Multimodal Fusion-Driven Pesticide Residue Detection: Principles, Applications, and Emerging Trends
Abstract
1. Introduction
2. Classification of Pesticide Detection Technologies
2.1. Chromatography-Mass Spectrometry
2.2. Spectral Analysis Techniques
2.2.1. Hyperspectral Imaging Technology
Principle
Hyperspectral Imaging Technology in Agricultural Product Safety Testing
2.2.2. SERS Technology
Principles and Classification
- (1)
- Flexible smart substrates utilize environmentally responsive materials (e.g., polyacrylamide hydrogels) to dynamically modulate nanoparticle spacing through solvent-induced deformation. This significantly enhances hot-spot density, making them particularly suitable for irregular surface detection [30,31].
- (2)
- Rigid nanostructured substrates (e.g., dendritic AgNPs, Au@Ag core–shell structures) optimize electromagnetic field distribution through precise morphological control (e.g., dendritic architecture, core–shell thickness). This approach elevates detection sensitivity by three orders of magnitude [32,33,34].
- (3)
- (4)
- Porous adsorptive substrates (e.g., activated carbon-supported AC@AgNPs, mesoporous scaffold-based AuNPs/MSN) leverage the enrichment capability of high-surface-area materials (e.g., mesoporous silica) combined with plasmonic effects. This synergy achieves detection limits as low as 0.01 ppb for certain organophosphorus pesticides [28,37].
SERS in Agricultural Product Safety Testing

2.2.3. Polarization Spectroscopy and Fluorescence Technology
2.3. Biosensing Technology
2.3.1. Adaptive Sensor
2.3.2. Enzyme Sensor
AChE Sensors
Oxidase Sensor
3. Emerging Technologies and Trends
3.1. Nanotechnology
3.1.1. Innovative Applications of Quantum Dot Fluorescent Probes
3.1.2. Integrated Innovation of Multifunctional Nano-Detection Platforms
3.1.3. Exploration of Emerging Detection Principles
3.2. Artificial Intelligence and Big Data
3.2.1. Intelligent Spectral Analysis Technology
3.2.2. Innovative Applications of Electronic Sensory Systems
3.2.3. Algorithm Innovation and Engineering Optimization
3.3. Development of On-Site Rapid Detection Technologies
Innovative Applications of Microfluidic Technology
4. Comparison of Various Detection Methods
5. Research Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Duan, J.; Gao, Q.; Zhang, Z. Degradation of pesticides in wheat flour during noodle production and storage. Food Addit. Contam. Part A 2022, 39, 1239–1247. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Li, Y.; Yang, W.; Jiang, W.; Liang, Y.; Zhang, Z. Residue behaviors of six pesticides during apple juice production and storage. Food Res. Int. 2024, 177, 113894. [Google Scholar] [CrossRef]
- Duan, J.; Gao, Q.; Shi, L.; Li, Y.; Zhang, Z.; Liang, Y. Residue changes of five pesticides during the production and storage of rice flour. Food Addit. Contam. Part A 2022, 39, 542–550. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Okonkwo, C.E.; Chen, L.; Zhou, C. Multimode ultrasonic-assisted decontamination of fruits and vegetables: A review. Food Chem. 2024, 450, 139356. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Uttl, L.; Dzuman, Z.; Pulkrabova, J.; Hajslova, J. A critical comparison between an ultra-high-performance liquid chromatography triple quadrupole mass spectrometry (UHPLC-QqQ-MS) method and an enzyme assay for anti-cholinesterase pesticide residue detection in cereal matrices. Anal. Methods 2022, 14, 1479–1489. [Google Scholar] [CrossRef]
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Stanley, S.L.; Siddique, M.A.B.; Mujumdar, A.S.; Zhu, J. Multi-frequency multi-mode ultrasound treatment for removing pesticides from lettuce (Lactuca sativa L.) and effects on product quality. LWT 2021, 143, 111147. [Google Scholar] [CrossRef]
- Farooq, S.; Chen, B.; Gao, F.; Muhammad, I.; Ahmad, S.; Wu, H. Development of Molecularly Imprinted Polymers for Fenthion Detection in Food and Soil Samples. Nanomaterials 2022, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Hu, P.; Dai, R.; Wu, B.; Yang, L.; Huang, Y.; Zhuang, G. Recent developments in the spectrometry of fluorescence, ultraviolet visible and surface-enhanced Raman scattering for pesticide residue detection. Bull. Mater. Sci. 2022, 45, 202. [Google Scholar] [CrossRef]
- Sharma, A.; Dubey, J.K.; Katna, S.; Shandil, D.; Brar, G.S.; Singh, S. Validation of Analytical Methods Used for Pesticide Residue Detection in Fruits and Vegetables. Food Anal. Methods 2021, 14, 1919–1926. [Google Scholar] [CrossRef]
- Dong, Z.; Lu, J.; Wu, Y.; Meng, M.; Yu, C.; Sun, C.; Chen, M.; Da, Z.; Yan, Y. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, X.; Yuan, T.; Wang, F.; Hu, D.; Lu, P. Absolute Configuration, Enantioselective Bioactivity, and Mechanism Study of the Novel Chiral Fungicide Benzovindiflupyr. J. Agric. Food. Chem. 2023, 71, 8808–8815. [Google Scholar] [CrossRef]
- Affricano, A.; Serra, S.; Bernardo, A.D.; Aigotti, R.; Floris, F.; Bello, F.D.; Medana, C. Stir Bar Sorptive Extraction (SBSE)-HPLC-Tandem MS-Based Method for Multi-Residue Determination of Pesticides in Drinking Water. Mass Spectrom. 2025, 14, A0172. [Google Scholar] [CrossRef] [PubMed]
- Vicari, M.C.; Facco, J.F.; Peixoto, S.C.; de Carvalho, G.S.; Floriano, L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Simultaneous Determination of Multiresidues of Pesticides and Veterinary Drugs in Agricultural Soil Using QuEChERS and UHPLC–MS/MS. Separations 2024, 11, 188. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Ren, X.; Li, W.; Chen, L.; Zhao, L. Fate and occurrence of indoxacarb during radish cultivation for multi-risk assessment. Ecotoxicol. Environ. Saf. 2023, 259, 115065. [Google Scholar] [CrossRef]
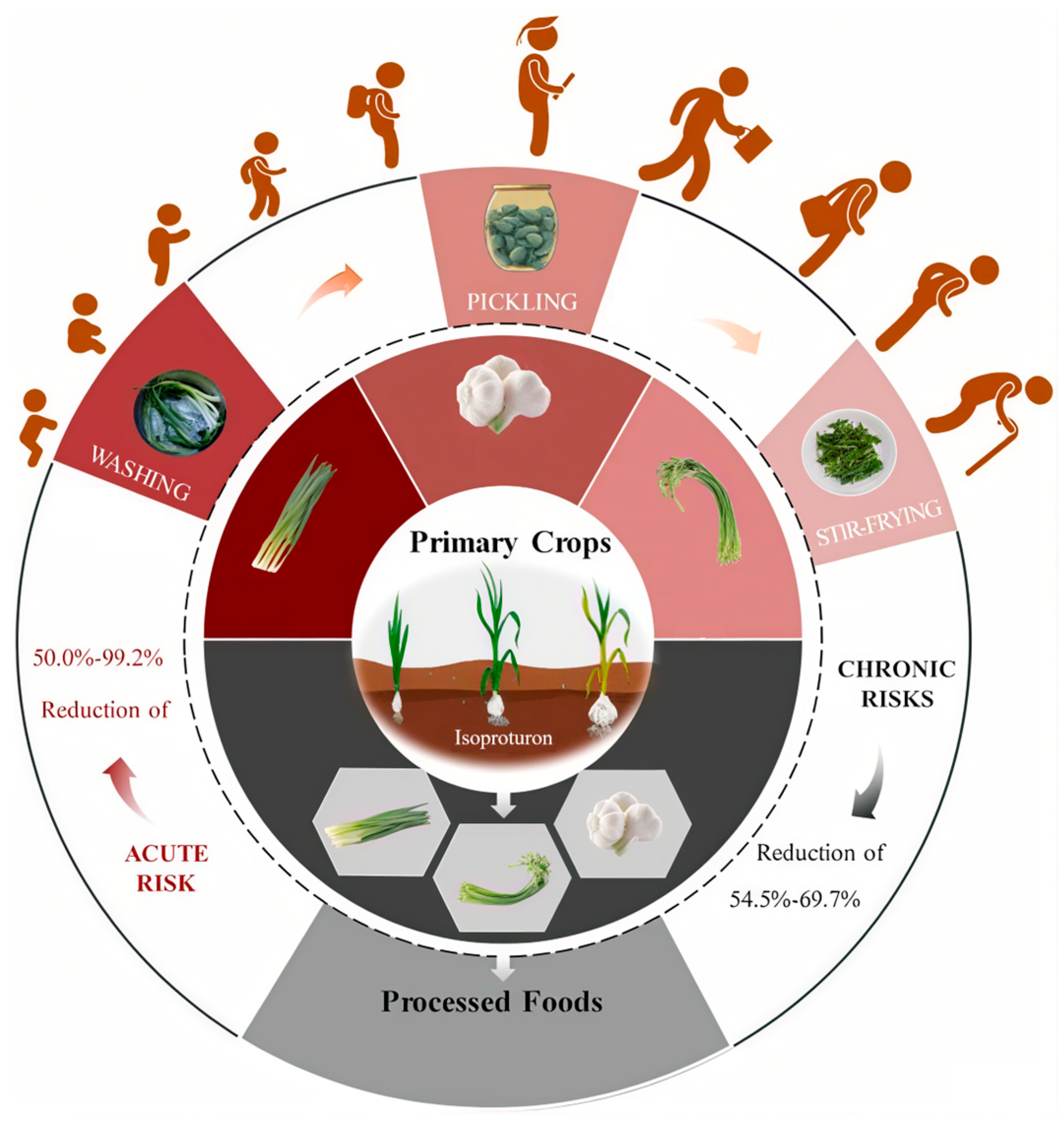
- Kang, S.; Li, L.; Ren, X.; Zhang, M.; Li, W.; Chen, Z. Occurrence and fate characteristics of isoproturon from garlic cultivation to household processing: Implication for human exposure. J. Hazard. Mater. 2023, 448, 130936. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hu, L.; Ding, F.; Liu, J.; Su, J.; Tu, K.; Peng, J.; Lan, W.; Pan, L. Introducing high-performance star-shaped bimetallic nanotags into SERS aptasensor: An ultrasensitive and interference-free method for chlorpyrifos detection. Biosens. Bioelectron. 2024, 263, 116577. [Google Scholar] [CrossRef]
- Sun, J.; Ge, X.; Wu, X.; Dai, C.; Yang, N. Identification of pesticide residues in lettuce leaves based on near infrared transmission spectroscopy. J. Food Process Eng. 2018, 41, 12816. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Wu, B.; Zhou, H. Identification of lambda-cyhalothrin residues on Chinese cabbage using fuzzy uncorrelated discriminant vector analysis and MIR spectroscopy. Int. J. Agric. Biol. Eng. 2022, 15, 217–224. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Wu, B.; Tan, Y.; Liu, J. Qualitative Analysis of Lambda-Cyhalothrin on Chinese Cabbage Using Mid-Infrared Spectroscopy Combined with Fuzzy Feature Extraction Algorithms. Agriculture 2021, 11, 275. [Google Scholar] [CrossRef]
- Adade, S.Y.-S.S.; Lin, H.; Johnson, N.A.N.; Nunekpeku, X.; Aheto, J.H.; Ekumah, J.-N.; Kwadzokpui, B.A.; Teye, E.; Ahmad, W.; Chen, Q. Advanced food contaminant detection through multi-source data fusion: Strategies, applications, and future perspectives. Trends Food Sci. Technol. 2025, 156, 104851. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Tan, M.; Xia, H.; Peng, Y.; Fu, X.; Huang, Y.; Yang, X.; Ma, X. Rapid pesticide residues detection by portable filter-array hyperspectral imaging. Spectrochim. Acta Part A 2025, 330, 125703. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, J.; Xin, Z.; Mao, H.; Wu, X.; Li, Q. Visualizing distribution of pesticide residues in mulberry leaves using NIR hyperspectral imaging. J. Food Process Eng. 2016, 40, 12510. [Google Scholar] [CrossRef]
- Sun, J.; Cong, S.; Mao, H.; Wu, X.; Yang, N. Quantitative detection of mixed pesticide residue of lettuce leaves based on hyperspectral technique. J. Food Process Eng. 2017, 41, 12654. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Mao, H.; Wu, X.; Zhang, X.; Li, Q. Discrimination of pesticide residues in lettuce based on chemical molecular structure coupled with wavelet transform and near infrared hyperspectra. J. Food Process Eng. 2016, 40, 12509. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Mao, H.; Wu, X.; Zhang, X.; Gao, H. Identification of pesticide residue level in lettuce based on hyperspectra and chlorophyll fluorescence spectra. Int. J. Agric. Biol. Eng. 2016, 9, 231–239. [Google Scholar]
- Han, F.; Huang, X.; Mahunu, G.K. Exploratory review on safety of edible raw fish per the hazard factors and their detection methods. Trends Food Sci. Technol. 2017, 59, 37–48. [Google Scholar] [CrossRef]
- Li, H.; Hassan, M.M.; He, Z.; Haruna, S.A.; Chen, Q.; Ding, Z. A sensitive silver nanoflower-based SERS sensor coupled novel chemometric models for simultaneous detection of chlorpyrifos and carbendazim in food. LWT 2022, 167, 113804. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, X.; Jayan, H.; Yin, L.; Xue, S.; El-Seedi, H.R.; Zou, X. Recent developments and applications of surface enhanced Raman scattering spectroscopy in safety detection of fruits and vegetables. Food Chem. 2024, 434, 137469. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, J.; Sun, M.; He, J.; Liu, J.; Mi, J.M.; Zhao, K.; Su, J.; Tu, K.; Peng, J.; et al. Isotropic shrinkage-inspired strategy for plasmonic nanoparticle-loaded hydrogel SERS sensor towards robust and sensitive detection of pesticides. Chem. Eng. J. 2025, 509, 161380. [Google Scholar] [CrossRef]
- Ashiagbor, K.; Jayan, H.; Yosri, N.; Amaglo, N.K.; Zou, X.; Guo, Z. Advancements in SERS based systematic evolution of ligands by exponential enrichment for detection of pesticide residues in fruits and vegetables. Food Chem. 2025, 463, 141394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, L.; Jayan, H.; Jiang, S.; El-Seedi, H.R.; Zou, X.; Guo, Z. In situ self-cleaning PAN/Cu2O@Ag/Au@Ag flexible SERS sensor coupled with chemometrics for quantitative detection of thiram residues on apples. Food Chem. 2025, 473, 143032. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2021, 338, 127796. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, W.; Hassan, M.M.; Zhang, Z.; Chen, Q. A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2018, 13, 259–268. [Google Scholar] [CrossRef]
- Ma, L.; Han, E.; Yin, L.; Xu, Q.; Zou, C.; Bai, J.; Wu, W.; Cai, J. Simultaneous detection of mixed pesticide residues based on portable Raman spectrometer and Au@Ag nanoparticles SERS substrate. Food Control 2023, 153, 109951. [Google Scholar] [CrossRef]
- Yi, X.; Yuan, Z.; Yu, X.; Zheng, L.; Wang, C. Novel Microneedle Patch-Based Surface-Enhanced Raman Spectroscopy Sensor for the Detection of Pesticide Residues. ACS Appl. Mater. Interfaces 2023, 15, 4873–4882. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Zhang, X.; Shen, Y.; Khalifa, S.A.M.; Huang, X.; Shi, J.; Li, Z.; Zou, X. Green and sustainable self-cleaning flexible SERS base: Utilized for cyclic-detection of residues on apple surface. Food Chem. 2024, 441, 138345. [Google Scholar] [CrossRef]
- Yang, H.; Qian, H.; Xu, Y.; Zhai, X.; Zhu, J. A Sensitive SERS Sensor Combined with Intelligent Variable Selection Models for Detecting Chlorpyrifos Residue in Tea. Foods 2024, 13, 2363. [Google Scholar] [CrossRef]
- Navitski, I.; Ramanaviciute, A.; Ramanavicius, S.; Pogorielov, M.; Ramanavicius, A. MXene-Based Chemo-Sensors and Other Sensing Devices. Nanomaterials 2024, 14, 447. [Google Scholar] [CrossRef]
- Jiao, T.; Mehedi Hassan, M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of deltamethrin residues in wheat by Ag@ZnO NFs-based surface-enhanced Raman spectroscopy coupling chemometric models. Food Chem. 2021, 337, 127652. [Google Scholar] [CrossRef]
- Sun, G.; Li, N.; Wang, D.; Xu, G.; Zhang, X.; Gong, H.; Li, D.; Li, Y.; Pang, H.; Gao, M.; et al. A Novel 3D Hierarchical Plasmonic Functional Cu@Co3O4@Ag Array as Intelligent SERS Sensing Platform with Trace Droplet Rapid Detection Ability for Pesticide Residue Detection on Fruits and Vegetables. Nanomaterials 2021, 11, 3460. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, G.; Zhu, Y.; Wang, Y.; Ma, W. Au-Decorated Dragonfly Wing Bioscaffold Arrays as Flexible Surface-Enhanced Raman Scattering (SERS) Substrate for Simultaneous Determination of Pesticide Residues. Nanomaterials 2018, 8, 289. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Tian, X.; Zhang, X.; Zhang, W.; Yu, S. Activated carbon@silver nanoparticles conjugates as SERS substrate for capturing malathion analyte molecules for SERS detection. J. Food Saf. 2023, 43, 13072. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, Y.; Shang, Z.; Liu, X.; Lu, C.; Chen, H.; Liang, P.; Ma, G. Fabrication of core shell Au@Ag supraparticles with 3D hotspots via evaporation self-assembly for sensitive surface enhanced Raman scattering detection. Sens. Actuators B 2023, 382, 133529. [Google Scholar] [CrossRef]
- Zhu, J.; Agyekum, A.A.; Kutsanedzie, F.Y.H.; Li, H.; Chen, Q.; Ouyang, Q.; Jiang, H. Qualitative and quantitative analysis of chlorpyrifos residues in tea by surface-enhanced Raman spectroscopy (SERS) combined with chemometric models. LWT 2018, 97, 760–769. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, D.; Wu, Z.; Ni, D.; Yu, Z.; Liang, P. Construction of an efficient molecular enrichment and magnetic separation flow with Fe3O4@Ag@COF for ratiometric SERS detection of methamidophos. Chem. Eng. J. 2024, 496, 154177. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Ali, S.; Li, H.; Ouyang, Q.; Chen, Q. Self-Cleaning-Mediated SERS Chip Coupled Chemometric Algorithms for Detection and Photocatalytic Degradation of Pesticides in Food. J. Agric. Food. Chem. 2021, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Everard, C.D.; Kim, M.S.; Siemens, M.C.; Cho, H.; Lefcourt, A.; O’Donnell, C.P. A multispectral imaging system using solar illumination to distinguish faecal matter on leafy greens and soils. Biosyst. Eng. 2018, 171, 258–264. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Yin, L.; Han, E.; Wang, C.; Zhou, R.; Bai, J.; Wang, Y.; Guo, Z.; Cai, J. Rapid dual-modal detection of two types of pesticides in fruits using SERS-based immunoassay. J. Food Compos. Anal. 2024, 136, 106781. [Google Scholar] [CrossRef]
- Fang, G.; Hasi, W.; Lin, X.; Han, S. Automated identification of pesticide mixtures via machine learning analysis of TLC-SERS spectra. J. Hazard. Mater. 2024, 474, 134814. [Google Scholar] [CrossRef]
- Xin, Z.; Jun, S.; Bing, L.; Xiaohong, W.; Chunxia, D.; Ning, Y. Study on pesticide residues classification of lettuce leaves based on polarization spectroscopy. J. Food Process Eng. 2018, 41, 12903. [Google Scholar] [CrossRef]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2025, 463, 141288. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Liu, N.; Shang, F. A detection method of two carbamate pesticides residues on tomatoes utilizing excitation-emission matrix fluorescence technique. Microchem. J. 2021, 164, 105920. [Google Scholar] [CrossRef]
- Ji, R.; Ma, S.; Yao, H.; Han, Y.; Yang, X.; Chen, R.; Yu, Y.; Wang, X.; Zhang, D.; Zhu, T.; et al. Multiple kinds of pesticide residue detection using fluorescence spectroscopy combined with partial least-squares models. Appl. Opt. 2020, 59, 1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Y.; Zhang, Z.; Shen, Y.; Li, Y.; Ma, T.; Zhang, Q.; Ying, Y.; Fu, Y. Portable and durable sensor based on porous MOFs hybrid sponge for fluorescent-visual detection of organophosphorus pesticide. Biosens. Bioelectron. 2022, 216, 114659. [Google Scholar] [CrossRef] [PubMed]
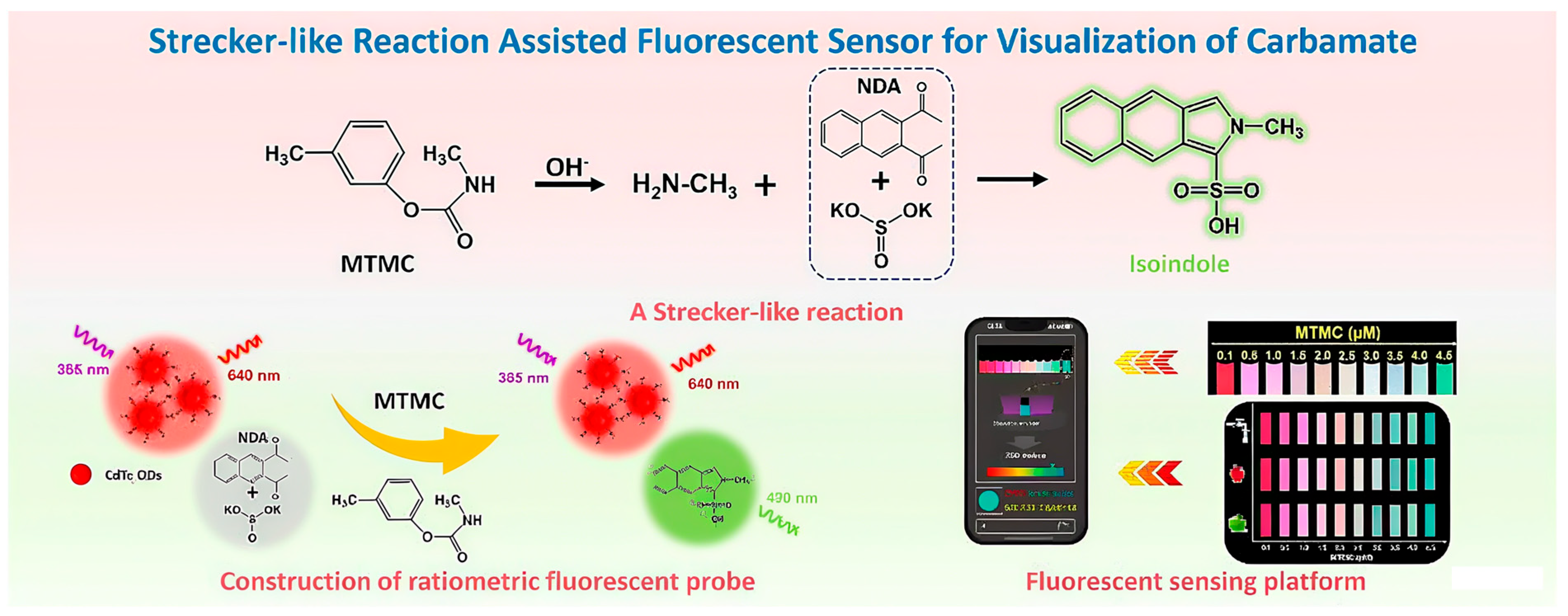
- Qin, L.; Cao, J.; Lin, D.; Xu, S.; Li, Y.; Jiang, C. A Strecker-like reaction triggering fluorescent sensing platform for enzyme-free and visual quantitative monitoring of carbamates. Chem. Eng. J. 2023, 464, 142550. [Google Scholar] [CrossRef]
- Lin, D.; Li, L.; Song, X.; Xu, S.; Zhang, Q.; Hu, Z.; Yang, L.; Jiang, C. “Light Up” Fluorescence Visual Sensitive Detection of Organophosphorus with a Smartphone-Based Platform Utilizing a Composite Rhodamine B-Ag@Au Nanoprobe. ACS Sustain. Chem. Eng. 2021, 9, 14579–14587. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef]
- Shoaib, M.; Li, H.; Zareef, M.; Khan, I.M.; Iqbal, M.W.; Niazi, S.; Raza, H.; Yan, Y.; Chen, Q. Recent Advances in Food Safety Detection: Split Aptamer-Based Biosensors Development and Potential Applications. J. Agric. Food. Chem. 2025, 73, 4397–4424. [Google Scholar] [CrossRef]
- Gao, S.; Yang, W.; Zheng, X.; Wang, T.; Zhang, D.; Zou, X. Advances of nanobody-based immunosensors for detecting food contaminants. Trends Food Sci. Technol. 2025, 156, 104871. [Google Scholar] [CrossRef]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent advances in foodborne pathogen detection using photoelectrochemical biosensors: From photoactive material to sensing strategy. Front. Sustain. Food Syst. 2024, 8, 1432555. [Google Scholar] [CrossRef]
- Wei, W.; Hassan, M.M.; Wu, J.; Mu, X.; Li, H.; Chen, Q. Competitive Ratiometric Aptasensing with Core-Internal Standard-Shell Structure Based on Surface-Enhanced Raman Scattering. J. Agric. Food. Chem. 2022, 71, 857–866. [Google Scholar] [CrossRef]
- Song, S.; Gao, Z.; Guo, X.; Chen, G. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Wang, L.; Haruna, S.A.; Ahmad, W.; Wu, J.; Chen, Q.; Ouyang, Q. Tunable multiplexed fluorescence biosensing platform for simultaneous and selective detection of paraquat and carbendazim pesticides. Food Chem. 2022, 388, 132950. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Beta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Muthu, A.; Elsakhawy, T.; Sheta, M.H.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials 2025, 15, 725. [Google Scholar] [CrossRef]
- Yin, L.; Hu, X.; Hao, M.; Shi, J.; Zou, X.; Dusabe, K.D. Upconversion nanoparticles-based background-free selective fluorescence sensor developed for immunoassay of fipronil pesticide. J. Food Meas. Charact. 2023, 17, 3125–3133. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Zou, X.; Shi, J.; Mao, H.; Zhao, J.; Hao, L.; Mel, H. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Wu, Y. A simple and rapid chemosensor for colorimetric detection of dimethoate pesticide based on the peroxidase-mimicking catalytic activity of gold nanoparticles. Anal. Methods 2019, 11, 5337–5347. [Google Scholar] [CrossRef]
- Xue, S.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. A film-like SERS aptasensor for sensitive detection of patulin based on GO@Au nanosheets. Food Chem. 2024, 441, 138364. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, W.; You, F.; Yuan, R.; Ding, L.; Hao, N.; Wei, J.; Wang, K. Ultrasensitive photoelectrochemical aptasensor for carbendazim detection based on in-situ constructing Schottky junction via photoreducing Pd nanoparticles onto CdS microsphere. Biosens. Bioelectron. 2022, 203, 114036. [Google Scholar] [CrossRef]
- Almenhali, A.Z.; Kanagavalli, P.; Abd-Ellah, M.; Khazaal, S.; El Darra, N.; Eissa, S. Reduced graphene oxide-based electrochemical aptasensor for the multiplexed detection of imidacloprid, thiamethoxam, and clothianidin in food samples. Sci. Rep. 2025, 15, 10329. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Lai, J.; Ding, L.; Liu, Y.; Fan, C.; You, F.; Wei, J.; Qian, J.; Wang, K. A miniaturized organic photoelectrochemical transistor aptasensor based on nanorod arrays toward high-sensitive T-2 toxin detection in milk samples. Food Chem. 2023, 423, 136285. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Rong, X.; Liu, L.; Yin, Y.; Zhao, X.; Weng, Y. DNA aptamer-crosslinked hydrogel sensor: Design, mechanism and application for food safety analysis. Trends Food Sci. Technol. 2025, 156, 104846. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Wu, J.; Khan, I.M.; Chen, M.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Upconversion fluorescence nanosensor based on enzymatic inhibited and copper-triggered o-phenylenediamine oxidation for the detection of dimethoate pesticides. Food Chem. 2024, 453, 139666. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; Yu, H.; She, Y.; Cao, Z.; Ye, J.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J.; Lao, S. An Overview on the Mechanisms and Applications of Enzyme Inhibition-Based Methods for Determination of Organophosphate and Carbamate Pesticides. J. Agric. Food. Chem. 2020, 68, 7298–7315. [Google Scholar] [CrossRef] [PubMed]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, X.; Yu, D.; Jiao, S.; Han, X.; Zhang, S.; Yin, H.; Mao, H. Pesticide residues identification by impedance time-sequence spectrum of enzyme inhibition on multilayer paper-based microfluidic chip. J. Food Process Eng. 2020, 43, 13544. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Hassan, M.M.; Guo, Z.; Zhang, Z.; Chen, Q. Fabricating an Acetylcholinesterase Modulated UCNPs-Cu2+ Fluorescence Biosensor for Ultrasensitive Detection of Organophosphorus Pesticides-Diazinon in Food. J. Agric. Food. Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef]
- Li, Q.; Hao, Z.; Zhang, C.; Ni, S.; Jiang, P.; Fan, P.; Li, L. Dual-Mode Detection of Glyphosate Based on DNAzyme-Mediated Click Chemistry and DNAzyme-Regulated CeO2 Peroxidase-like Activity. J. Agric. Food. Chem. 2025, 73, 7496–7503. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; She, Y.; Zheng, L.; Jin, F.; Shao, Y.; Wang, J.; Abd El-Aty, A.M. Highly Sensitive and Rapid Screening Technique for the Detection of Organophosphate Pesticides and Copper Compounds Using Bifunctional Recombinant TrxA-PvCarE1. J. Agric. Food. Chem. 2024, 72, 5003–5013. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Wang, H.; Luan, Y.; Zhang, Z. Porphyrin-based covalent organic framework as oxidase mimic for highly sensitive colorimetric detection of pesticides. Microchim. Acta 2024, 191, 296. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Waterhouse, G.I.N.; Wang, R.; Qiao, X.; Sun, Y.; Xu, Z. Bifunctional ZrO2@ZIF-90 nanozyme with high phosphohydrolase activity for sensitive electrochemical detection of methyl parathion. Food Sci. Hum. Wellness 2025, 14, 9250095. [Google Scholar] [CrossRef]
- Liu, S.; Nie, C.; He, F.; Wu, G.; Wang, H.; Li, S.; Du, C.; Zheng, Z.; Cheng, J.; Shen, Y.; et al. Oxidase-like nanozymes-driven colorimetric, fluorescence and electrochemiluminescence assays for pesticide residues. Trends Food Sci. Technol. 2024, 150, 104597. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Yang, S.; Fa, H.; Wang, Y.; Huo, D.; Hou, C.; Yang, M. Development of a novel ternary MOF nanozyme-based smartphone-integrated colorimetric and microfluidic paper-based analytical device for trace glyphosate detection. Food Chem. 2025, 464, 141780. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Tao, S.; Mao, H.; Wei, M.; Fu, J.; Song, W. An Integrated Platform and Method for Rapid High-Throughput Quantitative Detection of Organophosphorus Pesticide Residues. IEEE Trans. Instrum. Meas. 2024, 73, 1–11. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Wang, C.; Tian, X.; Yi, R.; Yuena, W. Fabrication and evaluation of chitosan modified filter paper for chlorpyrifos detection in wheat by surface-enhanced Raman spectroscopy. J. Sci. Food Agric. 2022, 102, 7323–7330. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Y.; Huang, Y.; Zhou, A.; Han, J.; Yang, K.; Zhao, Y.; Zhou, J.; Wang, J.; Chen, G.; et al. A pH-responsive MOFs@ MPN nanocarrier with enhancing antifungal activity for sustainable controlling myclobutanil release. Chem. Eng. J. 2024, 497, 155713. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, X.; Song, S.; Chen, G. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food. Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Y.; Li, L.; Lin, D.; Li, Y.; Xu, S.; Jiang, C. Ratiometric Fluorescent Sensor Based on Hydrogen-Bond Triggering the Internal Filter Effect for Enzyme-Free and Visual Monitoring Pesticide Residues. ACS Sustain. Chem. Eng. 2023, 11, 11032–11040. [Google Scholar] [CrossRef]
- Li, R.; Mu, X.; Xu, J.; Zeng, F. Silicon quantum dots based fluorescent probes for detecting methyl parathion pesticide residues in potato, tap water and Yellow River. Spectrochim. Acta Part A 2025, 325, 125071. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Hang, X.; Li, Z.; Zou, X. Convenient self-assembled PDADMAC/PSS/Au@Ag NRs filter paper for swift SERS evaluate of non-systemic pesticides on fruit and vegetable surfaces. Food Chem. 2023, 424, 136232. [Google Scholar] [CrossRef]
- Luo, D.; Huang, X.; Liu, B.; Zou, W.; Wu, Y. Facile Colorimetric Nanozyme Sheet for the Rapid Detection of Glyphosate in Agricultural Products Based on Inhibiting Peroxidase-Like Catalytic Activity of Porous Co3O4 Nanoplates. J. Agric. Food. Chem. 2021, 69, 3537–3547. [Google Scholar] [CrossRef]
- Yang, W.; Gao, M.; Zhang, Y.; Dai, Y.; Peng, W.; Ji, S.; Ji, Y.; Huang, W.; Xu, W. Self-driven photoelectrochemical sensor based on Z-type perovskite heterojunction for profenofos detection in milk and cabbage. J. Food Compos. Anal. 2024, 136, 106738. [Google Scholar] [CrossRef]
- Dai, J.; Chen, X.; Zhang, Y.; Zhang, M.; Dong, Y.; Zheng, Q.; Liao, J.; Zhao, Y. Machine learning-enhanced color recognition of test strips for rapid pesticide residue detection in fruits and vegetables. Food Control 2025, 174, 111256. [Google Scholar] [CrossRef]
- Adade, S.Y.-S.S.; Lin, H.; Johnson, N.A.N.; Afang, Z.; Chen, Z.; Haruna, S.A.; Ekumah, J.-N.; Agyekum, A.A.; Li, H.; Chen, Q. Rapid quantitative analysis of acetamiprid residue in crude palm oil using SERS coupled with random frog (RF) algorithm. J. Food Compos. Anal. 2024, 125, 105818. [Google Scholar] [CrossRef]
- Jiang, H.; Xue, Y.; Chen, Q. Quantitative analysis of residues of chlorpyrifos in corn oil based on Fourier transform near-infrared spectroscopy and deep transfer learning. Infrared Phys. Technol. 2023, 133, 104814. [Google Scholar] [CrossRef]
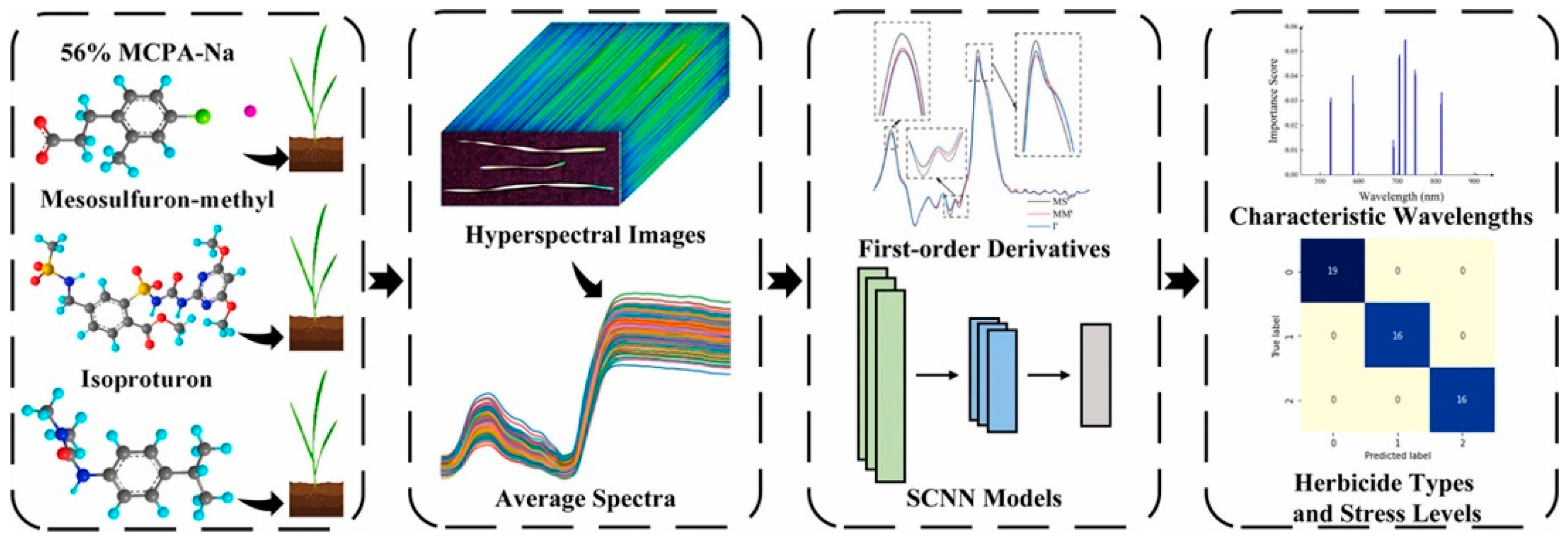
- Chu, H.; Zhang, C.; Wang, M.; Gouda, M.; Wei, X.; He, Y.; Liu, Y. Hyperspectral imaging with shallow convolutional neural networks (SCNN) predicts the early herbicide stress in wheat cultivars. J. Hazard. Mater. 2022, 421, 126706. [Google Scholar] [CrossRef]
- Durán Acevedo, C.M.; Cárdenas Niño, D.D.; Carrillo Gómez, J.K. Sensory Perception Systems and Machine Learning Methods for Pesticide Detection in Fruits. Appl. Sci. 2024, 14, 8074. [Google Scholar] [CrossRef]
- Jiang, R.; Zhuang, G.; Xie, S.; Wang, Y.; Zhang, G.; Qu, D.; Wen, W. Hyperspectral Detection of Pesticide Residues in Black Vegetable Based on Multi-Classifier Entropy Weight Method. IEEE Access 2025, 13, 55701–55711. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, H. Monitoring of Chlorpyrifos Residues in Corn Oil Based on Raman Spectral Deep-Learning Model. Foods 2023, 12, 2402. [Google Scholar] [CrossRef]
- Sun, J.; Cao, Y.; Zhou, X.; Wu, M.; Sun, Y.; Hu, Y. Detection for lead pollution level of lettuce leaves based on deep belief network combined with hyperspectral image technology. J. Food Saf. 2020, 41, 12866. [Google Scholar] [CrossRef]
- Wu, M.; Sun, J.; Lu, B.; Ge, X.; Zhou, X.; Zou, M. Application of deep brief network in transmission spectroscopy detection of pesticide residues in lettuce leaves. J. Food Process Eng. 2019, 42, 13005. [Google Scholar] [CrossRef]
- Li, H.; Luo, X.; Haruna, S.A.; Zareef, M.; Chen, Q.; Ding, Z.; Yan, Y. Au-Ag OHCs-based SERS sensor coupled with deep learning CNN algorithm to quantify thiram and pymetrozine in tea. Food Chem. 2023, 428, 136798. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Hua, L.; Guo, Z.; Jia, F.; Xu, X.; Wang, S.; Lu, Z. Nano-engineered fiber-based sensing frontiers: Revolutionizing on-site pesticide detection for global food-environment nexus challenges. Coord. Chem. Rev. 2025, 538, 216710. [Google Scholar] [CrossRef]
- Xie, J.; Pang, H.; Sun, R.; Wang, T.; Meng, X.; Zhou, Z. Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip. Micromachines 2021, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Xie, J.; Meng, X.; Sun, R.; Chen, J.; Guo, C.; Zhou, T. Portable organophosphorus pesticide detection device based on controllable microfluidic and luminol composite nanofibers. J. Food Eng. 2024, 364, 111810. [Google Scholar] [CrossRef]
- Yang, N.; Wang, P.; Xue, C.Y.; Sun, J.; Mao, H.P.; Oppong, P.K. A portable detection method for organophosphorus and carbamates pesticide residues based on multilayer paper chip. J. Food Process Eng. 2018, 41, 12867. [Google Scholar] [CrossRef]
- Xu, J.; Xie, J.; Zhang, X.; Pang, H.; Sun, R.; Zhou, F. Rapid fabrication of microfluidic mixer chip by non-isothermal molding method based on resistance heating principle and its application in pesticide residue detection. J. Micromech. Microeng. 2022, 32, 115002. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, M.; Li, R.; Zhao, Y.; Lu, Y.; Wang, T.; Du, L.; Wan, L. Research progress on pesticide residue detection based on microfluidic technology. Electrophoresis 2023, 44, 1377–1404. [Google Scholar] [CrossRef] [PubMed]


| Detection Scheme | Data Analysis Methods | Pesticides | Recovery Rate (%) | RSD (%) | LOD | Detection Time | Reference |
|---|---|---|---|---|---|---|---|
| SERS | Portable Raman spectrometer (785 nm), ImageJ (Bethesda, Maryland, USA) for nanoparticle sizing | Thiram, Thiabendazole (TBZ) | 82.78–115.35 | <10 | Thiram: 5.26 × 10−10 g/mL; TBZ: 3.00 × 10−8 g/mL | 12 min (dehydration) + 2 min (stand) | [30] |
| SERS with self-cleaning flexible sensor | CNN, CARS-PLS algorithms (R2 = 0.9963 for CNN) | Thiram | 88.32–111.80 | 2.92–4.91 | 0.020 mg/L | <10 min | [31] |
| Microneedle (MN) patch SERS | Confocal Raman spectrometer (633 nm) | Thiram, TBZ | - | - | Thiram: 10−7 M; TBZ: 10−8 M | 3 min | [35] |
| SERS-based immunoassay | Portable Raman spectrometer (785 nm), HALCON software (version:17.12, MVTec, München, Germany) for grayscale analysis | Acetamiprid, Carbendazim | 82.78–115.35 | <9 | Acetamiprid: 0.27 μg/kg; Carbendazim: 1.71 μg/kg | - | [49] |
| Fluorescent sensors | FRET, PET, IFE, AIE mechanisms, Stern-Volmer equation, lifetime measurements | OPs, carbamates, organochlorines, pyrethroids | 83.93–108.16 | 1.04–9 | Glyphosate: 0.000207 nM; Thiram: 7 nM | Seconds to minutes | [52] |
| Fluorescent MOF Sponge | Fluorescence spectroscopy | Methyl parathion | 90.1–107.5 | ≤5.9 | 4.95 ppb | 10 min | [55] |
| Ratiometric Fluorescent Probe | Smartphone RGB analysis | Carbamates (MTMC) | 90.1–107.5 | ≤5.9 | 18.6 nM | 8 min | [56] |
| ”Light Up” Fluorescent Sensor | Smartphone color recognition | Organophosphorus (OPs) | 89.4–110.5 | ≤6.2 | 7.89 nM | 20 min | [57] |
| Upconversion FRET Immunosensor | Fluorescence resonance energy transfer | Fipronil | 95.95–137.07 | ≤5.9 | 0.01 ppb | 30 min | [67] |
| Peroxidase-Mimicking Chemosensor | Differential pulse voltammetry (DPV) | Dimethoate | 89.4–110.5 | ≤6.2 | 4.7 ppb | 30 min | [70] |
| Photoelectrochemical Aptasensor | Photocurrent response | Carbendazim (CBZ) | 98.93–106.10 | ≤5.9 | 0.33 pM | 50 min | [72] |
| Electrochemical Aptasensor | DPV/CV | Imidacloprid, Thiamethoxam, Clothianidin | 76.0–118.7 | ≤8.16 | 6.3–7.1 pg/mL | 30 min | [73] |
| Impedimetric Aptasensor | Electrochemical impedance spectroscopy | Acetamiprid | 92.0–107.5 | ≤6.1 | 0.34 nM | 40 min | [76] |
| Upconversion fluorescence | Fluorescence resonance energy transfer (FRET), enzyme inhibition | Dimethoate | 78.8–105.9 | 2.5–9.5 | 0.008 ng/mL | 90 min | [78] |
| AChE-modulated fluorescence | Fluorescence “off-on-off” strategy | Diazinon | 84.3–105.9 | 2.5–9.5 | 0.05 ng/mL | 61 min | [82] |
| Bifunctional enzyme sensor | Fluorescence (IDA hydrolysis) and colorimetry (DTNB reduction) | 10 OPs (e.g., dichlorvos, paraoxon) + copper compounds | 80.08–112.38 | 0.6–15.99 | 0.00024 mg/L | 30–60 min | [84] |
| COF-based colorimetry | Oxidase-mimetic catalysis, superoxide radical/1O2 generation | Fipronil, chlorfenapyr, flufenoxuron, etc. | 80.0–82.4 | - | 2.7 ng/mL | 30 min | [85] |
| Ratiometric fluorescence probe | Smartphone RGB analysis, internal filter effect (IFE) | Methyl parathion (MP) | 98.9–105.0 | ≤4.5 | 8.9 nM | <2 s | [94] |
| SERS (Surface-Enhanced Raman Spectroscopy) | Portable Raman spectrometer (785 nm), UV-Vis, XRD, TEM/SEM | Methyl-parathion, thiram, chlorpyrifos | 81.77–126.80 | 8.58–9.29 | 0.051 ng/cm2 (thiram) | <10 min | [96] |
| Colorimetric nanozyme sheet | Smartphone imaging, UV-Vis spectrophotometry | Glyphosate | 89.0–96.1 | 1.89–5.38 | 0.175 mg/kg | 10 min | [97] |
| Paper-based SERS | “Drop-wipe-measure” method, Raman mapping | Imidacloprid, ferbam | 83.2–125 | - | 4.1 × 10−6 μg/mL | <5 min | [109] |
| Microfluidic mixer + Luminol | Photodetector voltage response | Quinalphos | - | - | 0.035 mg/L | 30 s | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Liu, Z.; Yang, F.; Bu, Q.; Song, X.; Yuan, S. Multimodal Fusion-Driven Pesticide Residue Detection: Principles, Applications, and Emerging Trends. Nanomaterials 2025, 15, 1305. https://doi.org/10.3390/nano15171305
Wang M, Liu Z, Yang F, Bu Q, Song X, Yuan S. Multimodal Fusion-Driven Pesticide Residue Detection: Principles, Applications, and Emerging Trends. Nanomaterials. 2025; 15(17):1305. https://doi.org/10.3390/nano15171305
Chicago/Turabian StyleWang, Mei, Zhenchang Liu, Fulin Yang, Quan Bu, Xianghai Song, and Shouqi Yuan. 2025. "Multimodal Fusion-Driven Pesticide Residue Detection: Principles, Applications, and Emerging Trends" Nanomaterials 15, no. 17: 1305. https://doi.org/10.3390/nano15171305
APA StyleWang, M., Liu, Z., Yang, F., Bu, Q., Song, X., & Yuan, S. (2025). Multimodal Fusion-Driven Pesticide Residue Detection: Principles, Applications, and Emerging Trends. Nanomaterials, 15(17), 1305. https://doi.org/10.3390/nano15171305







