Tuning Nanofibrous Sensor Performance in Selective Detection of B-VOCs by MIP-NP Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecularly Imprinted Nanoparticle (MIPNP) Synthesis
2.3. MWCNT Dispersion
2.4. Electrospun Layer Fabrication
2.5. UV-Crosslinking Process
2.6. Interdigitated Microelectrode Layout
2.7. Scanning Electron Microscopy (SEM)
2.8. Atomic Force Microscopy (AFM)
2.9. Electrical and Sensing Measurements
3. Results and Discussion
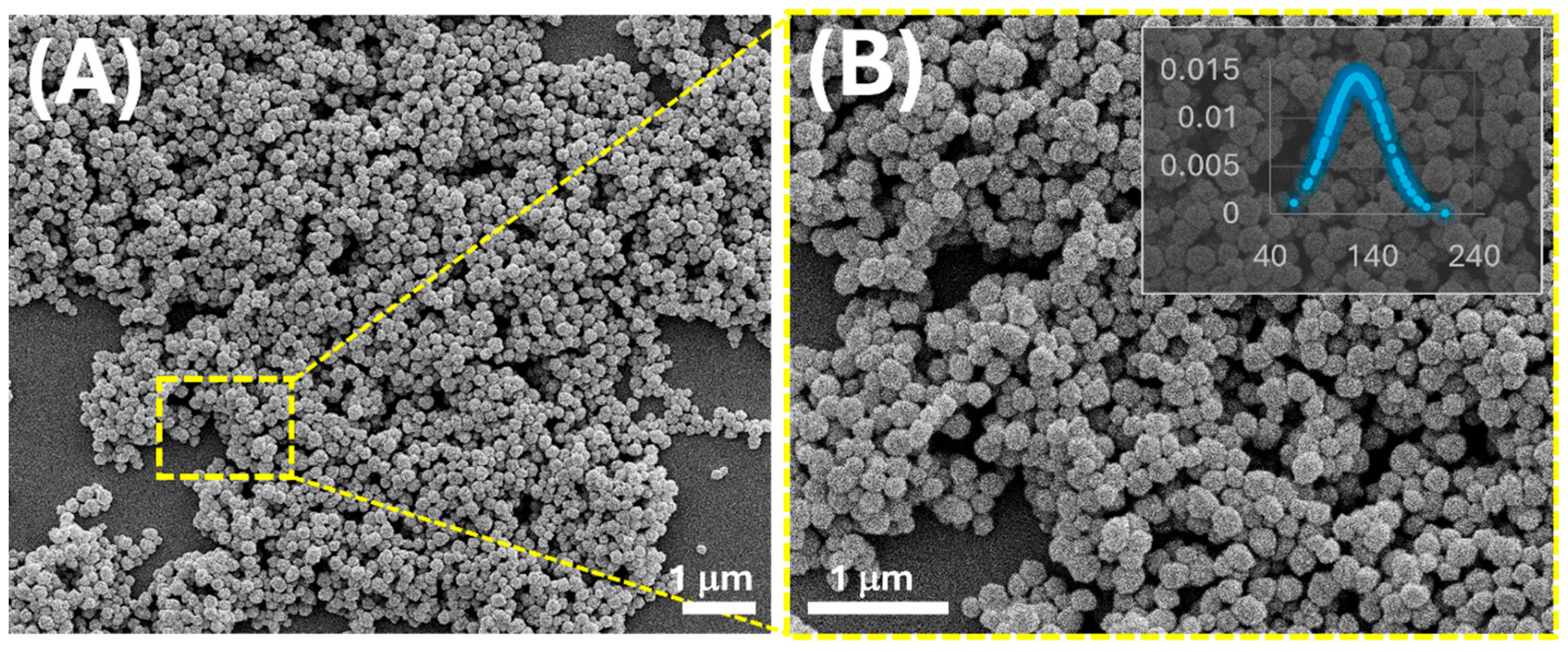
3.1. MIP-NP Characterization
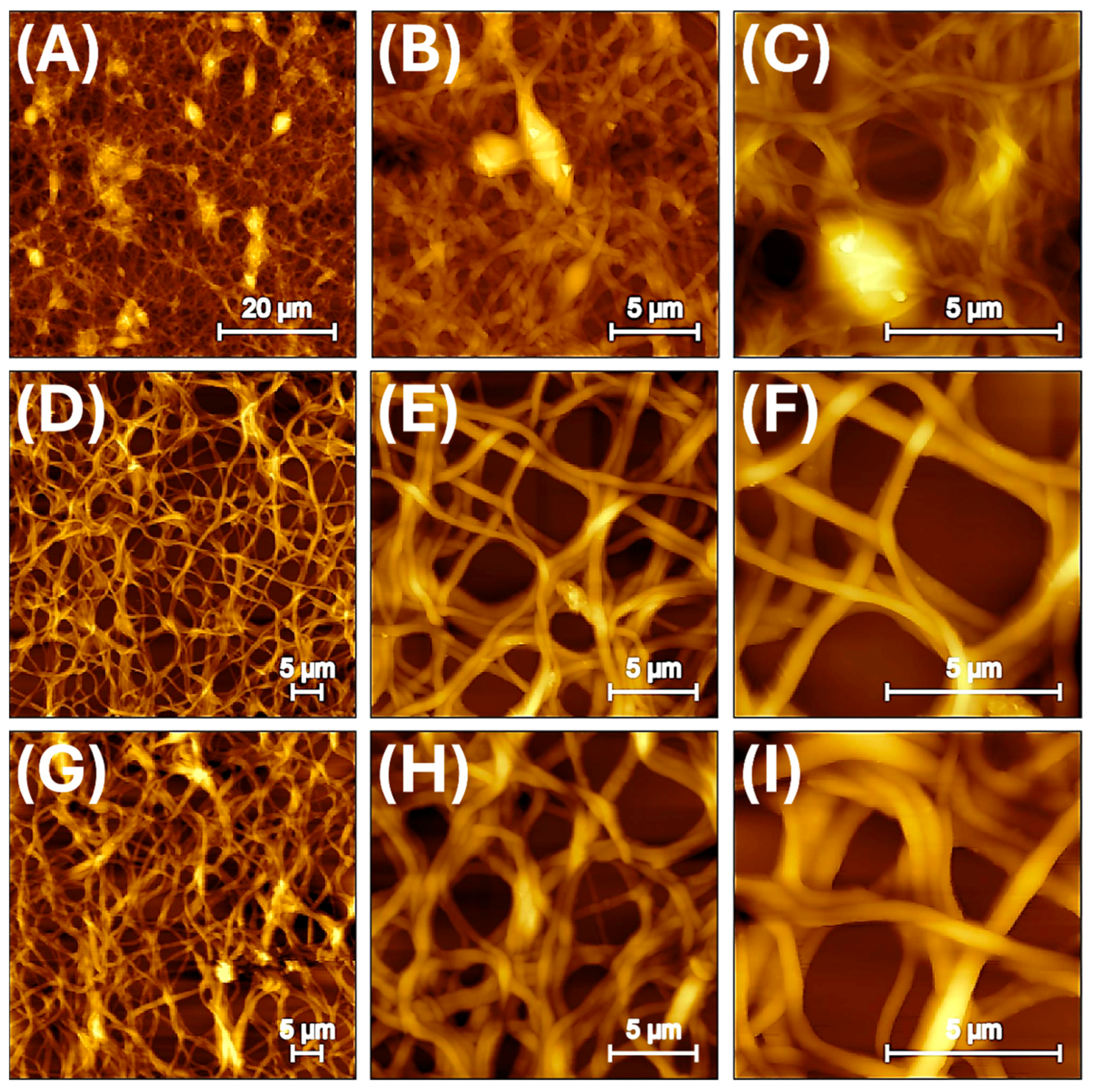
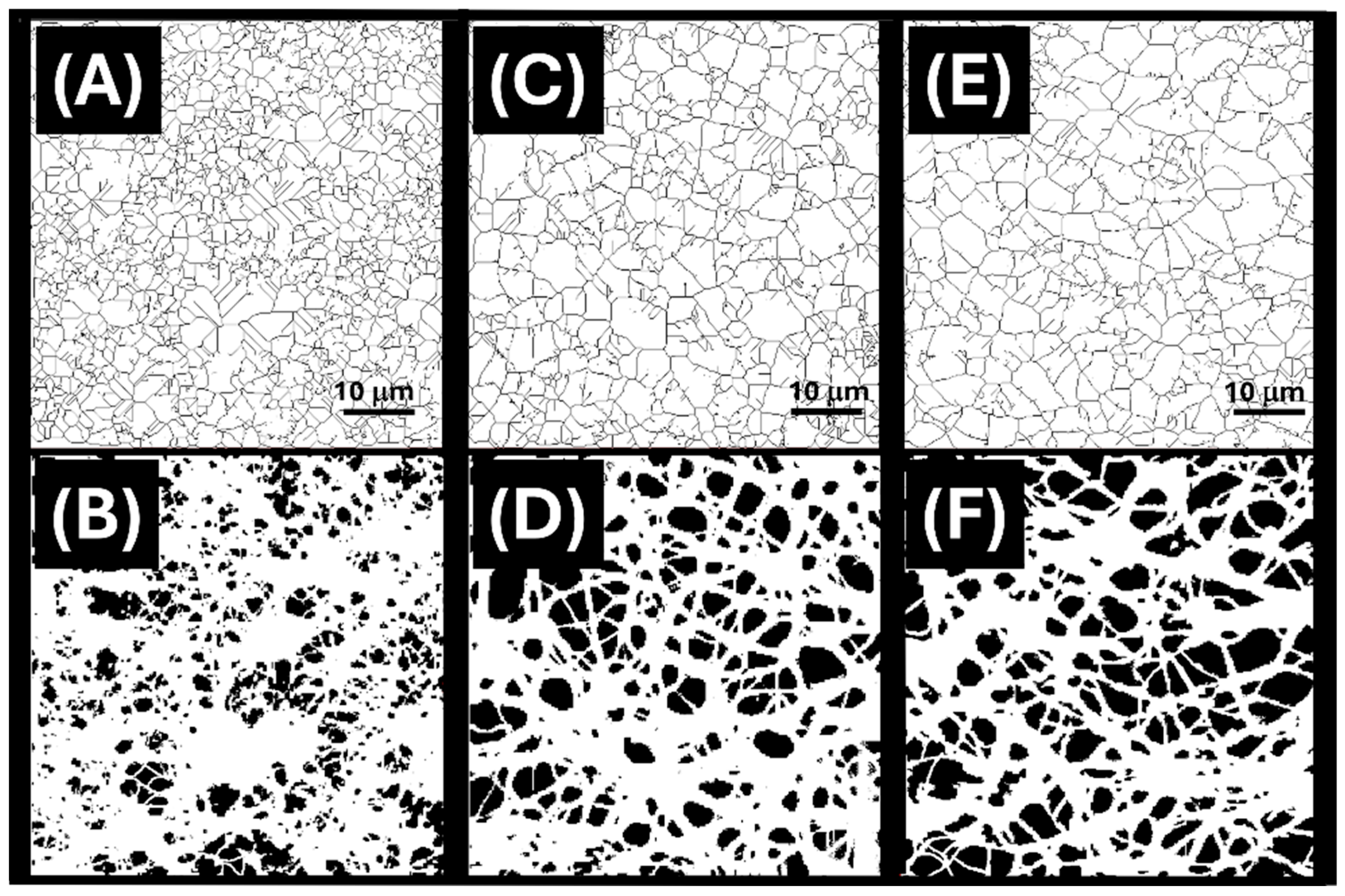
3.2. Nanofibrous Layer Characterization
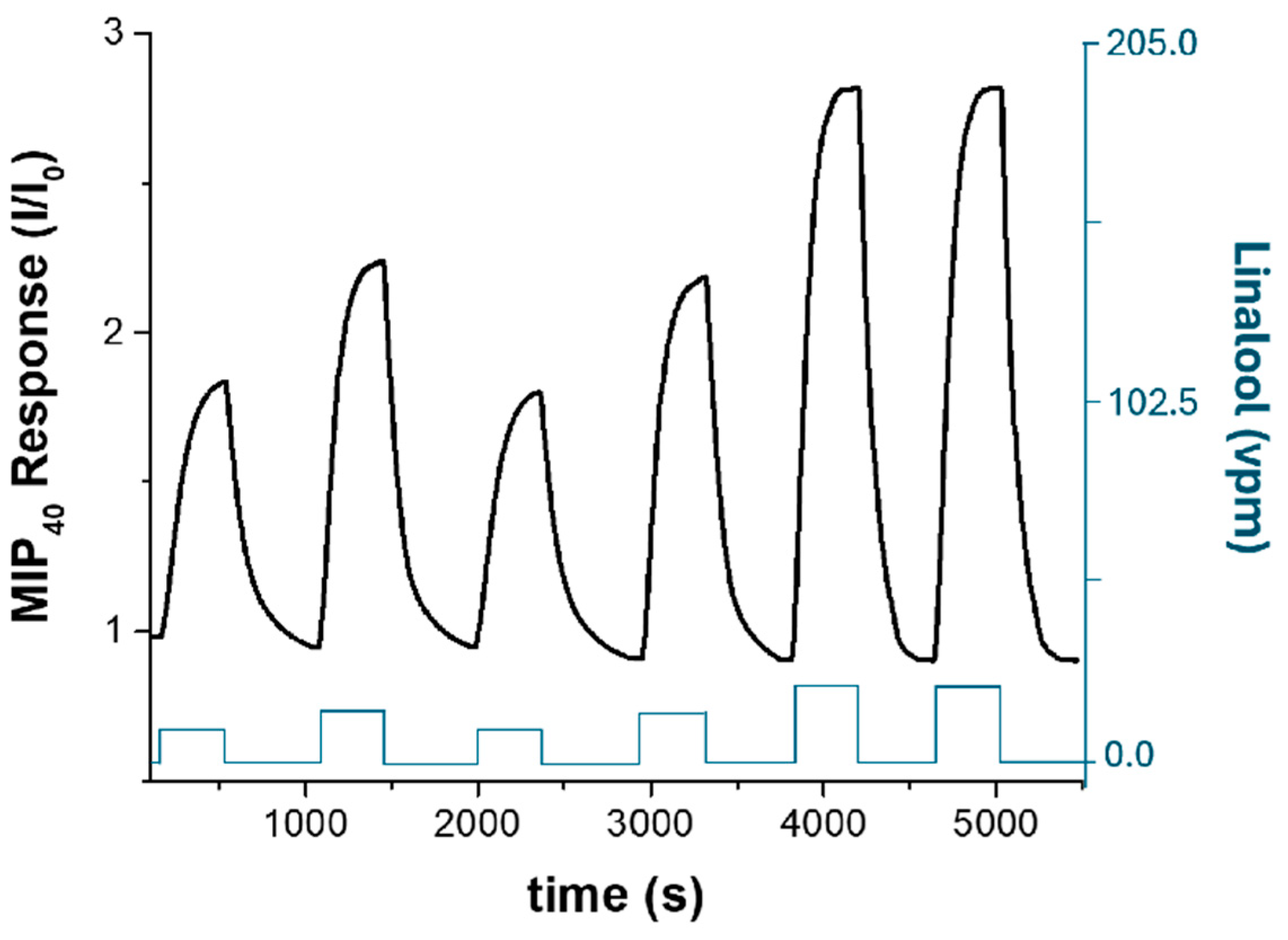
3.3. Electrical and Sensing Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, R.; Lun, X.; Gao, R.; Wang, L.; Yang, Y.; Su, X.; Habibullah-Al-Mamun, M.; Xu, X.; Li, H.; Li, J. A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects. Toxics 2025, 13, 364. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of Synthesis, Biochemistry and Functional Role of Plant Volatiles. South Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene-A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Qiu, C.L.; Li, W.; Wang, L.N.; Wang, S.C.; Falert, S.; Wang, C.; Yu, S.Y.; Abdelkhalek, S.T.; Lu, J.; Lin, Y.J.; et al. Limonene Enhances Rice Plant Resistance to a Piercing-Sucking Herbivore and Rice Pathogens. Plant Biotechnol. J. 2025, 23, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Heidari, M. Impact of Drought Stress on Biochemical and Molecular Responses in Lavender (Lavandula angustifolia Mill.): Effects on Essential Oil Composition and Antibacterial Activity. Front. Plant Sci. 2025, 16, 1506660. [Google Scholar] [CrossRef]
- de Lima Silva, J.R.; dos Santos, L.B.; Hassan, W.; Kamdem, J.P.; Duarte, A.E.; Soufan, W.; El Sabagh, A.; Ibrahim, M. Exploring the Therapeutic Potential of the Oxygenated Monoterpene Linalool in Alleviating Saline Stress Effects on Allium Cepa L. Environ. Sci. Pollut. Res. 2024, 31, 47598–47610. [Google Scholar] [CrossRef]
- Ilc, T.; Parage, C.; Boachon, B.; Navrot, N.; Werck-Reichhart, D. Monoterpenol Oxidative Metabolism: Role in Plant Adaptation and Potential Applications. Front. Plant Sci. 2016, 7, 509. [Google Scholar] [CrossRef]
- Yue, R.; Li, Y.; Qi, Y.; Liang, X.; Zheng, Z.; Ye, Z.; Tong, W.; Si, X.; Zhang, Y.; Xia, E.; et al. Divergent MYB Paralogs Determine Spatial Distribution of Linalool Mediated by JA and DNA Demethylation Participating in Aroma Formation and Cold Tolerance of Tea Plants. Plant Biotechnol. J. 2025, 23, 1455–1475. [Google Scholar] [CrossRef]
- Lahmar, I.; Yotova, L.; Belghith, K. Environmental Impact on Phonological Stages in Lavandula officinalis: Chemical Profiling of Essential Oil and Extract, Antioxidant Activity, and Acetylcholinesterase Inhibition Potential. Euro-Mediterr. J. Environ. Integr. 2025, 10, 1–12. [Google Scholar] [CrossRef]
- Eghlima, G.; Saeed-Abadi, B.; Sonboli, A.; Rezadoost, H.; Mirjalili, M.H. Phenotypic Yield-Attributed Traits, Essential Oil Content and Composition of Iranian Grammosciadium platycarpum (Apiaceae) Populations: A Rich Source of (S)-(+)-Linalool. BMC Plant Biol. 2025, 25, 208. [Google Scholar] [CrossRef]
- Jiao, C.; Gong, J.; Guo, Z.; Li, S.; Zuo, Y.; Shen, Y. Linalool Activates Oxidative and Calcium Burst and CAM3-ACA8 Participates in Calcium Recovery in Arabidopsis Leaves. Int. J. Mol. Sci. 2022, 23, 5357. [Google Scholar] [CrossRef]
- Wang, K.; Ren, W.; Hong, L.; Wang, Q.; Ghimire, R.; Haapanen, M.; Kivimäenpää, M.; Wu, P.; Ma, X.; Asiegbu, F.O. Linalool and 1,8-Cineole as Constitutive Disease-Resistant Factors of Norway Spruce Against Necrotrophic Pathogen Heterobasidion parviporum. Plant Cell Environ. 2025, 48, 1993–2008. [Google Scholar] [CrossRef]
- Ramanpong, J.; Tsao, C.; Yin, J.; Wu, C.D.; Huang, Y.C.; Yu, C.P. Effects of Forest Bathing and the Influence of Exposure Levels on Cognitive Health in the Elderly: Evidence from a Suburban Forest Recreation Area. Urban For. Urban Green 2025, 104, 128667. [Google Scholar] [CrossRef]
- Haluza, D.; Kersten, P.; Lazic, T.; Steinparzer, M.; Godbold, D. Unlocking the Power of Nature: Insights from a 20-Minute Forest Visit on Well-Being. Forests 2025, 16, 792. [Google Scholar] [CrossRef]
- Srivastava, D.; Vu, T.V.; Tong, S.; Shi, Z.; Harrison, R.M. Formation of Secondary Organic Aerosols from Anthropogenic Precursors in Laboratory Studies. NPJ Clim. Atmos. Sci. 2022, 5, 22. [Google Scholar] [CrossRef]
- Li, M.; Cappellin, L.; Xu, J.; Biasioli, F.; Varotto, C. High-Throughput Screening for in Planta Characterization of VOC Biosynthetic Genes by PTR-ToF-MS. J. Plant Res. 2020, 133, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Loreto, F.; Aprea, E.; Romano, A.; Sánchez del Pulgar, J.; Gasperi, F.; Biasioli, F. PTR-MS in Italy: A Multipurpose Sensor with Applications in Environmental, Agri-Food and Health Science. Sensors 2013, 13, 11923–11955. [Google Scholar] [CrossRef]
- Wasilewski, T.; Orbay, S.; Brito, N.F.; Sikora, K.; Melo, A.C.A.; Melendez, M.E.; Szulczyński, B.; Sanyal, A.; Kamysz, W.; Gębicki, J. Molecularly Imprinted Polymers for the Detection of Volatile Biomarkers. TrAC Trends Anal. Chem. 2024, 177, 117783. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Kim, K.-H. Molecularly Imprinted Polymers for Sensing Gaseous Volatile Organic Compounds: Opportunities and Challenges. Environ. Pollut. 2022, 311, 119931. [Google Scholar] [CrossRef]
- Macagnano, A.; Molinari, F.N.; Papa, P.; Mancini, T.; Lupi, S.; D’Arco, A.; Taddei, A.R.; Serrecchia, S.; De Cesare, F. Nanofibrous Conductive Sensor for Limonene: One-Step Synthesis via Electrospinning and Molecular Imprinting. Nanomaterials 2024, 14, 1123. [Google Scholar] [CrossRef]
- Cowen, T.; Cheffena, M. Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. Int. J. Mol. Sci. 2022, 23, 9642. [Google Scholar] [CrossRef]
- Caldara, M.; van Wissen, G.; Cleij, T.J.; Diliën, H.; van Grinsven, B.; Eersels, K.; Lowdon, J.W. Deposition Methods for the Integration of Molecularly Imprinted Polymers (MIPs) in Sensor Applications. Adv. Sens. Res. 2023, 2, 2200059. [Google Scholar] [CrossRef]
- Gavrilă, A.M.; Stoica, E.B.; Iordache, T.V.; Sârbu, A. Modern and Dedicated Methods for Producing Molecularly Imprinted Polymer Layers in Sensing Applications. Appl. Sci. 2022, 12, 3080. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for Commercial Application in Low-Cost Sensors and Assays—An Overview of the Current Status Quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP Sensors—The Electrochemical Approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- González-Vila, Á.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly Imprinted Electropolymerization on a Metal-Coated Optical Fiber for Gas Sensing Applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Emam, S.; Adedoyin, A.; Geng, X.; Zaeimbashi, M.; Adams, J.; Ekenseair, A.; Podlaha-Murphy, E.; Sun, N.X. A Molecularly Imprinted Electrochemical Gas Sensor to Sense Butylated Hydroxytoluene in Air. J. Sens. 2018, 2018, 437149. [Google Scholar] [CrossRef]
- Debliquy, M.; Dony, N.; Lahem, D.; Tang, X.; Zhang, C.; Raskin, J.P.; Olivier, M.G. Acetaldehyde Chemical Sensor Based on Molecularly Imprinted Polypyrrole. Procedia Eng. 2016, 168, 569–573. [Google Scholar] [CrossRef]
- Ye, X.; Ge, L.; Jiang, T.; Guo, H.; Chen, B.; Liu, C.; Hayashi, K. Fully Inkjet-Printed Chemiresistive Sensor Array Based on Molecularly Imprinted Sol-Gel Active Materials. ACS Sens. 2022, 7, 1819–1828. [Google Scholar] [CrossRef]
- Jin, M.; Shi, P.; Sun, Z.; Zhao, N.; Shi, M.; Wu, M.; Ye, C.; Lin, C.T.; Fu, L. Advancements in Polymer-Assisted Layer-by-Layer Fabrication of Wearable Sensors for Health Monitoring. Sensors 2024, 24, 2903. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly Imprinted Polymers by the Surface Imprinting Technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Azhdary, P.; Janfaza, S.; Fardindoost, S.; Tasnim, N.; Hoorfar, M. Highly Selective Molecularly Imprinted Polymer Nanoparticles (MIP NPs)-Based Microfluidic Gas Sensor for Tetrahydrocannabinol (THC) Detection. Anal. Chim. Acta 2023, 1278, 341749. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, Y.; Yang, X.; Guo, L.; Man, C.; Jiang, Y.; Zhang, W.; Zhang, X. Emerging Biosensors Integrated with Microfluidic Devices: A Promising Analytical Tool for on-Site Detection of Mycotoxins. NPJ Sci. Food 2025, 9, 84. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Tavakkoli, O.; Mesbah, M.; Bhutto, J.K.; Khademi, T.; Kirpichnikova, I.; Ahmad, A.; ALJohani, A.A. A Review on Carbon-Based Molecularly-Imprinted Polymers (CBMIP) for Detection of Hazardous Pollutants in Aqueous Solutions. Chemosphere 2022, 308, 136471. [Google Scholar] [CrossRef]
- Macagnano, A.; Zampetti, E.; Kny, E. (Eds.) Electrospinning for High Performance Sensors; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-14405-4. [Google Scholar] [CrossRef]
- Molinari, F.N.; Marelli, M.; Berretti, E.; Serrecchia, S.; Coppola, R.E.; De Cesare, F.; Macagnano, A. Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture. Polymers 2025, 17, 326. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly Imprinted Polymer Nanoparticles in Chemical Sensing—Synthesis, Characterisation and Application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Nagalingam, S.; Seco, R.; Kim, S.; Guenther, A. Heat Stress Strongly Induces Monoterpene Emissions in Some Plants with Specialized Terpenoid Storage Structures. Agric. For. Meteorol. 2023, 333, 109400. [Google Scholar] [CrossRef]
- Kask, K.; Kännaste, A.; Talts, E.; Copolovici, L.; Niinemets, Ü. How Specialized Volatiles Respond to Chronic and Short-Term Physiological and Shock Heat Stress in Brassica Nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Bardehle, L.; Seguel, I.; Espinoza, J.; Lizama, M.; Quiroz, A. Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring. Metabolites 2023, 13, 616. [Google Scholar] [CrossRef]
- Stringari, G.; Villanueva, J.; Appolloni, E.; Orsini, F.; Villalba, G.; Gabarrell Durany, X. Measuring BVOC Emissions Released by Tomato Plants Grown in a Soilless Integrated Rooftop Greenhouse. Heliyon 2024, 10, e23854. [Google Scholar] [CrossRef] [PubMed]
- Boachon, B.; Junker, R.R.; Miesch, L.; Bassard, J.E.; Höfer, R.; Caillieaudeaux, R.; Seidel, D.E.; Lesot, A.; Heinrich, C.; Ginglinger, J.F.; et al. CYP76C1 (Cytochrome P450)-Mediated Linalool Metabolism and the Formation of Volatile and Soluble Linalool Oxides in Arabidopsis Flowers: A Strategy for Defense against Floral Antagonists. Plant Cell 2015, 27, 2972–2990. [Google Scholar] [CrossRef] [PubMed]
- Avesani, S.; Lazazzara, V.; Robatscher, P.; Oberhuber, M.; Perazzolli, M. Volatile Linalool Activates Grapevine Resistance against Downy Mildew with Changes in the Leaf Metabolome. Curr. Plant Biol. 2023, 35–36, 100298. [Google Scholar] [CrossRef]
- McCallum, E.J.; Cunningham, J.P.; Lücker, J.; Zalucki, M.P.; De Voss, J.J.; Botella, J.R. Increased Plant Volatile Production Affects Oviposition, but Not Larval Development, in the Moth Helicoverpa Armigera. J. Exp. Biol. 2011, 214, 3672–3677. [Google Scholar] [CrossRef]
- Molinari, F.; Medrano, A.V.; Bacigalupe, A.; Escobar, M.M.; Monsalve, L.N. Different Dispersion States of MWCNT in Aligned Conductive Electrospun PCL/MWCNT Composites. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 667–674. [Google Scholar] [CrossRef]
- Maciejewska, B.M.; Wychowaniec, J.K.; Woźniak-Budych, M.; Popenda, Ł.; Warowicka, A.; Golba, K.; Litowczenko, J.; Fojud, Z.; Wereszczyńska, B.; Jurga, S. UV Cross-Linked Polyvinylpyrrolidone Electrospun Fibres as Antibacterial Surfaces. Sci. Technol. Adv. Mater. 2019, 20, 979–991. [Google Scholar] [CrossRef]
- Djunaidi, M.C.; Putri, V.R.; Maharani, N.D.; Lusiana, R.A.; Siahaan, P.; Sunarno, S. Precipitation Polymerization-Based Molecularly Imprinted Polymers: A Novel Approach for Transdermal Curcumin Delivery. Polymers 2024, 16, 3456. [Google Scholar] [CrossRef]
- Holdsworth, C.I.; Lim, K.F.; Muang-Non, P. Molecularly Imprinted Polymeric Nanoparticles by Precipitation Polymerization and Characterization by Quantitative NMR Spectroscopy. In Molecularly Imprinted Polymers. Methods in Molecular Biology; Martín-Esteban, A., Ed.; Humana: New York, NY, USA, 2021; Volume 2359, pp. 9–18. [Google Scholar] [CrossRef]
- Karnka, R.; Chaiyasat, P.; Chaiyasat, A. Synthesis of Uniform and Stable Molecularly Imprinted Polymer Particles by Precipitation Polymerization. Orient. J. Chem. 2017, 33, 37995. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Aglikov, A.S.; Zhukov, M.V.; Aliev, T.A.; Kozodaev, D.A.; Nosonovsky, M.; Skorb, E.V. New Metrics for Describing Atomic Force Microscopy Data of Nanostructured Surfaces through Topological Data Analysis. Appl. Surf. Sci. 2024, 670, 160640. [Google Scholar] [CrossRef]
- Langwald, S.V.; Ehrmann, A.; Sabantina, L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes 2023, 13, 488. [Google Scholar] [CrossRef]
- Raza, S.; Li, X.; Soyekwo, F.; Liao, D.; Xiang, Y.; Liu, C. A Comprehensive Overview of Common Conducting Polymer-Based Nanocomposites; Recent Advances in Design and Applications. Eur. Polym. J. 2021, 160, 110773. [Google Scholar] [CrossRef]
- Alam, N.; Abid, N.; Islam, S.S. Advancements in Trace and Low Humidity Sensors Technologies Using Nanomaterials: A Review. ACS Appl. Nano Mater. 2024, 7, 13836–13864. [Google Scholar] [CrossRef]
- Alam, M.W.; Bhat, S.I.; Al Qahtani, H.S.; Aamir, M.; Amin, M.N.; Farhan, M.; Aldabal, S.; Khan, M.S.; Jeelani, I.; Nawaz, A.; et al. Recent Progress, Challenges, and Trends in Polymer-Based Sensors: A Review. Polymers 2022, 14, 2164. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S.; Krishnamurthy, K. Electrical Conductivity of a Single Electrospun Fiber of Poly(Methyl Methacrylate) and Multiwalled Carbon Nanotube Nanocomposite. Appl. Phys. Lett. 2006, 88, 143114. [Google Scholar] [CrossRef]
- Yin, Z.; Ding, A.; Zhang, H.; Zhang, W. The Relevant Approaches for Aligning Carbon Nanotubes. Micromachines 2022, 13, 1863. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Hossain, M.M.; Islam, M.A.; Shima, H.; Hasan, M.; Lee, M. Alignment of Carbon Nanotubes in Carbon Nanotube Fibers Through Nanoparticles: A Route for Controlling Mechanical and Electrical Properties. ACS Appl. Mater. Interfaces 2017, 9, 5530–5542. [Google Scholar] [CrossRef]
- Feldner, A.; Völkle, J.; Lieberzeit, P.; Fruhmann, P. Conductive Molecularly Imprinted Polymers (CMIPs): Rising and Versatile Key Elements in Chemical Sensing. Chemosensors 2023, 11, 299. [Google Scholar] [CrossRef]
- Zhang, H. Water-Compatible Molecularly Imprinted Polymers: Promising Synthetic Substitutes for Biological Receptors. Polymer 2014, 55, 699–714. [Google Scholar] [CrossRef]
- Horemans, F.; Weustenraed, A.; Spivak, D.; Cleij, T.J. Towards Water Compatible MIPs for Sensing in Aqueous Media. Mol. Recognit. 2012, 25, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Vial, J.; Jardy, A. Experimental Comparison of the Different Approaches to Estimate LOD and LOQ of an HPLC Method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]
- Taleuzzaman, M. Limit of Blank (LOB), Limit of Detection (LOD), and Limit of Quantification (LOQ). Org. Med. Chem. Int. J. 2018, 7, 555722. [Google Scholar] [CrossRef]
- Sharma, P.; Ghosh, A.; Tudu, B.; Bhuyan, L.P.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R.; Chatterjee, A. Detection of Linalool in Black Tea Using a Quartz Crystal Microbalance Sensor. Sens. Actuators B Chem. 2014, 190, 318–325. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Chen, S.; Liu, C.; Zhang, W.; Yang, M.; Cheng, J.; Lu, G.; Wang, Z.; Chen, W.; et al. Rapid Detection of Linalool by QCM Gas Sensor Based on HKUST-1/MWCNT-Gel@MIP in Early Sweetpotato Black Spot Disease. Microchem. J. 2025, 212, 113232. [Google Scholar] [CrossRef]
- Mushannavar, S.; Ghosh, R. Selective Detection of Linalool Using WO3-WS2 Composite Based Resistive Sensors. Sens. Actuators B Chem. 2025, 439, 137844. [Google Scholar] [CrossRef]
- Kulkarni, S.; Kummara, S.; Gorthala, G.; Ghosh, R. CuO Nanoflake-Based Sensors for Detecting Linalool, Hexanal, and Methyl Salicylate. ACS Agric. Sci. Technol. 2022, 2, 1285–1291. [Google Scholar] [CrossRef]
- Bhuyan, A.; Tudu, B.; Bandyopadhyay, R.; Gogoi, S.; Singh, A. Preanodized Screen Printed Carbon Electrode for Detection of Linalool Using Three Terminal Network. Int. J. Eng. Adv. Technol. 2019, 9, 477–482. [Google Scholar] [CrossRef]
- Li, M.; Dong, S.; Cao, S.; Cui, Q.; Chen, Q.; Ning, J.; Li, L. A Rapid Aroma Quantification Method: Colorimetric Sensor-Coupled Multidimensional Spectroscopy Applied to Black Tea Aroma. Talanta 2023, 263, 124622. [Google Scholar] [CrossRef]
- Zhou, C.; Fan, J.; Tan, R.; Peng, Q.; Cai, J.; Zhang, W. Prediction of Linalool Content in Osmanthus Fragrans Using E-Nose Technology. J. Sens. 2022, 2022, 7349030. [Google Scholar] [CrossRef]
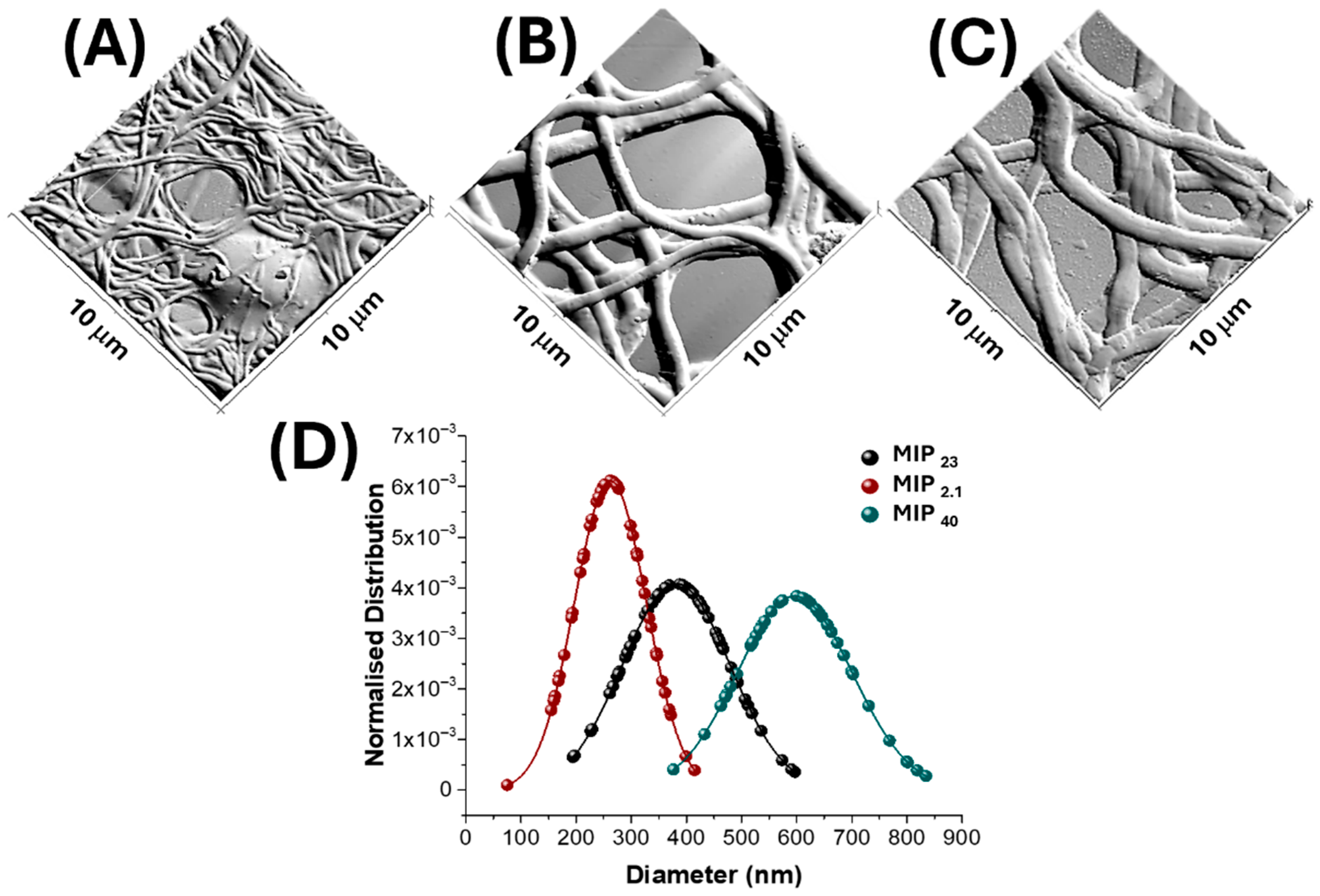
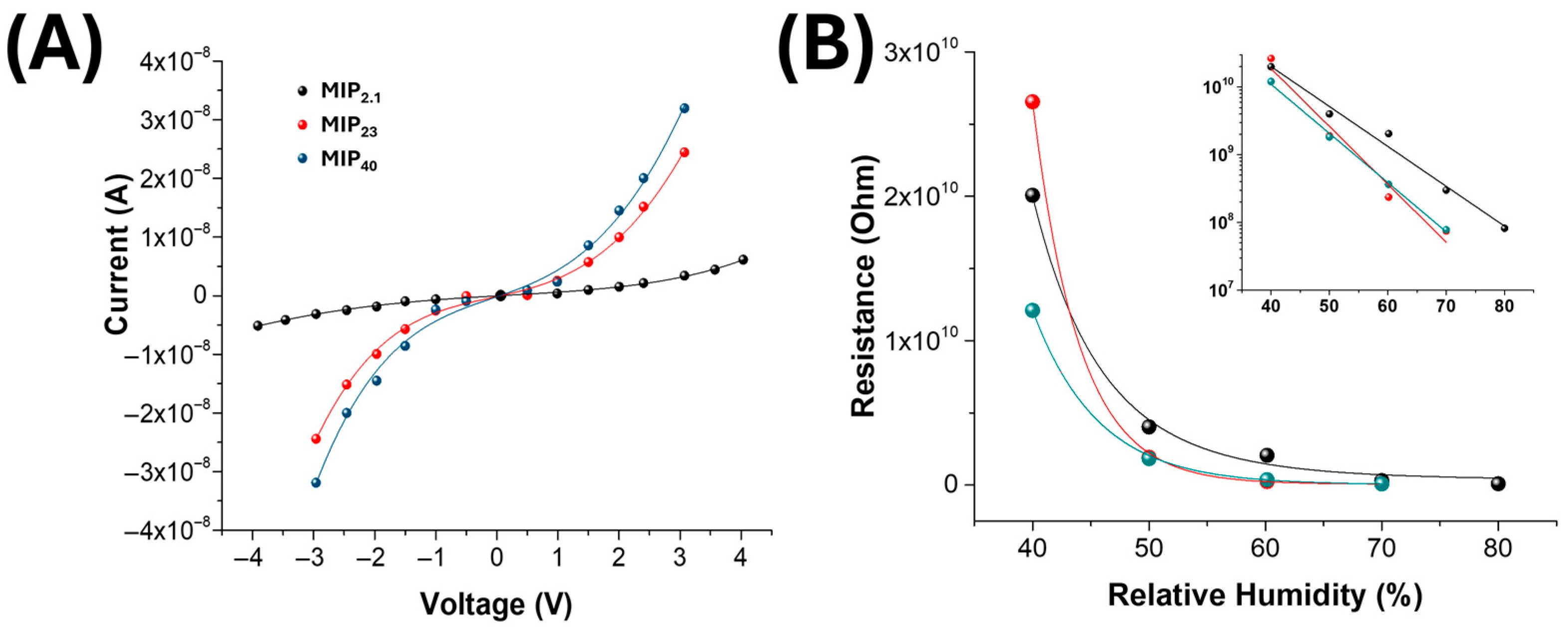
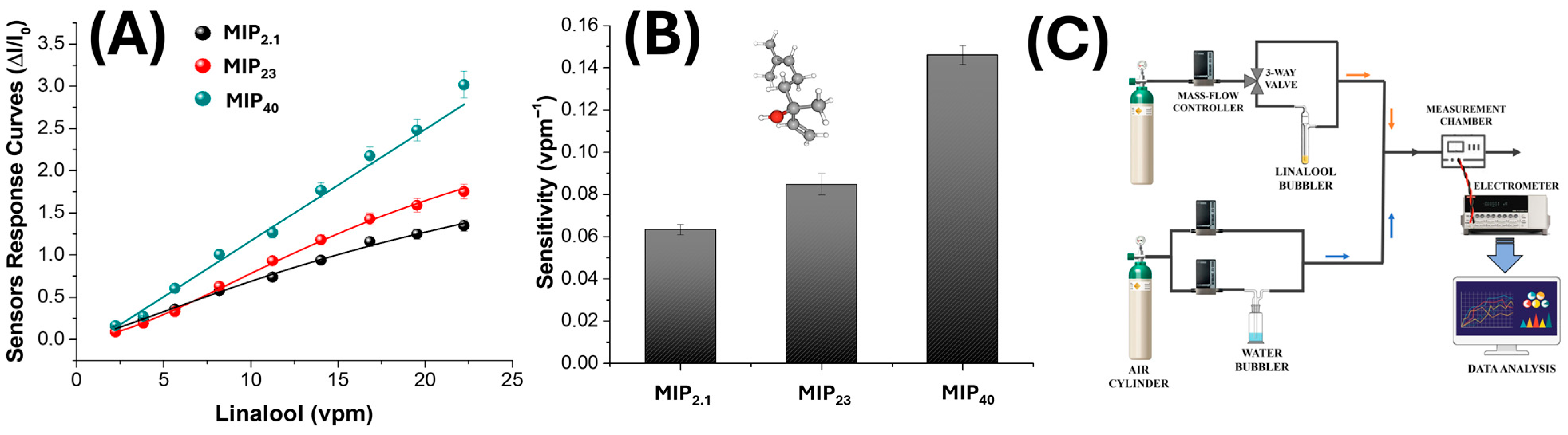
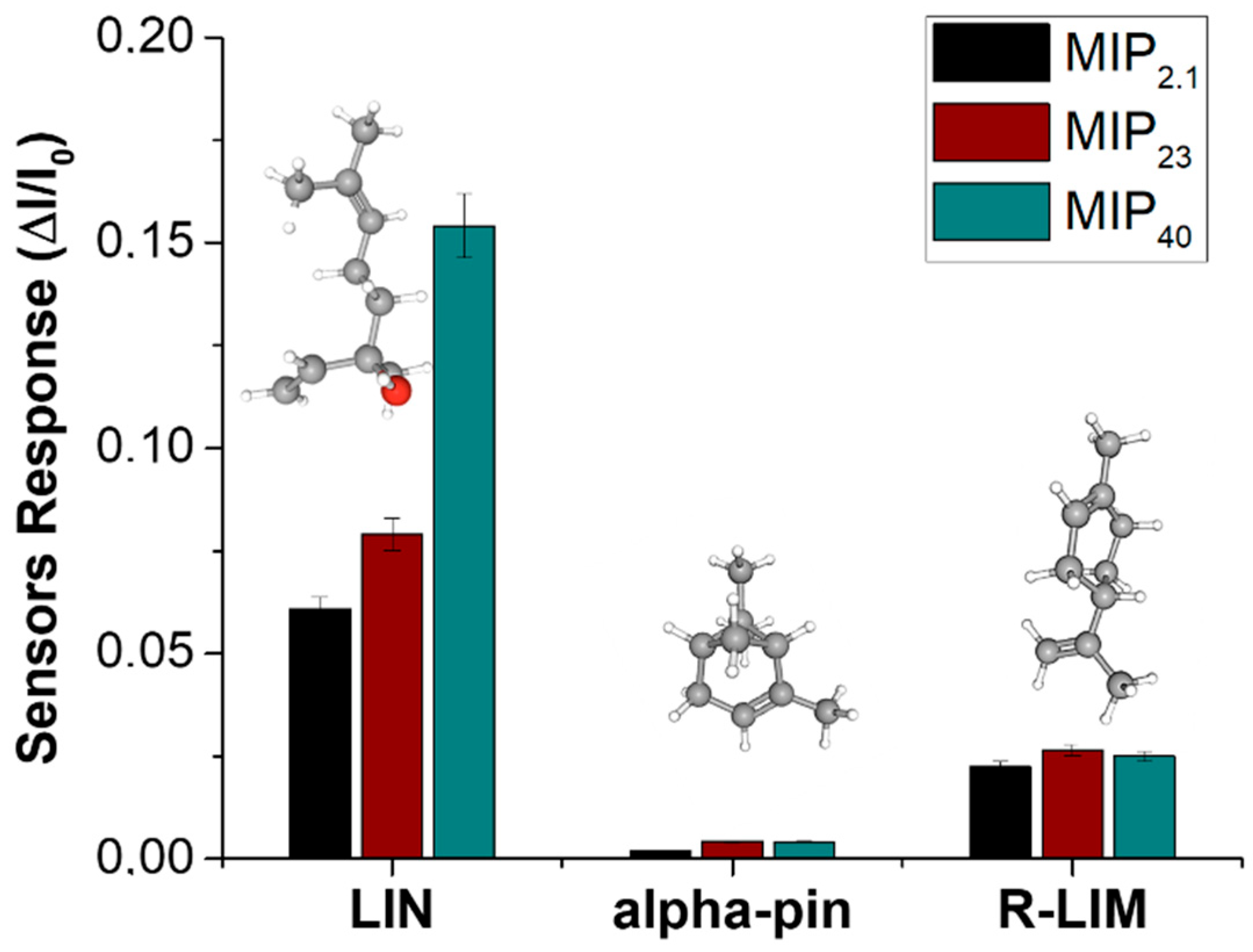
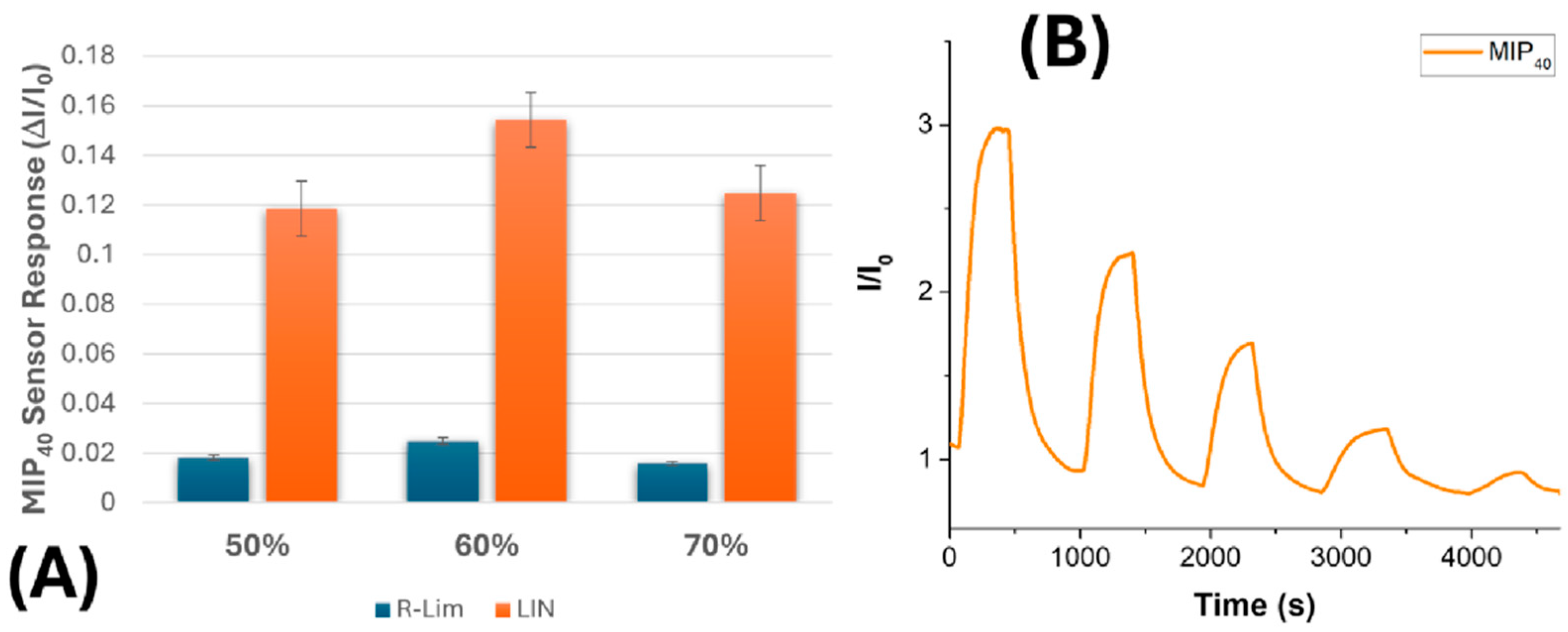
| MIP Loading (%) | Fibre Diameter (nm) | Mean Pore Area (µm2) | Percent Porosity (%) |
|---|---|---|---|
| 2.1 | 262 ± 65 | 1.5 ± 3.2 | 22 |
| 23 | 381 ± 98 | 2.9 ± 4.2 | 27 |
| 40 | 596 ± 104 | 4.29± 5.1 | 36 |
| Sensor Type | LOD | Operating Temp | Response Time | Selectivity | Ref. |
|---|---|---|---|---|---|
| MIP-nanofibre chemiresistor | ~8 ppb (±1) | RT (60% RH) | <60 s (t90) | SI ~73% (vs R-limonene) | This work |
| QCM (PEG-coated) | ~200 ppb | RT (dry) | ~minutes | Limited VOC selectivity | [67] |
| QCM (HKUST-1/MWCNT@MIP) | ~200 ppb | RT | ~10 min exposure | High template specificity | [68] |
| MOS (WO3, WS2, WO3–WS2) | 107–270 ppb | 200–300 °C | Seconds (typical) | Non-specific to linalool | [69] |
| CuO nanoflake resistive sensor | ~28 ppb | 200–300 °C | Seconds (typical) | Low molecular specificity | [70] |
| Electrochemical (SPCE) | ~12 vpm (liquid) | RT (liquid phase) | Seconds–minutes | Not applicable to gas-phase | [71] |
| Colorimetric sensors | (qualitative) | RT | Minutes | Qualitative, non-specific | [72] |
| E-nose (sensor array) | ~ppm (broad VOCs) | RT | Seconds–minutes | Requires algorithmic processing | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macagnano, A.; Molinari, F.N.; Serrecchia, S.; Papa, P.; Taddei, A.R.; De Cesare, F. Tuning Nanofibrous Sensor Performance in Selective Detection of B-VOCs by MIP-NP Loading. Nanomaterials 2025, 15, 1220. https://doi.org/10.3390/nano15161220
Macagnano A, Molinari FN, Serrecchia S, Papa P, Taddei AR, De Cesare F. Tuning Nanofibrous Sensor Performance in Selective Detection of B-VOCs by MIP-NP Loading. Nanomaterials. 2025; 15(16):1220. https://doi.org/10.3390/nano15161220
Chicago/Turabian StyleMacagnano, Antonella, Fabricio Nicolas Molinari, Simone Serrecchia, Paolo Papa, Anna Rita Taddei, and Fabrizio De Cesare. 2025. "Tuning Nanofibrous Sensor Performance in Selective Detection of B-VOCs by MIP-NP Loading" Nanomaterials 15, no. 16: 1220. https://doi.org/10.3390/nano15161220
APA StyleMacagnano, A., Molinari, F. N., Serrecchia, S., Papa, P., Taddei, A. R., & De Cesare, F. (2025). Tuning Nanofibrous Sensor Performance in Selective Detection of B-VOCs by MIP-NP Loading. Nanomaterials, 15(16), 1220. https://doi.org/10.3390/nano15161220









