Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
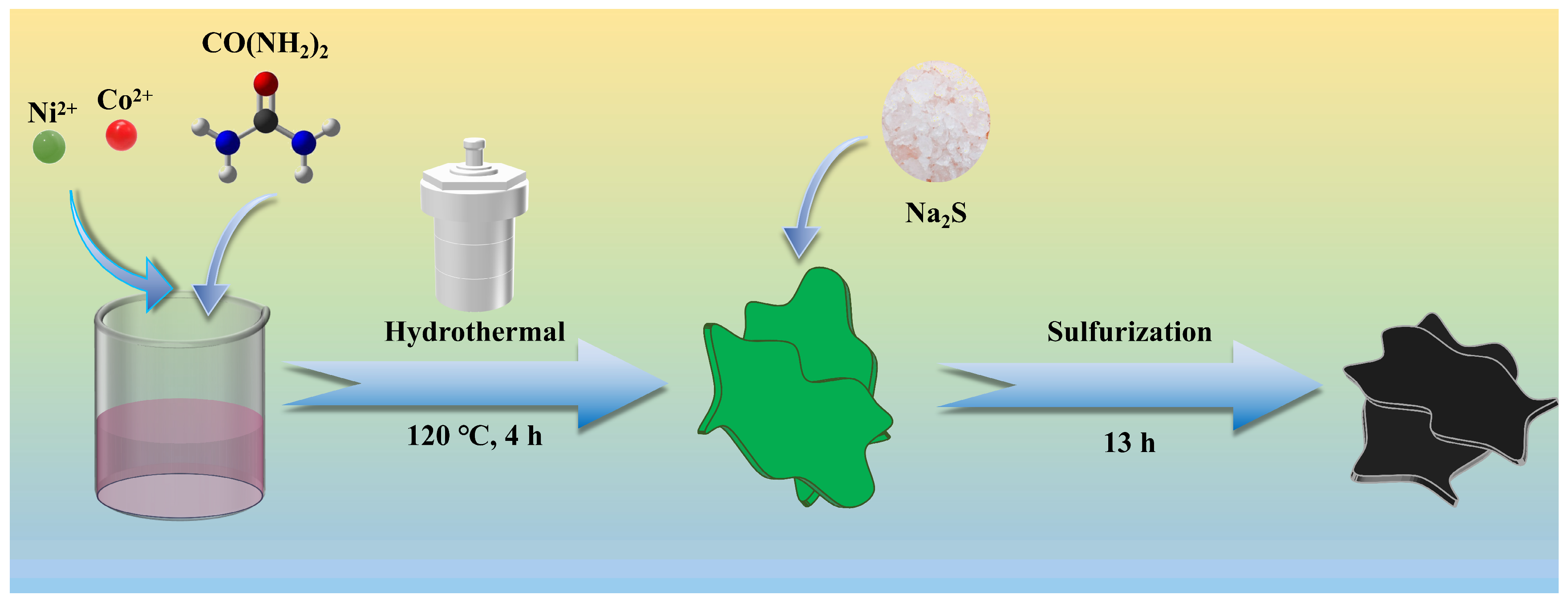
2.2. Preparation of CoS, NiS and CoS@NiS-x Nanosheets
2.3. Preparation of Electrolyte
- Alkaline-simulated seawater electrolyte
- b.
- Alkaline seawater electrolyte
2.4. Electrochemical Measurements
3. Results and Discussion
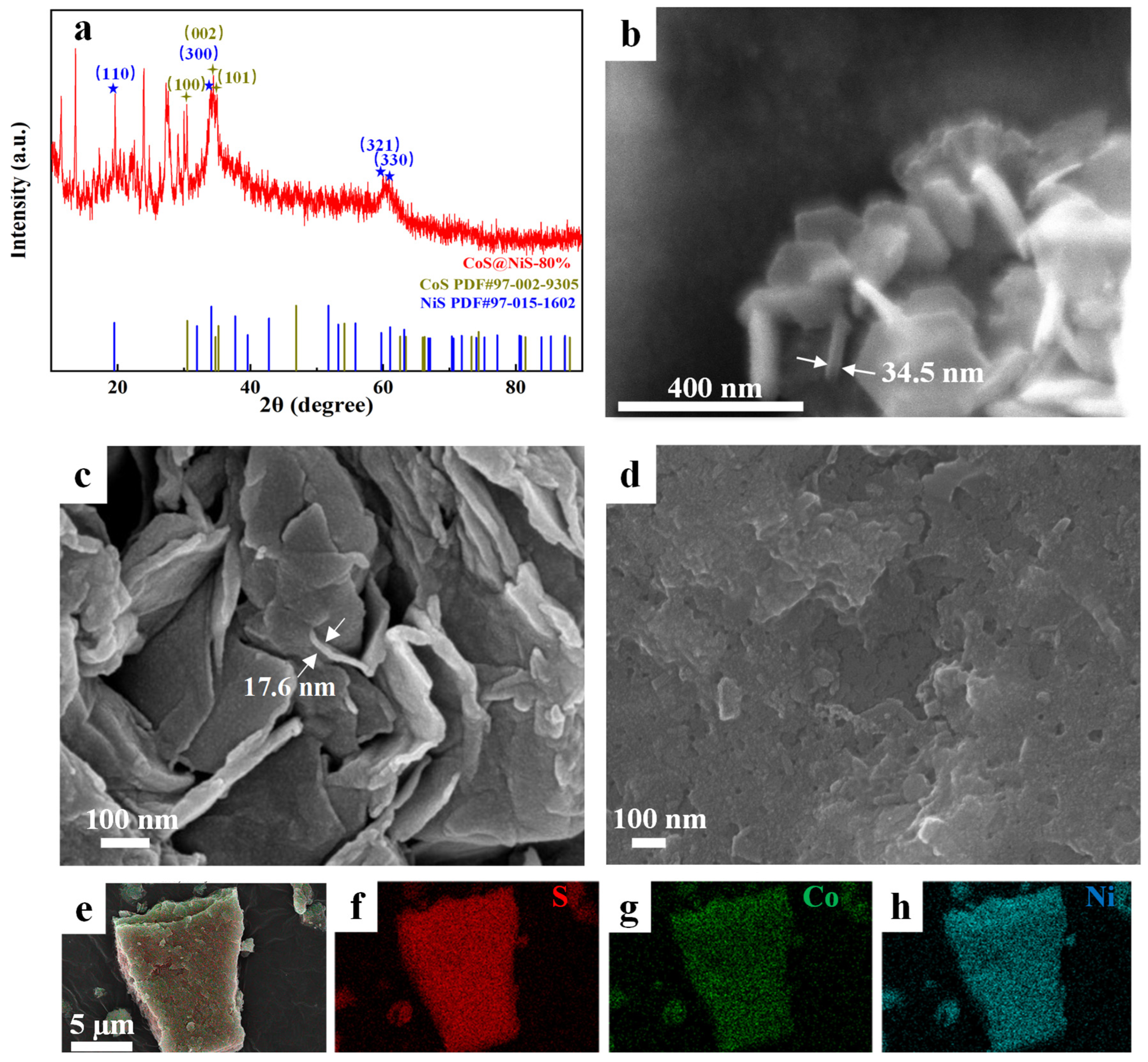
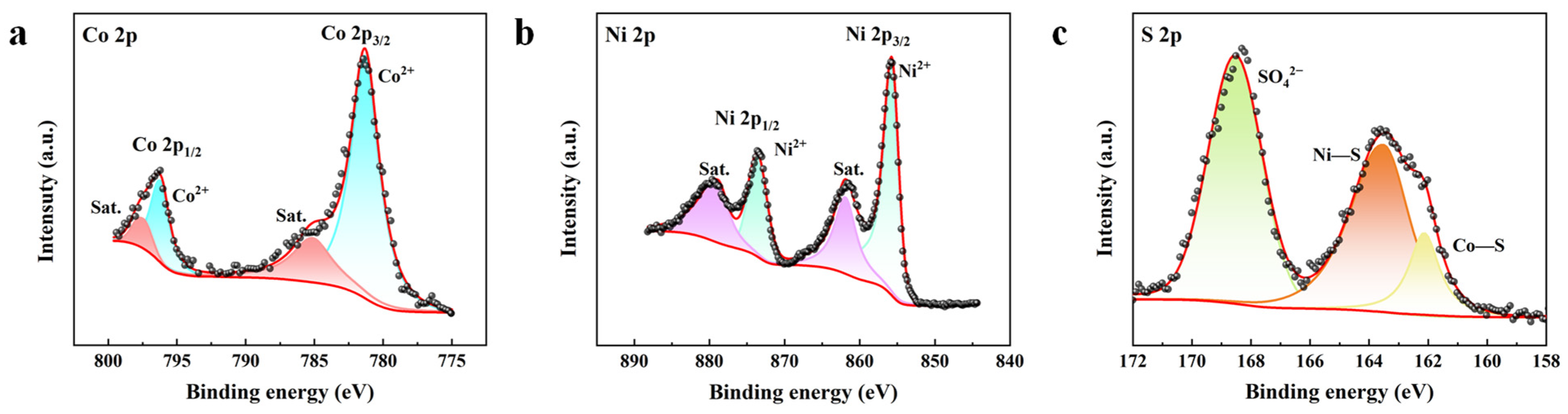
3.1. Characterization
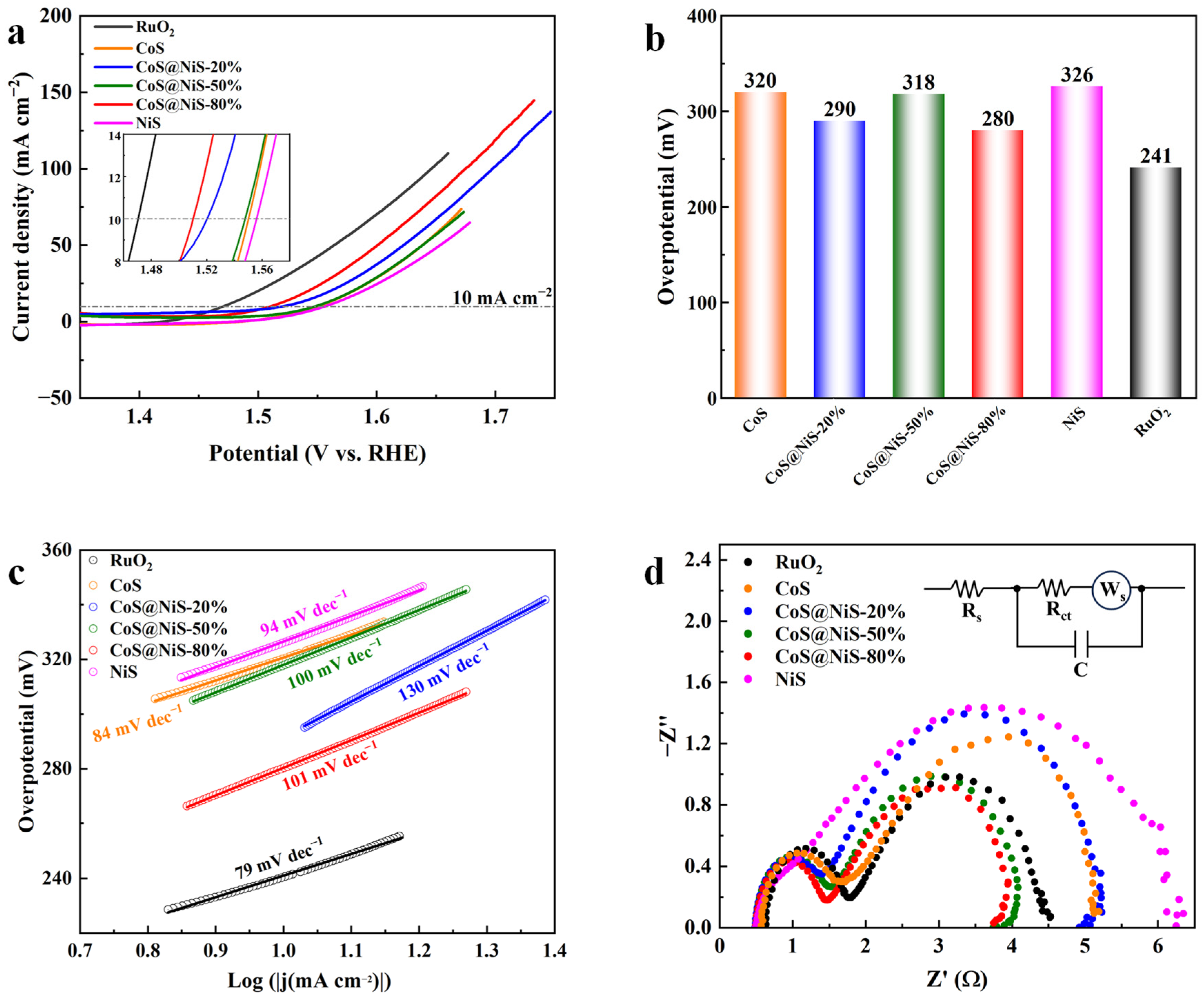
3.2. Electrochemical Characterizations of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Talha Aqueel Ahmed, A.; Ho Lee, C.; Saad Ansari, A.; Pawar, S.M.; Han, J.; Park, S.; Shin, G.; Yeon, S.; Cho, S.; Seol, J.; et al. Hybridized Heterostructure of CoS and MoS2 Nanoparticles for Highly-Efficient and Robust Bifunctional Water Electrolysis. Appl. Surf. Sci. 2022, 592, 153196. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, T.; Jin, C.; Zhang, S.; Cui, Y.; Chen, J.; Zeng, X. Synergistically Coupling CoS/FeS2 Heterojunction Nanosheets on a MXene via a Dual Molten Salt Etching Strategy for Efficient Oxygen Evolution Reaction. J. Mater. Chem. A 2024, 12, 14517–14530. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Chen, J.; Wang, R.; Zhang, B.; Song, B.; Xu, P. In Situ Raman Study of Surface Reconstruction of FeOOH/Ni3S2 Oxygen Evolution Reaction Electrocatalysts. Small 2024, 20, 2309371. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cao, K.-W.; He, J.-W.; Li, F.-M.; Huang, H.; Chen, P.; Chen, Y. Nitrogen-Doped Graphene Aerogel-Supported Ruthenium Nanocrystals for pH-Universal Hydrogen Evolution Reaction. Chin. J. Catal. 2022, 43, 1535–1543. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 201600371. [Google Scholar] [CrossRef]
- Peng, L.; Shah, S.S.A.; Wei, Z. Recent Developments in Metal Phosphide and Sulfide Electrocatalysts for Oxygen Evolution Reaction. Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Song, S.; An, B.; Li, N.; Gao, Y.; Ge, L. Recent Progress on Design and Applications of Transition Metal Chalcogenide-Associated Electrocatalysts for the Overall Water Splitting. Chin. J. Catal. 2023, 44, 7–49. [Google Scholar] [CrossRef]
- Zhao, T.; Gong, B.; Xu, G.; Jiang, J.; Zhang, L. In Situ Surface Reconstruction of Heterostructure Ni2P/CoP/FeP4 Nanowires Network Catalyst for High-Current-Density Overall Water Splitting. Chin. J. Catal. 2024, 61, 269–280. [Google Scholar] [CrossRef]
- Qin, R.; Chen, G.; Feng, X.; Weng, J.; Han, Y. Ru/Ir-Based Electrocatalysts for Oxygen Evolution Reaction in Acidic Conditions: From Mechanisms, Optimizations to Challenges. Adv. Sci. 2024, 11, 202309364. [Google Scholar] [CrossRef]
- Zhang, L.; Jang, H.; Liu, H.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Sodium-Decorated Amorphous/Crystalline RuO2 with Rich Oxygen Vacancies: A Robust pH-Universal Oxygen Evolution Electrocatalyst. Angew. Chem.-Int. Ed. 2021, 60, 18821–18829. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, M.; Feng, X. Electronic Structure Regulation of Noble Metal-Free Materials toward Alkaline Oxygen Electrocatalysis. eScience 2023, 3, 100141. [Google Scholar] [CrossRef]
- Huang, W.-H.; Li, X.-M.; Yang, X.-F.; Zhang, H.-B.; Wang, F.; Zhang, J. Highly Efficient Electrocatalysts for Overall Water Splitting: Mesoporous CoS/MoS2 with Hetero-Interfaces. Chem. Commun. 2021, 57, 4847–4850. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fu, L.; Yang, S.; Lu, Y.; Li, M.; Zhang, L.; Tang, J. Hydrothermal Synthesis of Co-MoS2 as a Bifunctional Catalyst for Overall Water Splitting. ACS Omega 2025, 10, 15129–15142. [Google Scholar] [CrossRef]
- Dong, A.; Lin, L.; Mu, R.; Li, R.; Li, K.; Wang, C.; Cao, Y.; Ling, Y.; Chen, Y.; Yang, F.; et al. Modulating the Formation and Evolution of Surface Hydrogen Species on ZnO through Cr Addition. ACS Catal. 2022, 12, 6255–6264. [Google Scholar] [CrossRef]
- Dong, A.; Li, K.; Mu, R.; Liu, C.; Li, R.; Jia, H.; Lin, L.; Fu, Q. Effects of water adsorption on active site-dependent H2 activation over MgO nanoflakes. Nano Res. 2023, 16, 9059–9064. [Google Scholar] [CrossRef]
- Dong, A.; Yang, T.; Xue, J.; Gao, H.; Che, L. Transition metal phosphide electrocatalysts for efficient oxygen evolution reaction. Appl. Surf. Sci. 2025, 708, 163769. [Google Scholar] [CrossRef]
- Cheng, Y.; Pang, K.; Xu, X.; Yuan, P.; Zhang, Z.; Wu, X.; Zheng, L.; Zhang, J.; Song, R. Borate Crosslinking Synthesis of Structure Tailored Carbon-Based Bifunctional Electrocatalysts Directly from Guar Gum Hydrogels for Efficient Overall Water Splitting. Carbon 2020, 157, 153–163. [Google Scholar] [CrossRef]
- Kawashima, K.; Márquez, R.A.; Smith, L.A.; Vaidyula, R.R.; Carrasco-Jaim, O.A.; Wang, Z.; Son, Y.J.; Cao, C.L.; Mullins, C.B. A Review of Transition Metal Boride, Carbide, Pnictide, and Chalcogenide Water Oxidation Electrocatalysts. Chem. Rev. 2023, 123, 12795–13208. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, R.; Zhu, W.; Ke, W.; Li, E. CoS Nanowires Mediated by Superionic Conductor Ag2S for Boosted Oxygen Evolution. Appl. Surf. Sci. 2020, 518, 146106. [Google Scholar] [CrossRef]
- Li, Z.; Gao, H.; Che, L.; Dong, A. Innovative Strategies for Oxygen Evolution Reaction Using Layered Double Hydroxide-Based Electrocatalysts. ChemistrySelect 2025, 10, e02680. [Google Scholar] [CrossRef]
- Wang, P.; Han, X.; Bai, P.; Mu, J.; Zhao, Y.; He, J.; Su, Y. Utilizing an Electron Redistribution Strategy to Inhibit the Leaching of Sulfur from CeO2/NiCo2S4 Heterostructure for High-Efficiency Oxygen Evolution. Appl. Catal. B-Environ. 2024, 344, 123659. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Song, M.; Qiu, Z.; Wang, S.; Sun, L. Hierarchical Interfaces Engineering-Driven of the CoS2/MoS2/Ni3S2/NF Electrode for High-Efficient and Stable Oxygen Evolution and Urea Oxidation Reactions. Appl. Surf. Sci. 2023, 617, 156621. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, C.; Chen, X.; He, X.; Chen, H.; Du, X.; Wang, D.; Fang, W.; Zhang, H.; Li, W. ZIF-67 Derived Mo-CoS2 Nanoparticles Embedded in Hierarchically Porous Carbon Hollow Sphere for Efficient Overall Water Splitting. Appl. Surf. Sci. 2023, 623, 157030. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Zhang, Q.; Zhang, Q.; Wu, C.; Yu, J.; Ma, Y.; An, H.; Wang, H.; Zou, Y.; et al. High-Spin Co3+ in Cobalt Oxyhydroxide for Efficient Water Oxidation. Nat. Commun. 2024, 15, 1383. [Google Scholar] [CrossRef]
- Yao, N.; Wang, G.; Jia, H.; Yin, J.; Cong, H.; Chen, S.; Luo, W. Intermolecular Energy Gap-Induced Formation of High-Valent Cobalt Species in CoOOH Surface Layer on Cobalt Sulfides for Efficient Water Oxidation. Angew. Chem.-Int. Ed. 2022, 61, e202117178. [Google Scholar] [CrossRef]
- Fan, K.; Zou, H.; Lu, Y.; Chen, H.; Li, F.; Liu, J.; Sun, L.; Tong, L.; Toney, M.F.; Sui, M.; et al. Direct Observation of Structural Evolution of Metal Chalcogenide in Electrocatalytic Water Oxidation. ACS Nano 2018, 12, 12369–12379. [Google Scholar] [CrossRef]
- Huang, W.; Su, C.; Zhu, C.; Bo, T.; Zuo, S.; Zhou, W.; Ren, Y.; Zhang, Y.; Zhang, J.; Rueping, M.; et al. Isolated Electron Trap-Induced Charge Accumulation for Efficient Photocatalytic Hydrogen Production. Angew. Chem.-Int. Ed. 2023, 62, e202304634. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and β-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem.-Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef]
- Zang, S.; Hou, Y.; Chang, J.; Xu, F.; Wu, D.; Jiang, K.; Gao, Z. Amorphous-Crystalline Heterostructures Enable Energy-Level Matching of Cobalt Sulfide/Nickel Iron Layered Double Hydroxide for Efficient Oxygen Evolution Reaction. J. Colloid Interface Sci. 2024, 656, 485–494. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, J.; Zhang, F.; Fei, Z.; Shi, H.; Kang, D.J.; Pang, H. Hierarchical Binary Metal Sulfides Nanoflakes Decorated on Graphene with Precious-Metal-Like Activity for Water Electrolysis. Chem. Eng. J. 2023, 470, 144372. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, T.; Wu, Y.; Zhou, T.; Zhou, B.; Shang, C.; Guo, Z. High-Valence Oxides for High Performance Oxygen Evolution Electrocatalysis. Adv. Sci. 2023, 10, 2301706. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, Y.; Chu, H.; Dong, P.; Baines, R.; Pei, Y.; Ye, M.; Shen, J. Dual-Functional Starfish-like P-Doped Co–Ni–S Nanosheets Supported on Nickel Foams with Enhanced Electrochemical Performance and Excellent Stability for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, X.; Yang, L.; Yang, W.; Sun, W.; Tuo, Y.; Zhou, Y.; Wang, S.; Pan, Y.; Yan, W.; et al. Highly Efficient CoMoS Heterostructure Derived from Vertically Anchored Co5Mo10 Polyoxometalate for Electrocatalytic Overall Water Splitting. Chem. Eng. J. 2020, 394, 124849. [Google Scholar] [CrossRef]
- Luo, W.; Yu, Y.; Wu, Y.; Wang, W.; Jiang, Y.; Shen, W.; He, R.; Su, W.; Li, M. Strong Interface Coupling Enables Stability of Amorphous Meta-Stable State in CoS/Ni3S2 for Efficient Oxygen Evolution. Small 2024, 20, 2310387. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-Like NiCo2S4@NiFe LDH Hollow Spheres as an Effective Oxygen Bifunctional Electrocatalyst in Alkaline Solution. Appl. Catal. B-Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Zheng, X.; Padmajan Sasikala, S.; Kim, Y.; Kim, T.-D.; Lee, G.S.; Kim, J.T.; Kim, S.O.; Prabhakaran, P. Dimension-Engineered Gold Heterostructures with Transition Metal Dichalcogenide for Efficient Overall Water Splitting. J. Colloid Interface Sci. 2025, 686, 516–524. [Google Scholar] [CrossRef]
- Qin, C.; Lu, Y.; Wei, L.; Xue, H.; Dang, Y.; Fan, A. MoS2/NiS Heterostructure Nanosheets as Bifunctional Electrocatalysts for Efficient Water Splitting. Ionics 2025. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lin, H.; Yin, Z.; Li, J.; Lu, M.; Guo, L.; Xi, P.; Tang, Y.; Yan, C.H. Optimized Metal Chalcogenides for Boosting Water Splitting. Adv. Sci. 2020, 7, 1903070. [Google Scholar] [CrossRef]
- Ji, Q.; Kong, Y.; Tan, H.; Duan, H.; Li, N.; Tang, B.; Wang, Y.; Feng, S.; Lv, L.; Wang, C.; et al. Operando Identification of Active Species and Intermediates on Sulfide Interfaced by Fe3O4 for Ultrastable Alkaline Oxygen Evolution at Large Current Density. ACS Catal. 2022, 12, 4318–4326. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Wu, Z.; Ding, W.; Xue, T.; Wang, J.; Chen, Z.; Han, X.; Deng, Y.; Hu, W. Rational Design and Spontaneous Sulfurization of NiCo-(oxy)Hydroxysulfides Nanosheets with Modulated Local Electronic Configuration for Enhancing Oxygen Electrocatalysis. Adv. Energy Mater. 2022, 12, 2103275. [Google Scholar] [CrossRef]
- Masa, J.; Andronescu, C.; Schuhmann, W. Electrocatalysis as the Nexus for Sustainable Renewable Energy: The Gordian Knot of Activity, Stability, and Selectivity. Angew. Chem.-Int. Ed. 2020, 59, 15298–15312. [Google Scholar] [CrossRef]
- Yang, J.; Xuan, H.; Zhang, G.; Wang, R.; Yang, J.; Liang, X.; Li, Y.; Han, P. The 3D Core-Shell Heterostructure Catalysts by Conis Nanosheets Interfacial Assembled on CuO Nanorods for Efficient Water Electrolysis. Appl. Surf. Sci. 2021, 570, 151181. [Google Scholar] [CrossRef]
- Min, K.; Kim, S.; Lee, E.; Yoo, G.; Ham, H.C.; Shim, S.E.; Lim, D.; Baeck, S.-H. A Hierarchical Co3O4/CoS Microbox Heterostructure as a Highly Efficient Bifunctional Electrocatalyst for Rechargeable Zn-Air Batteries. J. Mater. Chem. A 2021, 9, 17344–17352. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Li, W.; Tian, P.; Zhou, S.; Gao, H.; Tian, X.; Zang, J. Amorphous MoS2 Coated Ni3S2 Nanosheets as Bifunctional Electrocatalysts for High-Efficiency Overall Water Splitting. Electrochim. Acta 2020, 332, 135454. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, X.; You, W.; Peng, Z.; Luo, J.-L.; Fu, X.-Z. Amorphous Cobalt Hydroxysulfide Nanosheets with Regulated Electronic Structure for High-Performance Electrochemical Energy Storage. Sci. China-Mater. 2020, 63, 2303–2313. [Google Scholar] [CrossRef]
- Shilpa, R.; Anand, A.C.; Sibi, K.S.; Kumar, S.R.S.; Rakhi, R.B. MnO2/MoS2 Heterostructured Self-Standing Bifunctional Electrodes for Efficient Alkaline Simulated Seawater Electrolysis. Int. J. Hydrogen Energy 2025, 117, 73–85. [Google Scholar] [CrossRef]
- Padmajan Sasikala, S.; Singh, Y.; Bing, L.; Yun, T.; Koo, S.H.; Jung, Y.; Kim, S.O. Longitudinal Unzipping of 2D Transition Metal Dichalcogenides. Nat. Commun. 2020, 11, 5032. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Song, E.; Zhao, W.; Xu, S.; Zhao, W.; Lei, Y.; Fang, Y.; Liu, J.; Huang, F. Charge Self-Regulation in 1T’’’-MoS2 Structure with Rich S Vacancies for Enhanced Hydrogen Evolution Activity. Nat. Commun. 2022, 13, 5954. [Google Scholar] [CrossRef] [PubMed]
- Subashini, C.; Sivasubramanian, R.; Sundaram, M.M.; Priyadharsini, N. The Evolution of Allotropic Forms of Na2CoP2O7 Electrode and Its Role in Future Hybrid Energy Storage. J. Energy Storage 2025, 130, 117390. [Google Scholar] [CrossRef]
- Vasudevan, S.; D, S.T.; Manickam, M.; Sivasubramanian, R. A Sol–Gel Derived LaCoO3 Perovskite as an Electrocatalyst for Al–Air Batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Tian, W.; Lv, X.; Zhang, H.; Hu, Z.; Zhang, Y.; Ji, J.; Jiang, W. Co-Doped Ni3S2@CNT Arrays Anchored on Graphite Foam with a Hierarchical Conductive Network for High-Performance Supercapacitors and Hydrogen Evolution Electrodes. J. Mater. Chem. A 2018, 6, 10490–10496. [Google Scholar] [CrossRef]
- Chen, Y.; Rong, J.; Fan, Q.; Sun, M.; Deng, Q.; Ni, Z.; Li, X.; Hu, T. Facile Engineering of CoS/rGO Heterostructures on Carbon Cloth for Efficient All-pH Hydrogen Evolution Reaction and Alkaline Water Electrolysis. J. Mater. Chem. A 2025, 13, 486–498. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of Hierarchical NiS Microflowers for High Performance Asymmetric Supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Zhang, J.; Liu, X.; Zhang, K.; Zhang, Y.; Song, X.-m. Charge Separation at BiVO4/Co3O4 and BiVO4/CoOOH Interfaces: Differences between Dense and Permeable Cocatalysts. Appl. Surf. Sci. 2023, 624, 156965. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Huang, X.; Zhu, Y.; Wang, D. Bimetallic Phosphide Heterostructure Coupled with Ultrathin Carbon Layer Boosting Overall Alkaline Water and Seawater Splitting. Small 2023, 19, 2206533. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhang, Y.; Zhou, Y.; Zhu, C.M.; Xie, Y.D.; Lv, L.W.; Chen, W.L.; He, Y.Y.; Hu, Z.G. Design and Synthesis of NiS@Cos@CC with Abundant Heterointerfaces as High-Efficiency Hydrogen Evolution Electrocatalyst. Int. J. Hydrogen Energy 2019, 44, 26753–26763. [Google Scholar] [CrossRef]
- Jiang, L.; Hao, Y.; Gu, M.; Deng, L.; Chen, Q.; Zhang, X.; Li, L.; Peng, S.; Wang, G. Manipulating Charge Distribution at Organic-inorganic Interface via Optimizing Substituents for Sustainable Water Electrolysis. Angew. Chem.-Int. Ed. 2025, 64, e202423145. [Google Scholar] [CrossRef]
- Thangamathi, R.; Kumaresan, N.; Praveen Kumar, M.; Mangalaraja, R.V.; Herrera Diaz, F.V.; Farhang Sahlevani, S.; Ferreira de Oliveira, T.; Pabba, D.P.; Sivakumar, P. Heterostructure-Induced Interfacial Charge Transfer Interaction in CoS/CoO@NAC Nanosheets as a Bi-Functional Electrocatalyst for Water-Splitting Application. Int. J. Hydrogen Energy 2024, 64, 69–79. [Google Scholar] [CrossRef]
- Lu, W.; Song, Y.; Dou, M.; Ji, J.; Wang, F. Self-Supported Ni3S2@MoS2 Core/Shell Nanorod Arrays via Decoration with CoS as a Highly Active and Efficient Electrocatalyst for Hydrogen Evolution and Oxygen Evolution Reactions. Int. J. Hydrogen Energy 2018, 43, 8794–8804. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.-J.; Durst, J.; Bozza, F.; Graule, T.; Schäublin, R.; Wiles, L.; et al. Dynamic Surface Self-Reconstruction is the Key of Highly Active Perovskite Nano-Electrocatalysts for Water Splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef]
- Bao, W.; Liu, J.; Ai, T.; Han, J.; Hou, J.; Li, W.; Wei, X.; Zou, X.; Deng, Z.; Zhang, J. Unveiling the Role of Surface Self-Reconstruction of Metal Chalcogenides on Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2024, 34, 2408364. [Google Scholar] [CrossRef]
- Huang, R.; Chen, W.; Zhang, Y.; Huang, Z.; Dai, H.; Zhou, Y.; Wu, Y.; Lv, X. Well-Designed Cobalt-Nickel Sulfide Microspheres with Unique Peapod-Like Structure for Overall Water Splitting. J. Colloid Interface Sci. 2019, 556, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dongfang, N.; Triana, C.A.; Huang, C.; Erni, R.; Wan, W.; Li, J.; Stoian, D.; Pan, L.; Zhang, P.; et al. Dynamics and Control of Active Sites in Hierarchically Nanostructured Cobalt Phosphide/Chalcogenide-Based Electrocatalysts for Water Splitting. Energy Environ. Sci. 2022, 15, 727–739. [Google Scholar] [CrossRef] [PubMed]


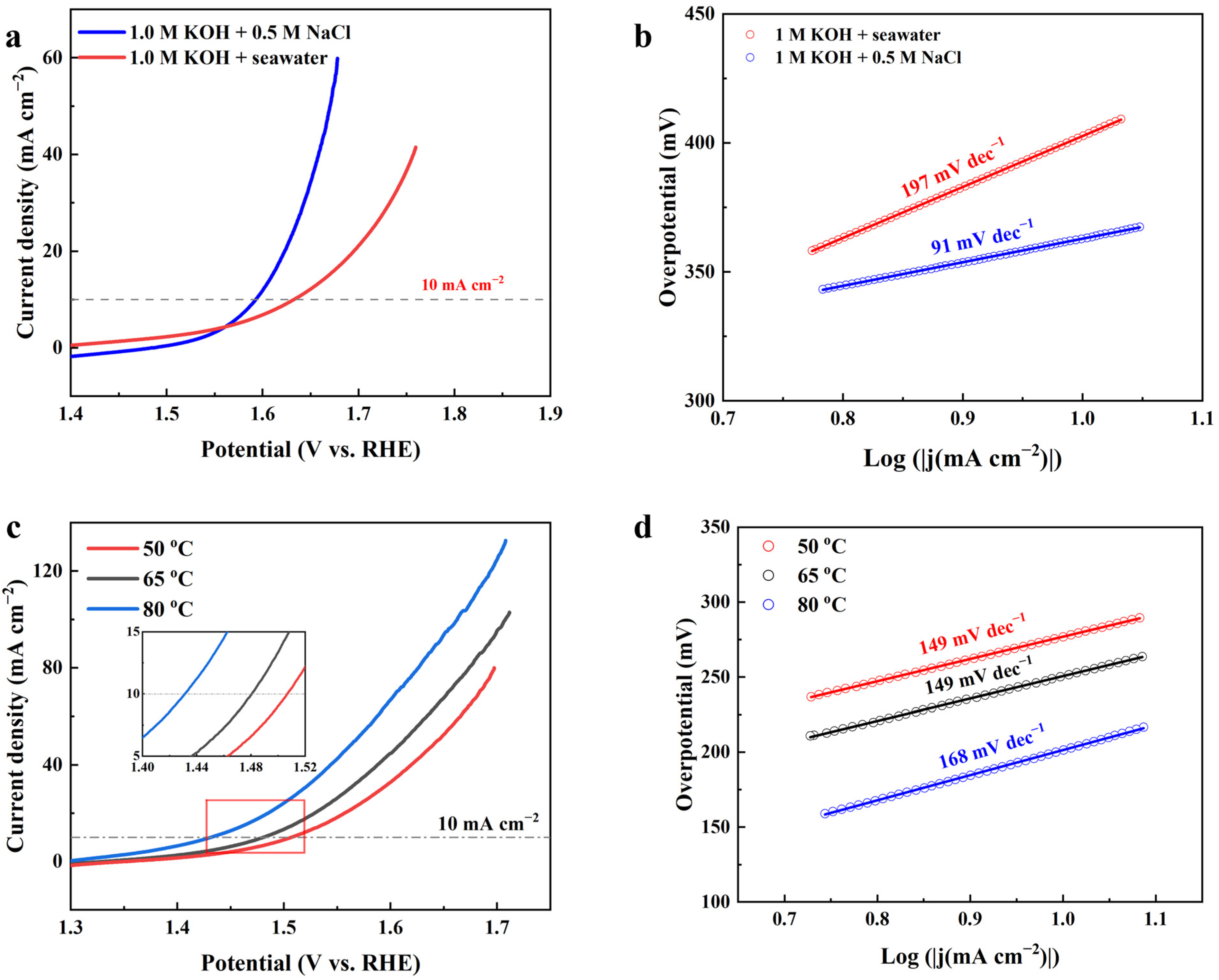
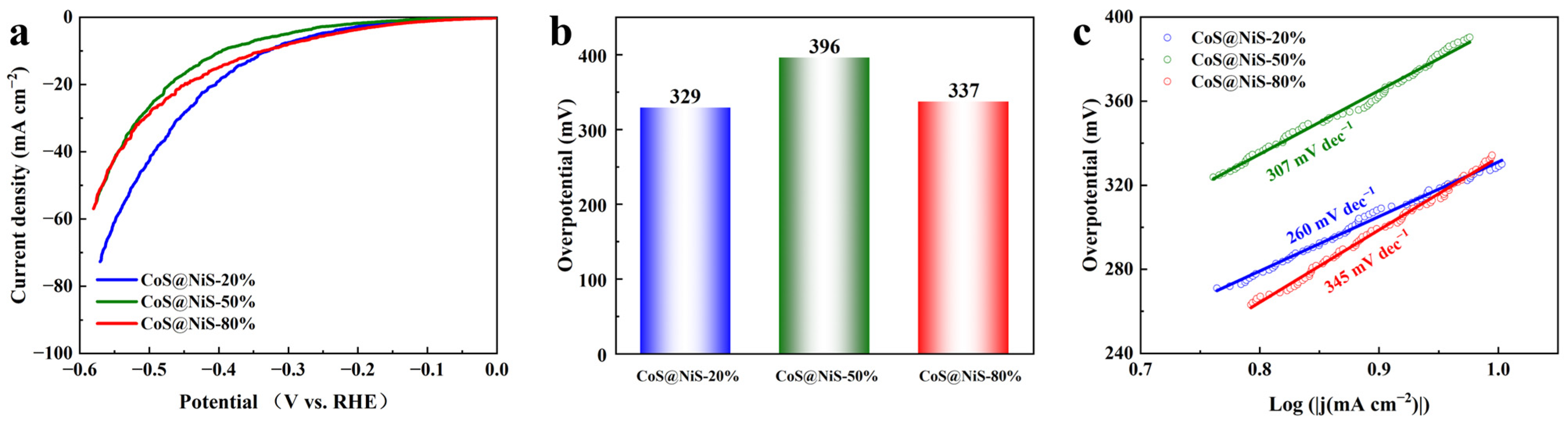
| Catalysts | Current Density j (mA cm−2) | Overpotential at Corresponding j (mV) | Tafel Slope (mV dec−1) | Stability (h) | Refs. |
|---|---|---|---|---|---|
| CoS-Ag-3% | 10 | 293 | 55.3 | 30 | [20] |
| CoS/rGO | 10 | 290 | 71 | 24 | [53] |
| CoS/MoS2 | 10 | 281 | 79 | 12 | [13] |
| Co-MoS2 | 10 | 312 | 126.07 | 100 | [14] |
| MoS2/NiS | 100 | 400 | 181 | 30 | [38] |
| Mo-CoS2/NC | 10 | 296 | 65 | 40 | [24] |
| MXene@CoS/FeS2 | 10 | 278 | 52.7 | 40 | [2] |
| CoS@NiS-80% | 10 | 280 | 101 | 100 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Dong, A.; Liao, W.; Zhang, X.; Ma, Y.; Che, L.; Gao, H. Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction. Nanomaterials 2025, 15, 1216. https://doi.org/10.3390/nano15161216
Yang T, Dong A, Liao W, Zhang X, Ma Y, Che L, Gao H. Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction. Nanomaterials. 2025; 15(16):1216. https://doi.org/10.3390/nano15161216
Chicago/Turabian StyleYang, Ting, Aiyi Dong, Weimin Liao, Xun Zhang, Yinhua Ma, Li Che, and Honglin Gao. 2025. "Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction" Nanomaterials 15, no. 16: 1216. https://doi.org/10.3390/nano15161216
APA StyleYang, T., Dong, A., Liao, W., Zhang, X., Ma, Y., Che, L., & Gao, H. (2025). Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction. Nanomaterials, 15(16), 1216. https://doi.org/10.3390/nano15161216






