Synthesis of Nb-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and DFT Approach
Abstract
1. Introduction
2. Experiments and Computational Methods
2.1. Experimental Details
2.1.1. Synthesis of the Nb-Doped Nanocomposites Through Pechini Sol–Gel Process
2.1.2. Characterizations
2.1.3. Photochemical Characterization—Quantification of Hydroxyl Radicals (•OH)
2.1.4. Photocatalytic Activity Evaluation
2.2. Computational Methods
3. Results and Discussion
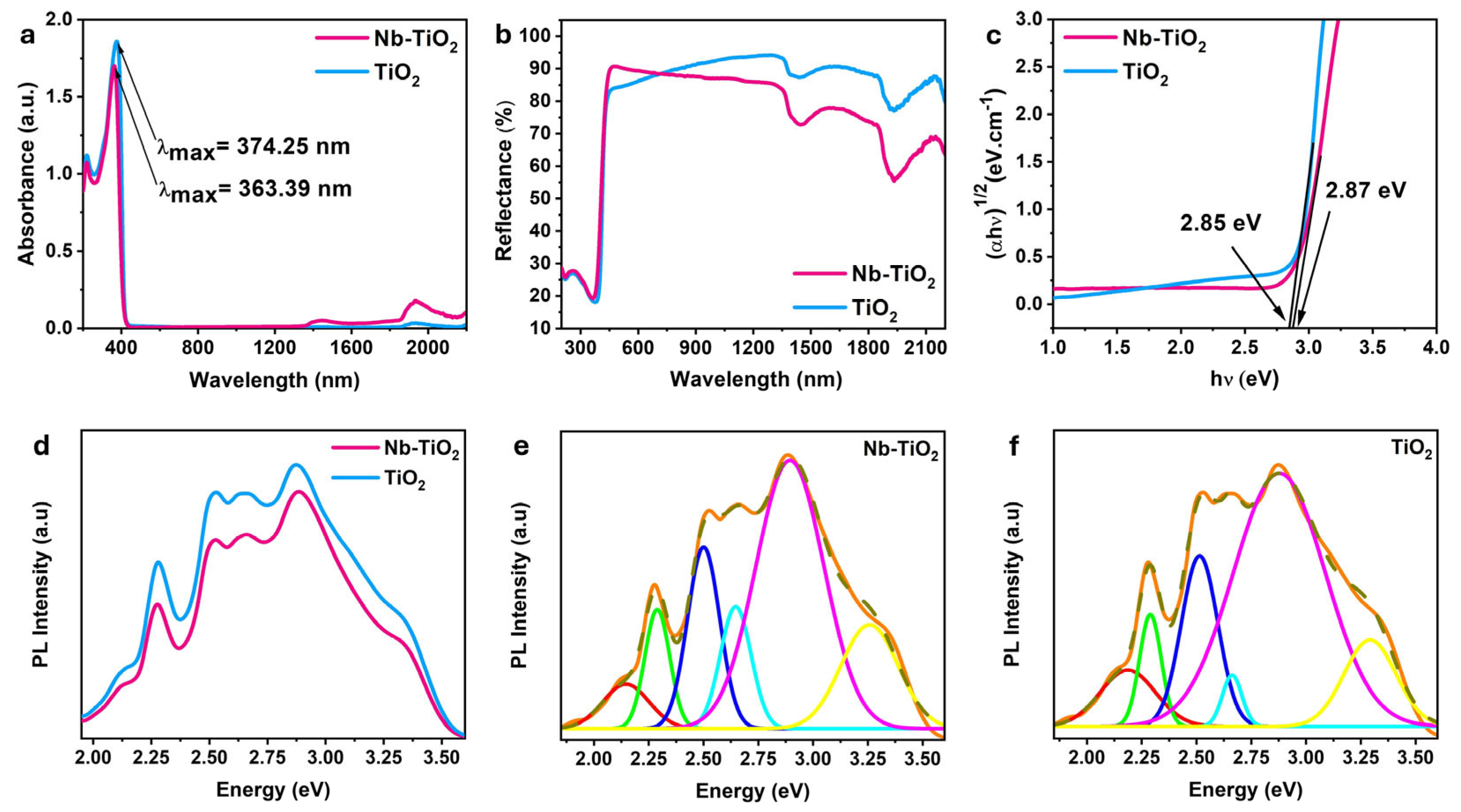
3.1. Physical-Chemical Properties
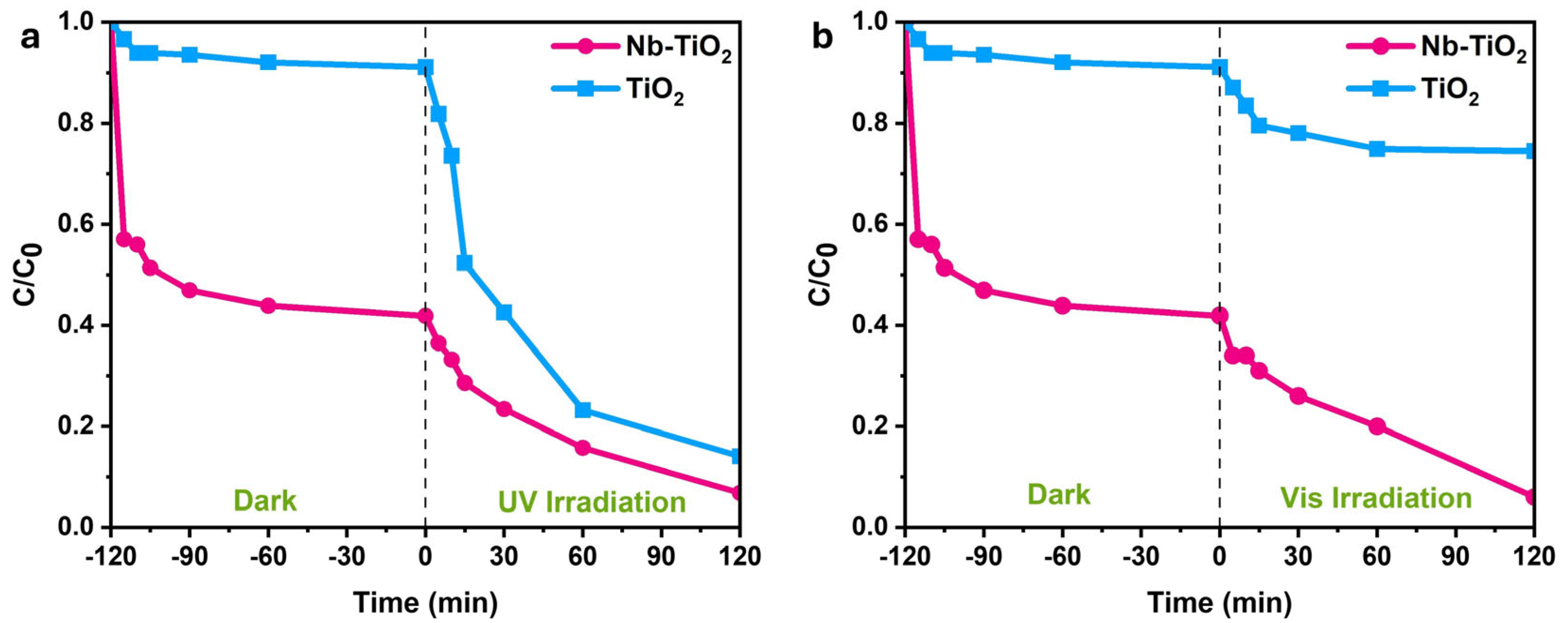
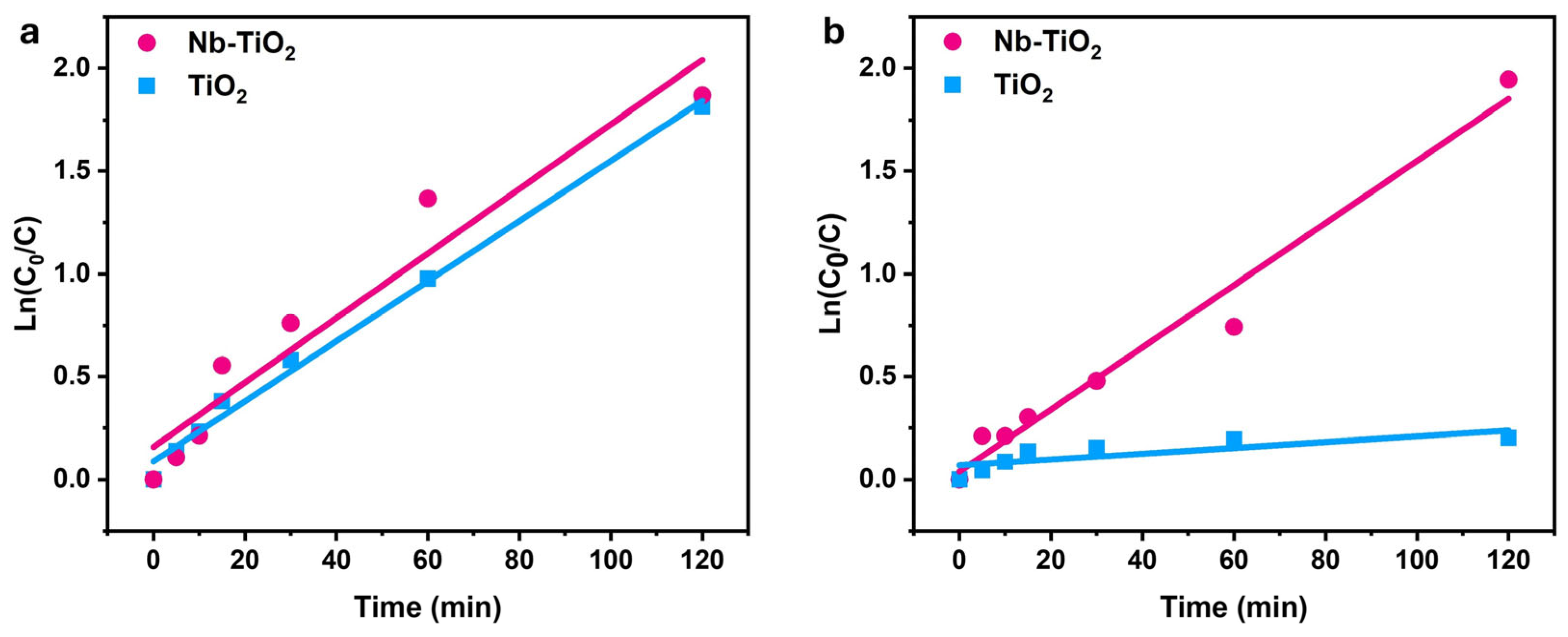
3.2. Photocatalytic Efficiency of Nb-TiO2 and TiO2 Nanoparticles
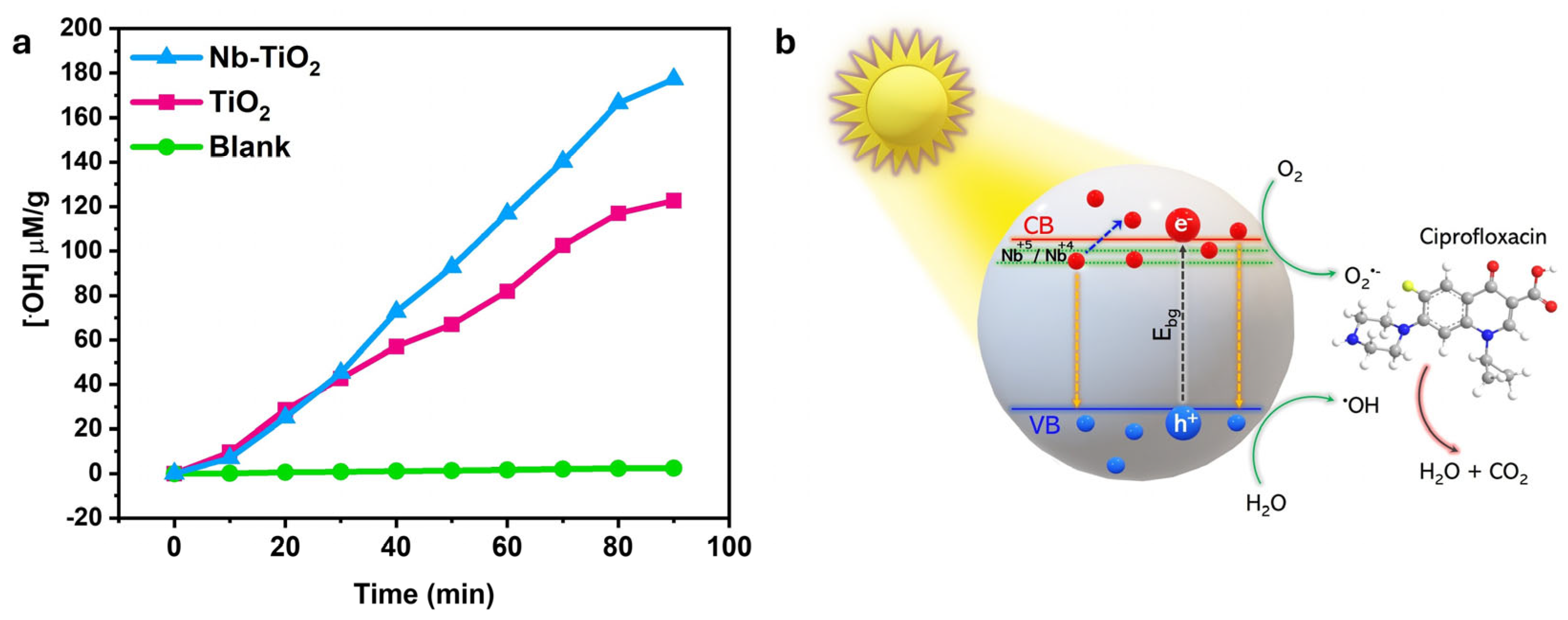
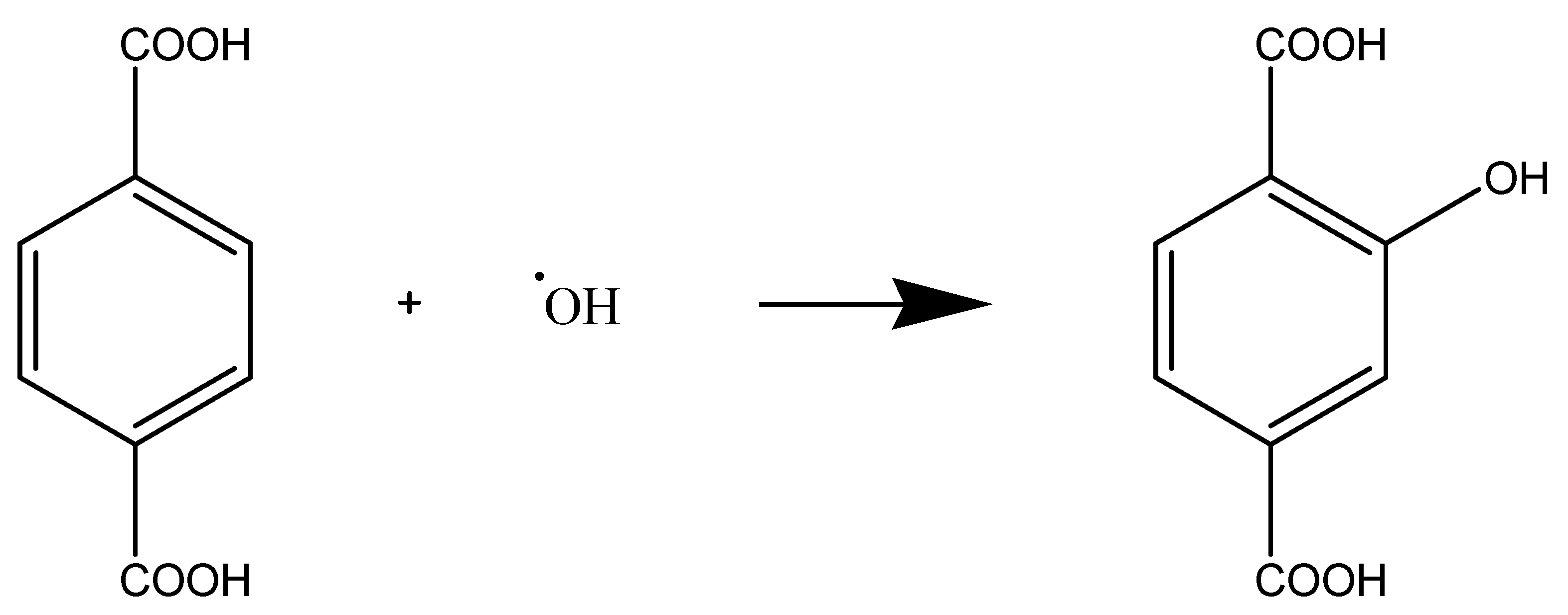
Photochemical Mechanism: Hydroxyl Radical Generation
4. Theoretical Results
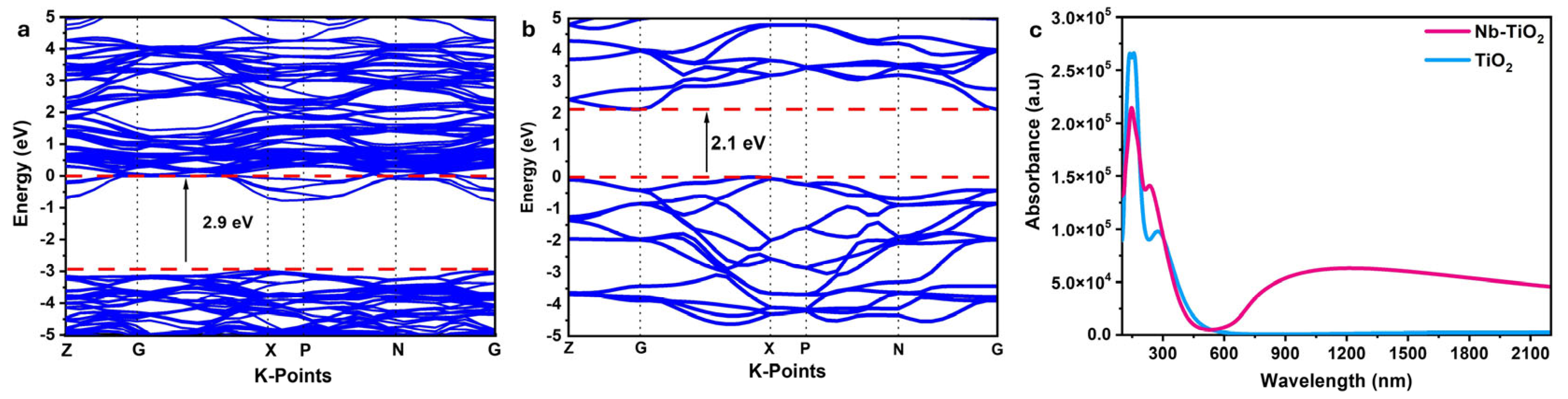
4.1. Electronic Properties
4.2. Optical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, K.R.; Cherif, Y.; Pazhani, G.P.; Anantharaj, S.; Azzi, H.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. The upsurge of photocatalysts in antibiotic micropollutants treatment: Materials design, recovery, toxicity and bioanalysis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100437. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Hsu, W.; Sutter-Fella, C.M.; Hettick, M.; Cheng, L.; Chan, S.; Chen, Y.; Zeng, Y.; Zheng, M.; Wang, H.-P.; Chiang, C.-C.; et al. Electron-Selective TiO2 Contact for Cu(In,Ga)Se2 Solar Cells. Sci. Rep. 2015, 5, 16028. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Mohammed, S.; Shnain, Z.; Abid, M. Use of TiO2 in Photocatalysis for Air Purification and Wastewater Treatment: A Review. Eng. Technol. J. 2022, 40, 1131–1143. [Google Scholar] [CrossRef]
- Espada, I.C.G.; González-Ballesteros, N.; Tavares, C.J.; Lanceros-Méndez, S.; Martins, P.M. Towards green visible range active photocatalytic Au/TiO2 nanocomposites through rutin-based synthesis and their application in the degradation of ciprofloxacin. RSC Sustain. 2024, 2, 3090–3099. [Google Scholar] [CrossRef]
- Xu, C.; Wu, J.; Desai, U.V.; Gao, D. High-Efficiency Solid-State Dye-Sensitized Solar Cells Based on TiO2-Coated ZnO Nanowire Arrays. Nano Lett. 2012, 12, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Tubio, C.R.; Pereira, N.; Campos-Arias, L.; Martins, P.M.; Vilas-Vilela, J.L.; Costa, C.M.; Lanceros-Mendez, S. Multifunctional Ternary Composites with Silver Nanowires and Titanium Dioxide Nanoparticles for Capacitive Sensing and Photocatalytic Self-Cleaning Applications. ACS Appl. Electron. Mater. 2022, 4, 3815–3824. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Chang, X.; Gong, J. Effective Charge Carrier Utilization in Photocatalytic Conversions. Acc. Chem. Res. 2016, 49, 911–921. [Google Scholar] [CrossRef]
- Ndabankulu, V.O.; Maddila, S.; Jonnalagadda, S.B. Synthesis of lanthanide-doped TiO2 nanoparticles and their photocatalytic activity under visible light. Can. J. Chem. 2019, 97, 672–681. [Google Scholar] [CrossRef]
- Yu, C.; He, H.; Fan, Q.; Xie, W.; Liu, Z.; Ji, H. Novel B-doped BiOCl nanosheets with exposed (001) facets and photocatalytic mechanism of enhanced degradation efficiency for organic pollutants. Sci. Total Environ. 2019, 694, 133727. [Google Scholar] [CrossRef]
- Yu, C.; He, H.; Liu, X.; Zeng, J.; Liu, Z. Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting high photocatalytic performances for the removal of organic pollutants. Chin. J. Catal. 2019, 40, 1212–1221. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, J.; Song, K.; Wang, X.; Yu, K.; Liang, C. Enhanced photocatalytic activities of Nd-doped TiO2 under visible light using a facile sol-gel method. J. Rare Earths 2020, 38, 148–156. [Google Scholar] [CrossRef]
- Zedek, R.; Djedjiga, H.; Megherbi, M.; Belkaid, M.S.; Ntsoenzok, E. Effects of slight Fe (III)-doping on structural and optical properties of TiO2 nanoparticles. J. Sol-Gel Sci. Technol. 2021, 100, 44–54. [Google Scholar] [CrossRef]
- Larumbe, S.; Monge, M.; Gómez-Polo, C. Comparative study of (N, Fe) doped TiO2 photocatalysts. Appl. Surf. Sci. 2015, 327, 490–497. [Google Scholar] [CrossRef]
- Kuranov, D.; Grebenkina, A.; Bogdanova, A.; Platonov, V.; Polomoshnov, S.; Krivetskiy, V.; Rumyantseva, M. Effect of Donor Nb(V) Doping on the Surface Reactivity, Electrical, Optical and Photocatalytic Properties of Nanocrystalline TiO2. Materials 2024, 17, 375. [Google Scholar] [CrossRef]
- Farzaneh, A.; Javidani, M.; Esrafili, M.D.; Mermer, O. Optical and photocatalytic characteristics of Al and Cu doped TiO2: Experimental assessments and DFT calculations. J. Phys. Chem. Solids 2022, 161, 110404. [Google Scholar] [CrossRef]
- Wen, L.; Liu, B.; Zhao, X.; Nakata, K.; Murakami, T.; Fujishima, A. Synthesis, Characterization, and Photocatalysis of Fe-Doped: A Combined Experimental and Theoretical Study. Int. J. Photoenergy 2012, 2012, 368750. [Google Scholar] [CrossRef]
- Rad, F.A.; Mehrabad, J.T.; Esrafili, M.D. A Communal Experimental and DFT Study on Structural and Photocatalytic Properties of Nitrogen-Doped TiO2. Adv. J. Chem. Sect. A 2023, 6, 244–252, no. Online First. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M. Synthesis and characterization of Nb-La co-doped TiO2 nanoparticles by sol-gel process for dye-sensitized solar cells. Ceram. Int. 2019, 45, 6994–7000. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Zoltan, T.; Rosales, M.C.; Yadarola, C. Reactive oxygen species quantification and their correlation with the photocatalytic activity of TiO2 (anatase and rutile) sensitized with asymmetric porphyrins. J. Environ. Chem. Eng. 2016, 4, 3967–3980. [Google Scholar] [CrossRef]
- Rosales, M.; Zoltan, T.; Yadarola, C.; Mosquera, E.; Gracia, F.; García, A. The influence of the morphology of 1D TiO2 nanostructures on photogeneration of reactive oxygen species and enhanced photocatalytic activity. J. Mol. Liq. 2019, 281, 59–69. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pakma, O.; Serin, N.; Serin, T. The effect of repeated annealing temperature on the structural, optical, and electrical properties of TiO2 thin films prepared by dip-coating sol–gel method. J. Mater. Sci. 2009, 44, 401–407. [Google Scholar] [CrossRef]
- Marami, M.B.; Farahmandjou, M.; Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. A spectroscopic ellipsometry study of TiO2 thin films prepared by ion-assisted electron-beam evaporation. Curr. Appl. Phys. 2009, 9, 707–712. [Google Scholar] [CrossRef]
- Arbiol, J.; Cerdà, J.; Dezanneau, G.; Cirera, A.; Peiró, F.; Cornet, A.; Morante, J.R. Effects of Nb doping on the TiO2 anatase-to-rutile phase transition. J. Appl. Phys. 2002, 92, 853–861. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Zidi, Y.; Khaldi, O.; Patru, R.E.; Leonat, L.N.; Enculescu, M.; Toma, V.; Stepanova, A.; Ben Younes, R.; Galca, A.C. Experimental and theoretical perspective on band gap modulation in Sr2+ modified BaTiO3 capacitors. Ceram. Int. 2025, 51, 18166–18177. [Google Scholar] [CrossRef]
- Rathore, N.; Kulshreshtha, A.; Shukla, R.K.; Sharma, D. Study on morphological, structural and dielectric properties of sol-gel derived TiO2 nanocrystals annealed at different temperatures. Phys. B Condens. Matter 2020, 582, 411969. [Google Scholar] [CrossRef]
- Xu, W.; Russo, P.A.; Schultz, T.; Koch, N.; Pinna, N. Niobium-Doped Titanium Dioxide with High Dopant Contents for Enhanced Lithium-Ion Storage. ChemElectroChem 2020, 7, 4016–4023. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; de Jesus, M.A.M.L.; Ferlauto, A.S.; Viana, M.M.; Mohallem, N.D.S. Characterization and application of niobium-doped titanium dioxide thin films prepared by sol–gel process. Appl. Phys. A 2021, 127, 641. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater. Sci. Semicond. Process. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Bhembe, Y.A.; Dlamini, L.N. Photoreduction of chromium (VI) by a composite of niobium (V) oxide impregnated with a Ti-based MOF. J. Environ. Sci. Health Part A 2020, 55, 1003–1020. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; Olusegun, S.J.; Gabriel, J.B.; Costa, R.C.V.; Mohallem, N.D.S. The role of crystalline Nb2O5 nanoparticles for enhanced dye adsorption and photodegradation. Ceram. Int. 2023, 49, 6164–6176. [Google Scholar] [CrossRef]
- Zani, V.; Pedron, D.; Pilot, R.; Signorini, R. Contactless Temperature Sensing at the Microscale Based on Titanium Dioxide Raman Thermometry. Biosensors 2021, 11, 102. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Alakhras, L.A.; Al-Bataineh, Q.M.; Telfah, A. Impact of metal doping on the physical characteristics of anatase titanium dioxide (TiO2) films. J. Mater. Sci. Mater. Electron. 2023, 34, 1552. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Su, M.; Li, D.; Li, G.; Li, C.; Tian, Y.; Hao, C.; Lei, Q. Enhanced Electrorheological Performance of Nb-Doped TiO2 Microspheres Based Suspensions and Their Behavior Characteristics in Low-Frequency Dielectric Spectroscopy. ACS Appl. Mater. Interfaces 2015, 7, 26624–26632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wen, Y.; Jiang, P.; Peng, X.; Zeng, F.; Lin, H.; Mu, L.; Lu, X.; Ji, T.; Zhu, J. Nb-Doping-Induced Transformation of Lewis and Brønsted Acidic Sites on Sulfonated TiO2 for Highly Efficient HMF Synthesis. ACS Sustain. Chem. Eng. 2025, 13, 3374–3383. [Google Scholar] [CrossRef]
- Yue, J.; Suchomski, C.; Voepel, P.; Ellinghaus, R.; Rohnke, M.; Leichtweiss, T.; Elm, M.T.; Smarsly, B.M. Mesoporous niobium-doped titanium dioxide films from the assembly of crystalline nanoparticles: Study on the relationship between the band structure, conductivity and charge storage mechanism. J. Mater. Chem. A 2016, 5, 1978–1988. [Google Scholar] [CrossRef]
- Rosales, M.; Orive, J.; Espinoza-González, R.; de Luis, R.F.; Gauvin, R.; Brodusch, N.; Rodríguez, B.; Gracia, F.; García, A. Evaluating the bi-functional capacity for arsenic photo-oxidation and adsorption on anatase TiO2 nanostructures with tunable morphology. Chem. Eng. J. 2021, 415, 128906. [Google Scholar] [CrossRef]
- Hossain, M.F.; Pervez, M.S.; Nahid, M.A.I. Influence of Film Thickness on Optical and Morphological Properties of TiO2 Thin Films. Emerg. Mater. Res. 2020, 9, 186–191. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Martins, P.M.; Tavares, C.J.; Lanceros-Méndez, S. Quercetin-mediated green synthesis of Au/TiO2 nanocomposites for the photocatalytic degradation of antibiotic ciprofloxacin. J. Ind. Eng. Chem. 2025, 143, 526–537. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, W.; Lan, T.; Xie, J.; Xie, W.; Liu, Z.; Huang, Y.; Wei, M. Anatase TiO2 Quantum Dots with a Narrow Band Gap of 2.85 eV Based on Surface Hydroxyl Groups Exhibiting Significant Photodegradation Property. Eur. J. Inorg. Chem. 2018, 2018, 1506–1510. [Google Scholar] [CrossRef]
- Kubacka, A.; Colón, G.; Fernández-García, M. Cationic (V, Mo, Nb, W) doping of TiO2–anatase: A real alternative for visible light-driven photocatalysts. Catal. Today 2009, 143, 286–292. [Google Scholar] [CrossRef]
- Kamat, P.V.; Dimitrijevic, N.M.; Nozik, A.J. Dynamic Burstein-Moss shift in semiconductor colloids. J. Phys. Chem. 1989, 93, 2873–2875. [Google Scholar] [CrossRef]
- Mercado, C.; Seeley, Z.; Bandyopadhyay, A.; Bose, S.; McHale, J.L. Photoluminescence of Dense Nanocrystalline Titanium Dioxide Thin Films: Effect of Doping and Thickness and Relation to Gas Sensing. ACS Appl. Mater. Interfaces 2011, 3, 2281–2288. [Google Scholar] [CrossRef]
- Rosales, M.; Gauvin, R.; Espinoza-González, R.; Lanceros-Méndez, S.; de Luis, R.F.; Vicente, J.; Brodusch, N.; Bessette, S.; Rodríguez, B.; Estay, H.; et al. Unveiling autarchic photo-thermal capabilities of morphology-tailored SnO2 nanostructures for boosting arsenic photo-oxidation. J. Environ. Chem. Eng. 2025, 13, 115736. [Google Scholar] [CrossRef]
- Tong, Q.; Qiu, C.; Zheng, G.; Zhu, Q.; Zhou, S.; Wang, Y.; Shi, L.; Wang, H.; He, D.; Sadakane, M.; et al. Higher acidity promoted photodegradation of fluoroquinolone antibiotics under visible light by strong interaction with a niobium oxide based zeolitic octahedral metal oxide. Appl. Catal. A Gen. 2023, 662, 119284. [Google Scholar] [CrossRef]
- Yin, M.; Liu, X.; Hu, L.; Xu, L.; He, J. Effects of Nb doping on microstructure and photocatalytic properties of TiO2 thin film. Desalination Water Treat. 2016, 57, 6910–6915. [Google Scholar] [CrossRef]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Lanceros-Mendez, S.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Michalow, K.A.; Flak, D.; Heel, A.; Parlinska-Wojtan, M.; Rekas, M.; Graule, T. Effect of Nb doping on structural, optical and photocatalytic properties of flame-made TiO2 nanopowder. Environ. Sci. Pollut. Res. 2012, 19, 3696–3708. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Singh, S.V. La doped TiO2 nanoparticles for photocatalysis: Synthesis, activity in terms of degradation of Methylene Blue dye and regeneration of used nanoparticles. Arab. J. Sci. Eng. 2023, 48, 16431–16443. [Google Scholar] [CrossRef]
- Dash, P.; Panda, P.K.; Su, C.; Lin, Y.-C.; Sakthivel, R.; Chen, S.-L.; Chung, R.-J. Near-infrared-driven upconversion nanoparticles with photocatalysts through water-splitting towards cancer treatment. J. Mater. Chem. B 2024, 12, 3881–3907. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Liu, X.D.; Jiang, E.Y.; Li, Z.Q.; Song, Q.G. Electronic structure and optical properties of Nb-doped anatase TiO2. Applied Physics Letters 2008, 92, 252104. [Google Scholar] [CrossRef]
- Hou, Q.Y.; Liu, Q.L.; Zhao, C.W.; Zhang, Y. First-principle study of the electronic structure and optical property of Nb-doped anatase TiO2. Int. J. Mod. Phys. B 2014, 28, 1450091. [Google Scholar] [CrossRef]
- Zhang, R.-S.; Liu, Y.; Gao, Q.; Teng, F.; Song, C.-L.; Wang, W.; Han, G.-R. First-principles study on the electronic and optical properties of F- and Nb-doped anatase TiO2. J. Alloys Compd. 2011, 509, 9178–9182. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Zhu, L.; Song, J.; Qiang, Y. Performance enhancement of perovskite solar cells via Nb/Ta-doped TiO2 mesoporous layers. J. Mater. Sci. Mater. Electron. 2019, 30, 9038–9044. [Google Scholar] [CrossRef]

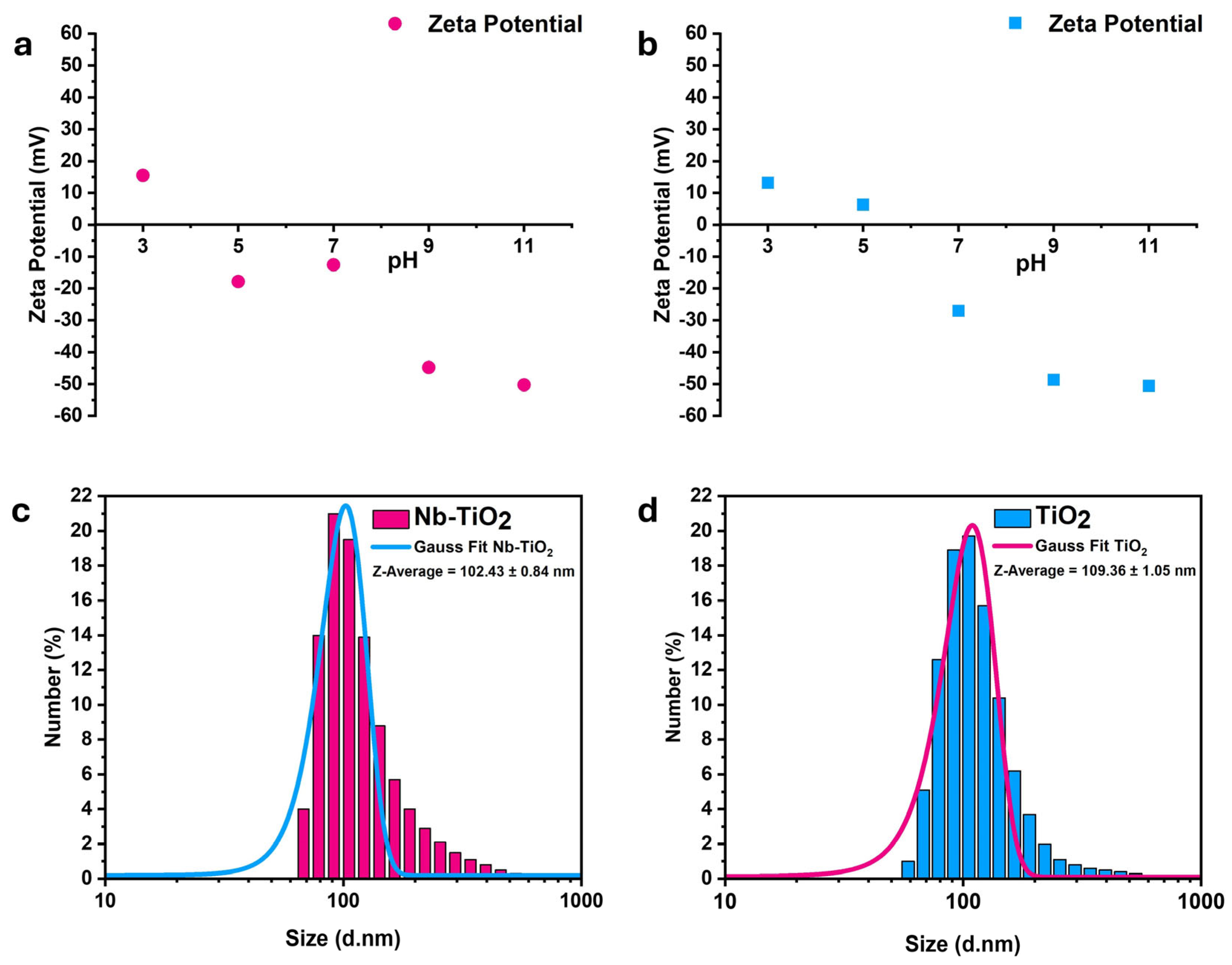
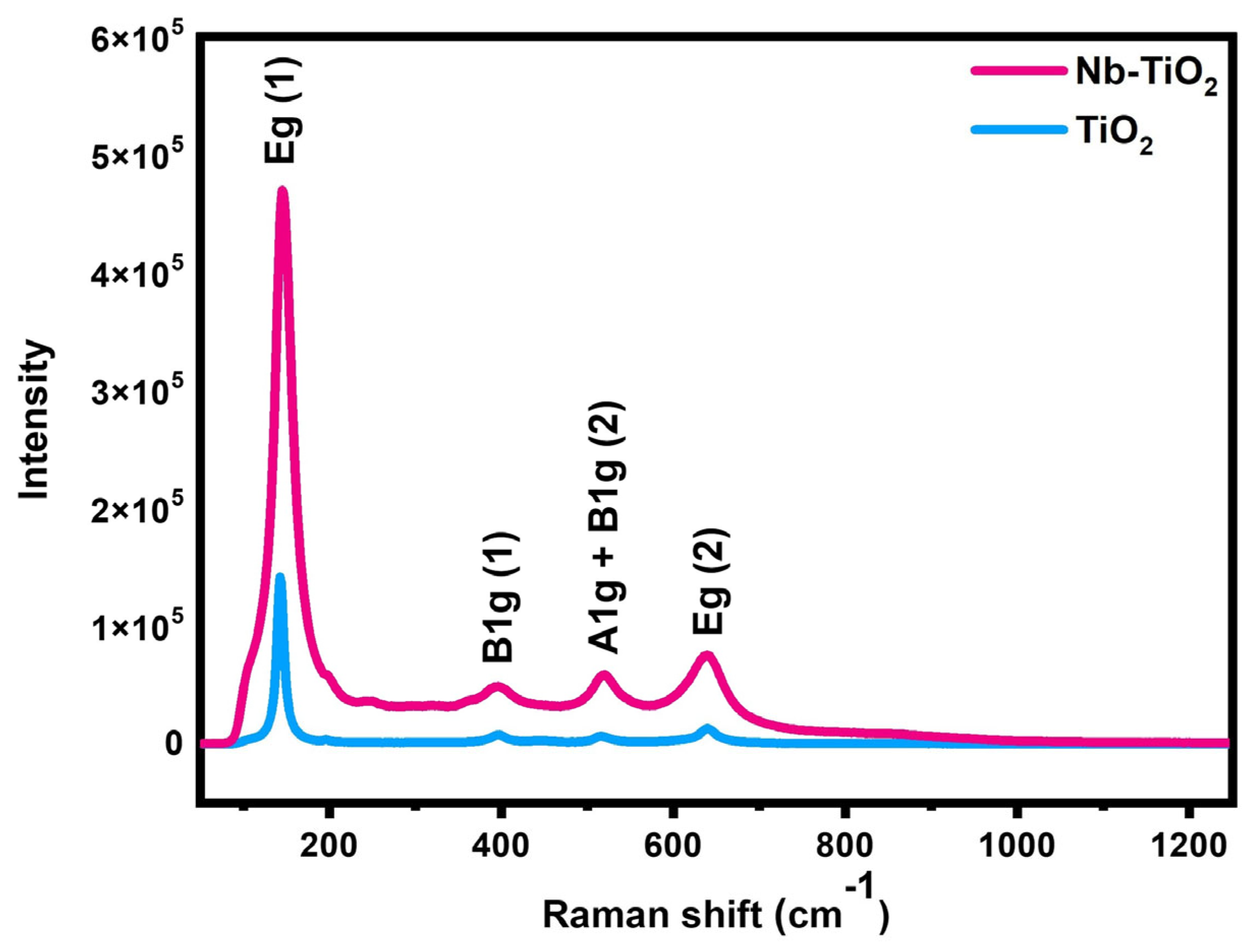
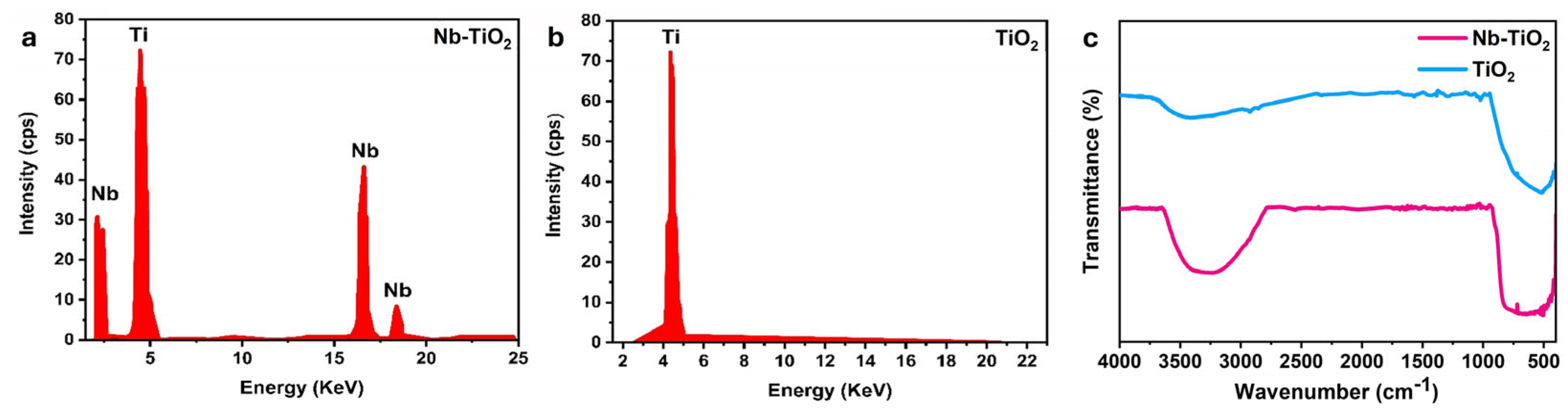
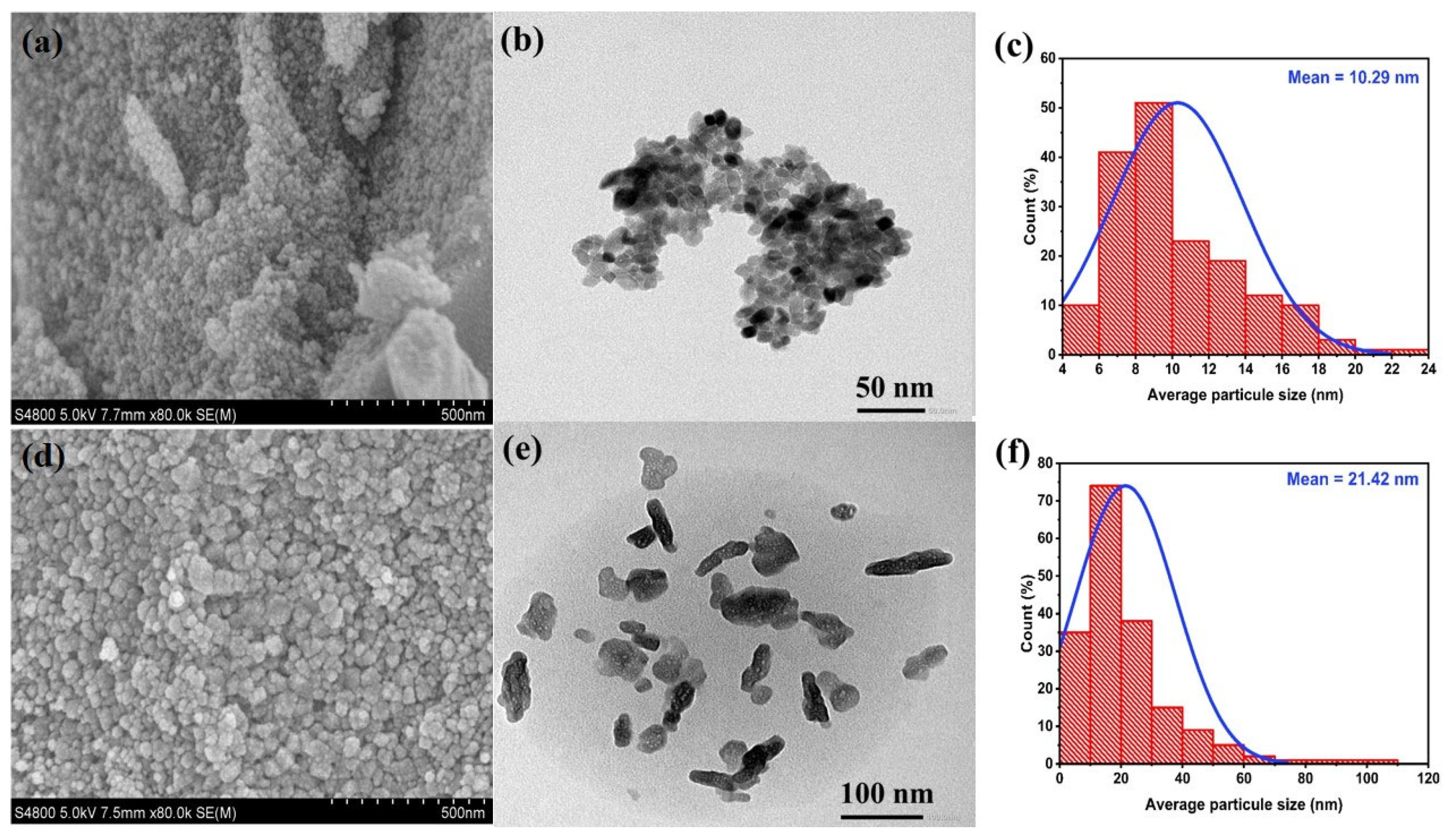
| Samples | a = b (Å) | C (Å) | Average D (nm) | Volume V (Å3) |
|---|---|---|---|---|
| Nb-TiO2 | 3.78 | 9.5 | 4.69 | 135.73 |
| TiO2 | 3.77 | 9.47 | 13.64 | 134.59 |
| Samples | Under UV Irradiation | Under Vis Irradiation | ||
|---|---|---|---|---|
| kapp × 10−2 (min−1) | R2 | kapp × 10−2 (min−1) | R2 | |
| Nb-TiO2 | 1.4 | 0.99 | 1.5 | 0.98 |
| TiO2 | 1.6 | 0.96 | 0.1 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shili, B.; Khaldi, O.; Mendes-Felipe, C.; Rosales, M.; Alves, D.C.; Martins, P.M.; Ben Younes, R.; Lanceros-Mendez, S. Synthesis of Nb-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and DFT Approach. Nanomaterials 2025, 15, 1307. https://doi.org/10.3390/nano15171307
Shili B, Khaldi O, Mendes-Felipe C, Rosales M, Alves DC, Martins PM, Ben Younes R, Lanceros-Mendez S. Synthesis of Nb-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and DFT Approach. Nanomaterials. 2025; 15(17):1307. https://doi.org/10.3390/nano15171307
Chicago/Turabian StyleShili, Bouthaina, Othmen Khaldi, Cristian Mendes-Felipe, Maibelin Rosales, Dinis C. Alves, Pedro M. Martins, Rached Ben Younes, and Senentxu Lanceros-Mendez. 2025. "Synthesis of Nb-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and DFT Approach" Nanomaterials 15, no. 17: 1307. https://doi.org/10.3390/nano15171307
APA StyleShili, B., Khaldi, O., Mendes-Felipe, C., Rosales, M., Alves, D. C., Martins, P. M., Ben Younes, R., & Lanceros-Mendez, S. (2025). Synthesis of Nb-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and DFT Approach. Nanomaterials, 15(17), 1307. https://doi.org/10.3390/nano15171307









