Ultrasensitive and Selective ZPNRs-H Sensor for Sulfur Gas Molecules Detection
Abstract
1. Introduction
2. Computational Methods
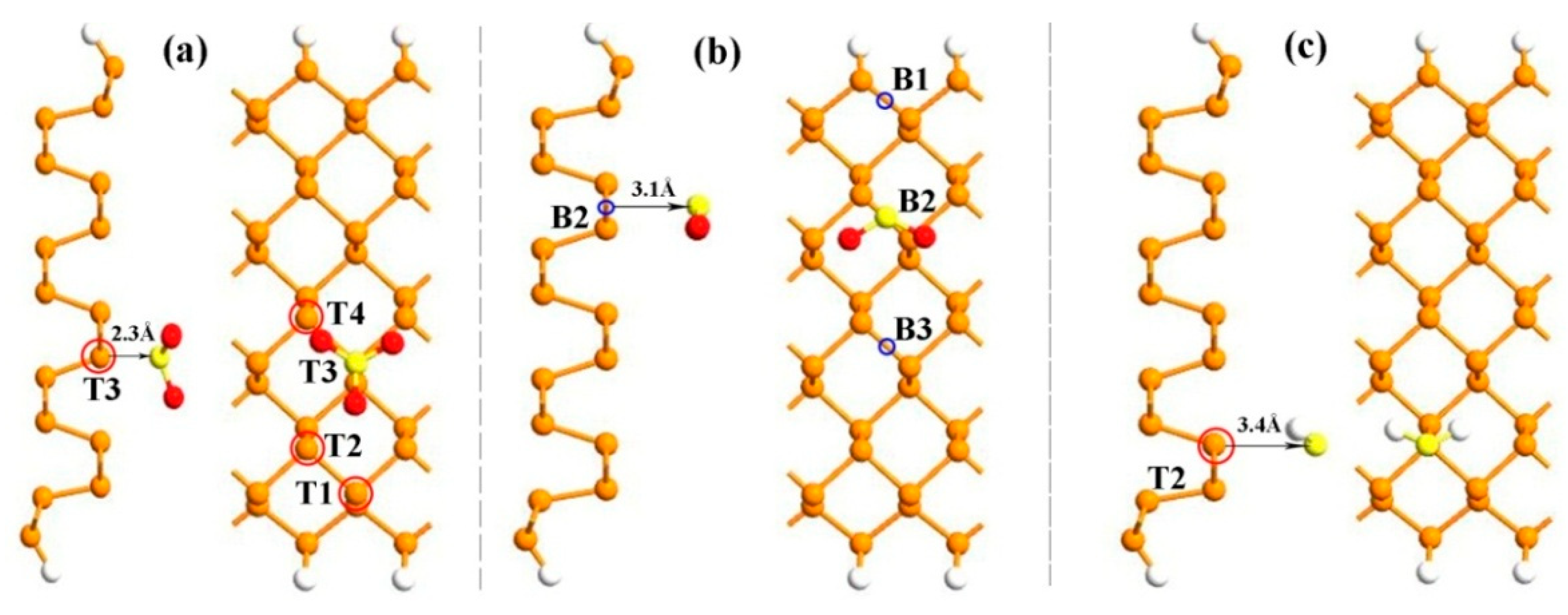
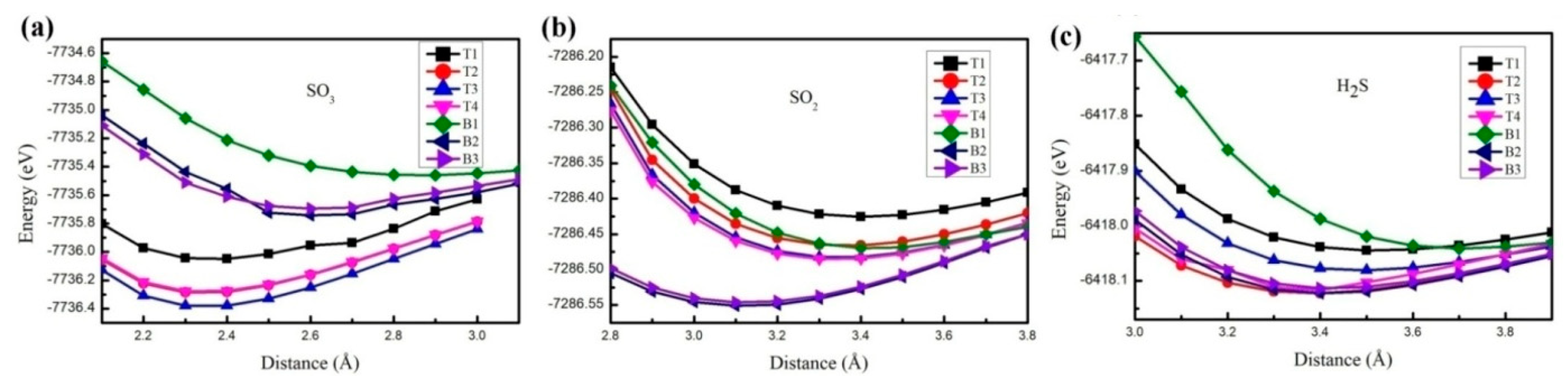
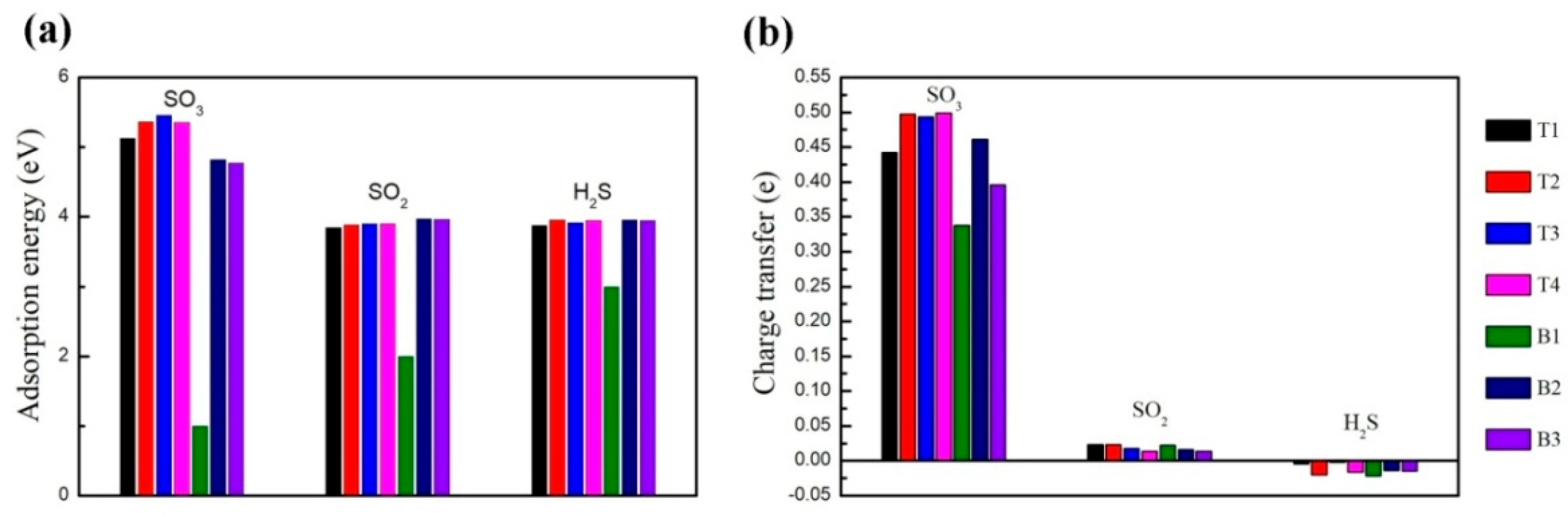
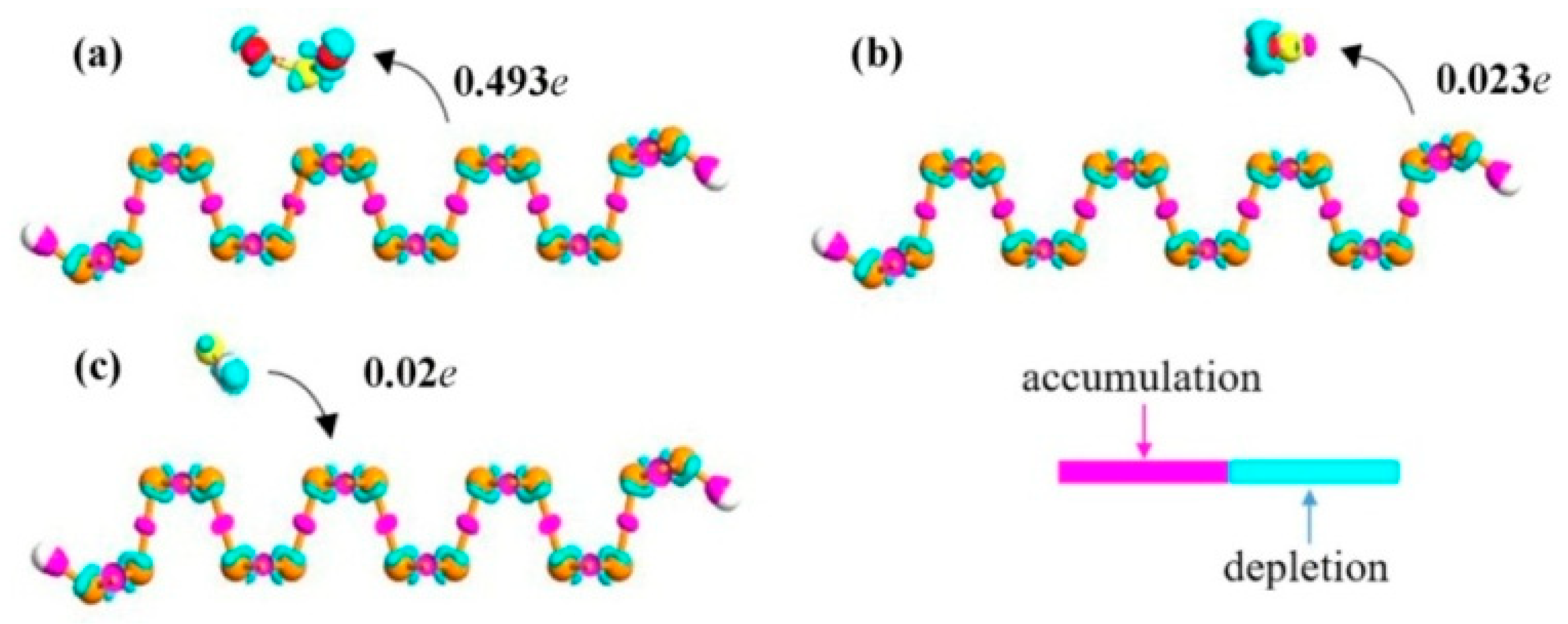
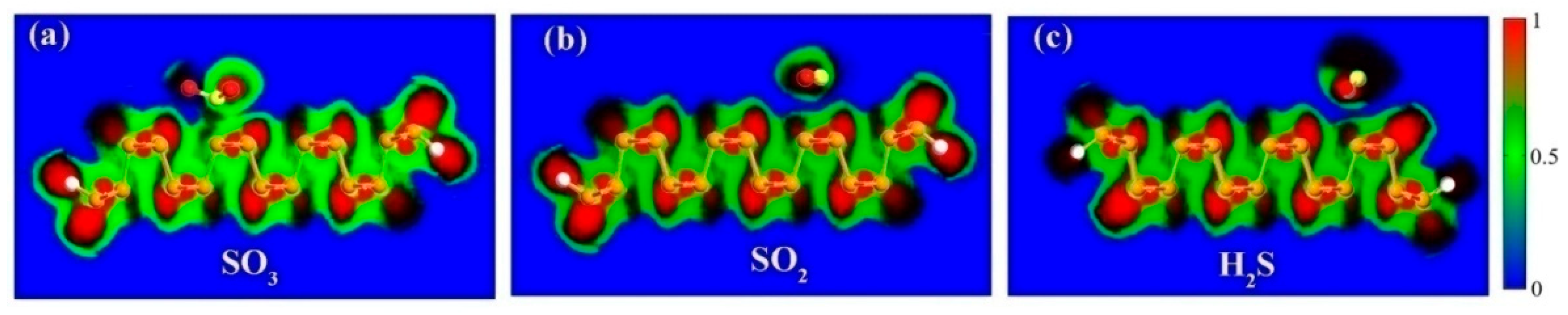
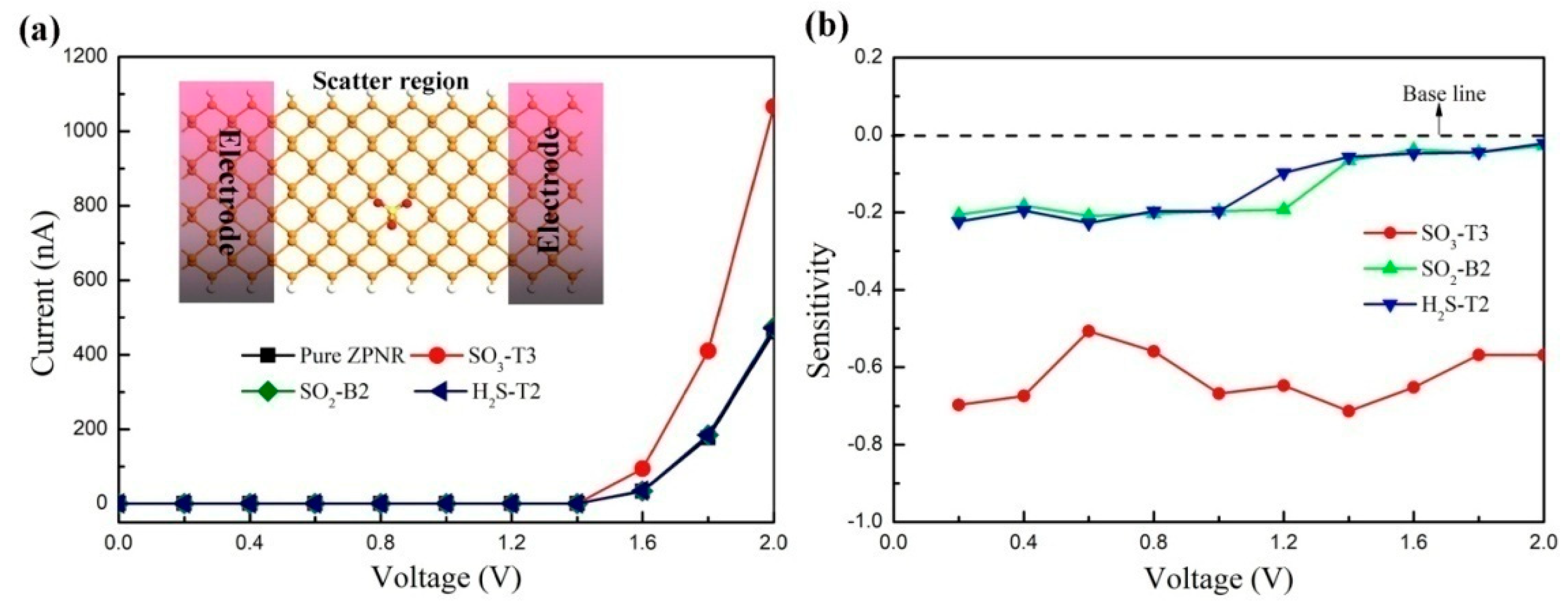
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Shimizu, Y. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuators B 2001, 77, 35–40. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Meng, R.S.; Tan, C.J.; Chen, X.P.; Xiao, J. Selective gas adsorption and I-V response of monolayer boron phosphide introduced by dopants: A first-principles study. Appl. Surf. Sci. 2018, 427, 176–188. [Google Scholar] [CrossRef]
- Lee, S.C.; Hwang, B.W.; Lee, S.J.; Choi, H.Y.; Kim, S.Y.; Jung, S.Y.; Ragupathy, D.; Lee, D.D.; Kim, J.C. A novel tin oxide-based recoverable thick film SO2 gas sensor promoted with magnesium and vanadium oxides. Sens. Actuator B-Chem. 2011, 160, 1328–1334. [Google Scholar] [CrossRef]
- Kou, L.Z.; Frauenheim, C.F.; Chen, T. Phosphorene as a superior gas sensor: Selective adsorption and distinct I-V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, L.; Liu, X.; Zhou, W.; Song, X.; Zhang, S. First-principles study of SO2 sensors based on phosphorene and its isoelectronic counterparts: GeS, GeSe, SnS, SnSe. Chem. Phys. Lett. 2017, 686, 83–87. [Google Scholar] [CrossRef]
- Imanaka, N.; Yamaguchi, Y.; Adachi, G.; Shiokawa, J. Sulfur dioxide gas detection with Na2SO4-Li2SO4-Y2(SO4)3-SiO2 solid electrolyte by a solid reference electrode method. J. Electrochem. Soc. 1987, 134, 725–728. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, S.; Parkash, O.; Kumar, D.; Bandyopadhyay, S.; Samudrala, S.K.; Sen, A.; Maiti, H.S. Vanadium doped tin dioxide as a novel sulfur dioxide sensor. Talanta 2008, 75, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Fergus, J.W. A review of electrolyte and electrode materials for high temperature electrochemical CO2 and SO2 gas sensors. Sens. Actuator B-Chem. 2008, 134, 1034–1041. [Google Scholar] [CrossRef]
- Yashina, L.V.; Zyubin, A.S.; Püttner, R.; Zyubina, T.S.; Neudachina, V.S.; Stojanov, P.; Riley, J.; Dedyulin, S.N.; Brzhezinskaya, M.M.; Shtanov, V.I. The Oxidation of PbS(001) surface with O2 and air studied with photoelectron spectroscopy and ab initio modelling. Surf. Sci. 2011, 605, 473–482. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.; Koh, H.J.; Jung, H.; Kim, J.S.; Yoo, H.W.; Kim, J.; Jung, H.T. Superior chemical sensing performance of black phosphorus: Comparison with MoS2 and graphene. Adv. Mater. 2016, 28, 7020. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.F.; Xue, J.J.; Lei, J.M.; Chen, D.J.; Lu, H.; Zhang, R.; Zheng, Y.D. High Sensitivity and Selectivity of AsP Sensor in Detecting SF6 Decomposition Gases. Sci. Rep. 2018, 13, 12011. [Google Scholar]
- Liu, Q.Q.; Li, J.J.; Wu, D.; Deng, X.Q.; Zhang, Z.H.; Fan, Z.Q.; Chen, K.Q. Gate-controlled reversible rectifying behavior investigated in a two-dimensional MoS2 diode. Phys. Rev. B 2021, 104, 045412. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Ryzhkov, S.A.; Eliseyev, I.A.; Stolyarova, D.Y.; Antonov, G.A.; Struchkov, N.S.; Brzhezinskaya, M.; Kirilenko, D.A.; Pavlov, S.I.; et al. A blueprint for the synthesis and characterization of thiolated Graphene. Nanomaterials 2022, 12, 45. [Google Scholar] [CrossRef]
- Yang, J.; Sun, R.S.; Bao, X.; Liu, J.; Ng, J.W.; Tang, B.; Liu, Z. Enhancing selectivity of two-dimensional materials-based gas sensors. Adv. Funct. Mater. 2025, 35, 2420393. [Google Scholar] [CrossRef]
- Kadam, S.A. Advancements in monolayer TMD-based gas sensors: Synthesis, mechanisms, elec tronic structure engineering, and flexible wearable sensors for real-world applications and future prospects. Chem. Eng. J. 2025, 517, 164223. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.L.; Perkins, F.K.; Cobas, E.; Jernigan, G.G.; Campbell, P.M.; Hanbicki, A.T.; Jonker, B.T. Chemical vapor sensing of two-dimensional MoS2 field effect transistor devices. Solid-State Electron 2014, 101, 2–7. [Google Scholar] [CrossRef]
- Salih, E.; Ayesh, A.I. Pt-doped armchair graphene nanoribbon as a promising gas sensor for CO and CO2: DFT study. Physica E 2021, 125, 114418. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A. Black phosphorus: Narrow gap, wide applications. J. Phys. Chem. Lett. 2015, 6, 4280–4291. [Google Scholar] [CrossRef] [PubMed]
- Li, L.K.; Yang, F.Y.; Ye, G.J.; Zhang, Z.C.; Zhu, Z.W.; Lou, W.K.; Zhou, X.Y.; Li, L.; Watanabe, K.; Taniguchi, T.; et al. Quantum Hall effect in black phosphorus two-dimensional electron system. Nat. Nanotechnol. 2016, 11, 592–596. [Google Scholar] [CrossRef]
- Xia, F.N.; Wang, H.; Jia, Y.C. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef]
- Ray, S.J. First-principles study of MoS2, phosphorene and graphene based single electron transistor for gas sensing applications. Sens. Actuator B-Chem. 2016, 222, 492–498. [Google Scholar] [CrossRef]
- Khan, M.S.; Srivastava, A.; Pandey, R. Electronic properties of a pristine and NH3/NO2 adsorbed buckled arsenene monolayer. RSC Adv. 2016, 6, 72634–72642. [Google Scholar] [CrossRef]
- Kuang, A.; Kuang, M.; Yuan, H.; Wang, G.; Chen, H.; Yang, X. Acidic gases (CO2, NO2 and SO2) cap ture and dissociation on metal decorated phosphorene. Appl. Surf. Sci. 2017, 410, 505–512. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. Adsorption of NO2 molecules on armchair phosphorene nanosheet for nano sensor applications–A first-principles study. J. Mol. Graphics Modell. 2017, 75, 365–374. [Google Scholar] [CrossRef]
- Huang, C.S.; Murat, A.; Babar, V.; Montes, E.; Schwingenschlögl, U. Adsorption of the gas molecules NH3, NO, NO2, and CO on borophene. J. Phys. Chem. C 2018, 122, 14665–14670. [Google Scholar] [CrossRef]
- Lei, S.Y.; Yu, Z.Y.; Shen, H.Y.; Sun, X.L.; Wan, N.; Yu, H. CO adsorption on metal-decorated phos phorene. ACS Omega 2018, 3, 3957–3965. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, S.; Gao, R.; Sun, X.; Chen, J. Effect of metal decoration on sulfur-based gas molecules adsorption on phosphorene. Sci. Rep. 2021, 11, 18179. [Google Scholar] [CrossRef] [PubMed]
- Kaewmaraya, T.; Ngarnwongwan, L.; Moontragoon, P.; Karton, A.; Hussain, T. Drastic improvement in gas-sensing characteristics of phosphorene nanosheets under vacancy defects and elemental functionalization. J. Phys. Chem. C 2018, 122, 20186–20193. [Google Scholar] [CrossRef]
- Su, S.L.; Gong, J.; Fan, Z.Q. Selective adsorption of harmful molecules on zigzag phosphorene nano ribbon for sensing applications. Physica E 2020, 117, 113838. [Google Scholar] [CrossRef]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of open systems: Charge transfer, electron conduction, and molecular switching of a C-60 device. Phys. Rev. B 2001, 63, 121104. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.L.; Ordejon, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165109. [Google Scholar] [CrossRef]
- Büttiker, M.; Imry, Y.; Landauer, R.; Pinhas, S. Generalized many-channel conductance formula with application to small rings. Phys. Rev. B 1985, 31, 6207–6215. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Wang, Z.Y.; Dai, X.Q.; Xiao, J.R.; Long, M.Q.; Chen, T. The electronic and transport properties of the phosphorene nanoribbons. Mater. Res. Express 2019, 6, 096317. [Google Scholar] [CrossRef]
- Nagarajan, V.; Bhuvaneswari, R.; Chandiramouli, R. Phosphoborane nanosheets as a sensing element for liquefied petroleum gas a first-principles study. Chem. Phys. Lett. 2024, 834, 140967. [Google Scholar] [CrossRef]
- Hussain, T.; Kaewmaraya, T.; Chakraborty, S.; Vovusha, H.; Amornkitbamrung, V.; Ahuja, R. Defected and functionalized germanene-based nanosensors under sulfur comprising gas exposure. ACS Sens. 2018, 3, 867–874. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Liu, G.; Yang, N.; Wu, Q.; Xiao, X.; Chen, T. Biaxila strain modulated high anisotropic gas-sensing performance of C5N-based two-dimensional devices: A first-principles study. Surf. Interfaces 2024, 48, 104277. [Google Scholar] [CrossRef]
- He, H.; Hao, Z.W.; Lu, X.Q.; Dong, M.M.; Li, Z.L.; Wang, C.K.; Fu, X.X. Black phosphorene with Au modification: Oxynitride remover and hydrogen sensor. Appl. Surf. Sci. 2024, 651, 159194. [Google Scholar] [CrossRef]
- Ye, H.Y.; Liu, L.; Xu, Y.X.; Wang, L.Y.; Chen, X.P.; Zhang, K.; Liu, Y.F.; Koh, S.W.; Zhang, G.Q. SnSe monolayer: A promising candidate of SO2 sensor with high adsorption quantity. Appl. Surf. Sci. 2019, 484, 33–38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Lv, X.; Gong, J.; Fan, Z.-Q. Ultrasensitive and Selective ZPNRs-H Sensor for Sulfur Gas Molecules Detection. Nanomaterials 2025, 15, 1273. https://doi.org/10.3390/nano15161273
Su S, Lv X, Gong J, Fan Z-Q. Ultrasensitive and Selective ZPNRs-H Sensor for Sulfur Gas Molecules Detection. Nanomaterials. 2025; 15(16):1273. https://doi.org/10.3390/nano15161273
Chicago/Turabian StyleSu, Shaolong, Xiaodong Lv, Jian Gong, and Zhi-Qiang Fan. 2025. "Ultrasensitive and Selective ZPNRs-H Sensor for Sulfur Gas Molecules Detection" Nanomaterials 15, no. 16: 1273. https://doi.org/10.3390/nano15161273
APA StyleSu, S., Lv, X., Gong, J., & Fan, Z.-Q. (2025). Ultrasensitive and Selective ZPNRs-H Sensor for Sulfur Gas Molecules Detection. Nanomaterials, 15(16), 1273. https://doi.org/10.3390/nano15161273




