Sustainable Green Synthesis of Fe3O4 Nanocatalysts for Efficient Oxygen Evolution Reaction
Abstract
1. Introduction
2. Results
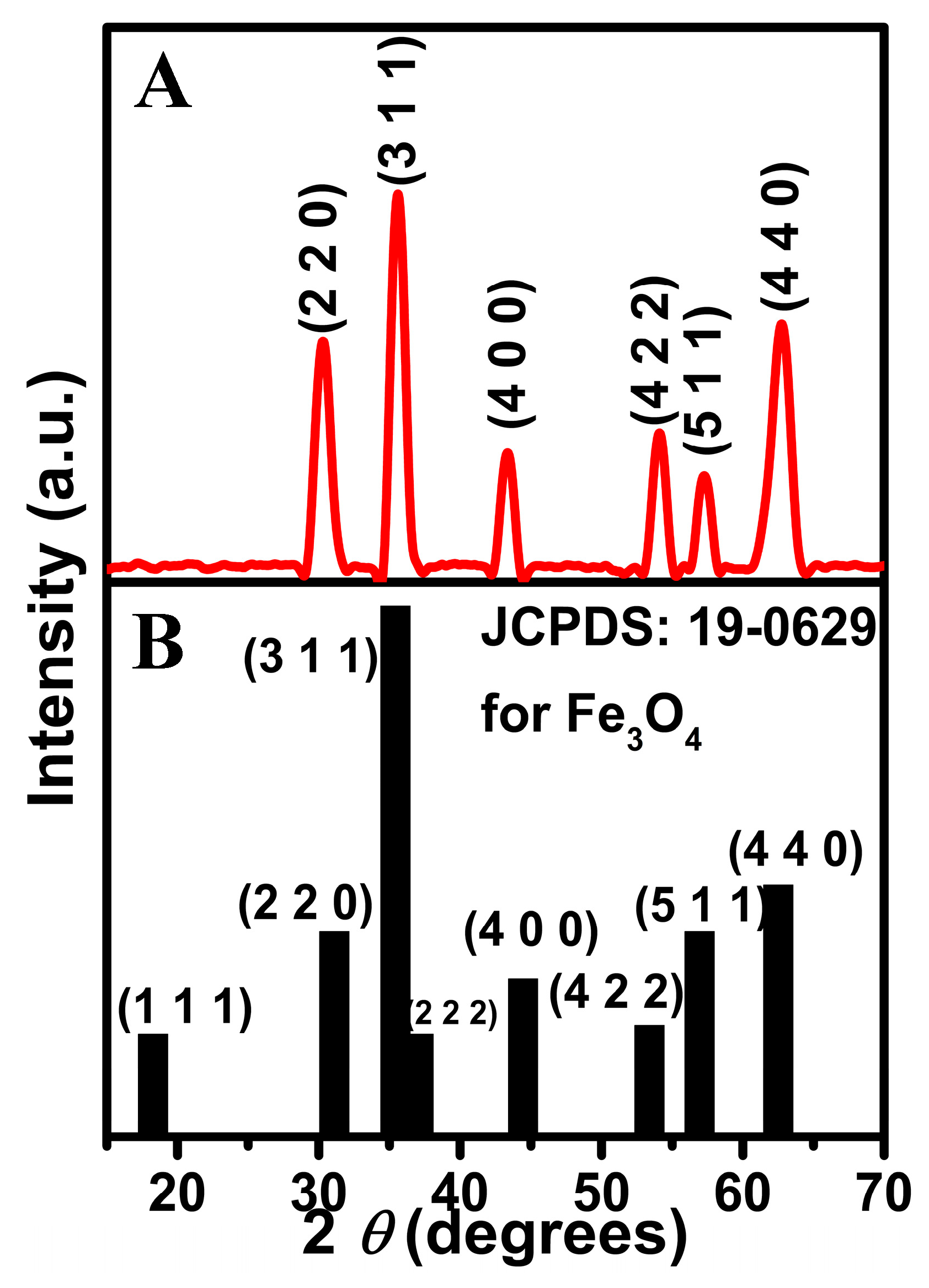
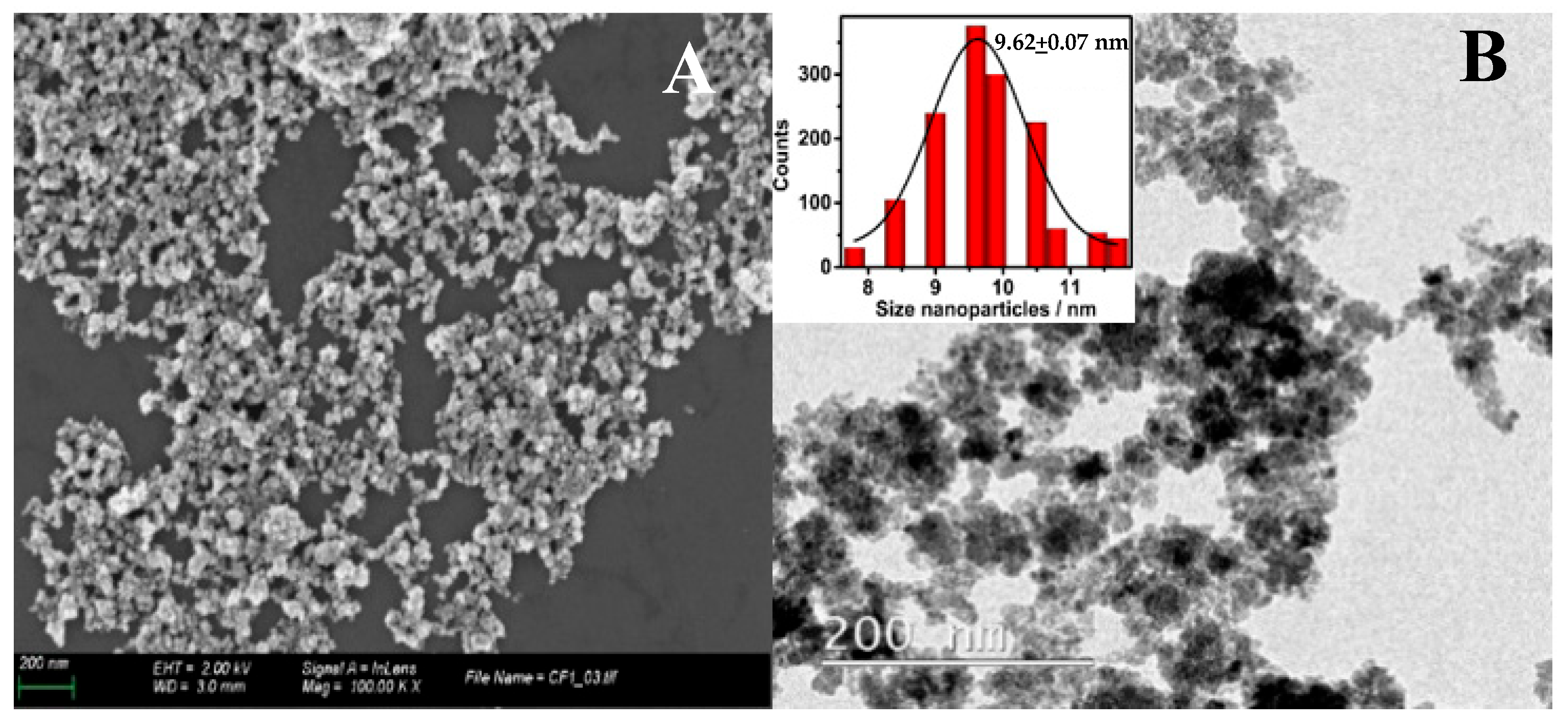
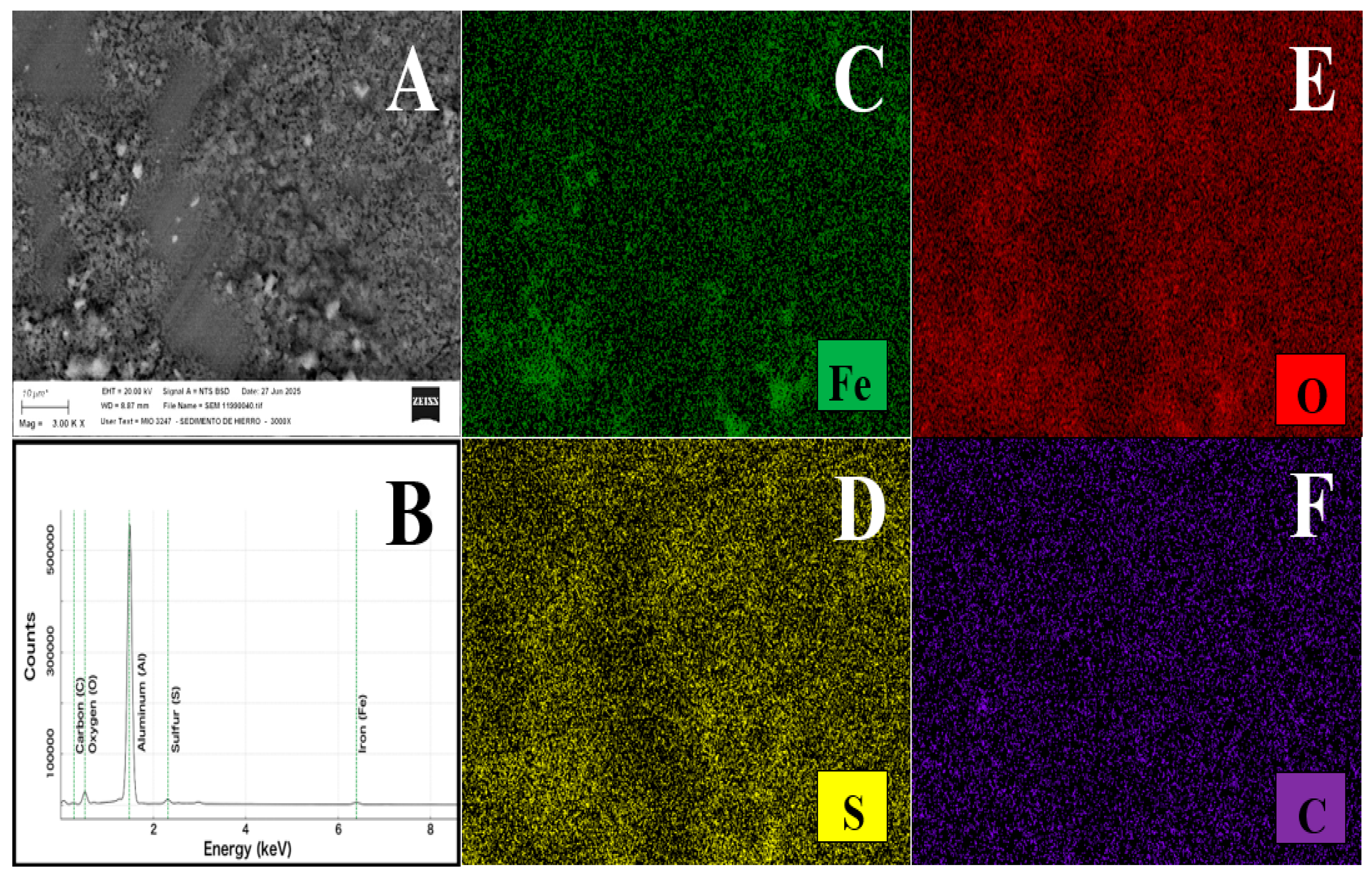
2.1. Materials Characterization Using X-Ray Diffraction and Microscopy Analysis
2.2. UV–Visible and FT-IR Analysis
2.3. Prepared Fe3O4NPs by BET Analysis
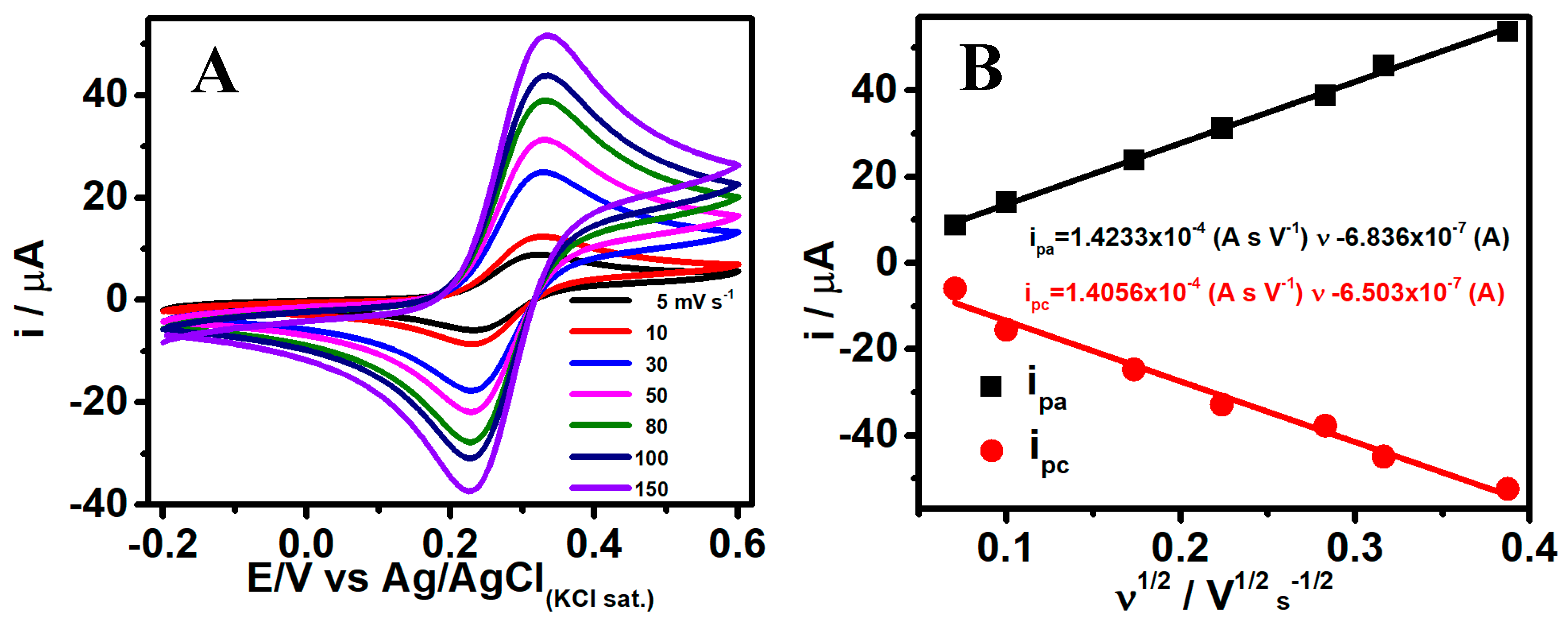
2.4. Electrochemical Characterization of Fe3O4NPs
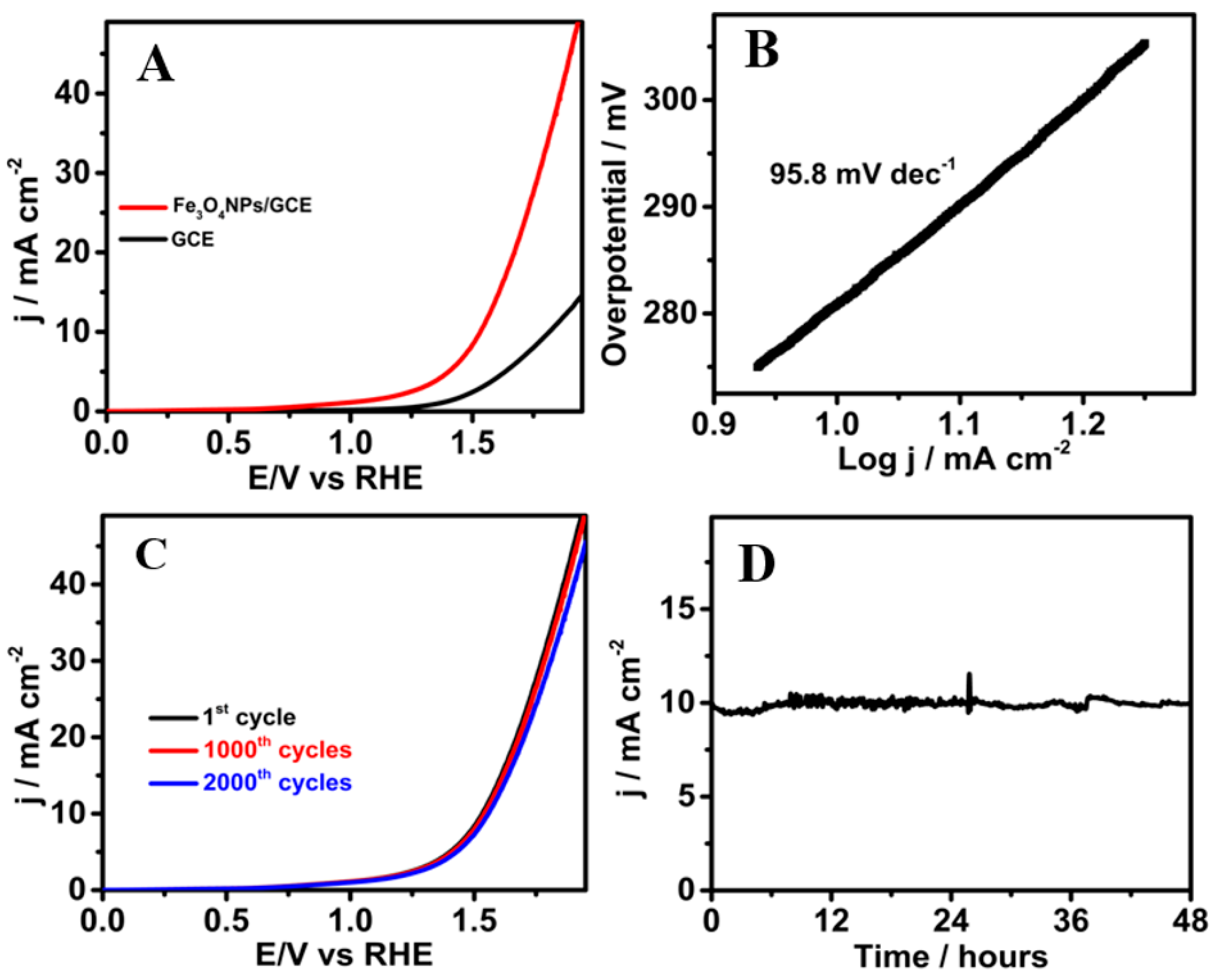
2.5. Electrochemical Studies for the Oxygen Evolution Reaction
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
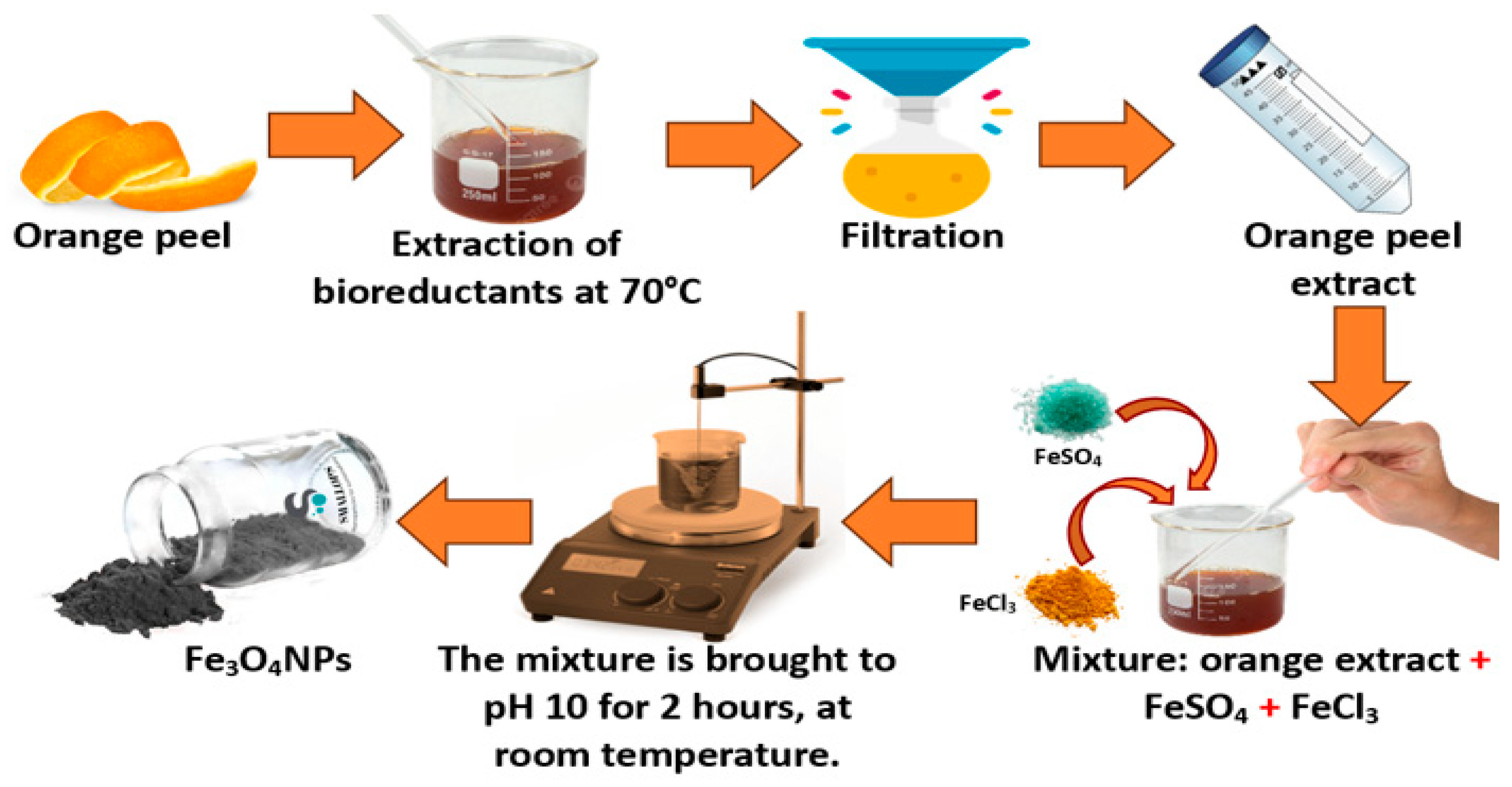
3.3. Preparation of Orange Peel Extract
3.4. Green Synthesis of Fe3O4 Nanoparticles
3.5. Preparation of Fe3O4 NPs Modified Glassy Carbon Electrode (GCE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Sharma, V.; Chatterjee, S. Contribution of Plastic and Microplastic to Global Climate Change and Their Conjoining Impacts on the Environment—A Review. Sci. Total Environ. 2023, 875, 162627. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, M.; Kumar, V.M.; Hariharan, V.S.; Narahari, T.; Kumar, P.A.; Madhesh, K.; Kumar, G.P.; Prabakaran, R. Fuelling the Future: A Review of Non-Renewable Hydrogen Production and Storage Techniques. Renew. Sustain. Energy Rev. 2023, 188, 113791. [Google Scholar] [CrossRef]
- Jamadar, A.S.; Sutar, R.; Patil, S.; Khandekar, R.; Yadav, J.B. Progress in Metal Oxide-Based Electrocatalysts for Sustainable Water Splitting. Mater. Rep. Energy 2024, 4, 100283. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Luo, W.; Zhang, Y.; Wang, Y.; Wu, Y. Rational Design Strategies for Covalent Organic Frameworks Toward Efficient Electrocatalytic Hydrogen Peroxide Production. Catalysts 2025, 15, 500. [Google Scholar] [CrossRef]
- Quezada, V.; Martinez, T.; Nelson, R.; Pérez-Fehrmann, M.; Zaragoza, G.; Vizcarra, A.; Kesternich, V.; Hernández-Saravia, L.P. A Novel Platform of Using Copper (II) Complex with Triazole-Carboxilated Modified as Bidentated Ligand SPCE for the Detection of Hydrogen Peroxide in Milk. J. Electroanal. Chem. 2020, 879, 114763. [Google Scholar] [CrossRef]
- Silva, T.R.; Raimundo, R.A.; Silva, V.D.; Santos, J.R.D.; Araújo, A.J.M.; Oliveira, J.F.G.d.A.; de Lima, L.C.; da Silva, F.F.; Ferreira, L.d.S.; Macedo, D.A. Green Synthesis of CuCo2O4–CuO Composite Nanoparticles Grown on Nickel Foam for High-Performance Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2023, 48, 17160–17176. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Sukeri, A.; Bertotti, M. Fabrication of Nanoporous Gold-Islands via Hydrogen Bubble Template: An Efficient Electrocatalyst for Oxygen Reduction and Hydrogen Evolution Reactions. Int. J. Hydrogen Energy 2019, 44, 15001–15008. [Google Scholar] [CrossRef]
- Aralekallu, S.; Hadimane, S.; Shantharaja; Nemakal, M.; Koodlur Sannegowda, L. Organic Hybrid of Cobalt Phthalocyanine Embedded Graphene as an Efficient Catalyst for Oxygen Reduction Reaction. Fuel 2024, 361, 130736. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Matias, T.A.; Saravia, L.P.H.; Nakamura, M.; Bernardes, J.S.; Bertotti, M.; Araki, K. Synergic Effects Enhance the Catalytic Properties of Alpha-Ni(OH)2-FeOCPc@rGO Composite for Oxygen Evolution Reaction. Electrochim. Acta 2018, 267, 161–169. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Z.; Zhao, C.; Ding, X.; Liu, X.; Wang, D.; Hui, Z.; Jia, R.; Liu, Y. Interface Engineering of Copper-Cobalt Based Heterostructure as Bifunctional Electrocatalysts for Overall Water Splitting. Ceram. Int. 2020, 46, 13125–13132. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Chandra Murmu, N.; Kuila, T. Progress in Theoretical and Experimental Investigation on Seawater Electrolysis: Opportunities and Challenges. Sustain. Energy Fuels 2021, 5, 5915–5945. [Google Scholar] [CrossRef]
- Huang, C.-J.; Xu, H.-M.; Shuai, T.-Y.; Zhan, Q.-N.; Zhang, Z.-J.; Li, G.-R. A Review of Modulation Strategies for Improving Catalytic Performance of Transition Metal Phosphides for Oxygen Evolution Reaction. Appl. Catal. B Environ. 2023, 325, 122313. [Google Scholar] [CrossRef]
- Jung, H.; Karmakar, A.; Adhikari, A.; Patel, R.; Kundu, S. Graphene-Based Materials as Electrocatalysts for the Oxygen Evolution Reaction: A Review. Sustain. Energy Fuels 2022, 6, 640–663. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Yan, H.; Yang, L.; Xu, S. Cobalt Phosphide Composite Encapsulated within N,P-Doped Carbon Nanotubes for Synergistic Oxygen Evolution. Small 2018, 14, e1800367. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Chen, G.; Zhou, Y.; Lin, H.; Chen, C.; Ran, R.; Zhou, W.; Shao, Z. Smart Control of Composition for Double Perovskite Electrocatalysts toward Enhanced Oxygen Evolution Reaction. ChemSusChem 2019, 12, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Bhanja, P.; Sharma, S.; Yamauchi, Y.; Alothman, Z.A.; Wang, Z.-L.; Bal, R.; Bhaumik, A. Morphologically Controlled Cobalt Oxide Nanoparticles for Efficient Oxygen Evolution Reaction. J. Colloid Interface Sci. 2021, 582, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Mohanty, B.; Paul, B.; Bhaumik, A.; Jena, B.K.; Basu, S. Novel Microporous Organic-Inorganic Hybrid Metal Phosphonates as Electrocatalysts towards Water Oxidation Reaction. Electrochim. Acta 2022, 416, 140277. [Google Scholar] [CrossRef]
- Chowde Gowda, C.; Mathur, A.; Parui, A.; Kumbhakar, P.; Pandey, P.; Sharma, S.; Chandra, A.; Singh, A.K.; Halder, A.; Tiwary, C.S. Understanding the Electrocatalysis OER and ORR Activity of Ultrathin Spinel Mn3O4. J. Ind. Eng. Chem. 2022, 113, 153–160. [Google Scholar] [CrossRef]
- Yang, L.; Yang, T.; Wang, E.; Yu, X.; Wang, K.; Du, Z.; Cao, S.; Chou, K.-C.; Hou, X. Bifunctional Hierarchical NiCoP@FeNi LDH Nanosheet Array Electrocatalyst for Industrial-Scale High-Current-Density Water Splitting. J. Mater. Sci. Technol. 2023, 159, 33–40. [Google Scholar] [CrossRef]
- Shen, P.; Guan, L.; Zhang, Z.; Zhao, J.; Yang, Y.; Wu, L.; Huang, Y.; Li, M.; Song, X. Fast Joule Heating Preparation and Performance Study of Carbon-Loaded Nano Tungsten-Based Electrocatalysts for Highly Efficient Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2025, 8, 7095–7106. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.; Li, M.; Geng, S.; Qiu, L.; He, L.; Li, L.; Chen, Z.; Yu, Y.; Yang, W.; et al. Engineering Lattice Oxygen Regeneration of NiFe Layered Double Hydroxide Enhances Oxygen Evolution Catalysis Durability. Angew. Chem. Int. Ed. 2025, 64, e202413250. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Y.; Yang, Z.-Q.; Zhao, J.; Liu, R. One-Pot Modulated Construction of Ni-MOF/NiFe2O4 Heterostructured Catalyst for Efficient Oxygen Evolution. Rare Met. 2024, 43, 6751–6757. [Google Scholar] [CrossRef]
- Wu, X.; Gu, X.; Tai, J.; Tang, J.; Yuan, K.; Liu, L.; Zheng, Y.; Altaf, A.; Shen, X.; Cui, S. Facile Synthesis of the NiFe Bimetallic Alloy Wrapped on the Porous N-Doped Graphene Aerogel via Thermal Reduction and Electronic Structure Modulation for Boosting Oxygen Evolution Reaction. J. Power Sources 2025, 633, 236430. [Google Scholar] [CrossRef]
- Liao, W.; Qing, F.; Liu, Q.; Wu, R.; Zhou, C.; Chen, L.; Chen, Y.; Li, X. Carbothermal Shock Synthesis of Lattice Oxygen-Mediated High-Entropy FeCoNiCuMo-O Electrocatalyst with a Fast Kinetic, High Efficiency, and Stable Oxygen Evolution Reaction. Nano Lett. 2025, 25, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Royer, L.; Guehl, J.; Zilbermann, M.; Dintzer, T.; Leuvrey, C.; Pichon, B.P.; Savinova, E.; Bonnefont, A. Influence of the Catalyst Layer Thickness on the Determination of the OER Activity of Fe3O4@CoFe2O4 Core-Shell Nanoparticles. Electrochim. Acta 2023, 446, 141981. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, H.; Hu, Y.; Wang, W.; Yang, X.; Kuang, C. Enhancing Catalytic Activity of Fe3O4 for Electrochemical Water Oxidation via the Coupling of OER-Inert Au. Int. J. Hydrogen Energy 2022, 47, 22731–22737. [Google Scholar] [CrossRef]
- Wei, M.; Han, Y.; Liu, Y.; Su, B.; Yang, H.; Lei, Z. Green Preparation of Fe3O4 Coral-like Nanomaterials with Outstanding Magnetic and OER Properties. J. Alloys Compd. 2020, 831, 154702. [Google Scholar] [CrossRef]
- Kashif Saleem, M.; Ahmad Niaz, N.; Fahmi Fawy, K.; Abdelmohsen, S.A.M.; Alanazi, M.M.; Hussain, F.; Naeem Ashiq, M.; Rasheed, U.; Abbas, Y.; Shuaib Khan, M. Electrocatalytic Behavior of Ni-Co-Fe3O4 Nanospheres for Efficient Oxygen Evolution Reaction. J. Electroanal. Chem. 2023, 940, 117503. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, Y.; Li, Q.; Liu, F.; Cui, L.; Li, C.; Liu, J. Sandwich Structured Fe3O4/NiFe LDH/Fe3O4 as a Bifunctional Electrocatalyst with Superior Stability for Highly Sustained Overall Water Splitting. J. Alloys Compd. 2023, 932, 167612. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, D.; Liao, J.; Bao, X.; Luo, S. One-Pot Synthesis of Fe3O4/Fe/C by Microwave Sintering as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions. J. Alloys Compd. 2019, 786, 134–138. [Google Scholar] [CrossRef]
- Liu, G.; Yao, R.; Zhao, Y.; Wang, M.; Li, N.; Li, Y.; Bo, X.; Li, J.; Zhao, C. Encapsulation of Ni/Fe3O4 Heterostructures inside Onion-like N-Doped Carbon Nanorods Enables Synergistic Electrocatalysis for Water Oxidation. Nanoscale 2018, 10, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Pan, D.; Zhang, H. Study on Formation Mechanism by Monitoring the Morphology and Structure Evolution of Nearly Monodispersed Fe3O4 Submicroparticles with Controlled Particle Sizes. Ind. Eng. Chem. Res. 2011, 50, 9009–9018. [Google Scholar] [CrossRef]
- Hu, H.; Yang, L.; Lin, Z.; Xiang, X.; Jiang, X.; Hou, L. Preparation and Characterization of Novel Magnetic Fe3O4/Chitosan/Al(OH)3 Beads and Its Adsorption for Fluoride. Int. J. Biol. Macromol. 2018, 114, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 Magnetic Nanoparticles Synthesis from Tailings by Ultrasonic Chemical Co-Precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Giri, S.K.; Das, N.N.; Pradhan, G.C. Synthesis and Characterization of Magnetite Nanoparticles Using Waste Iron Ore Tailings for Adsorptive Removal of Dyes from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 43–49. [Google Scholar] [CrossRef]
- Cao, W.; Ma, Y.; Zhou, W.; Guo, L. One-Pot Hydrothermal Synthesis of RGO-Fe3O4 Hybrid Nanocomposite for Removal of Pb(II) via Magnetic Separation. Chem. Res. Chin. Univ. 2015, 31, 508–513. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Yang, M.; Yuan, Y.; Yin, W.; Peng, Q.; Li, Y.; He, X. Electrospun Fe2O3 Nanotubes and Fe3O4 Nanofibers by Citric Acid Sol-gel Method. J. Am. Ceram. Soc. 2017, 100, 5460–5470. [Google Scholar] [CrossRef]
- Nayeem, J.; Al-Bari, M.A.A.; Mahiuddin, M.; Rahman, M.A.; Mefford, O.T.; Ahmad, H.; Rahman, M.M. Silica Coating of Iron Oxide Magnetic Nanoparticles by Reverse Microemulsion Method and Their Functionalization with Cationic Polymer P(NIPAm-Co-AMPTMA) for Antibacterial Vancomycin Immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125857. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Wang, X.; Xie, Y.; Wang, Y.; Dong, Q.; Zhu, L.; Zhang, G.; Xu, D. Electrospinning Fabrication of Flexible Fe3O4 Fibers by Sol-Gel Method with High Saturation Magnetization for Heavy Metal Adsorption. Mater. Des. 2020, 186, 108298. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Green Synthesis Protocols, Toxicity, and Recent Progress in Nanomaterial-Based for Environmental Chemical Sensors Applications. Trends Environ. Anal. Chem. 2023, 39, e00204. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Carmona, E.R.; Villacorta, A.; Carevic, F.S.; Marcos, R. Sustainable Use of Mining and Electronic Waste for Nanomaterial Synthesis with Technological Applications: State of the Art and Future Directions. Green Chem. Lett. Rev. 2023, 16, 2260401. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green Route for Recycling of Low-Cost Waste Resources for the Biosynthesis of Nanoparticles (NPs) and Nanomaterials (NMs)-A Review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and Green Synthesis of Carbon Nanomaterials: A Review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of Carbon Nanotubes Using Green Plant Extract as Catalyst: Unconventional Concept and Its Realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef]
- Paul, B.; Bhuyan, B.; Dhar Purkayastha, D.; Dey, M.; Dhar, S.S. Green Synthesis of Gold Nanoparticles Using Pogestemon Benghalensis (B) O. Ktz. Leaf Extract and Studies of Their Photocatalytic Activity in Degradation of Methylene Blue. Mater. Lett. 2015, 148, 37–40. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green Synthesis of Palladium Nanoparticles Mediated by Black Tea Leaves (Camellia sinensis) Extract: Catalytic Activity in the Reduction of 4-Nitrophenol and Suzuki-Miyaura Coupling Reaction under Ligand-Free Conditions. J. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green Synthesis of Copper Nanoparticles by Citrus medica Linn. (Idilimbu) Juice and Its Antimicrobial Activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green Synthesis of Carbon Quantum Dots from Lemon Peel Waste: Applications in Sensing and Photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Isnaeni, I.N.; Indriyati; Dedi; Sumiarsa, D.; Primadona, I. Green Synthesis of Different TiO2 Nanoparticle Phases Using Mango-Peel Extract. Mater. Lett. 2021, 294, 129792. [Google Scholar] [CrossRef]
- Rojo, C.; Carmona, E.R.; Hernández-Saravia, L.P.; Villacorta, A.; Marcos, R.; Carevic, F.S.; Apablaza, V.H.; Nelson, R. Utilization of Orange Peel Waste for the Green Synthesis of Iron Nanoparticles and Its Application to Stimulate Growth and Biofortification on Solanum lycopersicum. Waste Biomass Valorization 2024, 15, 6343–6356. [Google Scholar] [CrossRef]
- Selvaraj, R.; Murugesan, G.; Rangasamy, G.; Bhole, R.; Dave, N.; Pai, S.; Balakrishna, K.; Vinayagam, R.; Varadavenkatesan, T. As (III) Removal Using Superparamagnetic Magnetite Nanoparticles Synthesized Using Ulva Prolifera−Optimization, Isotherm, Kinetic and Equilibrium Studies. Chemosphere 2022, 308, 136271. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Villarreal, N.; Verástegui-Domínguez, L.; Rodríguez-Batista, R.; Gándara-Martínez, E.; Alcorta-García, A.; Martínez-Delgado, D.; Rodríguez-Castellanos, E.A.; Vázquez-Rodríguez, F.; Gómez-Rodríguez, C. Green Synthesis of Magnetic Nanoparticles of Iron Oxide Using Aqueous Extracts of Lemon Peel Waste and Its Application in Anti-Corrosive Coatings. Materials 2022, 15, 8328. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Iron Nanoparticles by Various Tea Extracts: Comparative Study of the Reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Ghazal, H.; Othman, H.A.; Hassabo, A.G. Synthesis of Different Nanometals Using Citrus Sinensis Peel (Orange Peel) Waste Extraction for Valuable Functionalization of Cotton Fabric. Chem. Pap. 2022, 76, 639–660. [Google Scholar] [CrossRef]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of Antioxidant and Antibacterial Activity of Iron Oxide Nanoparticles (IONPS) Synthesized from the Aqueous Extract of Penicillium spp. Sens. Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Afrouz, M.; Ahmadi-Nouraldinvand, F.; Elias, S.G.; Alebrahim, M.T.; Tseng, T.M.; Zahedian, H. Green Synthesis of Spermine Coated Iron Nanoparticles and Its Effect on Biochemical Properties of Rosmarinus Officinalis. Sci. Rep. 2023, 13, 775. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Kini, S.M.; Narasimhan, M.K.; Pugazhendhi, A.; Selvaraj, R. Structural Characterization and Adsorptive Ability of Green Synthesized Fe3O4 Nanoparticles to Remove Acid Blue 113 Dye. Surf. Interfaces 2021, 23, 100947. [Google Scholar] [CrossRef]
- Anupong, W.; On-uma, R.; Jutamas, K.; Joshi, D.; Salmen, S.H.; Alahmadi, T.A.; Jhanani, G.K. Cobalt Nanoparticles Synthesizing Potential of Orange Peel Aqueous Extract and Their Antimicrobial and Antioxidant Activity. Environ. Res. 2023, 216, 114594. [Google Scholar] [CrossRef] [PubMed]
- Keerthika Devi, R.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Akilarasan, M.; Shaju, A.; Rwei, S.-P.; Yu, J.; Yu, Y.-Y. In-Situ Formation of Niobium Oxide–Niobium Carbide–Reduced Graphene Oxide Ternary Nanocomposite as an Electrochemical Sensor for Sensitive Detection of Anticancer Drug Methotrexate. J. Colloid Interface Sci. 2023, 643, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, H.; Yuan, M.; Yu, Z.; Li, J.; Shi, K.; Liang, Z.; Yang, Y.; Zhu, T.; Sun, G.; et al. (NiFe)S2 Nanoparticles Grown on Graphene as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Electrochim. Acta 2018, 286, 195–204. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Xiang, R.; Liu, K.; Wang, H.; Chen, M.; Han, Y.; Cao, R. Porous Nickel–Iron Oxide as a Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Sci. 2015, 2, 1500199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, Y.; Dou, M.; Ji, J.; Wang, F. One-Step Conversion from Ni/Fe Polyphthalocyanine to N-Doped Carbon Supported Ni-Fe Nanoparticles for Highly Efficient Water Splitting. Nano Energy 2016, 30, 426–433. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Huang, J.; Xi, Y.; Gao, R.; Su, G.; Wang, W.; Cao, L.; Dong, B. Vertical Growth of 2D Amorphous FePO4 Nanosheet on Ni Foam: Outer and Inner Structural Design for Superior Water Splitting. Adv. Mater. 2017, 29, 1704574. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, W.; Zheng, X.; He, D.; Luo, Y.; Wang, X.; Yang, J.; Wu, Y.; Yan, W.; Zhuang, Z.; et al. Hierarchical Fe-Doped NiOx Nanotubes Assembled from Ultrathin Nanosheets Containing Trivalent Nickel for Oxygen Evolution Reaction. Nano Energy 2017, 38, 167–174. [Google Scholar] [CrossRef]
- Zou, H.-H.; Yuan, C.-Z.; Zou, H.-Y.; Cheang, T.-Y.; Zhao, S.-J.; Qazi, U.Y.; Zhong, S.-L.; Wang, L.; Xu, A.-W. Bimetallic Phosphide Hollow Nanocubes Derived from a Prussian-Blue-Analog Used as High-Performance Catalysts for the Oxygen Evolution Reaction. Catal. Sci. Technol. 2017, 7, 1549–1555. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Zhao, C.; Han, X.; Yang, J.; Liu, Z.; Li, S.; Zhang, M.; Qiu, J. Iron-Tuned Super Nickel Phosphide Microstructures with High Activity for Electrochemical Overall Water Splitting. Nano Energy 2017, 34, 472–480. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Ling, T.; Vasileff, A.; Qiao, S.-Z. S-NiFe2O4 Ultra-Small Nanoparticle Built Nanosheets for Efficient Water Splitting in Alkaline and Neutral PH. Nano Energy 2017, 40, 264–273. [Google Scholar] [CrossRef]
- Yan, K.; Sheng, M.; Sun, X.; Song, C.; Cao, Z.; Sun, Y. Microwave Synthesis of Ultrathin Nickel Hydroxide Nanosheets with Iron Incorporation for Electrocatalytic Water Oxidation. ACS Appl. Energy Mater. 2019, 2, 1961–1968. [Google Scholar] [CrossRef]
- Manso, R.H.; Acharya, P.; Deng, S.; Crane, C.C.; Reinhart, B.; Lee, S.; Tong, X.; Nykypanchuk, D.; Zhu, J.; Zhu, Y.; et al. Controlling the 3-D Morphology of Ni–Fe-Based Nanocatalysts for the Oxygen Evolution Reaction. Nanoscale 2019, 11, 8170–8184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional Iron Oxide Nanoflake/Graphene Composites Derived from Mechanochemical Synthesis for Enhanced Lithium Storage and Electrocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 14446–14455. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Yang, T.; Wang, E.; Yu, X.; Hou, Y.; Du, Z.; Cao, S.; Chou, K.-C.; Hou, X. Enhanced Overall Water Splitting by Morphology and Electronic Structure Engineering on Pristine Ultrathin Metal-Organic Frameworks. J. Mater. Sci. Technol. 2025, 220, 92–103. [Google Scholar] [CrossRef]

| Fe3O4 NPs Wavenumber (cm−1) | Functional Groups | Orange Peel Extract Wavenumber (cm−1) | Functional Groups |
|---|---|---|---|
| 547 | Strong Fe–O | 547 | - |
| 1033 | Medium S=O | 1033 | Medium S=O |
| 1635 | Medium C=C | 1635 | Medium C=C |
| 3540 | Board O–H | 3540 | Board O–H |
| Nanoparticles | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Fe3O4NPs | 80.37 | 0.274 | 4.66 |
| Materials | Electrolytes | Overpotential (V) (at 10 mA cm−2) | Ref. |
|---|---|---|---|
| (NiFe)S2 | 1.0 M KOH | 0.32 | [63] |
| porous Ni–FeO | 1.0 M KOH | 0.33 | [64] |
| NiFe@NC | 1.0 M KOH | 0.58 | [65] |
| FePO4/NF | 1.0 M KOH | 0.31 | [66] |
| Fe-doped NiOx | 1.0 M KOH | 0.31 | [67] |
| Ni0.62Fe0.38P | 1.0 M KOH | 0.31 | [68] |
| Ni1.5Fe0.5P/CF | 1.0 M KOH | 0.36 | [69] |
| S-NiFe2O4/NF | 1.0 M KOH | 0.42 | [70] |
| NiFeOCPc@rGO-K | 1.0 M KOH | 0.37 | [9] |
| Fe0.22Ni0.78(OH)2 | 1.0 M KOH | 0.32 | [71] |
| NiOx–NiOx/FeOx | 1.0 M KOH | 0.32 | [72] |
| Fe3O4/graphene | 0.1 M KOH | - | [73] |
| FeCoV-NiMOF | 0.1 M KOH | 0.238 | [74] |
| Fe3O4-600 | 1.0 M NaOH | 0.33 | [28] |
| Fe3O4NPs | 1.0 M KOH | 0.30 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, E.R.; Sukeri, A.; Nelson, R.; Rojo, C.; Vizcarra, A.; Villacorta, A.; Carevic, F.; Marcos, R.; Arriaza, B.; Lara, N.; et al. Sustainable Green Synthesis of Fe3O4 Nanocatalysts for Efficient Oxygen Evolution Reaction. Nanomaterials 2025, 15, 1317. https://doi.org/10.3390/nano15171317
Carmona ER, Sukeri A, Nelson R, Rojo C, Vizcarra A, Villacorta A, Carevic F, Marcos R, Arriaza B, Lara N, et al. Sustainable Green Synthesis of Fe3O4 Nanocatalysts for Efficient Oxygen Evolution Reaction. Nanomaterials. 2025; 15(17):1317. https://doi.org/10.3390/nano15171317
Chicago/Turabian StyleCarmona, Erico R., Anandhakumar Sukeri, Ronald Nelson, Cynthia Rojo, Arnoldo Vizcarra, Aliro Villacorta, Felipe Carevic, Ricard Marcos, Bernardo Arriaza, Nelson Lara, and et al. 2025. "Sustainable Green Synthesis of Fe3O4 Nanocatalysts for Efficient Oxygen Evolution Reaction" Nanomaterials 15, no. 17: 1317. https://doi.org/10.3390/nano15171317
APA StyleCarmona, E. R., Sukeri, A., Nelson, R., Rojo, C., Vizcarra, A., Villacorta, A., Carevic, F., Marcos, R., Arriaza, B., Lara, N., Martinez, T., & Hernández-Saravia, L. P. (2025). Sustainable Green Synthesis of Fe3O4 Nanocatalysts for Efficient Oxygen Evolution Reaction. Nanomaterials, 15(17), 1317. https://doi.org/10.3390/nano15171317







