Gold Nanoparticles Disrupt Mitochondrial Activity in Hypothalamic POMC Cells: Implications for Energy Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Treatment
2.3. Seahorse Measurement
2.4. Viability Test
2.5. ROS Assay
2.6. Data Analysis
3. Results
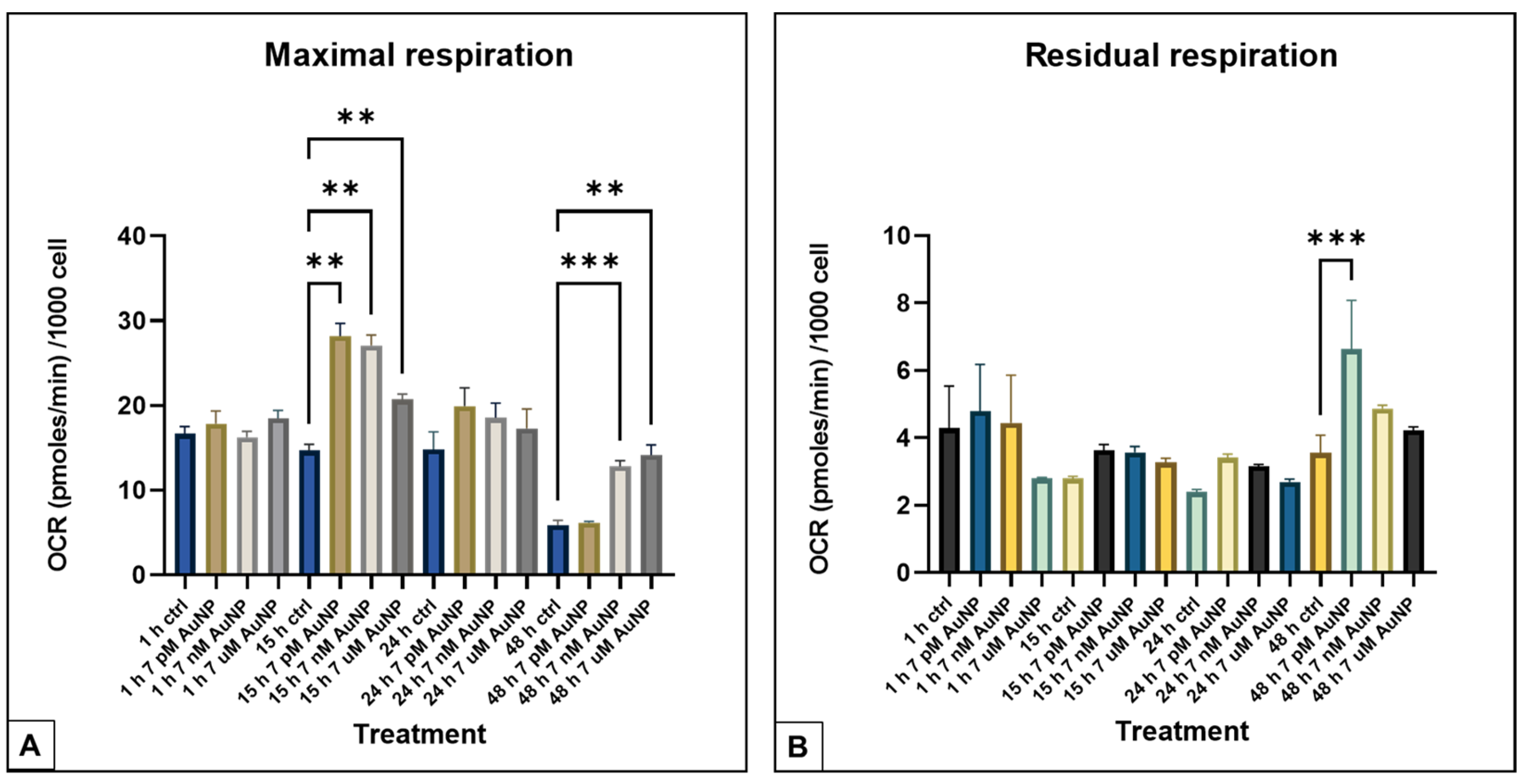
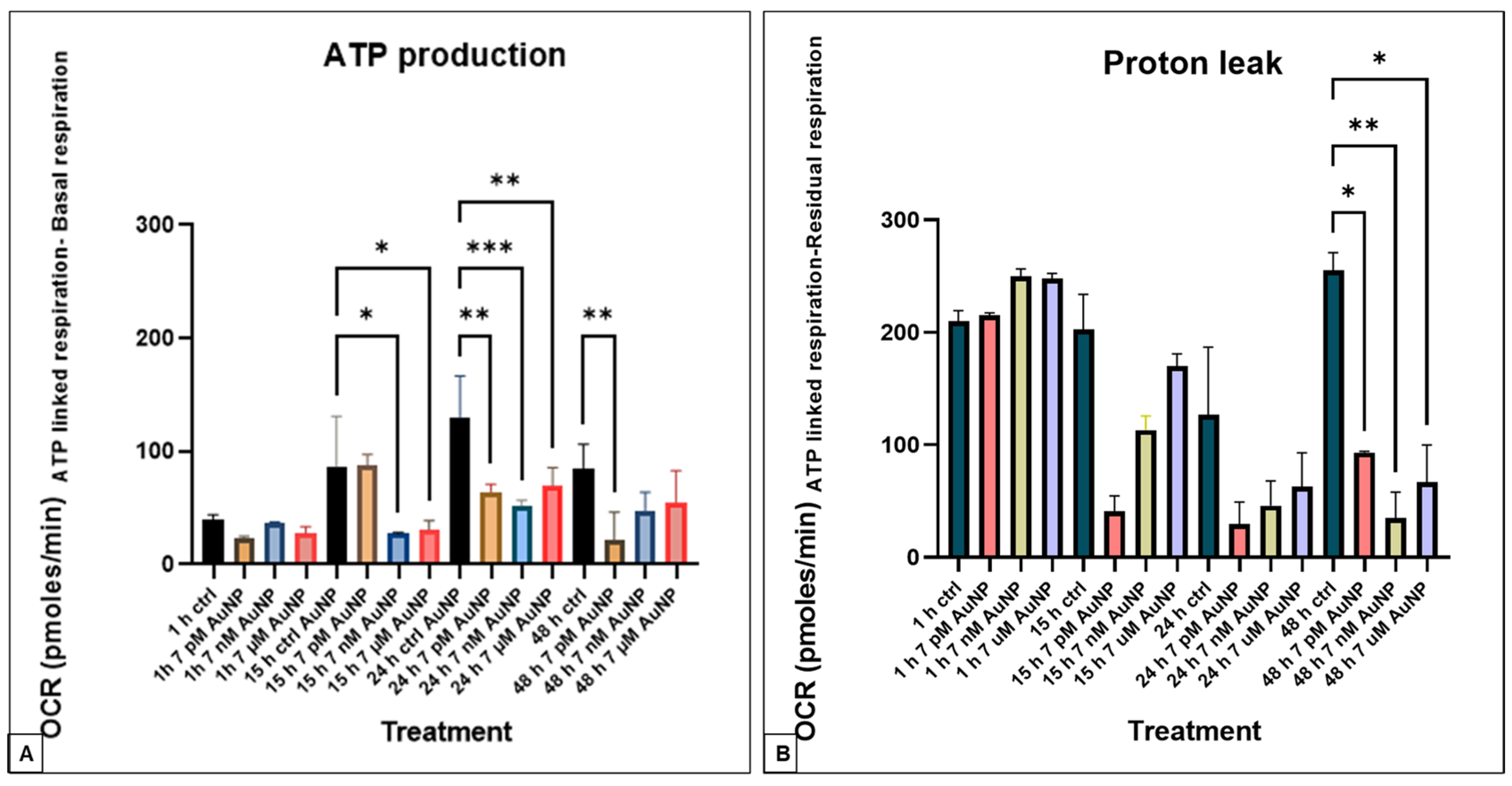
3.1. Seahorse XFp Measurements
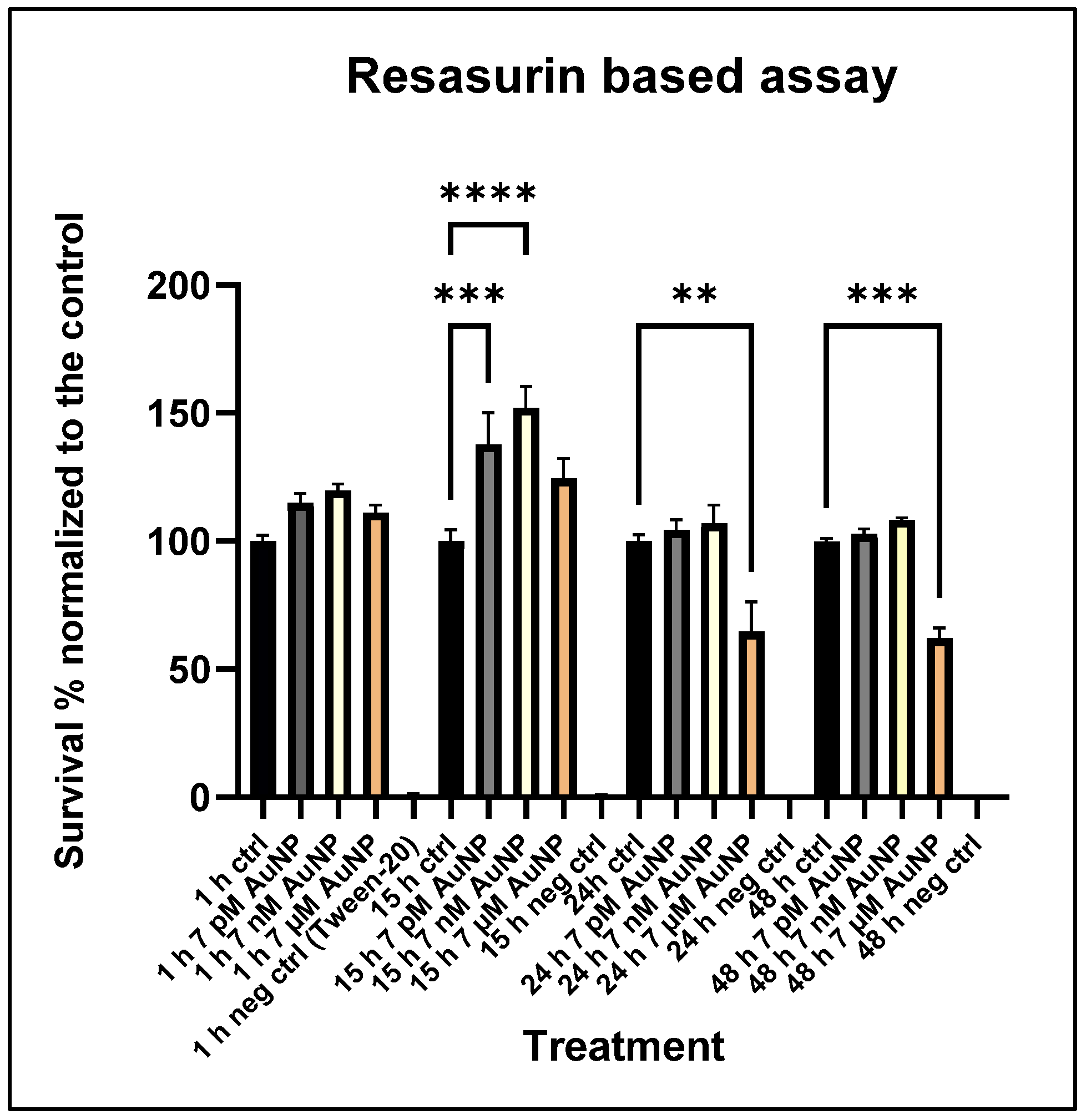
3.2. Cytotoxicity Assay
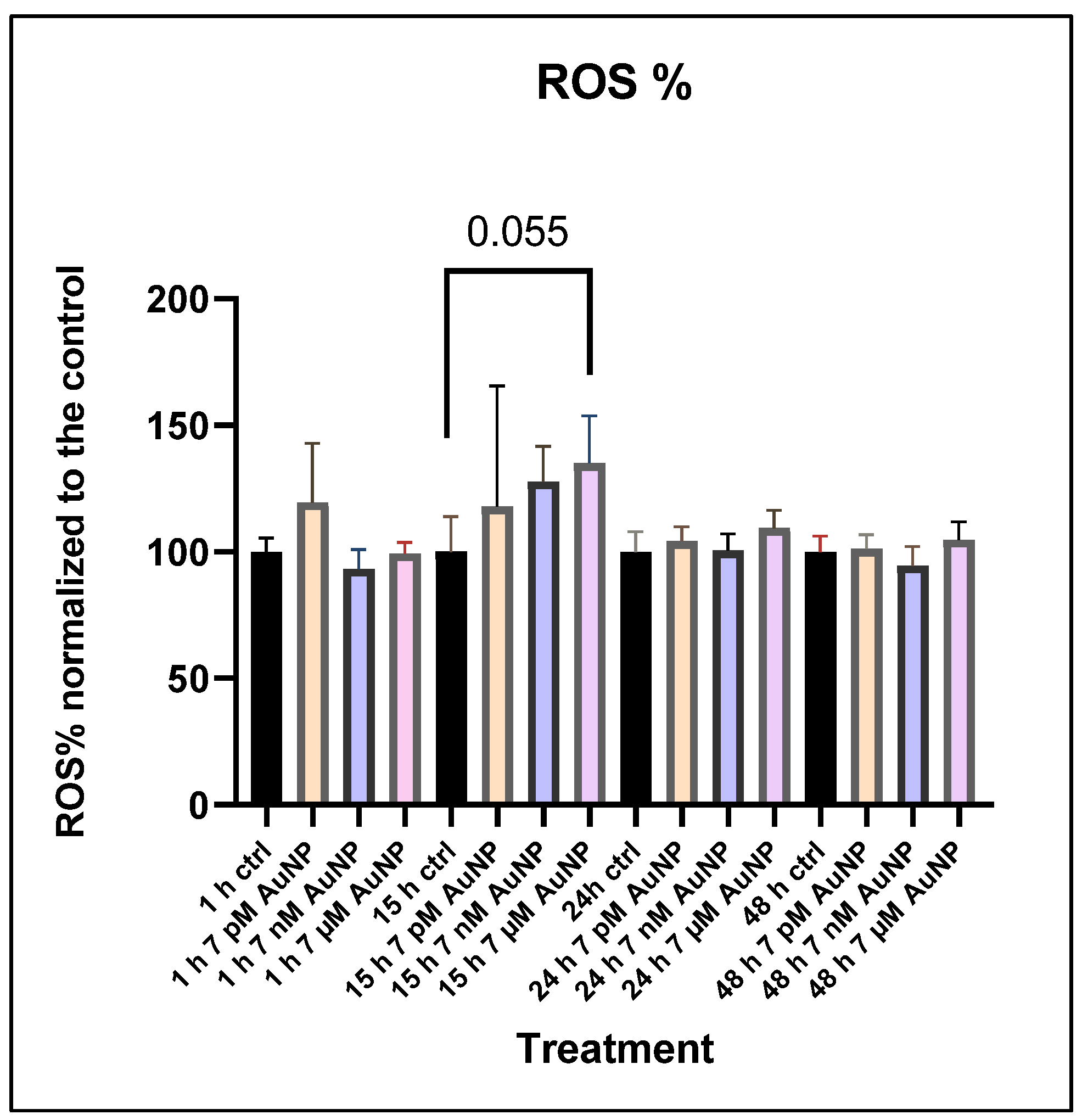
3.3. ROS Measurements
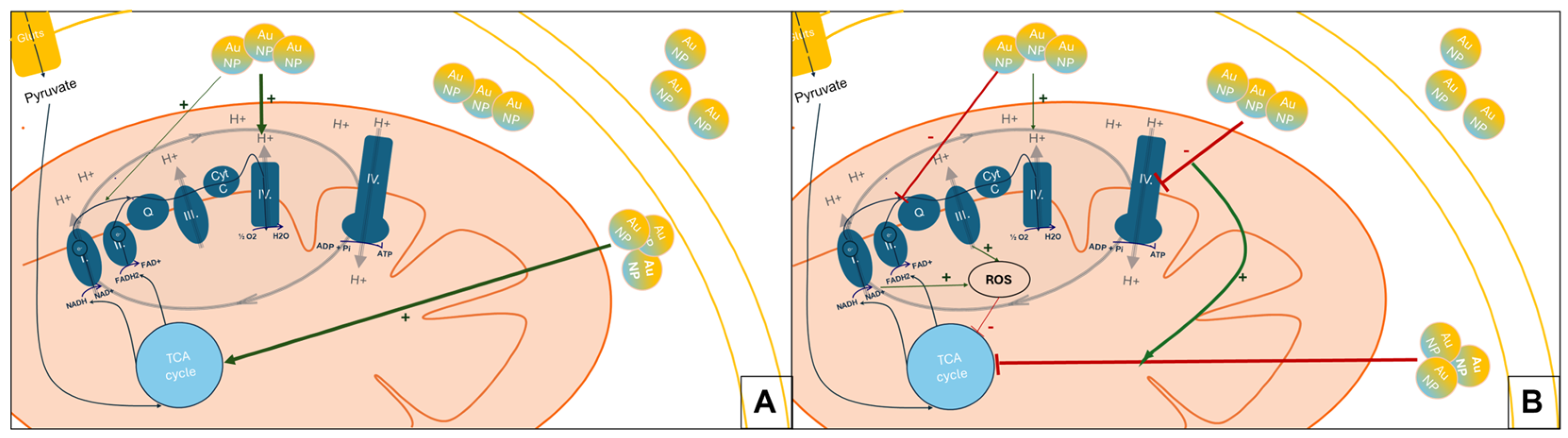
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold Nanoparticles |
| CNS | Central Nervous System |
| FDA | Food and Drug Administration, USA |
| HT | Hypothalamus |
| POMC | Pro-Opiomelanocortin |
| ROS | Reactive Oxygen Species |
| NPs | Nanoparticles |
| BBB | Blood–Brain Barrier |
| ARC | Arcuate Nucleus |
| MSH | Melatonin Stimulating Hormone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| P/S | Penicillin Streptomycin |
| OCR | Oxygen Consumption Rate |
| ECAR | Extracellular acidification rate |
| OM | Oligomycin |
| FCCP | Carbonyl Cyanide-p-trifluoromethoxy-phenylhydrazone |
| Rot | Rotanon |
| AM | Animycin |
| TCA | Tricarboxylic Acid Cycle |
| RotA | Rotanon/Antimycin |
References
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, L.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Gul, M.; Kashif, M.; Muhammad, S.; Azizi, S.; Sun, H. Various Methods of Synthesis and Applications of Gold-Based Nanomaterials: A Detailed Review. Cryst. Growth Des. 2025, 25, 2227–2266. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Multi-Functional Gold Nanoparticles for Drug Delivery. Adv. Exp. Med. Biol. 2007, 620, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Gold nanoparticles and gold nanoparticle-conjugates for delivery of therapeutic molecules. Progress and challenges. J. Mater. Chem. B 2014, 2, 4204–4220. [Google Scholar] [CrossRef]
- Liu, B.; Cao, W.; Qiao, G.; Yao, S.; Pan, S.; Wang, L.; Yue, C.; Ma, L.; Liu, Y.; Cui, D. Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer. Acta Biomater. 2019, 99, 307–319. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Wu, D.; Liu, Y.; Gao, Y.; Lian, H.; Zhang, H.; Yin, Z.; Wu, A.; Zeng, L. Ce6-Conjugated and polydopamine-coated gold nanostars with enhanced photoacoustic imaging and photothermal/photodynamic therapy to inhibit lung metastasis of breast cancer. Nanoscale 2020, 12, 22173–22184. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Lu, X.-M.; Zhu, F.; Huang, P.; Yu, Y.; Zeng, L.; Long, Z.-Y.; Wu, Y.-M. The use of a gold nanoparticle-based adjuvant to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials 2011, 32, 7988–7998. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef]
- Yue, J.; Feliciano, T.J.; Li, W.; Lee, A.; Odom, T.W. Gold Nanoparticle Size and Shape Effects on Cellular Uptake and Intracellular Distribution of siRNA Nanoconstructs. Bioconjugate Chem. 2017, 28, 1791–1800. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular Uptake and Cytotoxicity of Gold Nanorods: Molecular Origin of Cytotoxicity and Surface Effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dinesh, M.G.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B: Biointerfaces 2013, 106, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Aipire, A.; Dilxat, E.; Li, J.; Xia, G.; Jiang, Z.; Fan, Z.; Li, J. Research Progress of Polysaccharide-Gold Nanocomplexes in Drug Delivery. Pharmaceutics 2024, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shieh, M.J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Badir, A.; Refki, S.; Sekkat, Z. Utilizing gold nanoparticles in plasmonic photothermal therapy for cancer treatment. Heliyon 2025, 11, e42738. [Google Scholar] [CrossRef]
- Pinho, R.A.; Haupenthal, D.P.S.; Fauser, P.E.; Thirupathi, A.; Silveira, P.C.L. Gold Nanoparticle-Based Therapy for Muscle Inflammation and Oxidative Stress. J. Inflamm. Res. 2022, 15, 3219–3234. [Google Scholar] [CrossRef]
- Arnida, M.M.; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef]
- Lee, E.; Jeon, H.; Lee, M.; Ryu, J.; Kang, C.; Kim, S.; Jung, J.; Kwon, Y. Molecular origin of AuNPs-induced cytotoxicity and mechanistic study. Sci. Rep. 2019, 9, 2494. [Google Scholar] [CrossRef]
- Xue, J.; Liu, T.; Liu, Y.; Jiang, Y.; Seshadri, V.D.D.; Mohan, S.K.; Ling, L. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease–In vitro & In vivo model. J. Photochem. Photobiol. B: Biol. 2019, 200, 111635. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Elsayed, I.; Abourehab, M.A.; Khan, S.; Sohail, M.; Sarfraz, R.M.; Farooq, M.A. Nano-scaled materials may induce severe neurotoxicity upon chronic exposure to brain tissues: A critical appraisal and recent updates on predisposing factors, underlying mechanism, and future prospects. J. Control. Release 2020, 328, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Ramírez, E.; Montes, M.; Urrutia, R.A.; Reginensi, D.; González, E.A.S.; Estrada-Petrocelli, L.; Gutierrez-Vega, A.; Appaji, A.; Molino, J.; Ali, S. Metallic Nanoparticles Applications in Neurological Disorders: A Review. Int. J. Biomater. 2025, 2025, 4557622. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B: Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Li, C.-H.; Shyu, M.-K.; Jhan, C.; Cheng, Y.-W.; Tsai, C.-H.; Liu, C.-W.; Lee, C.-C.; Chen, R.-M.; Kang, J.-J. Gold Nanoparticles Increase Endothelial Paracellular Permeability by Altering Components of Endothelial Tight Junctions, and Increase Blood–Brain Barrier Permeability in Mice. Toxicol. Sci. 2015, 148, 192–203. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Tuna, B.G.; Yesilay, G.; Yavuz, Y.; Yilmaz, B.; Culha, M.; Maharramov, A.; Dogan, S. Electrophysiological effects of polyethylene glycol modified gold nanoparticles on mouse hippocampal neurons. Heliyon 2020, 6, e05824. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, J.; Yuan, R.; Zhou, Y.; Rao, J.; Wan, Y.; Li, Y.; Zhang, X.; Li, R. Gold nanoparticles: Diagnostic and therapeutic applications in neurodegenerative disorders. J. Drug Target. 2025, 1–18. [Google Scholar] [CrossRef]
- Chen, H.; Ng, J.P.M.; Tan, Y.; McGrath, K.; Bishop, D.P.; Oliver, B.; Chan, Y.L.; Cortie, M.B.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J. Nanobiotechnol. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Luo, X.; Tang, S.; Luo, X.; Quzhen, L.; Xia, R.; Wang, X. Mitochondrial regulation of obesity by POMC neurons. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2025, 1871, 167682. [Google Scholar] [CrossRef]
- Marcovici, I.; Coricovac, D.; Pinzaru, I.; Macasoi, I.G.; Popescu, R.; Chioibas, R.; Zupko, I.; Dehelean, C.A. Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research—A Review. Cancers 2022, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xin, Y.; Wang, S.; Zhang, X.; Ren, Y.; Qiao, B.; Li, H.; Wu, J.; Hao, X.; Xu, L.; et al. Hypothalamic POMC neuron-specific knockout of MC4R affects insulin sensitivity by regulating Kir2.1. Mol. Med. 2024, 30, 1–14. [Google Scholar] [CrossRef]
- Sabella, S.; Brunetti, V.; Vecchio, G.; Galeone, A.; Maiorano, G.; Cingolani, R.; Pompa, P.P. Toxicity of citrate-capped AuNPs: An in vitro and in vivo assessment. J. Nanopart. Res. 2011, 13, 6821–6835. [Google Scholar] [CrossRef]
- Bruszt, K.; Horvath, O.; Ordog, K.; Toth, S.; Juhasz, K.; Vamos, E.; Fekete, K.; Gallyas, F.; Toth, K.; Halmosi, R.; et al. Cardiac effects of OPA1 protein promotion in a transgenic animal model. PLoS ONE 2024, 19, e0310394. [Google Scholar] [CrossRef]
- Fasano, C.; Cavaliere, A.; Tiranti, V.; Peron, C. Protocol for evaluating mitochondrial respiration in iPSC-derived neurons by the Seahorse XF analyzer. STAR Protoc. 2024, 5, 103127. [Google Scholar] [CrossRef]
- Petiti, J.; Caria, S.; Revel, L.; Pegoraro, M.; Divieto, C. Standardized Protocol for Resazurin-Based Viability Assays on A549 Cell Line for Improving Cytotoxicity Data Reliability. Cells 2024, 13, 1959. [Google Scholar] [CrossRef]
- Zhang, H.; Du, G.; Zhang, J. Assay of mitochondrial functions by resazurin in vitro. Acta. Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar]
- Gu, X.; Ma, Y.; Liu, Y.; Wan, Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc. 2021, 2, 100245. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; Von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Pazin, M.; Franco-Bernardes, M.F.; Martins, A.d.C.; Barcelos, G.R.M.; Pereira, M.C.; Mesquita, J.P.; Rodrigues, J.L.; Barbosa, F.; Dorta, D.J. A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). J. Trace Elem. Med. Biol. 2018, 47, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, K.; Zhang, C.; Talukder, M.; Lv, M.-W.; Li, J.-Y.; Li, J.-L. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci. Total Environ. 2021, 773, 145442. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; De Almeida, M.P.; Araújo, A.M.; Bastos, M.D.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Franich, R.; Feltis, B.; Geso, M. Oxidative Damage to Mitochondria Enhanced by Ionising Radiation and Gold Nanoparticles in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6887. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Li, J.; Russe, A.C.M.; Kawazoe, N.; Yang, Y.; Chen, G. Influence of cell size on cellular uptake of gold nanoparticles. Biomater. Sci. 2016, 4, 970–978. [Google Scholar] [CrossRef]
- Ding, X.-L.; Liu, M.-D.; Cheng, Q.; Guo, W.-H.; Niu, M.-T.; Huang, Q.-X.; Zeng, X.; Zhang, X.-Z. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy. Biomaterials 2022, 281, 121369. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Nicol, C.J.B. GSH-AuNP anti-oxidative stress, ER stress and mitochondrial dysfunction in amyloid-beta peptide-treated human neural stem cells. Free. Radic. Biol. Med. 2022, 187, 185–201. [Google Scholar] [CrossRef]
- Jang, D.H.; Shofer, F.S.; Weiss, S.L.; Becker, L.B. Impairment of mitochondrial respiration following ex vivo cyanide exposure in peripheral blood mononuclear cells. Clin. Toxicol. 2016, 54, 303–307. [Google Scholar] [CrossRef]
- Nedel, W.; Deutschendorf, C.; Portela, L.V.C. Sepsis-induced mitochondrial dysfunction: A narrative review. World J. Crit. Care Med. 2023, 12, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Wang, Y.-F.; Li, X.L.; Wang, Y.-Q.; Huang, Q.-Q.; Ma, X.-W.; Liang, X.-J. Nanoparticle-Driven Controllable Mitochondrial Regulation through Lysosome–Mitochondria Interactome. ACS Nano 2022, 16, 12553–12568. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Xu, H.-L.; Li, Y.; Liu, Y.F.; Li, X.-Y.; Zhou, W.; Wang, J.; Guo, C.-X.; Sun, Z.-W.; Li, Y.-B. Silica nanoparticles perturbed mitochondrial dynamics and induced myocardial apoptosis via PKA-DRP1-Mitochondrial fission signaling. Sci. Total Environ. 2022, 842, 156854. [Google Scholar] [CrossRef]
- Dan, M.; Wen, H.-R.; Shao, A.L.; Xu, L.M. Silver Nanoparticle Exposure Induces Neurotoxicity in the Rat Hippocampus Without Increasing the Blood-Brain Barrier Permeability. J. Biomed. Nanotechnol. 2018, 14, 1330–1338. [Google Scholar] [CrossRef]
- Safaei, F.; Farimaneh, J.; Rajabi Mohammad Abad, A.; Iranmanesh, E.; Arabpour, F.; Doostishoar, F.; Taherizadeh, Z. The effect of silver nanoparticles on learning and memory in rodents: “A systematic review”. J. Occup. Med. Toxicol. 2023, 18, 15. [Google Scholar] [CrossRef]
- Zhang, B.-J.; Liu, N.; Liu, Q.-S.; Zhang, J.-Q.; Zhou, Q.-F.; Jiang, G.-B. Silver nanoparticles induce size-dependent and particle-specific neurotoxicity to primary cultures of rat cerebral cortical neurons. Ecotoxicol. Environ. Saf. 2020, 198, 110674. [Google Scholar] [CrossRef]
- Haigh, J.L.; New, L.E.; Filippi, B.M. Mitochondrial Dynamics in the Brain Are Associated With Feeding, Glucose Homeostasis, and Whole-Body Metabolism. Front. Endocrinol. 2020, 11, 580879. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Jo, Y.; Woo, J.S.; Lee, A.R.; Lee, S.-Y.; Shin, Y.; Lee, L.P.; Cho, M.-L.; Kang, T. Inner-Membrane-Bound Gold Nanoparticles as Efficient Electron Transfer Mediators for Enhanced Mitochondrial Electron Transport Chain Activity. Nano Lett. 2022, 22, 7927–7935. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Suh, S.B.; Jang, S.; Kim, S.; Kim, D.-G.; Park, J.K.; Lee, K.-W.; Choi, S.Y.; Lee, C.H. metformin ameliorates olanzapine-induced disturbances in POMC neuron number, axonal projection, and hypothalamic leptin resistance. BMB Rep. 2022, 55, 293–298. [Google Scholar] [CrossRef]
- Diano, S. Role of reactive oxygen species in hypothalamic regulation of energy metabolism. Endocrinol. Metab. 2013, 28, 3. [Google Scholar] [CrossRef]
- Yoboue, E.D.; Devin, A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int. J. Cell Biol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilling-Tóth, B.M.; Ondrašovičová, S.; Vámos, E.; Radnai, B.; Alymbaeva, D.; Bartha, T.; Tóth, I.; Kiss, D.S. Gold Nanoparticles Disrupt Mitochondrial Activity in Hypothalamic POMC Cells: Implications for Energy Homeostasis. Nanomaterials 2025, 15, 1291. https://doi.org/10.3390/nano15161291
Schilling-Tóth BM, Ondrašovičová S, Vámos E, Radnai B, Alymbaeva D, Bartha T, Tóth I, Kiss DS. Gold Nanoparticles Disrupt Mitochondrial Activity in Hypothalamic POMC Cells: Implications for Energy Homeostasis. Nanomaterials. 2025; 15(16):1291. https://doi.org/10.3390/nano15161291
Chicago/Turabian StyleSchilling-Tóth, Boglárka Mária, Silvia Ondrašovičová, Eszter Vámos, Balázs Radnai, Daiana Alymbaeva, Tibor Bartha, István Tóth, and Dávid Sándor Kiss. 2025. "Gold Nanoparticles Disrupt Mitochondrial Activity in Hypothalamic POMC Cells: Implications for Energy Homeostasis" Nanomaterials 15, no. 16: 1291. https://doi.org/10.3390/nano15161291
APA StyleSchilling-Tóth, B. M., Ondrašovičová, S., Vámos, E., Radnai, B., Alymbaeva, D., Bartha, T., Tóth, I., & Kiss, D. S. (2025). Gold Nanoparticles Disrupt Mitochondrial Activity in Hypothalamic POMC Cells: Implications for Energy Homeostasis. Nanomaterials, 15(16), 1291. https://doi.org/10.3390/nano15161291






