Enhanced Electrochromic Properties of NiOx Films Through Magnesium Doping Strategy
Abstract
1. Introduction
2. Materials and Experiment Section
2.1. Materials
2.2. Preparation Method of Precursor
2.3. Fabrication of Mg-Doped NiOx Films
2.4. Characterization
3. Results and Discussion
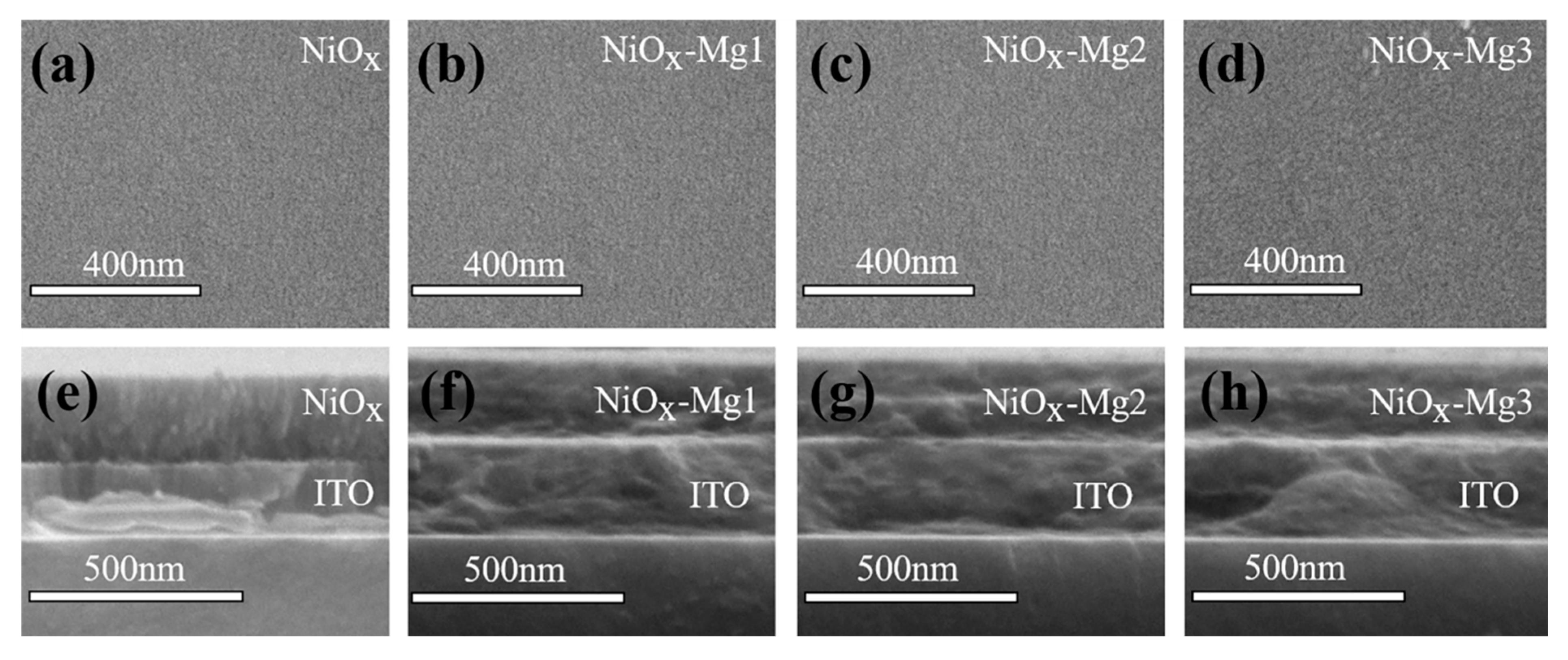
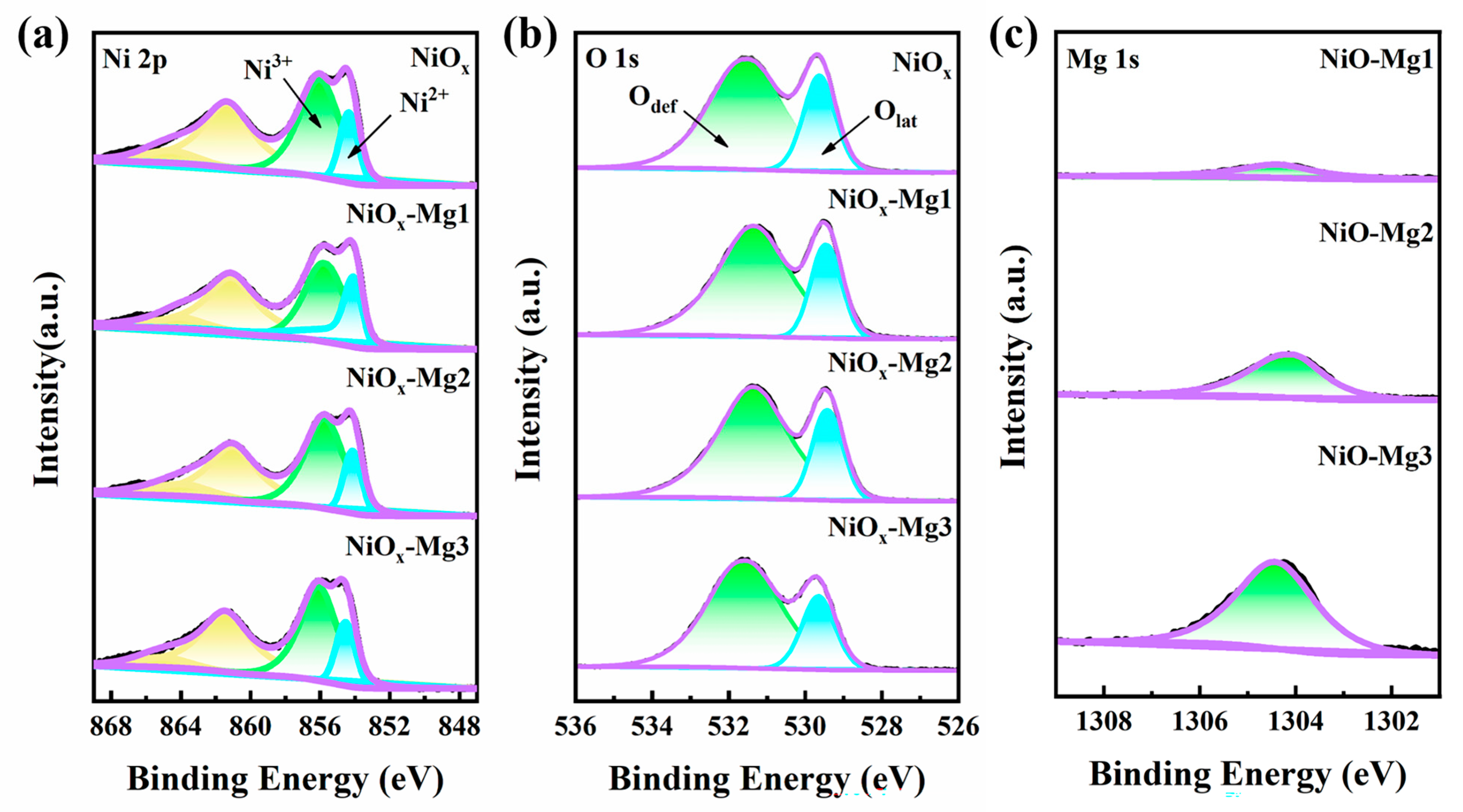
3.1. Sample Characterization
3.2. Electrochemical and Electrochromic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, C.; Jia, A.B.; Zhang, Y.M.; Zhang, S.X.A. Emerging Electrochromic Materials and Devices for Future Displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, B.; Zhang, W.; Cao, S.; Liu, L.; Elezzabi, A.Y.; Li, H.; Yu, W.W. Counterbalancing the interplay between electrochromism and energy storage for efficient electrochromic devices. Mater. Today 2023, 66, 431–447. [Google Scholar] [CrossRef]
- Su, L.; Lu, Z.; Wei, Y. An all solid-state electrochromic smart window. Chin. Sci. Bull. 1998, 43, 944–947. [Google Scholar] [CrossRef]
- Shao, Z.; Huang, A.; Cao, C.; Ji, X.; Hu, W.; Luo, H.; Bell, J.; Jin, P.; Yang, R.; Cao, X. Tri-band electrochromic smart window for energy savings in buildings. Nat. Sustain. 2024, 7, 796–803. [Google Scholar] [CrossRef]
- Meng, Q.; Cao, S.; Guo, J.; Wang, Q.; Wang, K.; Yang, T.; Zeng, R.; Zhao, J.; Zou, B. Sol-gel-based porous Ti-doped tungsten oxide films for high-performance dual-band electrochromic smart windows. J. Energy Chem. 2023, 77, 137–143. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Jang, H.; Auh, Y.; Kim, B.; Kim, E. Transparent photo-electrochromic capacitive windows with a Bi-dopant redox ionic liquids. Chem. Eng. J. 2022, 450, 138081. [Google Scholar] [CrossRef]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef]
- Wu, W.; Poh, W.C.; Lv, J.; Chen, S.; Gao, D.; Yu, F.; Wang, H.; Fang, H.; Wang, H.; Lee, P.S. Self-Powered and Light-Adaptable Stretchable Electrochromic Display. Adv. Energy Mater. 2023, 13, 2204103. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Yu, W.W.; Elezzabi, A.Y. Transparent inorganic multicolour displays enabled by zinc-based electrochromic devices. Light. Sci. Appl. 2020, 9, 121. [Google Scholar] [CrossRef]
- Oh, S.-J.; Choi, S.-E.; Woo, I.; Yoon, J.U.; Bae, J.; Bae, J.W. Stretchable Multicolored Electroluminescent Sound Display for Wearable and Interactive Textiles. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Zhou, Y.; Li, J.; Chen, X.; Wang, Y.; Lu, B. A multicolored polymer for dynamic military camouflage electrochromic devices. Sol. Energy Mater. Sol. Cells 2024, 278, 113180. [Google Scholar] [CrossRef]
- Fu, G.; Gong, H.; Xu, J.; Zhuang, B.; Rong, B.; Zhang, Q.; Chen, X.; Liu, J.; Wang, H. Highly integrated all-in-one electrochromic fabrics for unmanned environmental adaptive camouflage. J. Mater. Chem. A 2024, 12, 6351–6358. [Google Scholar] [CrossRef]
- An, J.; Cai, W.; Zheng, W.; Zheng, Z.; Ming, X.; Niu, H.; Wang, W. High-contrast electrochromic nano fiber films changing from yellow-green to black based on butterfly monomer for smart windows and self-adaptive camouflage. J. Colloid. Interface Sci. 2025, 693, 137559. [Google Scholar] [CrossRef]
- Uplane, M.M.; Mujawar, S.H.; Inamdar, A.I.; Shinde, P.S.; Sonavane, A.C.; Patil, P.S. Structural, optical and electrochromic properties of nickel oxide thin films grown from electrodeposited nickel sulphide. Appl. Surf. Sci. 2007, 253, 9365–9371. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Chen, Y.B.; Yang, J.L.; Guo, S.Y. Enhanced electrochromic properties of ordered porous nickel oxide thin film prepared by self-assembled colloidal crystal template-assisted electrodeposition. Electrochim. Acta 2011, 56, 1208–1212. [Google Scholar] [CrossRef]
- Yadav, A.A.; Chavan, U.J. Influence of substrate temperature on electrochemical supercapacitive performance of spray deposited nickel oxide thin films. J. Electroanal. Chem. 2016, 782, 36–42. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Boshta, M.; Farag, B.S.; Osman, M.B.S. Structural and optical properties of nickel oxide thin films prepared by chemical bath deposition and by spray pyrolysis techniques. J. Mater. Sci. Mater. Electron. 2016, 27, 711–717. [Google Scholar] [CrossRef]
- Taskopru, T.; Zor, M.; Turan, E. Structural characterization of nickel oxide/hydroxide nanosheets produced by CBD technique. Mater. Res. Bull. 2015, 70, 633–639. [Google Scholar] [CrossRef]
- Zhao, F.; He, H.; Cheng, Z.; Tang, Y.; Li, G.; Xu, G.; Liu, Y.; Han, G. Improving electrochromic performance of porous nickel oxide electrode via Cu doping. Electrochim. Acta 2022, 417, 140332. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, T.; Zeng, Y.; Chen, J.; Zheng, J.; Liu, Y.; Han, G. Nickel oxide electrochromic films: Mechanisms, preparation methods, and modification strategies-a review. J. Mater. Chem. C 2024, 12, 7126–7145. [Google Scholar] [CrossRef]
- Wang, C.; Cao, Z.; Xiang, S.; Liao, L.; Chen, W.; Zhang, H. Improved transparency and charge matching of electrochromic devices with Ce-doped NiO counter electrode. J. Alloys Compd. 2025, 1010, 177050. [Google Scholar] [CrossRef]
- Zhan, X.; Gao, F.; Zhuang, Q.; Zhang, Y.; Dang, J. Two-Dimensional Porous Structure of V-Doped NiO with Enhanced Electrochromic Properties. ACS Omega 2022, 7, 8960–8967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Long, X.; Liao, W.; Hu, B.; Miao, R.; Zhang, G.; Sun, G.; Xie, Y.; Miao, L. Manganese doped nickel oxide thin film with improved electrochromic performance towards smart window application. Ceram. Int. 2024, 50, 12810–12817. [Google Scholar] [CrossRef]
- Zargouni, S.; El Whibi, S.; Tessarolo, E.; Rigon, M.; Martucci, A.; Ezzaouia, H. Structural properties and defect related luminescence of Yb-doped NiO sol-gel thin films. Superlattices Microstruct. 2020, 138, 106361. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Awad, R. Influence of Ru dopants on the structural, optical, and magnetic properties of nickel oxide nanoparticles. Phys. B Condens. Matter 2022, 629, 413651. [Google Scholar] [CrossRef]
- Babu, G.A.; Ravi, G. Quantification of ferromagnetism in metal doped NiO nanostructures. Mater. Lett. 2015, 161, 149–152. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, G.; Zhou, D.; Tang, H.; Wang, X.; Gu, C.; Tu, J. Co-doped NiO nanoflake array films with enhanced electrochromic properties. J. Mater. Chem. C 2014, 2, 7013–7021. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Han, D.; Gu, F. Electron compensation in p-type 3DOM NiO by Sn doping for enhanced formaldehyde sensing performance. J. Mater. Chem. C 2017, 5, 3254–3263. [Google Scholar] [CrossRef]
- Cerqueira, M.F.; Viseu, T.; de Campos, J.A.; Rolo, A.G.; de Lacerda-Aroso, T.; Oliveira, F.; Bogdanovic-Radovic, I.; Alves, E.; Vasilevskiy, M.I. Raman study of insulating and conductive ZnO:(Al, Mn) thin films. Phys. Status Solidi A Appl. Mater. Sci. 2015, 212, 2345–2354. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Yan, D.; Deng, J.; Chen, M.; Zhang, H.; Sun, W.; Zhao, J.; Li, Y. Defect engineering of W6+-doped NiO for high-performance black smart windows. Nano Res. 2024, 17, 3043–3052. [Google Scholar] [CrossRef]
- Lee, N.; Vo, V.K.; Lim, H.-J.; Jin, S.; Dang, T.H.T.; Jang, H.; Choi, D.; Lee, J.-H.; Jeong, B.-S.; Heo, Y.-W. Effect of magnesium doping on NiO hole injection layer in quantum dot light-emitting diodes. Nanophotonics 2024, 13, 4615–4624. [Google Scholar] [CrossRef]
- Garrido-García, L.F.; Pérez-Martínez, A.L.; Reyes-Gasga, J.; Aguilar-Del-Valle, M.d.P.; Wong, Y.H.; Rodríguez-Gómez, A. A Simple Methodology to Gain Insights into the Physical and Compositional Features of Ternary and Quaternary Compounds Based on the Weight Percentages of Their Constituent Elements: A Proof of Principle Using Conventional EDX Characterizations. Ceramics 2024, 7, 2571–6131. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, Y.; Li, J.; Hong, Y.; Wang, Y.; Cui, J.; Shu, X.; Liu, J.; Tan, H.H.; Wu, Y. Hierarchical crystalline/defective NiO nanoarrays with (111) crystal orientation for effective electrochromic energy storage. Ceram. Int. 2024, 50, 47949–47962. [Google Scholar] [CrossRef]
- Usha, K.S.; Lee, S.Y. Fast-switching electrochromic smart windows based on WO3 doped NiO thin films. J. Alloys Compd. 2024, 1009, 176774. [Google Scholar] [CrossRef]
- Hou, J.; Gao, L.; Gu, X.; Li, Z.; Huang, M.; Su, G. Improvement of electrochromic performance of nickel oxide porous films by Sn doping. Mater. Chem. Phys. 2023, 306, 128079. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Yan, D.; Deng, J.; Sun, W.; Li, Z.; Xiao, Y.; Ding, Z.; Zhao, J.; Li, Y. Oxygen vacancy modulated amorphous tungsten oxide films for fast-switching and ultra-stable dual-band electrochromic energy storage smart windows. Mater. Horiz. 2023, 10, 2191–2203. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Probing the dominance of interstitial oxygen defects in ZnO nanoparticles through structural and optical characterizations. Ceram. Int. 2014, 40, 14569–14578. [Google Scholar] [CrossRef]
- Bouessay, I.; Rougier, A.; Tarascon, J.M. Electrochemically inactive nickel oxide as electrochromic material. J. Electrochem. Soc. 2004, 151, H145–H152. [Google Scholar] [CrossRef]
- Gao, L.; Hou, J.; Li, Z.; Gu, X.; Huang, M.; Su, G. Improved electrochromic performance of nickel oxide porous films by regulating their semiconductor type by titanium doping. J. Phys. Chem. Solids 2023, 174, 111168. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.; Shen, S.; Zhong, Y.; Liu, S.; Xia, X.; Tong, Y.; Lu, X. Enhancing the Capacitive Storage Performance of Carbon Fiber Textile by Surface and Structural Modulation for Advanced Flexible Asymmetric Supercapacitors. Adv. Funct. Mater. 2019, 29, 1806329. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 2017, 8, 15893. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Q.; Xiao, Y.; He, Y.; Diao, X. Improved electrochromic performance of NiO-based thin films by lithium and tantalum co-doping. J. Alloys Compd. 2018, 747, 416–422. [Google Scholar] [CrossRef]
- Braatz, M.-L.; Veith, L.; Koester, J.; Kaiser, U.; Binder, A.; Gradhand, M.; Klaeui, M. Impact of nitrogen doping on the band structure and the charge carrier scattering in monolayer graphene. Phys. Rev. Mater. 2021, 5, 084003. [Google Scholar] [CrossRef]
- Zou, J.; Xu, Y.; Miao, X.; Chen, H.; Zhang, R.; Tan, J.; Tang, L.; Cai, Z.; Zhang, C.; Kang, L.; et al. Raman spectroscopy and carrier scattering in 2D tungsten disulfides with vanadium doping. Mater. Chem. Front. 2023, 7, 2059–2067. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, M.; Mei, Z.; Liu, L.; Yang, J.; Zhong, X.; Wang, M.; Diao, X. Electrochromic Adaptability of NiOx Films Modified by Substrate Temperature in Aqueous and Non-Aqueous Electrolytes. Adv. Mater. Interfaces 2022, 9, 2102223. [Google Scholar] [CrossRef]






| Sample | Ni (%) | O (%) | Mg (%) | Mg/(Mg + Ni) (%) |
|---|---|---|---|---|
| NiOx | 30.3 | 69.7 | 0 | 0 |
| NiOx-Mg1 | 32.9 | 65.2 | 1.9 | 5.4 |
| NiOx-Mg2 | 28.1 | 68.9 | 3.0 | 9.6 |
| NiOx-Mg3 | 27.0 | 68.4 | 4.6 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Ding, S.; Shen, X.; Guo, C.; Liu, Y.; Xia, W.; Wu, G.; Zhang, Y. Enhanced Electrochromic Properties of NiOx Films Through Magnesium Doping Strategy. Nanomaterials 2025, 15, 1217. https://doi.org/10.3390/nano15161217
Yao X, Ding S, Shen X, Guo C, Liu Y, Xia W, Wu G, Zhang Y. Enhanced Electrochromic Properties of NiOx Films Through Magnesium Doping Strategy. Nanomaterials. 2025; 15(16):1217. https://doi.org/10.3390/nano15161217
Chicago/Turabian StyleYao, Xiaoyu, Shuai Ding, Xiaoyu Shen, Congkai Guo, Yao Liu, Wenjuan Xia, Guohua Wu, and Yaohong Zhang. 2025. "Enhanced Electrochromic Properties of NiOx Films Through Magnesium Doping Strategy" Nanomaterials 15, no. 16: 1217. https://doi.org/10.3390/nano15161217
APA StyleYao, X., Ding, S., Shen, X., Guo, C., Liu, Y., Xia, W., Wu, G., & Zhang, Y. (2025). Enhanced Electrochromic Properties of NiOx Films Through Magnesium Doping Strategy. Nanomaterials, 15(16), 1217. https://doi.org/10.3390/nano15161217







