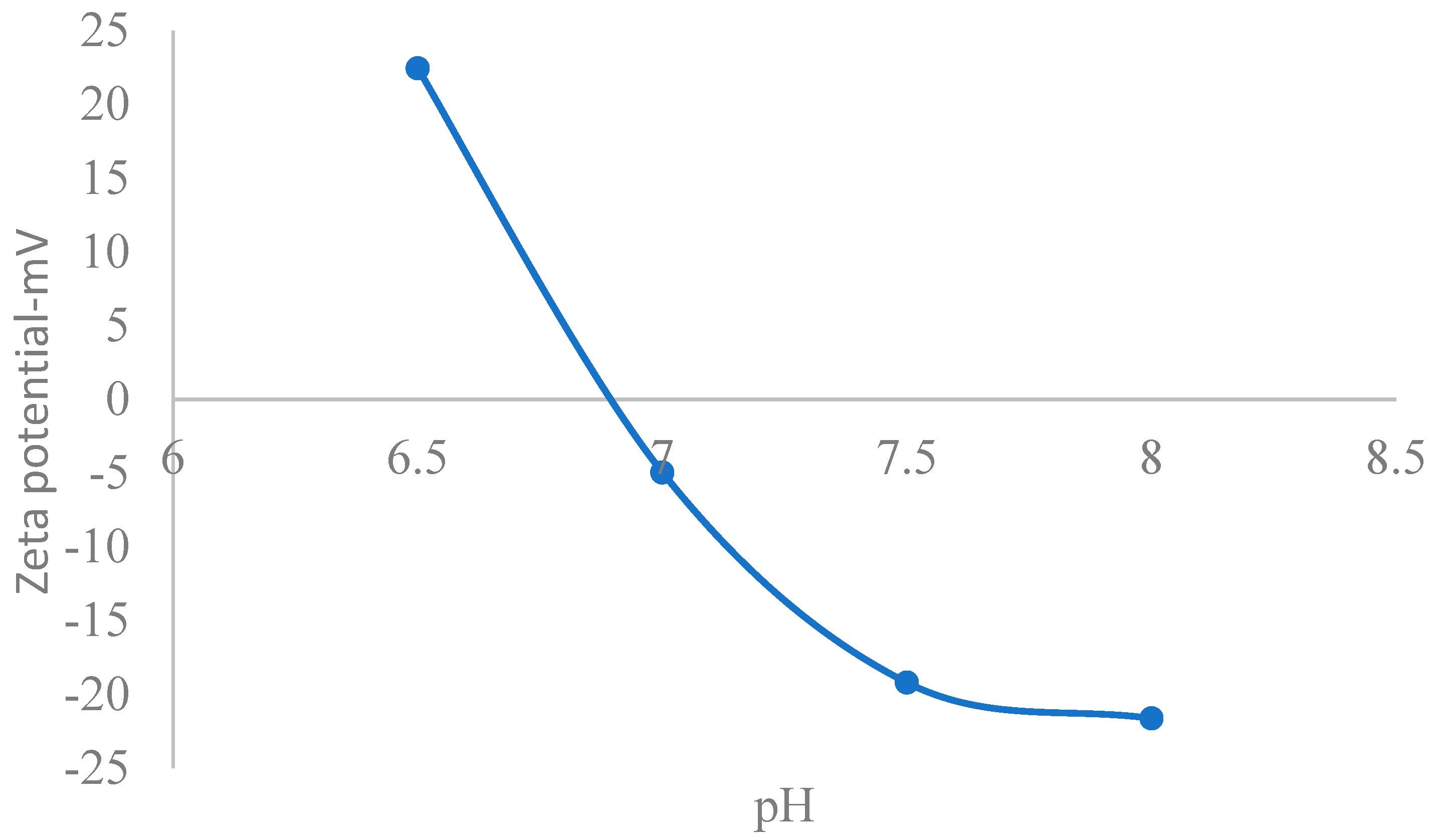
3.1.1. Zeta Potential Model
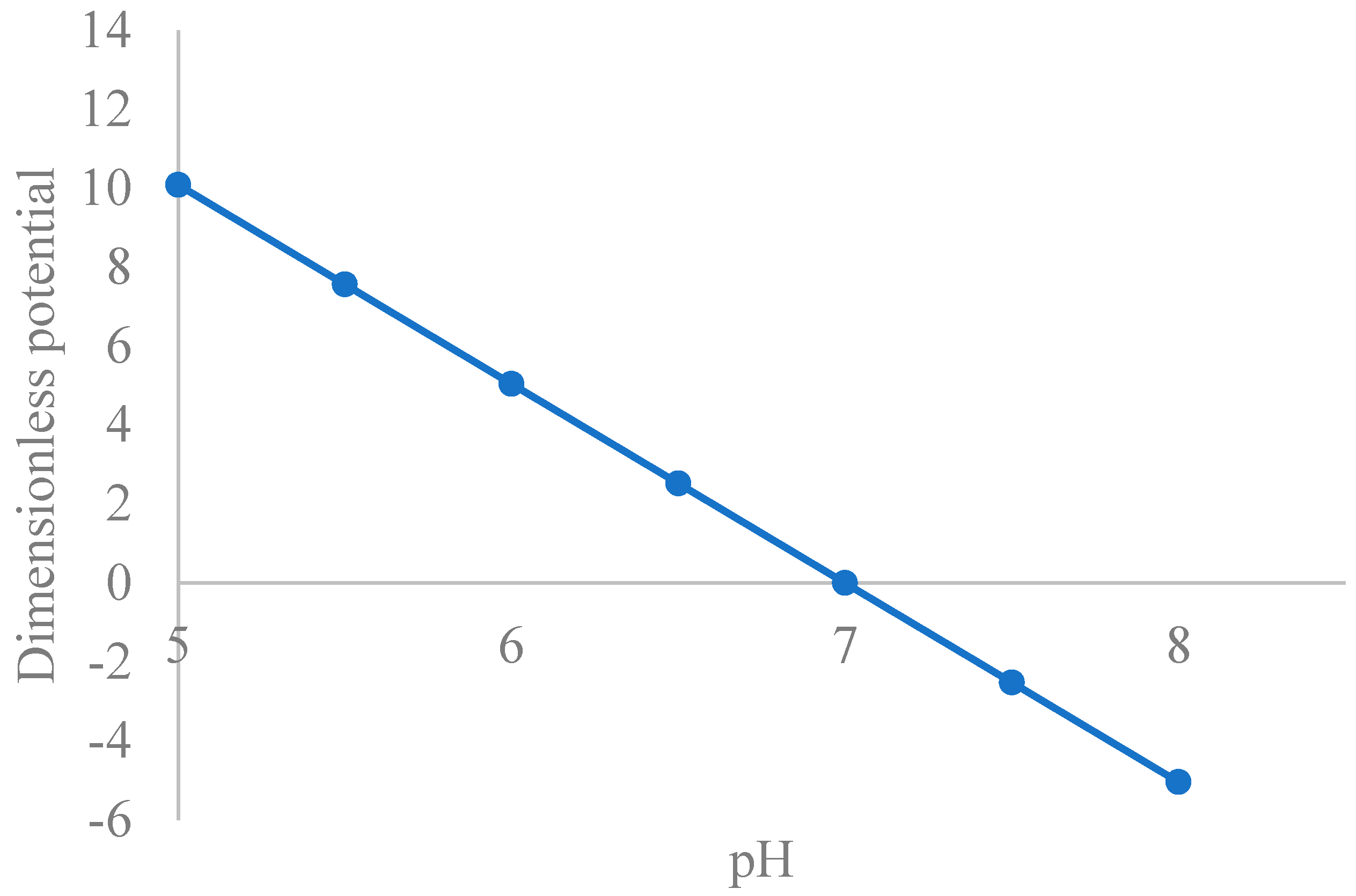
3.1.2. Zeta Potential Dependence on pH
3.1.3. Surface Charge Density Model
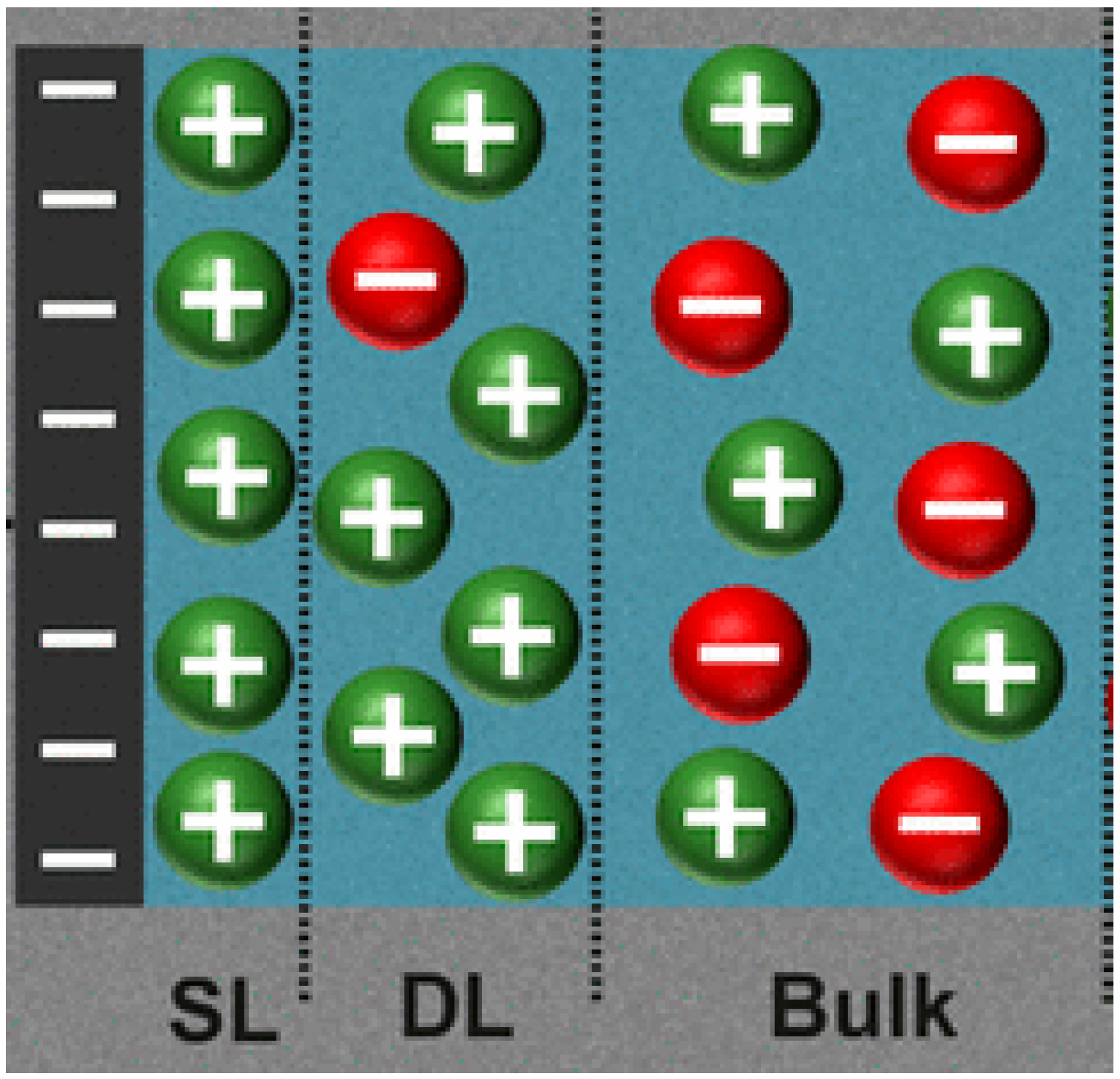
3.1.4. Double Layer Repulsion Model
4. Methodology
4.1. Dielectric Permittivity
4.2. Debye Length
4.3. Calculation of Double Layer Repulsion
4.4. Assumption for Zeta Potential Calculations
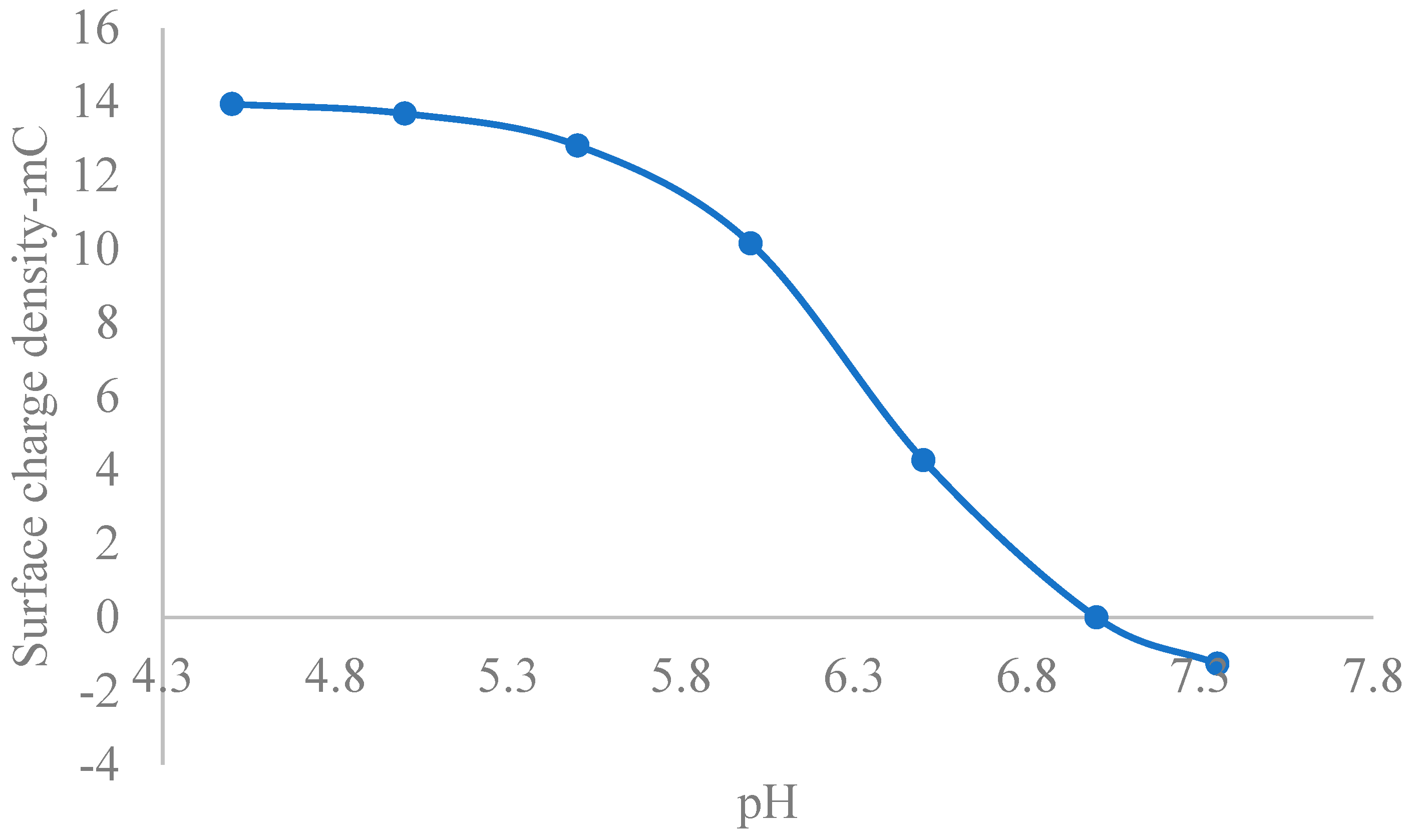
4.5. Point of Zero Charge pH of CONS
5. Results and Discussion
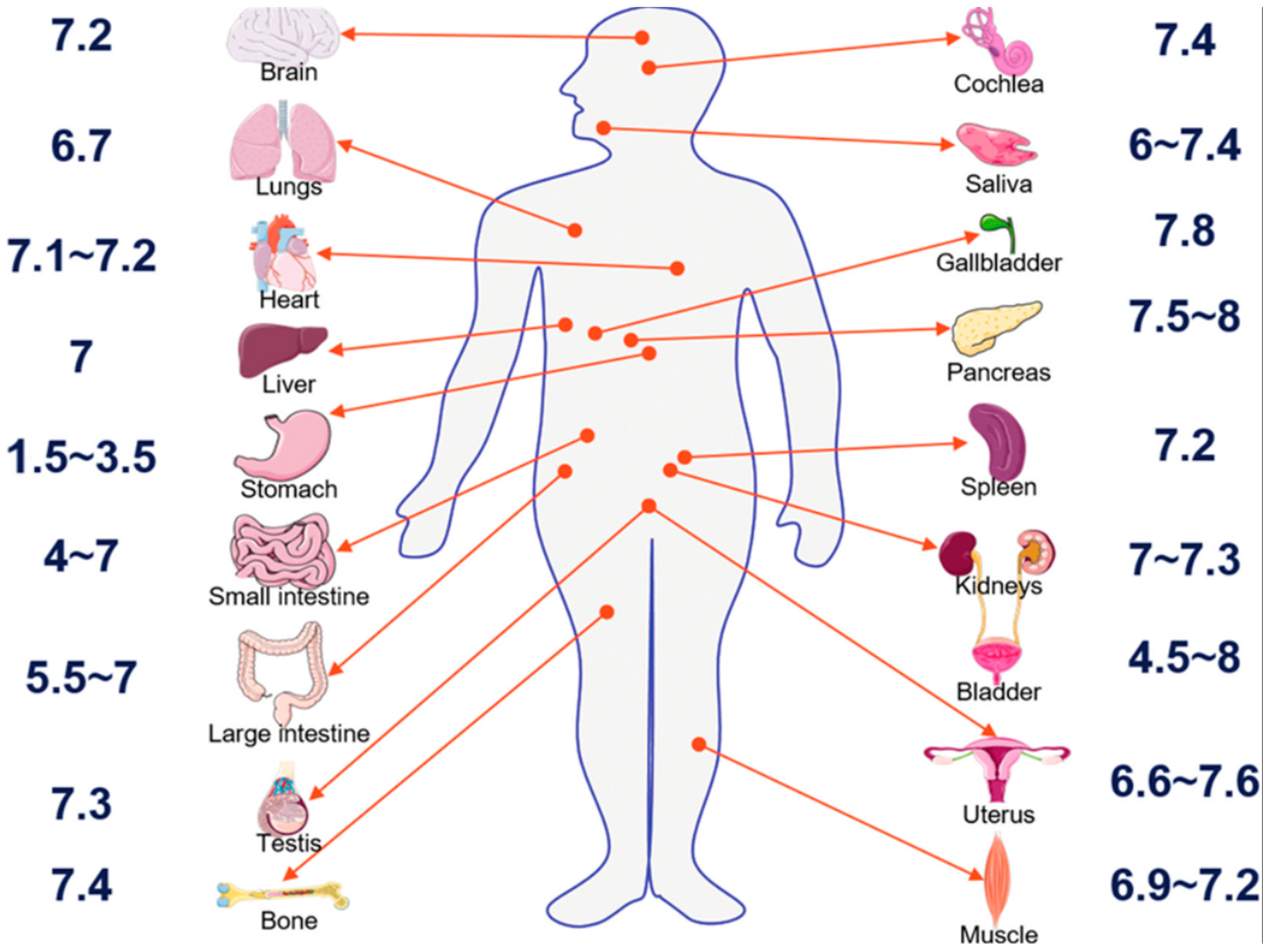
5.1. Theoretical Zeta Potential Characteristics of Extracellular Fluids
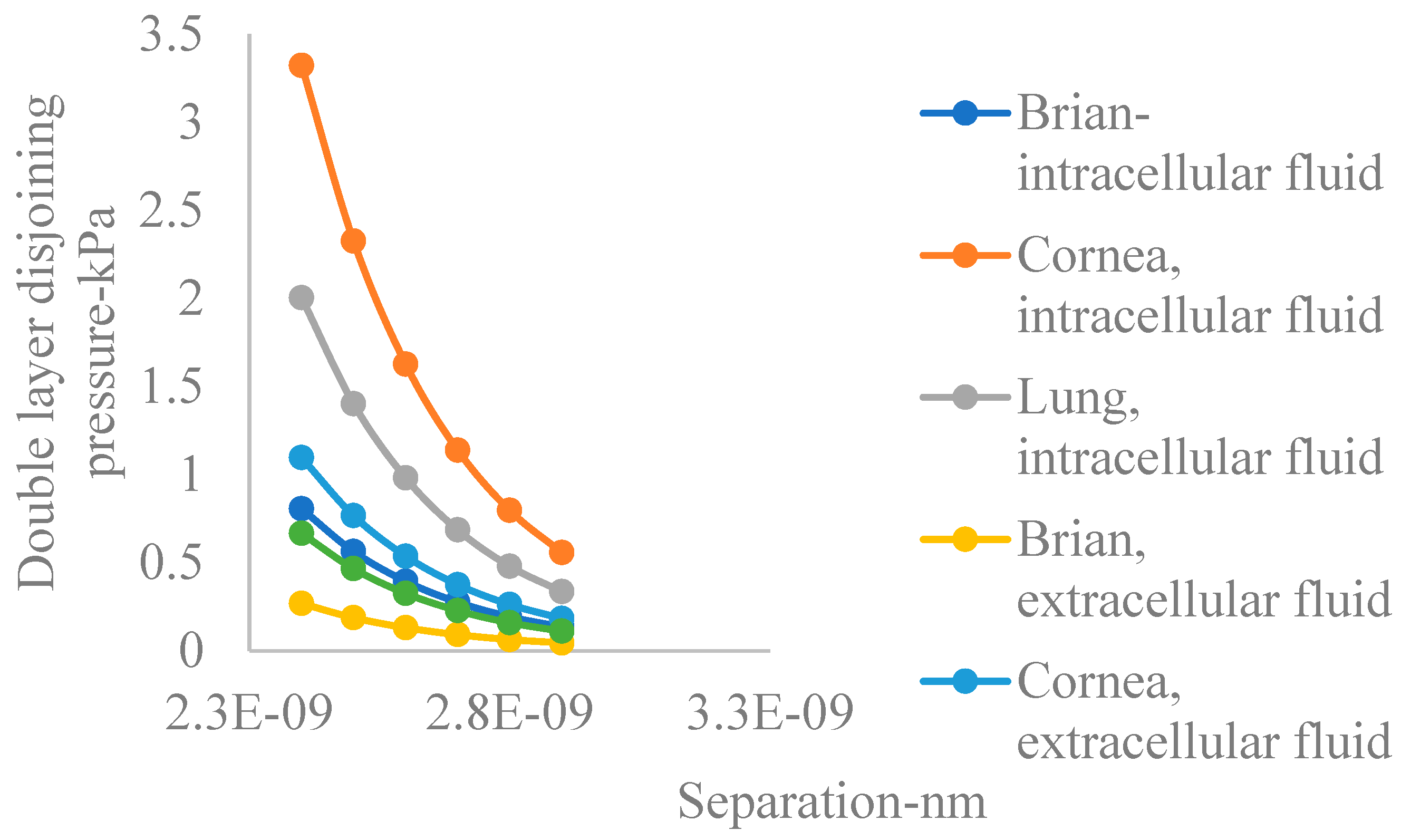
5.2. Stability of CONSs in Extracellular Fluids
5.3. Cellular Uptake
5.4. Implications of the Study for Bioassay
5.5. Clinical Implications for the Theoretical Findings
6. Summary
7. Conclusions
- Based on the NLMFPB electrostatic theory assessment of CONSs in the intracellular fluid environment, they have negative values of zeta potential under physiological pH regimes,
- Values of zeta potential under physiological pH implies less electrostatic repulsion, which translates to short term stability of CONSs in physiological fluids,
- Comparing the analytical predictions of zeta potential within physiological pH values obtained in this study to those in literature related to toxicity, CONSs will be non-toxic from the point of view of electrostatic interactions only,
- Electric double layer disjoining pressure is generally higher in intercellular fluids compared to extracellular fluids for the organs studied.
- On the average, and within intracellular fluids, the lung has the highest double layer disjoining pressure, followed by the brain, with the cornea having the lowest.
- In intracellular fluids, the cornea has the highest double layer disjoining pressure, followed by the lung, with the brain having the lowest.
- The trend revealed by conclusion number 4 also applies in the case of extracellular fluid.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBE | Poisson–Boltzmann Equation |
| MFPB | Mean Field Poisson–Boltzmann |
| NMFPB | Nonlinear Mean Field Poisson–Boltzmann |
| CONSs | Copper Oxide Nanosheets |
| EDL | Electric Double Layer |
Appendix A
Appendix B
| Constsnt | Value | Unit | |
|---|---|---|---|
| kb | Boltzmann constant | 1.38 × 10−23 | JK−1 |
| e | Electronic charge | 1.602 × 10−19 | C |
| E0 | permitivity of vacuo | 8.854 × 10−12 | Fm−1 |
| NA | Avogadro’s number | 6.022 × 1023 | Mol−1 |
References
- Yuan, G.; Yu, S.; Jie, C.; Wang, C.; Li, Q.; Pang, H. Cu/Cu2O nanostructures derived from copper oxalate as high performance electrocatalyst for glucose oxidation. Chin. Chem. Lett. 2020, 31, 1941–1945. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A review on electrochemical synthesized copper-based catalysts for electrochemical reduction of CO2 to C2+ products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, M.; Wang, Y.; Chen, P.R.; Liu, H. Facile Synthesis of CuO Nanosheets and Efficient Degradation of Rhodamine B in a Copper Oxide/Ascorbic Acid/Hydrogen Peroxide System: Kinetics, Fate of Ascorbic Acid, and Mechanism. ChemistrySelect 2020, 5, 6075–6082. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Sun, H.; Guo, X.; Xu, J.; Zhang, H.; Zhang, X. In situ Growth of Cu2O/CuO Nanosheets on Cu Coating Carbon Cloths as a Binder-Free Electrode for Asymmetric Supercapacitors. Front. Chem. 2019, 7, 420. [Google Scholar] [CrossRef]
- Sajid, M.; Qayyum, W.; Qamar, M.A.; Nawaz, H. Progress in the development of copper oxide-based materials for electrochemical water splitting. Int. J. Hydrogen Energy 2024, 62, 209–227. [Google Scholar] [CrossRef]
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agricultur. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
- Shende, S.; Rathod, D.; Gade, A.; Rai, M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotechnol. 2017, 11, 773–781. [Google Scholar] [CrossRef]
- Biskos, G.; Schmidt-Ott, A. Airborne Engineered Nanoparticles: Potential Risks and Monitoring Challenges for Assessing their Impacts on Children. Paediatr. Respir. Rev. 2012, 13, 79–83. [Google Scholar] [CrossRef]
- Nazarenko, Y.; Zhen, H.; Han, T.; Lioy, P.J.; Mainelis, G. Potential for Inhalation Exposure to Engineered Nanoparticles from Nanotechnology-Based Cosmetic Powders. Environ. Health Perspect. 2012, 120, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public. Health 2023, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandstrom, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef]
- Miller, M.R.; Shaw, C.A.; Langrish, J.P. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012, 8, 577–602. [Google Scholar] [CrossRef]
- Radiom, M.; Sarkis, O.M.; Brookes, E.; Oikonomou, K.; Baeza-Squiban, A.; Berret, J.F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cell. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Li, X.L.M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Ceña, V.; Játiva, P. Nanoparticle Crossing of Blood–Brain Barrier: A Road to New Therapeutic Approaches to Central Nervous. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Varenne, F.; Coty, J.-B.; Botton, J.; Legrand, F.-X.; Hillaireau, H.; Barratt, G.; Vauthier, C. Evaluation of zeta potential of nanomaterials by electrophoretic light scattering: Fast field reversal versus Slow field reversal modes. Talanta 2019, 205, 120062. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wan, Y.-J.; Wang, X.; Li, Y.; Yang, W.; Xu, C.W.S.Q. Sensitive bioassay for detection of PPARα potentially hazardous ligands with gold nanoparticle probe. J. Hazard. Mater. 2011, 192, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar]
- Sharma, S.; Tripathi, G. Toxicity Bioassay of Silver Nanoparticle Using a Piscine Model. J. Exp. Zool. India 2018, 2, 787–791. [Google Scholar]
- Vass, I.Z.; Deák, K.P.Z.; Kovács, S.; Vass, I. Interaction of nanoparticles with biological systems. Acta Biol. Szeged. 2015, 59, 225–245. [Google Scholar]
- Eisenberg, S.; Haimov, E.; Walpole, G.F.W.; Plumb, J.; Kozlov, M.M.; Grinstein, S. Mapping the electrostatic profiles of cellular membranes. Mol. Biol. Cell 2021, 32, 301–310. [Google Scholar] [CrossRef]
- Kittaka, S.; Morimoto, T. Isoelectric point of metal oxides and binary metal oxides having spinel structure. J. Colloid Interface Sci. 1980, 75, 398–403. [Google Scholar] [CrossRef]
- Stamatatos, L.; Leventis, R.; Zuckermann, M.J.; Silvius, J.R. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry 1988, 27, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, Y. Estimation of Nanodiamond Surface Charge Density from Zeta Potential and Molecular Dynamics Simulations. J. Phys. Chem. B 2017, 121, 3394–3402. [Google Scholar]
- Nagy, A.; Steinbruck, A.; Gao, J.; Doggett, N.; Hollingsworth, J.A.; Iyer, R. Comprehensive, Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano 2012, 6, 4748–4762. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Hamidi, M.; Makila, E.M.; Zhang, H.B.; Almeida, P.V.; Kaasalainen, M. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials 2013, 34, 7776–7789. [Google Scholar] [CrossRef]
- Gray, C.G.; Stiles, P.J. Nonlinear electrostatics: The Poisson–Boltzmann equation. Eur. J. Phys. 2018, 39, 053002. [Google Scholar] [CrossRef]
- Yike, H.; Xiaohong, L.; Tianying, L.S.Y. Development of mean-field electrical double layer theory. Chin. Physics B 2016, 25, 016801. [Google Scholar]
- Bohinc, K.; Bossa, G.V.; May, S. Incorporation of ion and solvent structure into mean-field modeling of the electric double layer. Adv. Colloid. Interface Sci. 2017, 249, 220–233. [Google Scholar] [CrossRef]
- Ben-Yaakov, D.; Andelman, D. Revisiting the Poisson–Boltzmann theory: Charge surfaces, multivalent ions and inter-plate forces. Phys. A Stat. Mech. Its Appl. 2010, 389, 2956–2961. [Google Scholar] [CrossRef]
- Panjukov, V. The surface charge density/surface potential relationship and the Poisson–Boltzmann equation. J. Colloid. Interface Sci. 1986, 110, 556–560. [Google Scholar] [CrossRef]
- Oberdörster, G.; Elder, A.; Rinderknecht, A. Nanoparticles and the brain: Cause for concern? J. Nanosci. Nanotechnol. 2009, 9, 4996–5007. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Darroudi, M.; Kazemi, O.R.; Gholami, L.; Khorsand, Z.A. Honey-based synthesis of ZnO nanopowders and their cytotoxicity effects. Adv. Powder Technol. 2015, 26, 991–996. [Google Scholar] [CrossRef]
- Gnatenko, Y.; Bukivskij, P.; Gamernyk, R.; Yevdokymenko, V.; Opanasyuk, A.; Bukivskii, A.; Furyer, M.; Tarakhan, L. Study of optical and photoelectric properties of copper oxide films. Mater. Chem. Phys. 2023, 370, 128175. [Google Scholar] [CrossRef]
- Fu, Q.; Li, W.; Kruis, F.E. Highly conductive copper films prepared by multilayer sintering of nanoparticles synthesized via arc discharge. Nanotechnology 2023, 34, 225601. [Google Scholar] [CrossRef]
- Thambidurai, C.; Jayaraju, N.; Kim, Y.-G.; Stickney, J.L. Copper Nano Film Formation Using Electrochemical ALD. ECS Trans. 2007, 11, 103. [Google Scholar] [CrossRef]
- Das, A.; Kushwaha, A.K.; Bansal, N.R.; Suresh, V.; Dinda, S.; Chattopadhyay, S.; Dalapati, G. Copper Oxide Nano-Particles Film On Glass By Using Sputter And Chemical Bath Deposition Technique. Adv. Mater. Lett. 2016, 7, 600–603. [Google Scholar] [CrossRef]
- Molero, M.; Outhwaite, C.W.; Bhuiyan, L.B. Application of the symmetric Poisson–Boltzmann theory to equilibrium thermodynamic properties of primitive model electrolyte mixtures. J. Mol. Liq. 2023, 390, 123025. [Google Scholar] [CrossRef]
- Levine, P. The solution of a modified Poisson—Boltzmann equation for colloidal particles in electrolyte solutions. J. Colloid. Interface Sci. 1975, 51, 72–86. [Google Scholar] [CrossRef]
- Bossa, G.V.; May, S. Debye-Hückel Free Energy of an Electric Double Layer with Discrete Charges Located at a Dielectric Interface. Membranes 2021, 11, 129. [Google Scholar] [CrossRef]
- Ruiz-Cabell, F.J.M.; Trefalt, G.; Maroni, P.; Borkovec, M. Accurate Predictions of Forces in the Presence of Multivalent Ions by Poisson–Boltzmann Theory. Langmuir 2014, 30, 4551–4555. [Google Scholar] [CrossRef]
- Carnie, S.L.; Chan, D.Y. Interaction Free Energy between Identical Spherical Colloidal Particles: The Linearized Poisson–Boltzmann Theory. J. Colloid. Interface Sci. 1993, 155, 297–312. [Google Scholar] [CrossRef]
- Su, M.; Xu, Z.; Wang, Y. Poisson–Boltzmann theory with non-linear ion correlations. J. Phys. Condens. Matter 2019, 31, 355101. [Google Scholar] [CrossRef]
- Fogolari, F.; Brigo, A.; Molinari, H. The Poisson–Boltzmann equation for biomolecular electrostatics: A tool for structural biology. J. Mol. Recognit. 2002, 15, 3773–3792. [Google Scholar] [CrossRef]
- Sun, S.; Poudel, P.; Alexov, E.; Li, L. Electrostatics in Computational Biophysics and Its Implications for Disease Effects. Int. J. Mol. Sci. 2022, 23, 10347. [Google Scholar] [CrossRef] [PubMed]
- Orucoglu, E.; Grangeon, S.; Gloter, A.; Robinet, J.-C.; Madé, B.; Tournassat, C. Competitive Adsorption Processes at Clay Mineral Surfaces: A Coupled Experimental and Modeling Approach. ACS Earth Space Chem. 2022, 6, 144–159. [Google Scholar] [CrossRef]
- Sposito, G. The Surface Chemistry of Soils; Clarendo Press: New York, NY, USA; Oxford Unibversity: Oxford, UK, 1984. [Google Scholar]
- Erickson, E. Cation-exchange equilibria on clay minerals. Soil. Sci. 1952, 74, 103–114. [Google Scholar] [CrossRef]
- Davis, K.A.; Gamelli, R.L. Fluids and Electrolytes. In General Surgery; Springer: London, UK, 2009; pp. 13–22. [Google Scholar]
- Tortora, G. Principles of Anatomy and Physiology, 5th ed.; Harper International Edition; Harper & Row: New York, NY, USA, 1987; pp. 61–62. [Google Scholar]
- WHO. Air Quality, Energy and Health. 2025. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-energy-and-health/health-impacts#:~:text=The%20main%20pathway%20of%20exposure,and%20ultimately%20leading%20to%20disease (accessed on 18 May 2025).
- Schradera, A.M.; Sheil, J.I.M.R.; Sheil, R.; Dobbs, H.A.; Keller, T.J.; Li, Y.; Jain, S.; Shella, M.S.; Israelachvili, J.N.; Han, S. Surface chemical heterogeneity modulates silica surface hydration. Proc. Natl. Acad. Sci. USA 2018, 115, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.M.; Skylaris, C.-K.; Green, N.G. Acid-base dissociation mechanisms and energetics at the silica–water interface: An activationless process. J. Colloid. Interface Sci. 2015, 451, 231–244. [Google Scholar] [CrossRef]
- Khademi, M.; Barz, D.P.J. Structure of the Electrical Double Layer Revisited: Electrode Capacitance in Aqueous Solutions. Langmuir 2020, 36, 4250–4260. [Google Scholar] [CrossRef]
- Janusz, W.; Gałgan, A.; Reszka, M. Electrical double layer at the Cu2O/aqueous solution of alkali metal chlorides interface. Physicochem. Probl. Miner. Process. 2006, 40, 161–174. [Google Scholar]
- Melkikh, A.S.M. Model of active transport of ions in cardiac cell. J. Theor. Biol. 2008, 252, 247–254. [Google Scholar] [CrossRef]
- Shkel, I.A.; Tsodikov, O.V.; Record, M.T., Jr. Asymptotic solution of the cylindrical nonlinear Poisson–Boltzmann equation at low salt concentration: Analytic expressions for surface potential and preferential interaction coefficient. Proc. Natl. Acad. Sci. USA 2002, 99, 2597–2602. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Joly, L. The Poisson Boltzman equation in micro and nanofluidics: A formulary. Phys. Fluids 2024, 36, 1–25. [Google Scholar]
- Yee, K.; Ghayesh, M.H. A review on the mechanics of graphene nanoplatelets reinforced structures. Int. J. Eng. Sci. 2023, 186, 2597–2602. [Google Scholar] [CrossRef]
- Sharp, K.A.; Honig, B. Calculating total electrostatics with the nonlinear Poisson-Boltzmann equation. J. Phys. Chem. 1990, 94, 7684–7692. [Google Scholar] [CrossRef]
- Liu, X.; Tian, R.; Tian, R. Analytical solutions of the nonlinear Poisson–Boltzmann equation in mixture of electrolytes. Surf. Sci. 2013, 607, 197–202. [Google Scholar] [CrossRef]
- Kim, D.-S. Measurement of point of zero charge pH of bentonite by solubization technique and its dependence of surface potential on pH. Envion. Eng. Res. 2003, 8, 222–227. [Google Scholar] [CrossRef]
- Tombácz, E. pH-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Mullet, M.; Fievet, P.; Reggiani, J.; Pagetti, J. Surface electrochemical properties of mixed oxide ceramic membranes: Zeta-potential and surface charge density. J. Membr. Sci. 1997, 123, 255–265. [Google Scholar] [CrossRef]
- Ohshima, H. Approximate expressions for the surface charge density/surface potential relationship and double-layer potential distribution for a spherical or cylindrical colloidal particle based on the modified Poisson–Boltzmann equation. Colloid. Polym. Sci. 2018, 296, 647–652. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Takeya, M.; Ubaidah, A.; Shimokawara, M.; Okano, H.; Nawa, T.; Elakneswaran, Y. Crude oil/brine/rock interface in low salinity waterflooding: Experiments, triple-layer surface complexation model, and DLVO theory. J. Colloid. Interface Sci. 2020, 188, 106913. [Google Scholar] [CrossRef]
- Mitchell, H.; Hamilton, T.; Steggerda, F.; Bean, H. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 158, 625–637. [Google Scholar] [CrossRef]
- Watanabe, T. Chapter 4—The Cell. In Biophysical Basis of Physiology and Calcium Signaling Mechanism in Cardiac and Smooth Muscle; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–137. [Google Scholar]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H. Anatomy and Physiology, 2nd ed.; OpenStax: Houston, TX, USA, 2023. [Google Scholar]
- Hall, J.E.; Guyton, A.C. Textbook of Medical Physiology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Sogryn, A. Equations for Calculating the Dielectric Constant of Saline Water (Correspondence). IEEE Trans. Microw. Theory Techbiques 1971, 19, 733–736. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J. Chapter 2. Fundamentals of Interface and Colloid Science. In Studies in Interface Science; Elsevier: Amsterdam, The Netherlands, 2010; Chapter 2; pp. 21–89. [Google Scholar]
- Solomon, T. The Definition and Unit of Ionic Strength. J. Chem. Educ. 2001, 78, 1691. [Google Scholar] [CrossRef]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef]
- Uematsu, Y. Ion adsorption and zeta potential of hydrophobic interfaces. In Encyclopedia of Solid-Liquid Interfaces; Elsevier: Amsterdam, The Netherlands, 2024; pp. 519–529. [Google Scholar]
- Glover, P.W.J.; Walker, E.; Jackson, M.D. Streaming-potential coefficient of reservoir rock: A theoretical model. Gophysics 2012, 77, D17–D43. [Google Scholar] [CrossRef]
- Hughes, M.P. The cellular zeta potential: Cell electrophysiology beyond the membrane. Integr. Biol. 2024, 16, zyae003. [Google Scholar] [CrossRef]
- Revil, A.; Glover, P.W.J. Theory of ionic surface electrical conduction in porous media. Phys. Rev. B 1997, 55, 1757–1773. [Google Scholar] [CrossRef]
- Israellashvili, J.N. Intehrmolecular and Surfaces Forces; Academic Press: London, UK, 1992. [Google Scholar]
- Herbowski, L.; Gurgul, H.; Staron, W. Experimental determination of the Stern layer thickness at the interface of the human arachnoid membrane and the cerebrospinal fluid. Z. Med. Phys. 2009, 19, 189–192. [Google Scholar] [CrossRef]
- Diamond, A.; Lye, C.T.; Prasad, D.; Abbott, D. One size does not fit all: Assuming the same normal body temperature for everyone is not justified. PLoS ONE 2021, 16, e0245257. [Google Scholar]
- Bene, V.E.D. Chapter 218: Temperature. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworth: Boston, MA, USA, 1990. [Google Scholar]
- Van, M.J.; Kaspers, G.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. Adv. Colloid Interface Sci. 2018, 319, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Kanan, S.M.; Tripp, C.P. Synthesis of high surface area monoclinic WO3 particles using organic ligands and emulsion based methods. J. Mater. Chem. 2002, 12, 983–989. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, D.; Jiang, H.; Zhao, Y.; Sun, Y.; Li, Z.; Yang, M.; Wang, P.; Ma, J. Peroxymonosulfate-based cleaning technology for metal oxide-coated ceramic ultrafiltration membrane polluted by Alcian Blue 8GX dye: Radical and non-radical Radical and non-radical oxidation cleaning mechanism. J. Membr. Sci. 2019, 573, 210–217. [Google Scholar] [CrossRef]
- Ejeta, B.A.; FikaduAaga, G.; Fereja, W.M.; Mengesha, B. Biofabrication of highly effective and easily regenerated CuO nanoparticles as adsorbents for Congo red and malachite green removal. Sci. Rep. 2024, 14, 1–20. [Google Scholar] [CrossRef]
- Butt, H.; Kapp, M. Physics and Chemistry of Interfaces, 2nd ed.; Wiley-VC: Weinheim, Germany, 2006. [Google Scholar]
- Bibi, H.; Iqbal, M.; Wahab, H.; Öztürk, M.; Ke, F.; Iqbal, Z.; Khan, M.I.; Alghanem, S.M. Green synthesis of multifunctional carbon coated copper oxide nanosheets and their photocatalytic and antibacterial activities. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Song, G.; Hou, W.; Gao, Y.; Wang, Y.; Lin, L.; Zhang, Z.; Niu, Q.; Ma, R.; Mu, L.; Wang, H. Effects of CuO nanoparticles on Lemna minor. Bot. Stud. 2016, 57, 3. [Google Scholar] [CrossRef]
- Russo, P.; Lauria, F.; Siani, A. Regulation of Potassium Homeostasis. In Encyclopedia of Endocrine Diseases, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 552–557. [Google Scholar]
- Večeř, M.; Pospisil, J. Stability and Rheology of Aqueous Suspensions. Procedia Eng. 2012, 42, 1720–1725. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.C.; Tortella, G.R.; Rubilar, O. Extracellular Biosynthesis of Copper and Copper Oxide Nanoparticles byStereum hirsutum, a Native White-Rot Fungus from Chilean Forests. J. Nano 2015, 10, 1–7. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications Ar Cells. Nan. Biotech. 2018, 46, 1–18. [Google Scholar]
- Mendes, L.; Amorim, M.; Scott-Fordsmand, J. Assessing the toxicity of safer by design CuO surface-modifications using. Sci. Total Environ. 2019, 678, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Colombo, G.; Chirico, G.; Capitani, G.; Dalle-Donne, I.; Mantecca, P. Biological mechanism of cell oxidative stress and death during short-term exposure to nano CuO. Sci. Rep. 2023, 13, 1–18. [Google Scholar] [CrossRef]
- Semisch, A.; Ohle, J.; Witt, B.; Hartwig, A. Cytotoxicity and genotoxicity of nano—and microparticulate copper oxide: Role of solubility and intracellular bioavailability. Part. Fibre Toxicol. 2014, 11, 1–16. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Fan, J.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021, 19, 5. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2023, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Human Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Shao, X.; Wei, X.; Song, X.; Hao, L.; Cai, X.; Zhang, Z.; Peng, Q.; Lin, Y. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lattekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Brown, M.A.; Goel, A.; Abbas, Z. Effect of Electrolyte Concentration on the Stern Layer Thickness at a Charged Interface. Angew. Chem. Int. Ed. Engl. 2016, 55, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 2013, 265–273. [Google Scholar]
- Gumustas, M.; Ozkan, S.-T.G.A.S.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tomak, A.; Cesmeli, S.; Hanoglu, B.D.; Winkler, D.; Karakus, C.O. Nanoparticle-protein corona complex: Understanding multiple interactions between environmental factors, corona formation, and biological activity. Nanotoxicology 2021, 15, 1331–1357. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Strauch, B.M.; Niemand, R.K.; Winkelbeiner, N.L.; Hartwig, A. Comparison between micro- and nanosized copper oxide and water soluble copper chloride: Interrelationship between intracellular copper concentrations, oxidative stress and DNA damage response in human lung cells. Part. Fibre Toxicol. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhiev, A.; Avramescu, M.-L.; Wu, D.; Williams, A.; Rasmussen, P.; Halappanavar, S. Impact of copper oxide particle dissolution on lung epithelial cell toxicity: Response characterization using global transcriptional analysis. Nanotoxicology 2021, 15, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Panicker, L.; Barick, K.C. Selective binding of proteins on functional nanoparticles via reverse charge parity model: An in vitro study. Mater. Res. Express 2014, 1, 015017. [Google Scholar] [CrossRef]
- Gupta, G.; Cappellini, F.; Farcal, L.; Gornati, R.; Bernardini, G.; Fadeel, B. Copper oxide nanoparticles trigger macrophage cell death with misfolding of Cu/Zn superoxide dismutase 1 (SOD1). Part. Fibre Toxicol. 2022, 19, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Nandhakumar, S.; Dhanaraju, M.D.; Sundar, V.D.; Heera, B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly(ε-caprolactone) nanoparticles. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 249–258. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Sato, K.-I. Protein pI and Intracellular Localization. Front. Mol. Biosci. Sec. Biophys. 2021, 8, 1–6. [Google Scholar] [CrossRef]
- Aoi, W.; Zou, X.; Xiao, J.B.; Marunaka, Y. Body Fluid pH Balance in Metabolic Health and Possible Benefits of Dietary Alkaline Foods. eFood 2020, 1, 12–23. [Google Scholar] [CrossRef]
- Schwartz, R.; Ting, C.S.; King, J. Whole proteome pI values correlate with subcellular localizations of proteins for organisms within the three domains of life. Genome Res. 2001, 11, 703–709. [Google Scholar] [CrossRef]
- Salvati, A. The biomolecular corona of nanomedicines: Effects on nanomedicine outcomes and emerging opportunities. Curr. Opin. Biotechnol. 2024, 87, 103101. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef]
- Mitchel, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Aliyandi, A.; Reker-Smit, C.; Bron, R.; Zuhorn, I.S.; Salvati, A. Correlating Corona Composition and Cell Uptake to Identify Proteins Affecting Nanoparticle Entry into Endothelial Cells. ACS Biomater. Sci. Eng. 2021, 7, 5573–5584. [Google Scholar] [CrossRef]
- Delay, M.; Frimmel, F. Nanoparticles in aquatic systems. Anal. Bianal. Chem. 2012, 402, 583–592. [Google Scholar] [CrossRef]
- Baalousha, M.; Manciulea, A.; Cumberland, S.; Kendall, K., Jr. Lead Aggregation and surface properties of iron oxide nanoparticles: Influence of pH and natural organic matterEnviron. Toxicol. Chem. 2008, 27, 1875–1882. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Sabat, D.; Singh, S.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of Al2O3 nanoparticles at low exposure levels to a freshwater bacterial isolate. Chem. Res. Toxicol. 2011, 24, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Limbach, L.; Duc, L.V.; Krumeich, F.; Athanassiou, E.; Gerber, L.; Moch, H.; Stark, W. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Anreddy, R.N.R. Copper oxide nanoparticles induces oxidative stress and liver toxicity in rats following oral exposure. Toxicol. Rep. 2018, 5, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Sutunkova, M.P.; Ryabova, Y.V.; Minigalieva, I.A.; Bushueva, T.V.; Sakhautdinova, R.R.; Bereza, I.A.; Shaikhova, D.R.; Amromina, A.M.; Chemezov, A.I.; Shelomencev, I.G.; et al. Features of the response to subchronic low-dose exposure to copper oxide nanoparticles in rats. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Lee, I.-C.; Ko, J.-W.; Park, S.-H.; Shin, N.-R.; Shin, I.-S.; Moon, C.; Kim, J.-H.; Kim, H.-C.; Kim, J.-C. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part. Fibre Toxicol. 2016, 13, 56. [Google Scholar] [CrossRef]
- Abdelazeim, S.A.; Shehata, N.I.; Aly, H.F.; Shams, S.G.E. Amelioration of oxidative stress-mediated apoptosis in copper oxide nanoparticles-induced liver injury in rats by potent antioxidants. Sci. Rep. 2020, 10, 10812. [Google Scholar] [CrossRef]
- Henson, T.E.; Navratilova, J.; Tennant, A.H.; Bradham, K.D.; Rogers, K.R.; Hughes, M.F. In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology 2019, 13, 795–811. [Google Scholar] [CrossRef]
- Sahin, Ö.; Bulutcu, A. Effect of surface charge distribution on the crystal growth ofsodiumperborate tetrahydrate. J. Cryst. Growth 2002, 241, 471–480. [Google Scholar] [CrossRef]
- Schaefer, J.; Backus, E.H.G.; Bonn, M. Evidence for auto-catalytic mineral dissolution from surface-specific vibrational spectroscopy. Nat. Commun. 2018, 3316, 1–6. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T.; Gebremeskel, F.G. Green and cost-effective biofabrication of copper oxide nanoparticles: Exploring antimicrobial and anticancer applications. Biotechnol. Rep. 2024, 41, 1–16. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticles. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, J.G.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 2, 3623–3632. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, M.I.; Foy, T.; Berggad, S.; Donnelly; Cagney, G.; Linse, S.; Dawson, K. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5754–5756. [Google Scholar] [CrossRef]
- Urbančič, L.; Garvas, M.; Kokot, B.; Majaron, H.; Umek, P.; Cassidy, H.; Škarabot, M.; Schneider, F.; Galiani, S.; Arsov, Z.; et al. Nanoparticles can wrap epithelial cell membranes and relocate them across the epithelial cell layer. Nano Lett. 2018, 18, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Langford, A.; Bruchsaler, M.; Gupta, M. Suspension properties and characterization of aluminum-adjuvanted vaccines. In Practical Aspects of Vaccine Development; Academic Press: Cambridge, MA, USA, 2022; pp. 225–266. [Google Scholar]
- Aoi, W.; Marunaka, Y. Importance of pH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. Biomed. Res. Int. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Weetman, P.; Goldman, S.; Gray, C.G. Use of the Poisson–Boltzmann Equation To Estimate The Electrostatic Free Energy Barrier for Dielectric Models of Biological Ion Channels. Phys. Chem. B 1997, 101, 6073–6078. [Google Scholar] [CrossRef]
- Amadu, M.; Miadonye, A. Applicability of the linearized Poisson–Boltzmann theory to contact angle problems and application to the carbon dioxide–brine–solid systems. Sci. Rep. 2022, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fixman, M. The Poisson–Boltzmann equation and its application to polyelectrolytes. J. Chem. Phys. 1979, 70, 4995–5005. [Google Scholar] [CrossRef]
- Ahmad, A.; Rasheed, N.; Banu, N.; Palit, G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress 2010, 13, 355–364. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles–a comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kaye, G.; Laby, T. Tables of Physical and Chemical Constants; Longmans, Green and Co.: London, UK, 1911. [Google Scholar]
| Ion | Intracellular Concentration—mM/L | Extracellular Concentration—mM/L |
|---|---|---|
| Sodium | 10 | 142 |
| Potassium | 149 | 4 |
| Calcium | 0.0001 | 2.4 |
| Chloride | 5 | 103 |
| Magnesium | 58 | 1.2 |
| Bicarbonate | 10 | 28 |
| Phosphate | 75 | 4 |
| Organ | Intracellular | Extracellular |
|---|---|---|
| Lung | High | Lower |
| Brain | High | Lower |
| Cornea | High | Lower |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadu, M.; Raheem, N.M.; Miadonye, A. A Mean Field Poisson–Boltzmann Theory Assessment of Copper Oxide Nanosheets Interaction Potential in Physiological Fluids. Nanomaterials 2025, 15, 1330. https://doi.org/10.3390/nano15171330
Amadu M, Raheem NM, Miadonye A. A Mean Field Poisson–Boltzmann Theory Assessment of Copper Oxide Nanosheets Interaction Potential in Physiological Fluids. Nanomaterials. 2025; 15(17):1330. https://doi.org/10.3390/nano15171330
Chicago/Turabian StyleAmadu, Mumuni, Nafisat Motunrayo Raheem, and Adango Miadonye. 2025. "A Mean Field Poisson–Boltzmann Theory Assessment of Copper Oxide Nanosheets Interaction Potential in Physiological Fluids" Nanomaterials 15, no. 17: 1330. https://doi.org/10.3390/nano15171330
APA StyleAmadu, M., Raheem, N. M., & Miadonye, A. (2025). A Mean Field Poisson–Boltzmann Theory Assessment of Copper Oxide Nanosheets Interaction Potential in Physiological Fluids. Nanomaterials, 15(17), 1330. https://doi.org/10.3390/nano15171330







