In Situ TEM Observation of Electric Field-Directed Self-Assembly of PbS and PbSe Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystal Growth and Preparation of TEM Specimens
2.2. TEM
2.3. Mass Spectrometry
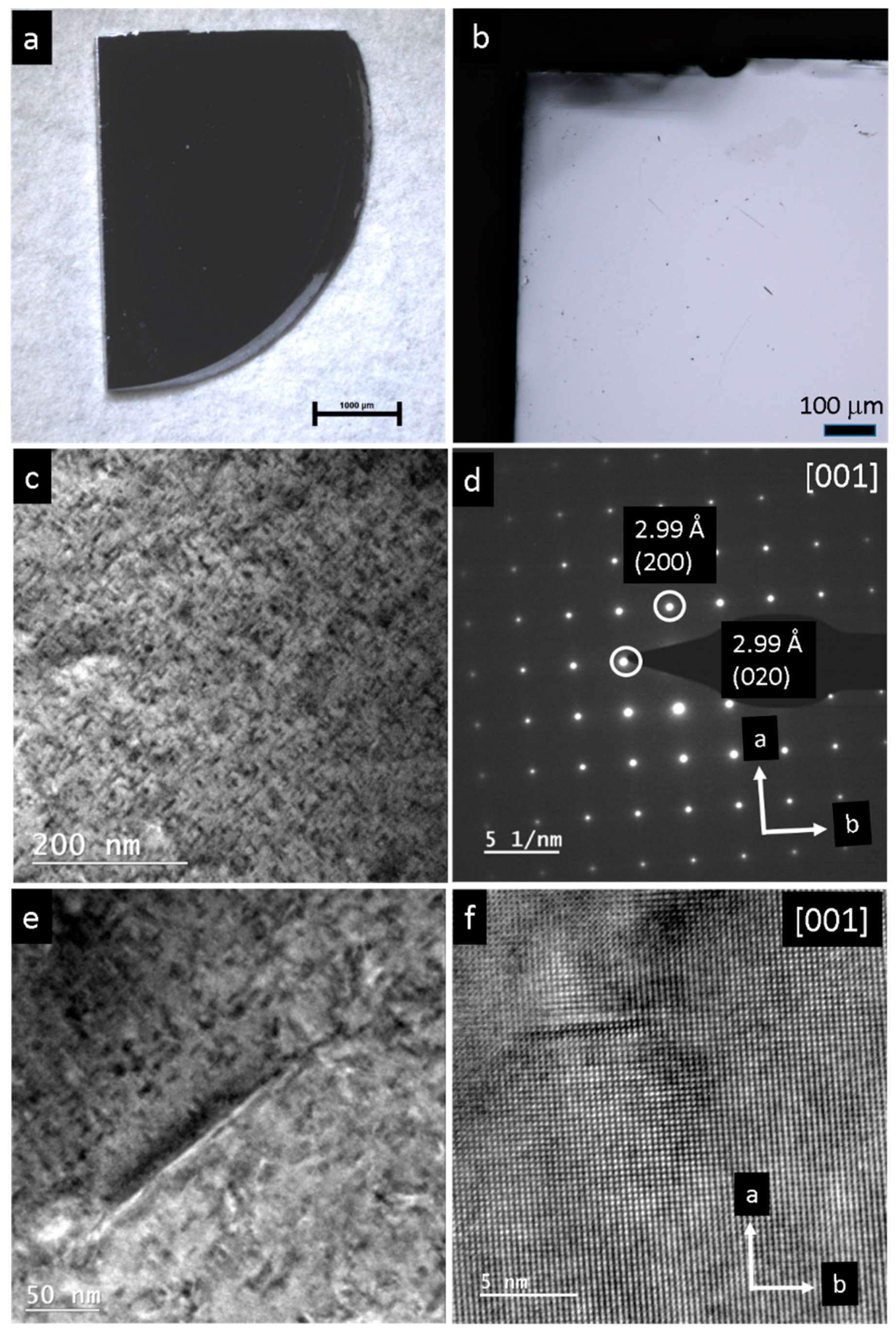
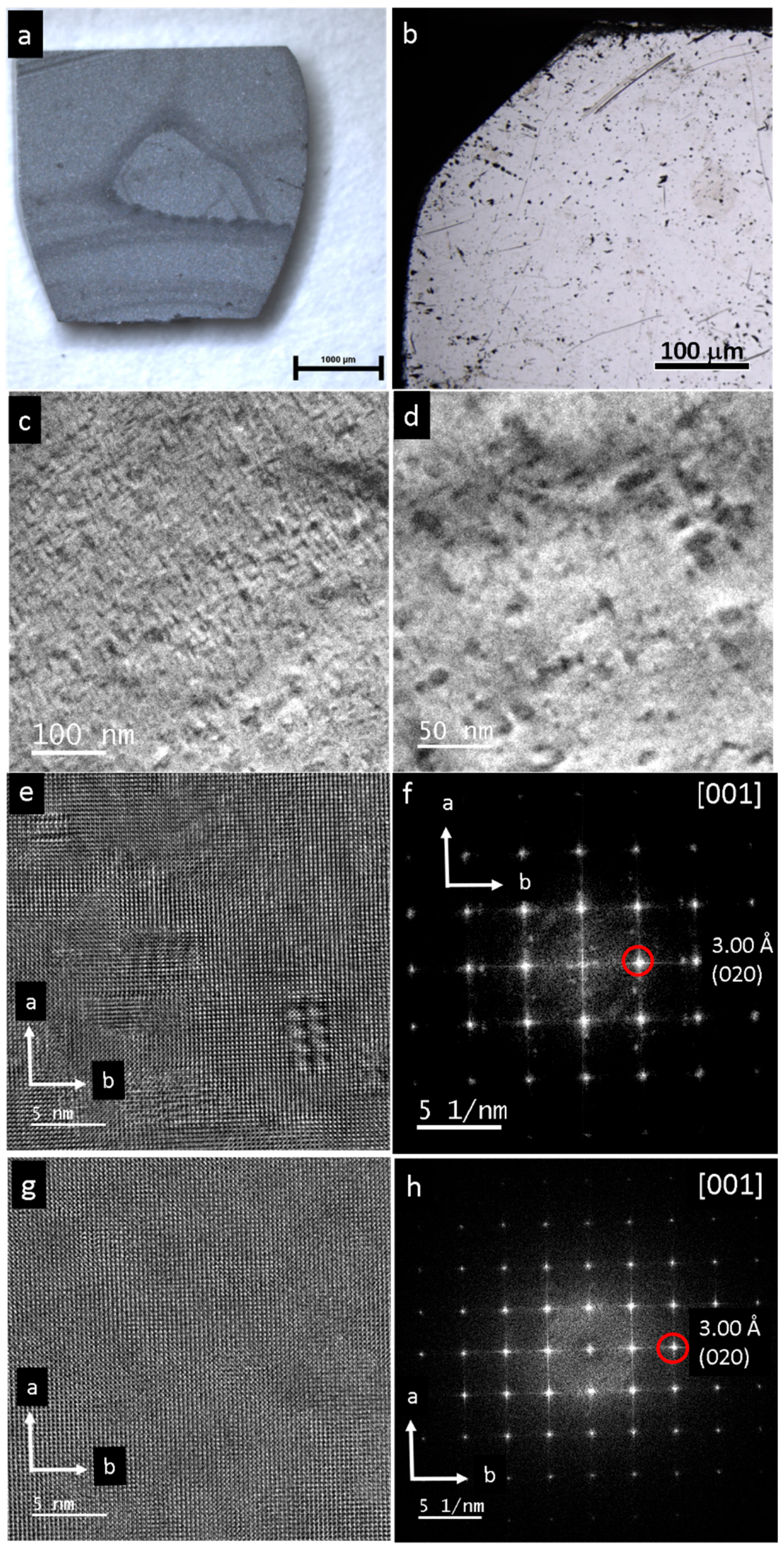
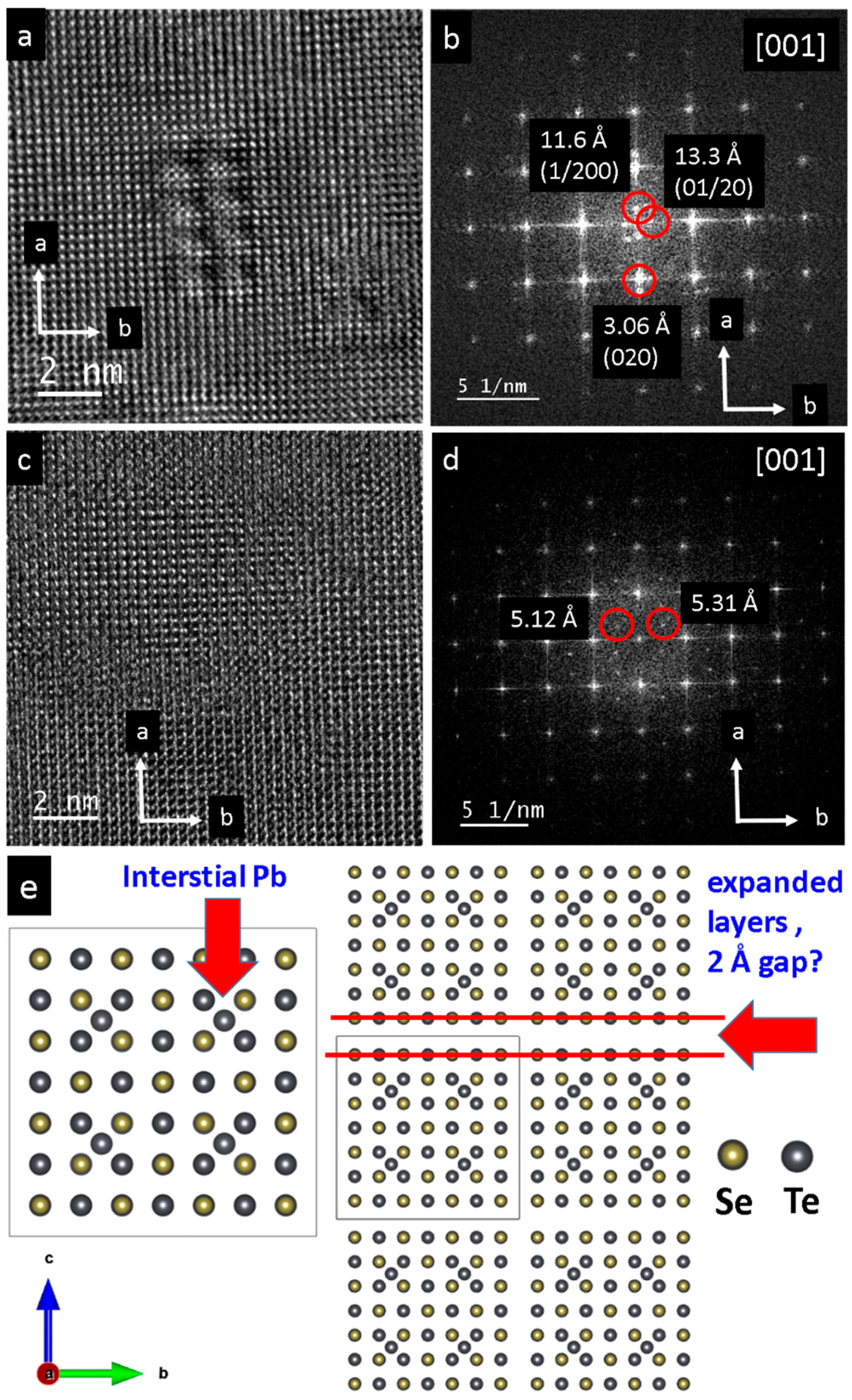
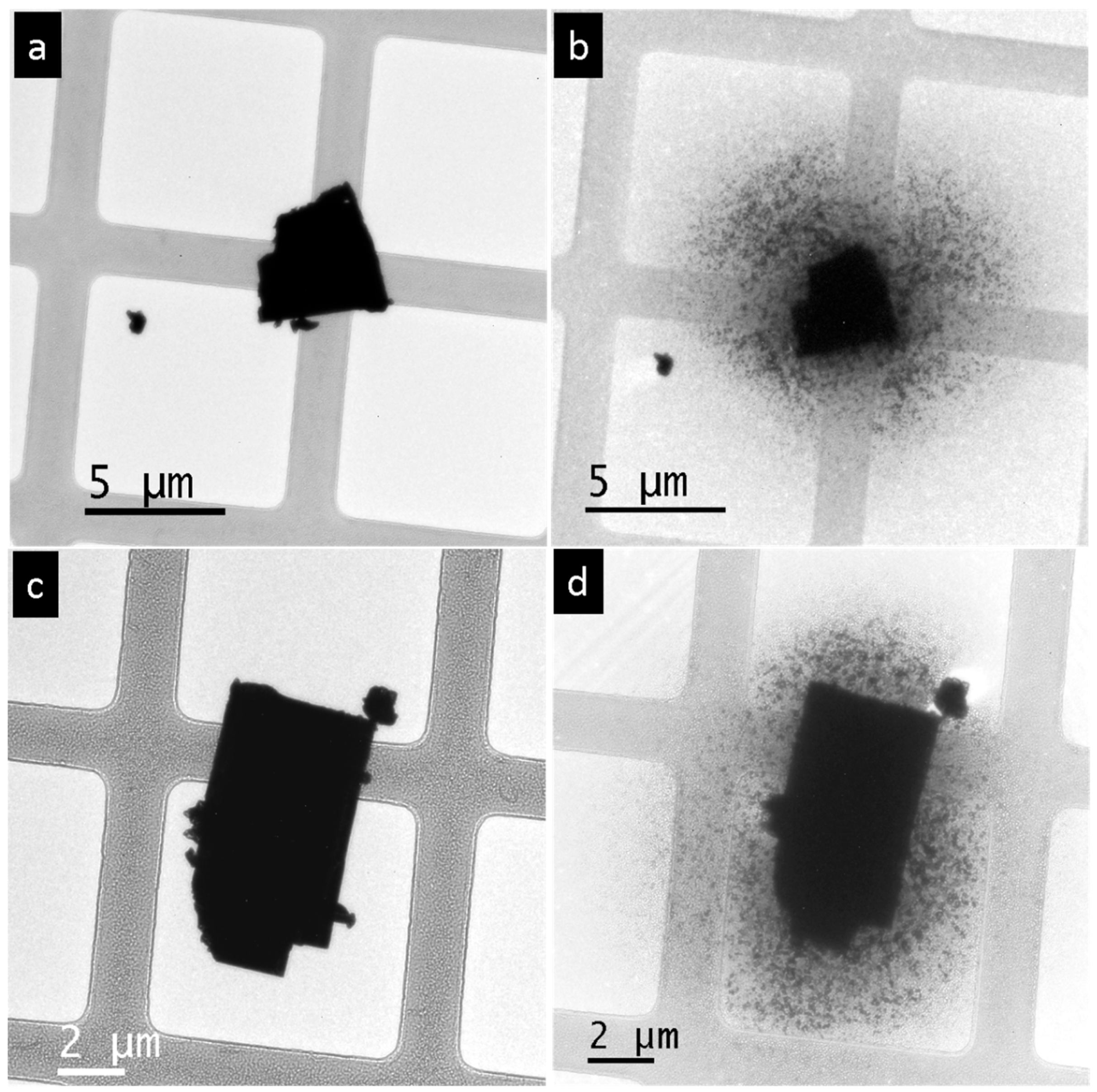
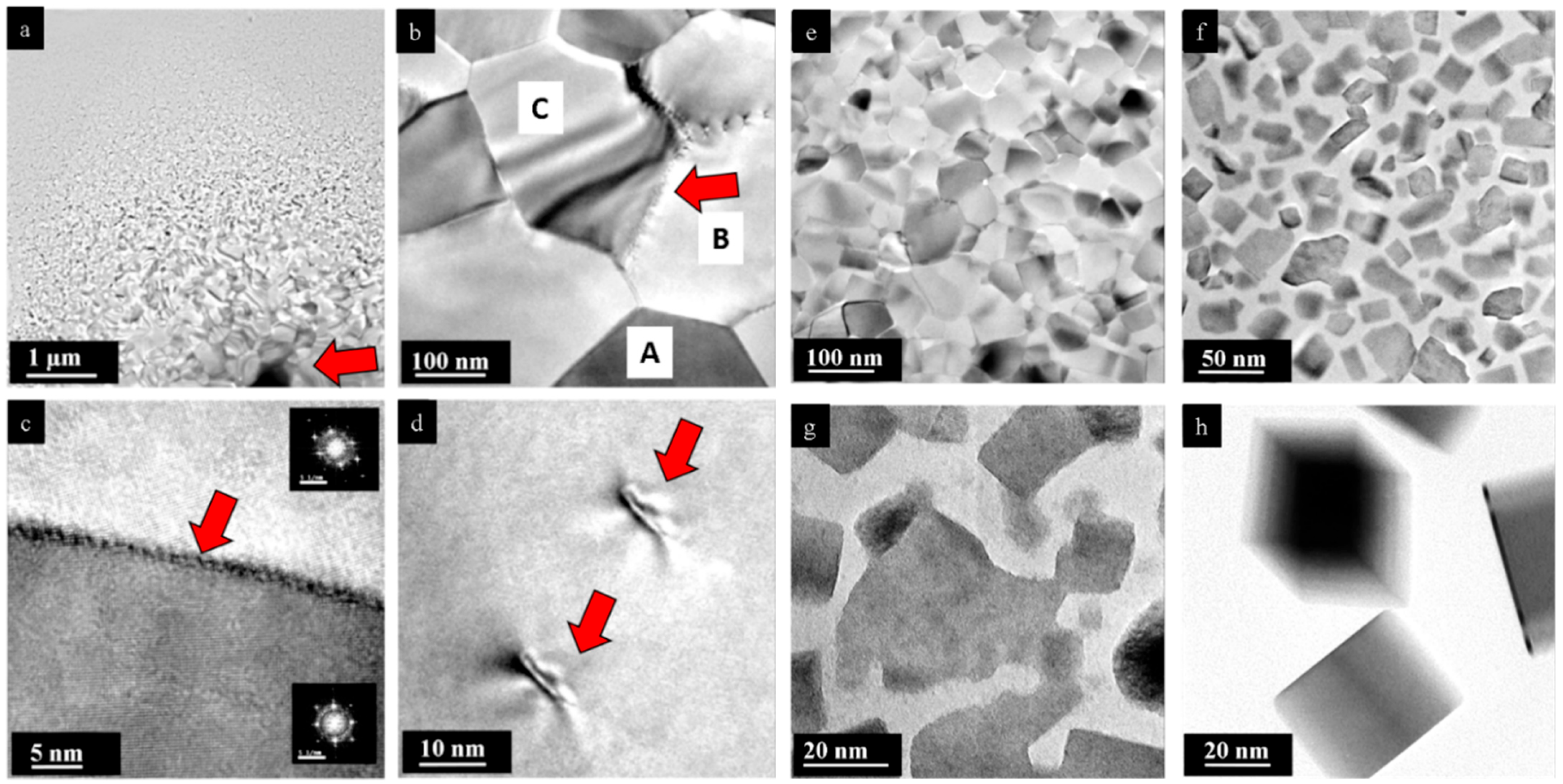
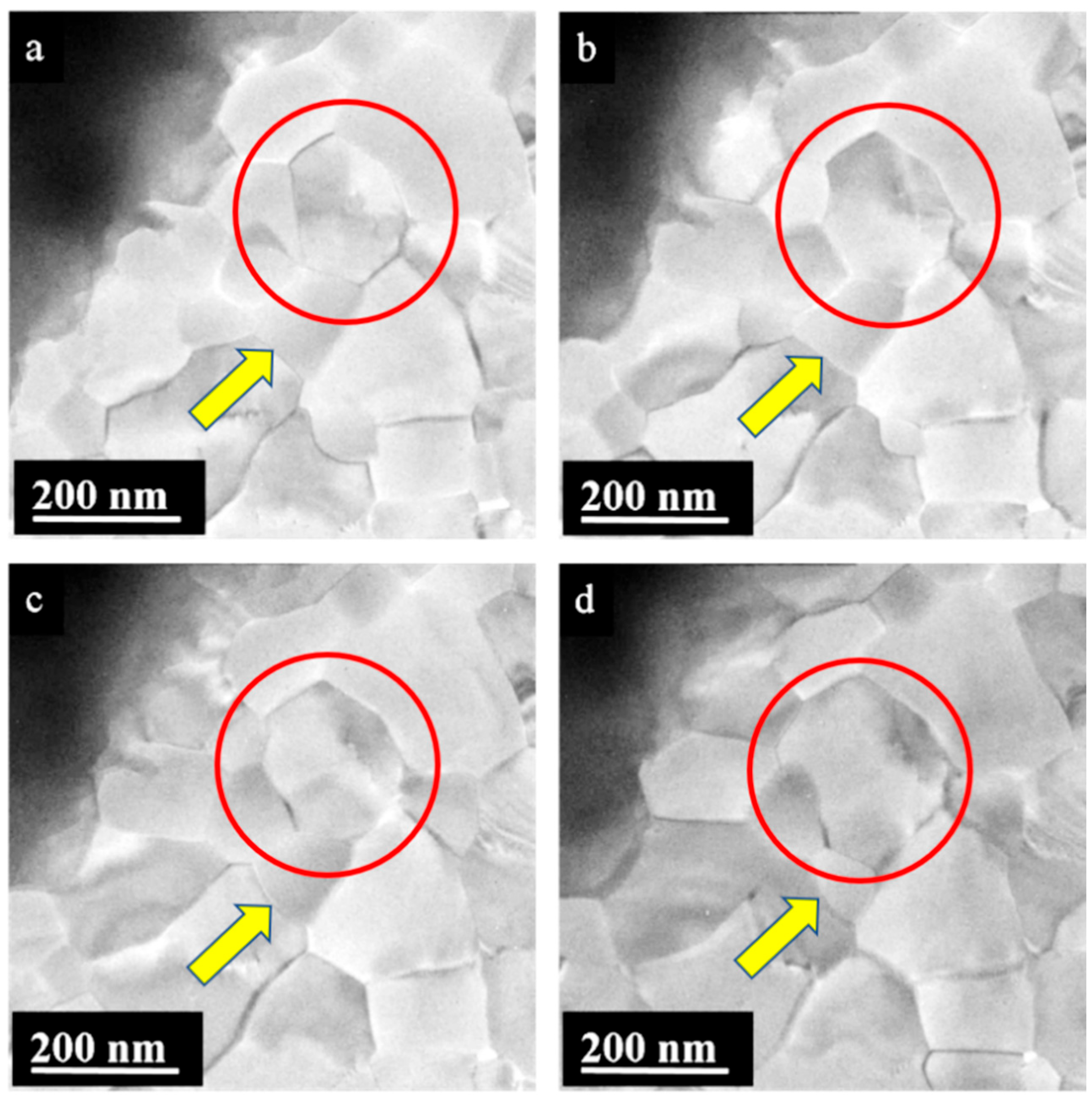
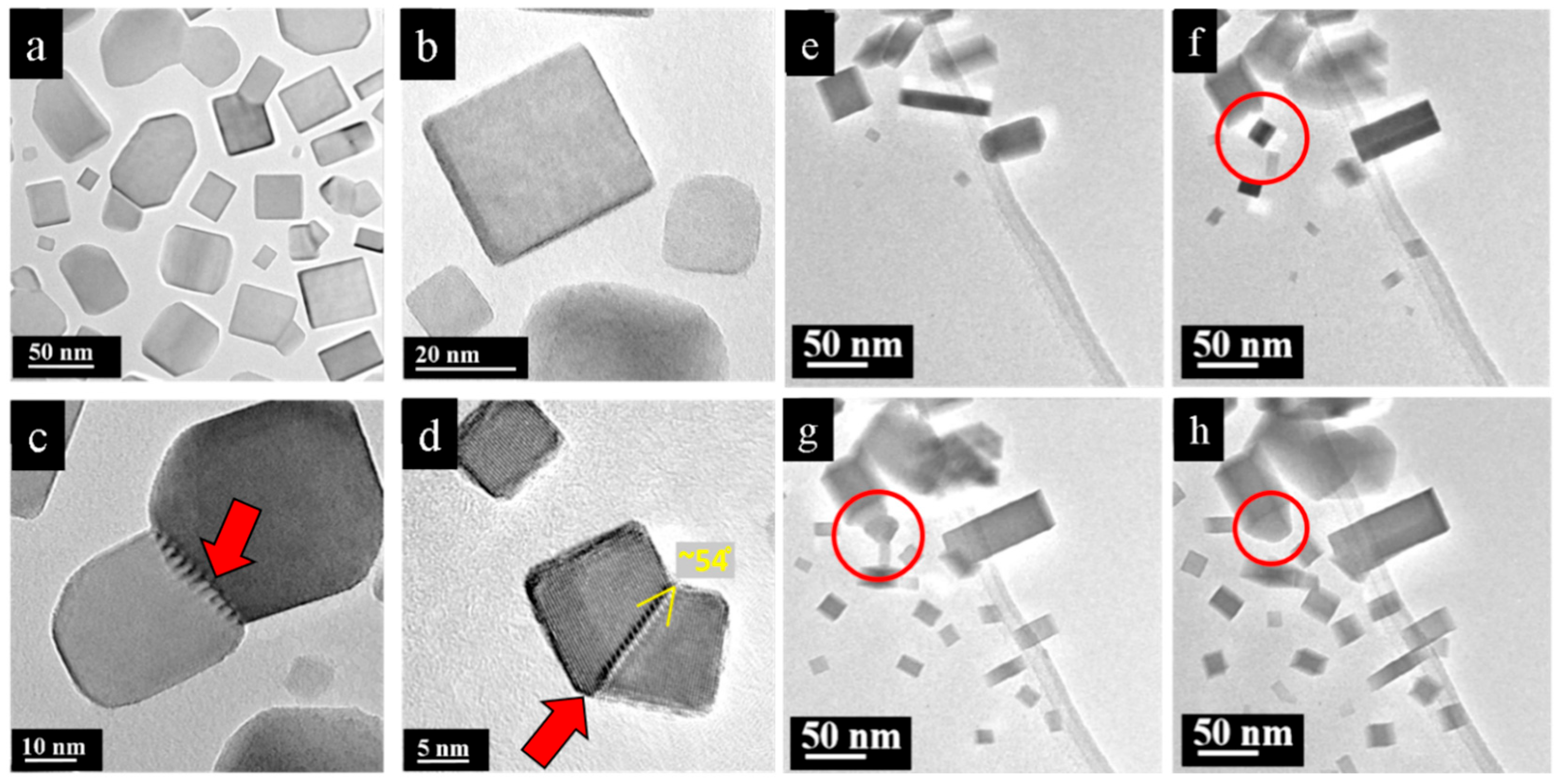
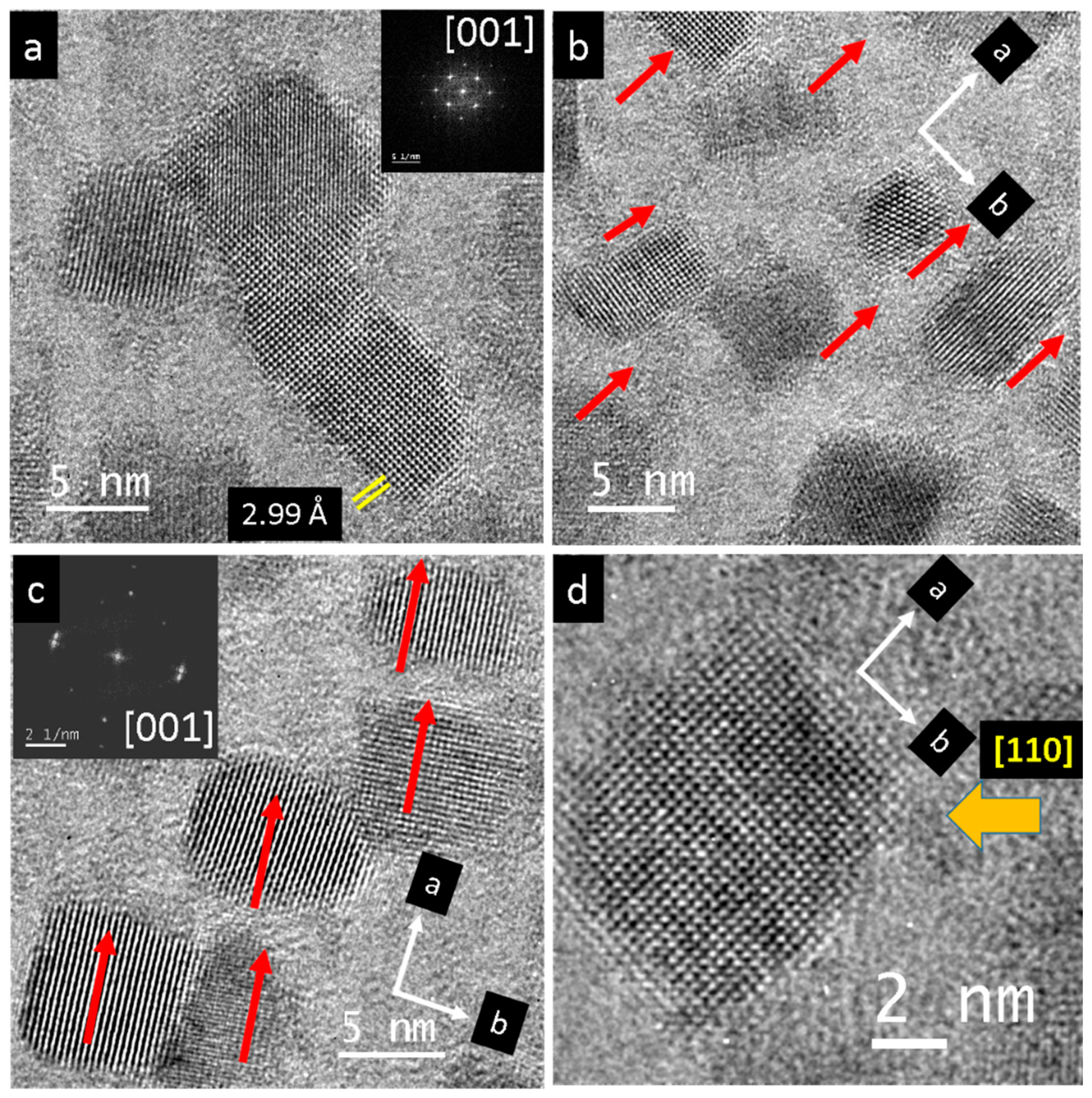
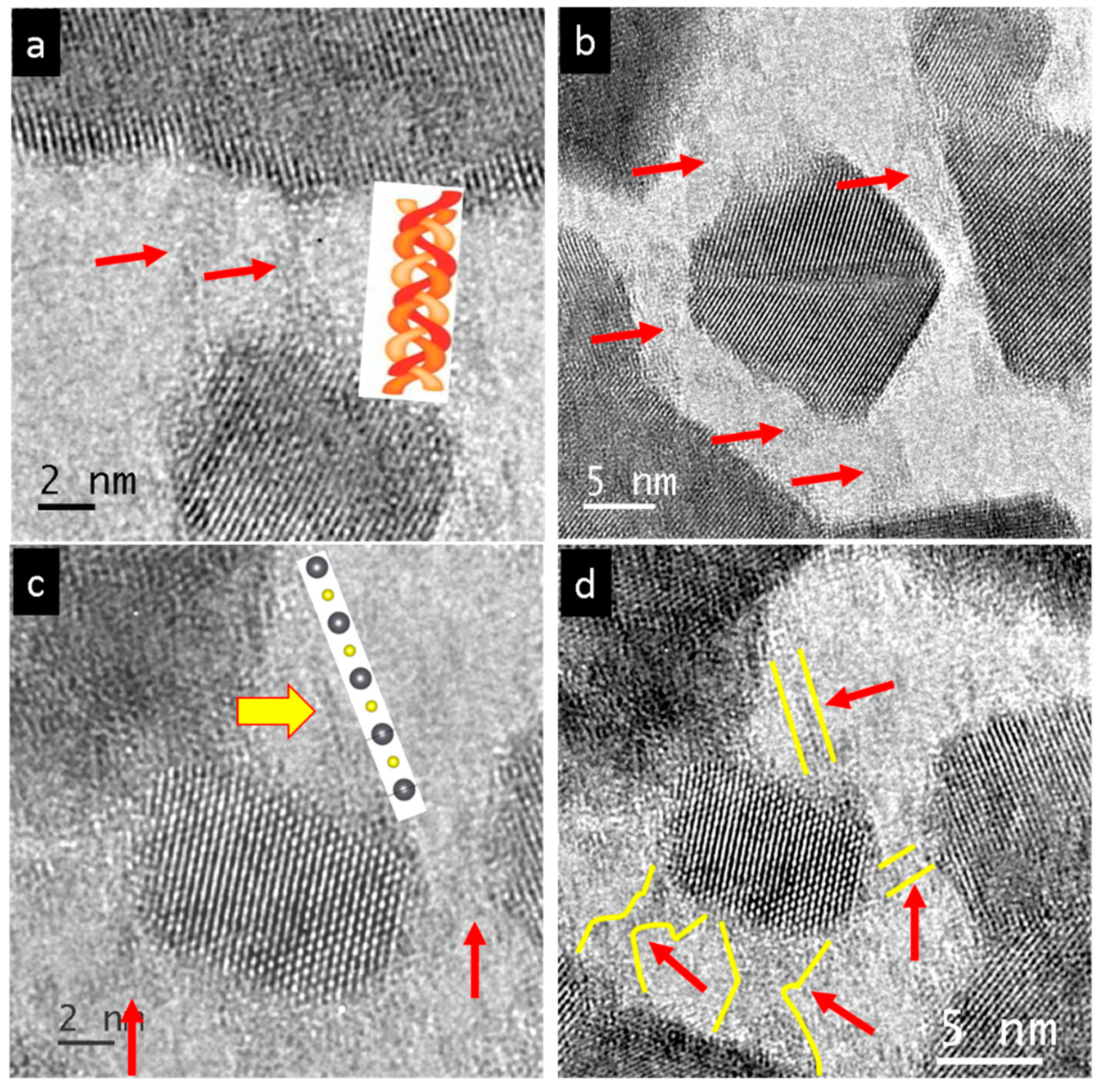
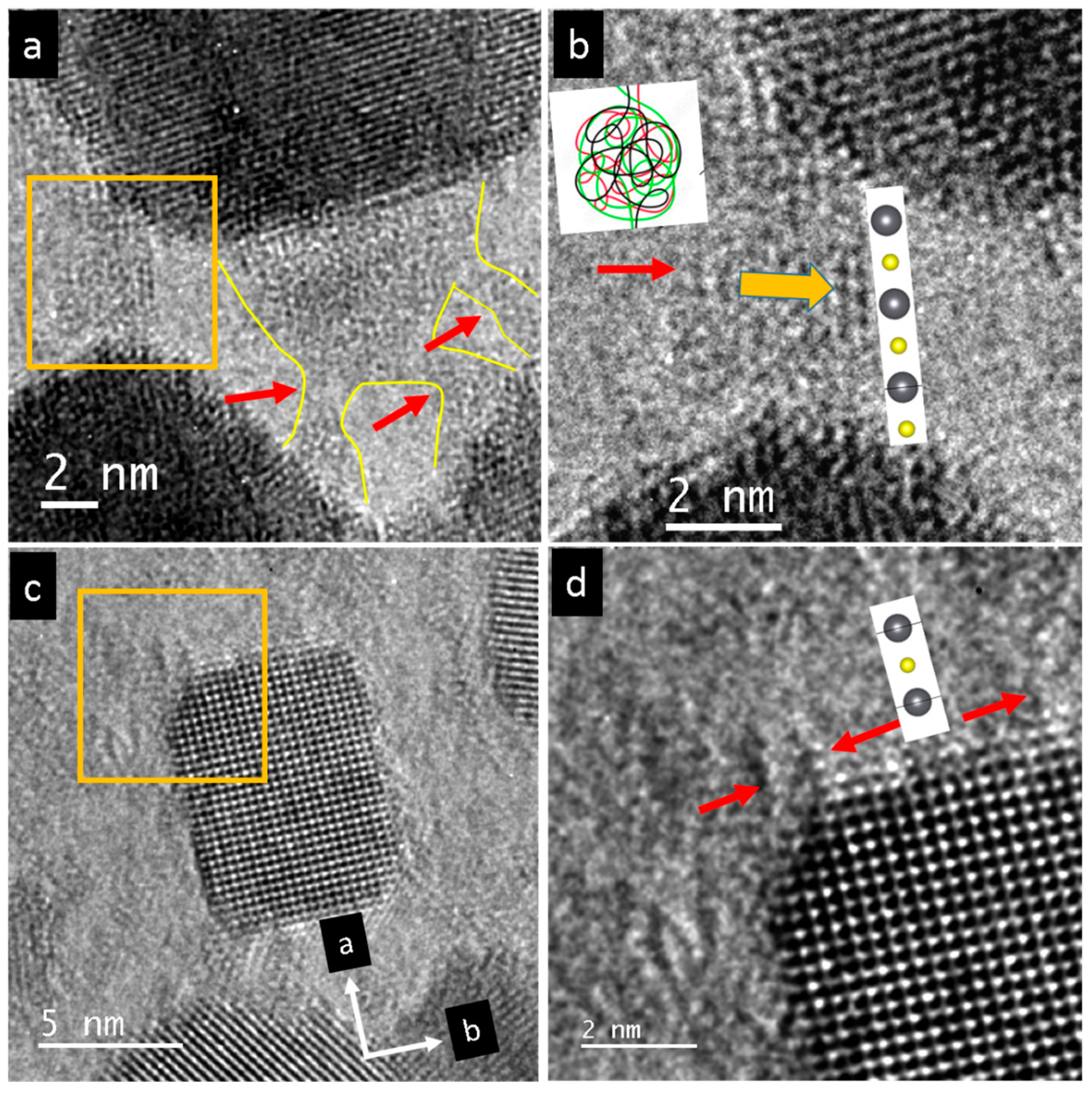
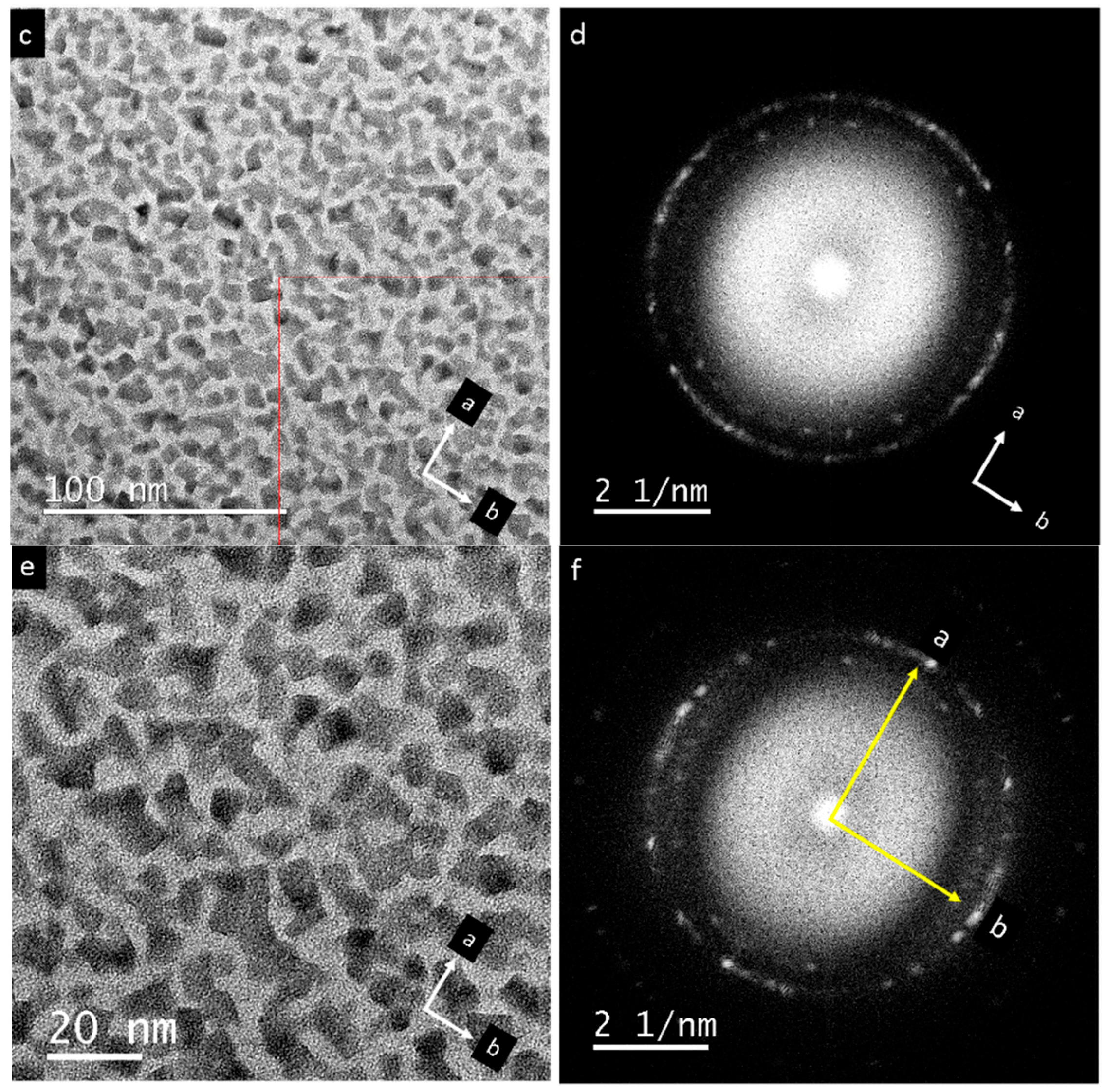
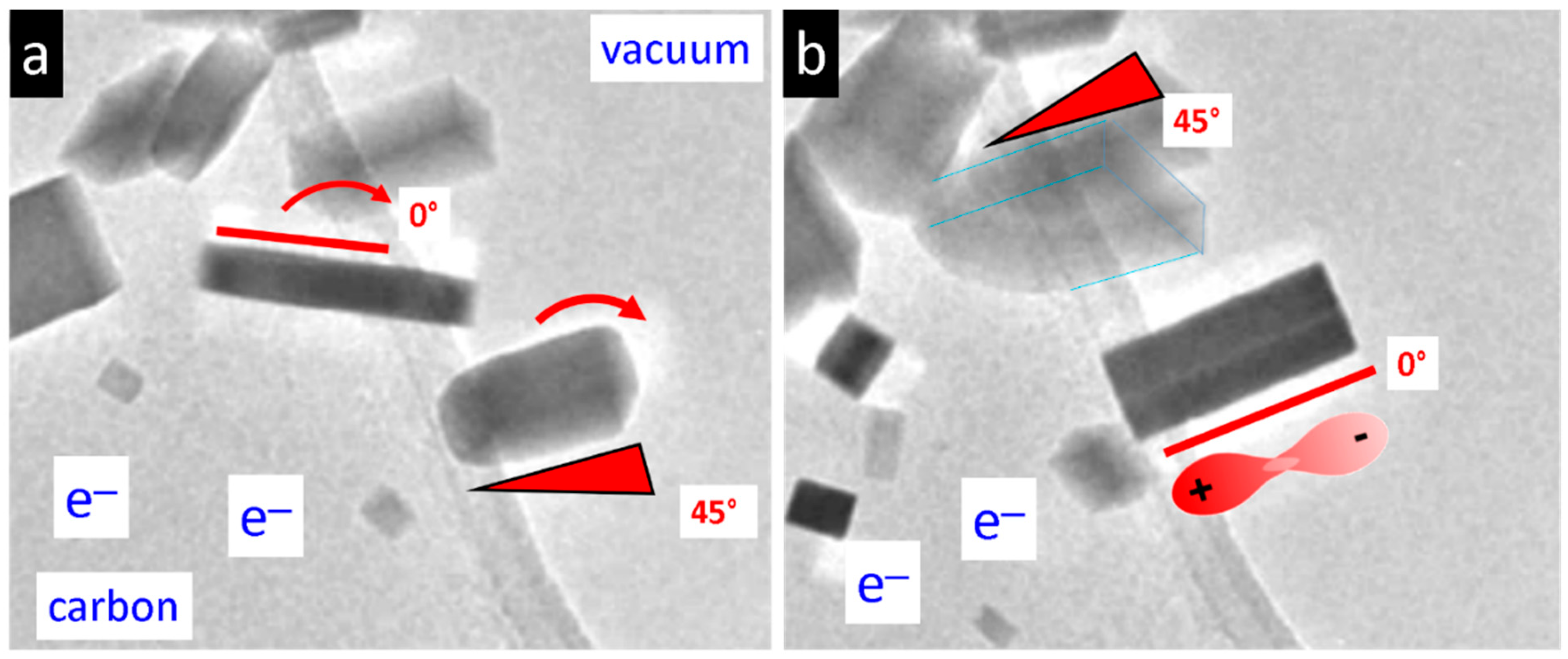
3. Results
4. Discussion
4.1. Stability of Lead Chalcogenide Compounds and Mass Spectrometry
4.2. Possible Electric Field Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.; Khan, A.H.; Acharya, S. Fabrication of highly stable, hybrid PbS nanocomposites in PAMAM dendrimer matrix for photodetection. J. Phys. Chem. C 2012, 116, 6022–6030. [Google Scholar] [CrossRef]
- Axnanda, S.; Scheele, M.; Crumlin, E.; Mao, B.; Chang, R.; Rani, S.; Faiz, M.; Wang, S.; Alivisatos, A.P.; Liu, Z. Direct Work Function Measurement by Gas Phase Photoelectron Spectroscopy and Its Application on PbS Nanoparticles. Nano Lett. 2013, 13, 6176–6182. [Google Scholar] [CrossRef]
- Souici, A.H.; Keghouche, N.; Delaire, J.A.; Remita, H.; Etcheberry, A.; Mostafavi, M. Structural and optical properties of PbS nanoparticles synthesized by the radiolytic method. J. Phys. Chem. C 2009, 113, 8050–8057. [Google Scholar] [CrossRef]
- Xu, Y.; Al-Salim, N.; Bumby, C.W.; Tilley, R.D. Synthesis of SnS Quantum Dots. J. Am. Chem. Soc. 2009, 131, 15990–15991. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, D.I.; De Leon-Covian, L.M.; Garcia-Gutierrez, D.F.; Treviño-Gonzalez, M.; Garza-Navarro, M.A.; Sepulveda-Guzman, S. On the role of Pb0 atoms on the nucleation and growth of PbSe and PbTe nanoparticles. J. Nanopart. Res. 2013, 15, 1620. [Google Scholar] [CrossRef]
- Shkir, M.; Chandekar, K.V.; Khan, A.; El-Toni, A.M.; AlFaify, S. A facile synthesis of Bi@PbS nanosheets and their key physical properties analysis for optoelectronic technology. Mater. Sci. Semicond. Process. 2020, 107, 104807. [Google Scholar] [CrossRef]
- Pandey, B.; Gopal, R. Synthesis and Mn doped band gap engineering in SnS nanoparticles. Mater. Lett. 2020, 272, 127842. [Google Scholar] [CrossRef]
- Schliehe, C.; Juarez, B.H.; Pelletier, M.; Jander, S.; Greshnykh, D.; Nagel, M.; Meyer, A.; Foerster, S.; Kornowski, A.; Klinke, C.; et al. Ultrathin PbS sheets by two-dimensional oriented attachment. Science 2010, 329, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, X.; Abelson, A.; Zhang, B.-K.; Qian, C.; Ercius, P.; Wang, L.-W.; Law, M.; Zheng, H. In situ TEM observation of neck formation during oriented attachment of PbSe nanocrystals. Nano Res. 2019, 12, 2549–2553. [Google Scholar] [CrossRef]
- Pop, I.; Nascu, C.; Ionescu, V.; Indrea, E.; Bratu, I. Structural and optical properties of PbS thin films obtained by chemical deposition. Thin Solid Film. 1997, 307, 240–244. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, H.; Chen, F. Impact of thickness on crystal structure and optical properties for thermally evaporated PbSe thin films. Vacuum 2015, 114, 82–85. [Google Scholar] [CrossRef]
- Gorer, S.; Albu-Yaron, A.; Hodes, G. Quantum size effects in chemically deposited, nanocrystalline lead selenide films. J. Phys. Chem. 1995, 99, 16442–16448. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, Z.H.; El-Sebaii, A.; Al-Marzouki, F.; Al-Ghamdi, A. Structural, optical and electrical properties of cadmium-doped lead chalcogenide (PbSe) thin films. Phys. B Condens. Matter 2010, 405, 3384–3390. [Google Scholar] [CrossRef]
- Bai, R.; Chaudhary, S.; Pandya, D.K. Temperature dependent charge transport mechanisms in highly crystalline p-PbS cubic nanocrystals grown by chemical bath deposition. Mater. Sci. Semicond. Process. 2018, 75, 301–310. [Google Scholar] [CrossRef]
- Acharya, H.N.; Misra, N.K. Structural defects in chemically deposited photoconducting films of PbS. J. Phys. D Appl. Phys. 1971, 4, 1968–1970. [Google Scholar] [CrossRef]
- Pintilie, I.; Pentia, E.; Pintilie, L.; Petre, D.; Constantin, C.; Botila, T. Growth and characterization of PbS deposited on ferroelectric ceramics. J. Appl. Phys. 1995, 78, 1713–1718. [Google Scholar] [CrossRef]
- Blount, G.H.; Schreiber, P.J.; Smith, D.K.; Yamada, R.T. Variation of the properties of chemically deposited lead sulfide film with the use of an oxidant. J. Appl. Phys. 1973, 44, 978–981. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Wang, H.; Xu, S.; Chen, H.-Y. Sonochemical method for the preparation of monodisperse spherical and rectangular lead selenide nanoparticles. Langmuir 2002, 18, 3306–3310. [Google Scholar] [CrossRef]
- Delekar, S.; Patil, M.; Jadhav, B.; Sanadi, K.; Hankare, P. Synthesis and characterization of Cd0.7Pb0.3Se thin films for photoelectrochemical solar cell. Sol. Energy 2010, 84, 394–400. [Google Scholar] [CrossRef]
- Nair, P.K.; Nair, M.T.S. PbS solar control coatings: Safety, cost and optimisation. J. Phys. D Appl. Phys. 1990, 23, 150. [Google Scholar] [CrossRef]
- Simon, P.; Bahrig, L.; Baburin, I.A.; Formanek, P.; Röder, F.; Sickmann, J.; Hickey, S.G.; Eychmüller, A.; Lichte, H.; Kniep, R.; et al. Interconnection of nanoparticles within 2D superlattices of PbS/oleic acid thin films. Adv. Mater. 2014, 26, 3042–3049. [Google Scholar] [CrossRef]
- Shyju, T.; Anandhi, S.; Sivakumar, R.; Garg, S.; Gopalakrishnan, R. Investigation on structural, optical, morphological and electrical properties of thermally deposited lead selenide (PbSe) nanocrystalline thin films. J. Cryst. Growth 2012, 353, 47–54. [Google Scholar] [CrossRef]
- Feng, W.; Wang, X.; Zhou, H.; Chen, F. Effects of sputtering power on properties of PbSe nanocrystalline thin films deposited by RF magnetron sputtering. Vacuum 2014, 109, 108–111. [Google Scholar] [CrossRef]
- Zelenina, I.; Veremchuk, I.; Grin, Y.; Simon, P. In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe. Nanomaterials 2021, 11, 163. [Google Scholar] [CrossRef]
- Stöber, D.; Hildmann, B.O.; Böttner, H.; Schelb, S.; Bachem, K.H.; Binnewies, M. Chemical transport reactions during crystal growth of PbTe and PbSe via vapour phase influenced by AgI. J. Cryst. Growth 1992, 121, 656–664. [Google Scholar] [CrossRef]
- Urban, J.J.; Talapin, D.V.; Shevchenko, E.V.; Murray, C.B. Self-assembly of PbTe quantum dots into nanocrystal superlattices and glassy films. J. Am. Chem. Soc. 2006, 128, 3248–3255. [Google Scholar] [CrossRef]
- Cho, K.-S.; Talapin, D.V.; Gaschler, W.; Murray, C.B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 2005, 127, 7140–7147. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Drowart, J. Thermodynamic Study of Tin Sulfide and Lead Sulfide Using a Mass Spectrometer. J. Chem. Phys. 1962, 37, 1120–1125. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Available online: https://legacy.materialsproject.org (accessed on 24 April 2024).
- Oshchapovskii, V.V. Interpolation determination of the lattice energy of ionic crystals within the framework of stereoatomic model. Russ. J. General Chem. 2008, 78, 532–542. [Google Scholar] [CrossRef]
- Tanaka, H.; Morita, A. The crystal binding energies of the IV–VI semiconductors. J. Phys. Soc. Jpn 1979, 46, 523–529. [Google Scholar] [CrossRef]
- Nanda, K. Paradox associated with cohesive energy of IV–VI semiconductors. Phys. Lett. A 2020, 384, 126645. [Google Scholar] [CrossRef]
- Zelenina, I.; Simon, P.; Veremchuk, I.; Wang, X.; Bobnar, M.; Lu, W.; Liebscher, C.H.; Grin, Y. Structural complexity and the metal-to-semiconductor transition in lead telluride. Commun. Mater. 2021, 2, 99. [Google Scholar] [CrossRef]
- Foitzik, A.; Haasen, P.; Skrotzki, W. High resolution TEM of dissociated dislocations in PbS. Philos. Mag. A 1991, 64, 29–37. [Google Scholar] [CrossRef]
- Liou, S.-C.; Chiou, W.-A.; Zhou, Y.; Shu, G.-J.; Chou, F.-C. Microstructure of Thermoelectric Material—Pb1-xSnxSe and PbSe. Microsc. Microanal. 2018, 24 (Suppl. 1), 2266–2267. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Han, M.-K.; Sootsman, J.R.; Girard, S.; Arachchige, I.U.; Kanatzidis, M.G.; Dravid, V.P. Electron-beam activated thermal sputtering of thermoelectric materials. J. Appl. Phys. 2011, 110, 044325. [Google Scholar] [CrossRef]
- Božin, E.S.; Malliakas, C.D.; Souvatzis, P.; Proffen, T.; Spaldin, N.A.; Kanatzidis, M.G.; Billinge, S.J.L. Entropically Stabilized Local Dipole Formation in Lead Chalcogenides. Science 2010, 330, 1660–1663. [Google Scholar] [CrossRef] [PubMed]

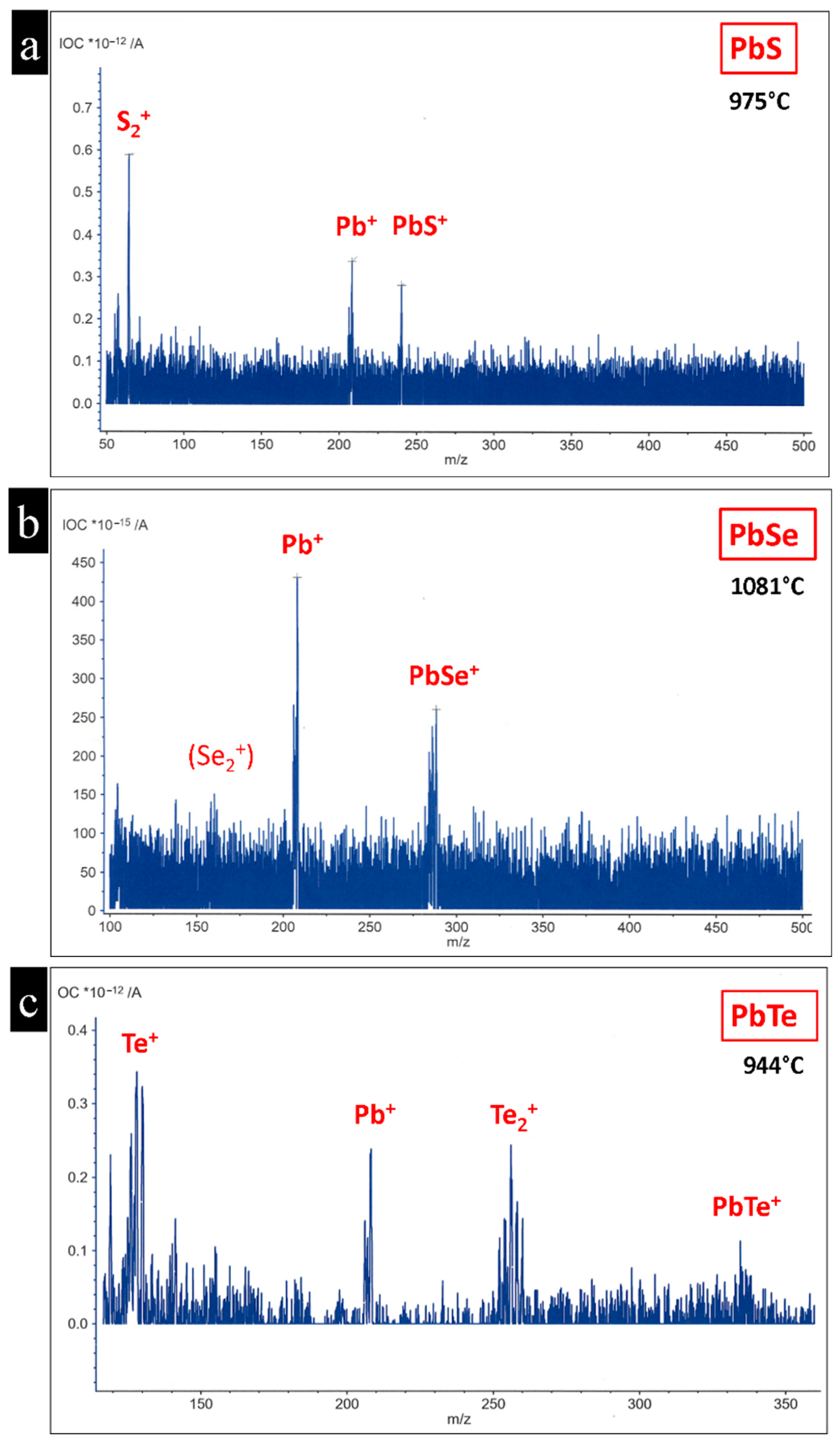

| Compound | Gas Ion (m/z) | Gas Ion/(m/z) | Gas Ion/(m/z) | Gas Ion/(m/z) |
|---|---|---|---|---|
| PbS | - | S2+ 64 | Pb+ 208 | PbS+ 240 |
| PbSe | - | Se2+ 160 | Pb+ 208 | PbSe+ 288 |
| PbTe | Te+ 130 | Te2+ 256 | Pb+ 208 | PbTe+ 336 |
| Compound | Sublimation Temp. |
|---|---|
| PbS | 970 °C ± 5 °C (Pb+) |
| PbSe | 960 °C ± 5 °C (Pb+) |
| PbTe | 925 °C ± 5 °C (Pb+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenina, I.; Böttner, H.; Schmidt, M.; Grin, Y.; Simon, P. In Situ TEM Observation of Electric Field-Directed Self-Assembly of PbS and PbSe Nanoparticles. Nanomaterials 2025, 15, 1275. https://doi.org/10.3390/nano15161275
Zelenina I, Böttner H, Schmidt M, Grin Y, Simon P. In Situ TEM Observation of Electric Field-Directed Self-Assembly of PbS and PbSe Nanoparticles. Nanomaterials. 2025; 15(16):1275. https://doi.org/10.3390/nano15161275
Chicago/Turabian StyleZelenina, Iryna, Harald Böttner, Marcus Schmidt, Yuri Grin, and Paul Simon. 2025. "In Situ TEM Observation of Electric Field-Directed Self-Assembly of PbS and PbSe Nanoparticles" Nanomaterials 15, no. 16: 1275. https://doi.org/10.3390/nano15161275
APA StyleZelenina, I., Böttner, H., Schmidt, M., Grin, Y., & Simon, P. (2025). In Situ TEM Observation of Electric Field-Directed Self-Assembly of PbS and PbSe Nanoparticles. Nanomaterials, 15(16), 1275. https://doi.org/10.3390/nano15161275






