Gold Nanoparticles for Wound Healing in Animal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporting and Registration
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Quality Assessment
2.6. Data Extraction
2.7. Statistical Analysis
2.8. Data Presentation
3. Results
3.1. Study Selection
3.2. Study Characteristics
| Study | Intervention Group, n | Control Group, n | Wound Type | Animal Model | Day of Peak Effect | Study Quality |
|---|---|---|---|---|---|---|
| 1. Chao et al., 2025 [76] | 5 | 5 | Full thickness | Mice | Day 7 | 7/14 |
| 2. Gerile et al., 2025 [82] | 5 | 5 | Full thickness | Mice | Day 7 | 7/14 |
| 3. Ma et al., 2025 [77] | 5 | 5 | Full thickness | Mice | Day 7 | 6/14 |
| 4. Biswal et al., 2025 [70] | 3 | 3 | Full thickness | Rats | Day 7 | 6/14 |
| 5. Cai et al., 2025 [69] | 4 | 4 | Full thickness | Rats | Day 8 | 8/14 |
| 6. Du et al., 2025 [65] | 4 | 4 | Full thickness | Mice | Day 9 | 7/14 |
| 7. Zhou et al., 2025 [73] | 4 | 4 | Full thickness | Mice | Day 3 | 5/14 |
| 8. Jiang et al., 2025 [83] | 3 | 3 | Full thickness | Mice | Day 13 | 7/14 |
| 9. Luo et al., 2025 [66] | 3 | 3 | Full thickness | Mice | Day 12 | 7/14 |
| 10. Salama et al., 2024 [84] | 6 | 6 | Full thickness | Rats | Day 9 | 7/14 |
| 11. Peng et al., 2024 [71] | 6 | 6 | Full thickness | Rats | Day 12 | 7/14 |
| 12. Jiang et al., 2024 [79] | 5 | 5 | Full thickness | Mice | Day 12 | 10/14 |
| 13. Li et al., 2023 [81] | 3 | 3 | Full thickness | Mice | Day 14 | 10/14 |
| 14. Yao et al., 2023 [75] | 6 | 6 | Full thickness | Mice | Day 15 | 7/14 |
| 15. Chen et al., 2023 [20] | 6 | 6 | Full thickness | Rats | Day 13 | 8/14 |
| 16. Wang et al., 2022 [64] | 7 | 7 | Full thickness | Rats | Day 7 | 10/14 |
| 17. Azlan et al., 2021 [63] | 8 | 8 | Full thickness | Rats | Day 3 | 8/14 |
| 18. Gharehpapagh et al., 2021 [67] | 18 | 18 | Full thickness | Mice | Day 7 | 9/14 |
| 19. Wang et al., 2020 [60] | 12 | 12 | Full thickness | Rats | Day 10 | 10/14 |
| 20. Al-Musawi et al., 2020 [62] | 5 | 5 | Full thickness | Rabbits | Day 7 | 7/14 |
| 21. Hu et al., 2020 [61] | 5 | 5 | Full thickness | Mice | Day 4 | 7/14 |
| 22. Li et al., 2019 [74] | 6 | 6 | Full thickness | Rats | Day 3 | 9/14 |
| 23. Sun et al., 2019 [72] | 15 | 15 | Full thickness | Mice | Day 7 | 7/14 |
| 24. Martínez et al., 2019 [59] | 12 | 12 | Full thickness | Mice | Day 10 | 10/14 |
| 25. Xu et al., 2018 [80] | 3 | 3 | Full thickness | Rats | Day 10 | 10/14 |
| 26. Pan et al., 2018 [58] | 6 | 6 | Full thickness | Rats | Day 10 | 10/14 |
| 27. Raghuwanshi et al., 2017 [68] | 6 | 6 | Full thickness | Rats | Day 9 | 10/14 |
| 28. Comune et al., 2017 [57] | 10 | 10 | Full thickness | Mice | Day 7 | 10/14 |
| 29. Lau et al., 2017 [7] | 5 | 5 | Full thickness | Rats | Day 7 | 9/14 |
| 30. Naraginti et al., 2016 [56] | 6 | 6 | Full thickness | Rats | Day 9 | 10/14 |
| 31. Leu et al., 2012 [78] | 6 | 6 | Full thickness | Mice | Day 7 | 10/14 |
3.3. Study Quality and Publication Bias
3.4. Dealing with Missing Data
3.5. Statistical Analysis and Pooling
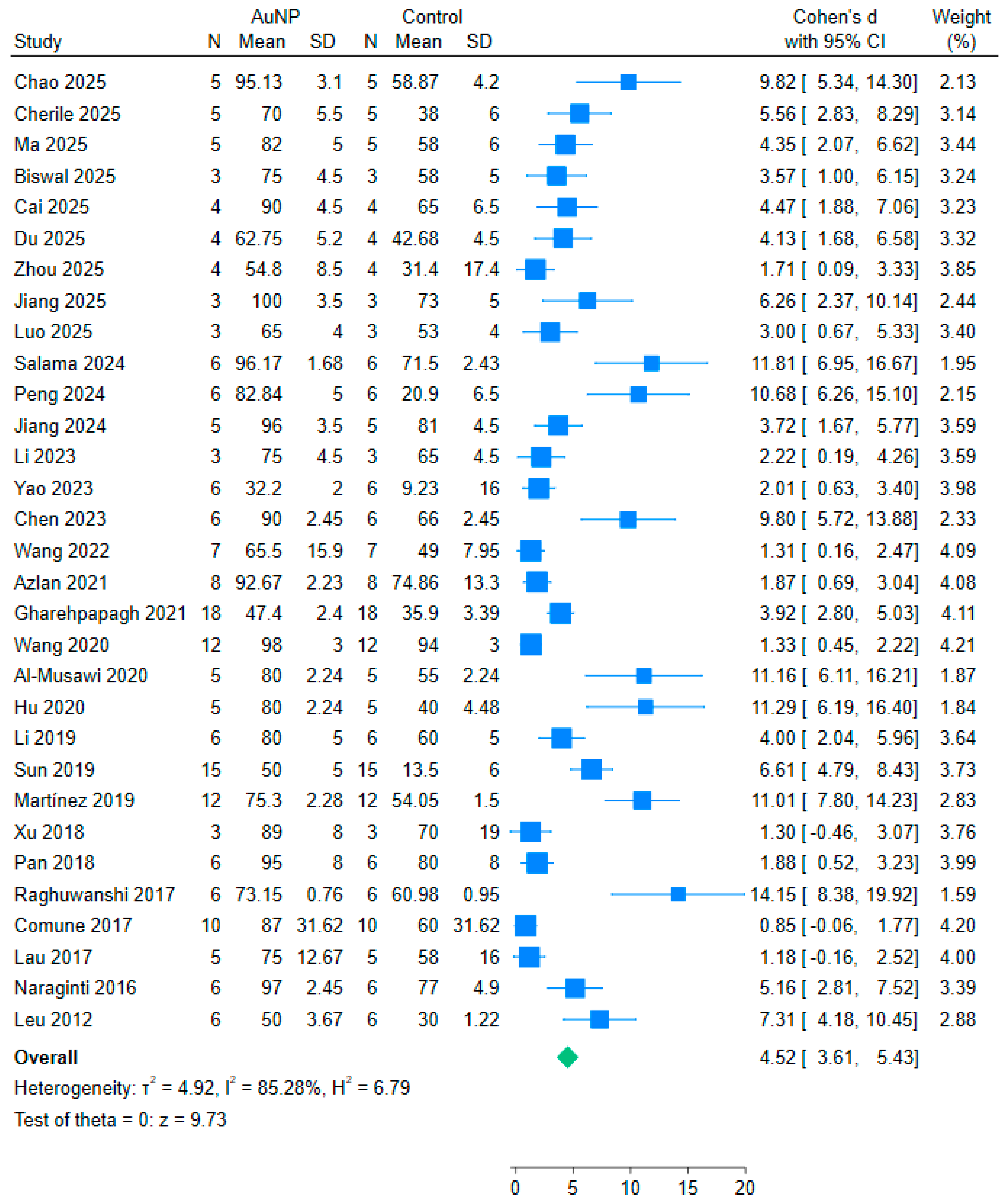
3.6. Meta-Analysis Results

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GNPs | Gold Nanoparticles |
| ROS | Reactive Oxygen Species |
| IL-4 | Interleukin 4 |
| SD | Standard Deviation |
| SE | Standard Error |
| CI | Confidence Interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CAMARADES | Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies |
Appendix A
Search Strategy
| Source | Thesaurus Headings/Free Text and Truncation | Results | Date of Search |
|---|---|---|---|
| Pubmed | “gold nanoparticle*”[Text Word] “gold nanoparticles”[Text Word] “gold nanorod*”[Text Word] “gold nanoshell*”[Text Word] “AuNPs”[Text Word] “GNPs”[Text Word] “gold nanostructure”[Text Word] “gold nanomaterials”[Text Word] | 39,136 | 14 May 2025 |
| “wound*”[Text Word] “wound healing”[Text Word] “wound repair”[Text Word] “wound treatment”[Text Word] “tissue regeneration”[Text Word] “tissue repair”[Text Word] “skin regeneration”[Text Word] “skin repair”[Text Word] “Wound Healing/drug effects”[MeSH] “Re-Epithelialization/drug effects”[MeSH] | 514,331 | ||
| “randomized controlled trial”[Text Word] “randomised controlled trial”[Text Word] “RCT”[Text Word] “randomized clinical trial”[Text Word] | 723,707 | ||
| ((“gold nanoparticle*” OR “gold nanoparticles” OR “gold nanorod*” OR “gold nanoshell*” OR “AuNPs” OR “GNPs” OR “gold nanostructure” OR “gold nanomaterials”) AND (wound* OR “wound healing” OR “wound repair” OR “wound treatment” OR “tissue regeneration” OR “tissue repair” OR “skin regeneration” OR “skin repair” OR “Wound Healing/drug effects” OR “Re-Epithelialization/drug effects” OR “randomized controlled trial” OR “randomised controlled trial” OR “RCT” OR “randomized clinical trial”)) | 519 | ||
| Embase | (gold nanoparticle* OR gold nanorod* OR gold nanoshell* OR “AuNPs” OR “GNPs”) | 15,897 | 14 May 2025 |
| (randomized controlled trial* OR randomised controlled trial* OR random allocation OR random* OR “RCT”) | 2,431,698 | ||
| (wound healing OR wound repair OR wound* OR tissue regeneration OR tissue repair OR skin regeneration OR skin repair) | 614,077 | ||
| (gold nanoparticle* OR gold nanorod* OR gold nanoshell* OR “AuNPs” OR “GNPs”) AND (randomized controlled trial* OR randomised controlled trial* OR random allocation OR random* OR “RCT”) AND (wound healing OR wound repair OR wound* OR tissue regeneration OR tissue repair OR skin regeneration OR skin repair) | 9 | ||
| Cochrane | ((“gold” NEXT nanoparticle*) OR (“gold” NEXT nanorod*) OR (“gold” NEXT nanoshell*) OR “AuNPs” OR “GNPs”) | 35 | 14 May 2025 |
| (randomized controlled trial OR randomised controlled trial OR randomized OR randomised OR RCT) | 8942 | ||
| (wound NEXT healing OR wound NEXT repair OR wound* OR tissue NEXT regeneration OR tissue NEXT repair OR skin NEXT regeneration OR skin NEXT repair) | 494 | ||
| ((“gold” NEXT nanoparticle*) OR (“gold” NEXT nanorod*) OR (“gold” NEXT nanoshell*) OR “AuNPs” OR “GNPs”) AND (randomized controlled trial OR randomised controlled trial OR randomized OR randomised OR RCT) AND (wound NEXT healing OR wound NEXT repair OR wound* OR tissue NEXT regeneration OR tissue NEXT repair OR skin NEXT regeneration OR skin NEXT repair) | |||
| Scopus | (TITLE-ABS-KEY(“gold nanoparticle*” OR “gold nanorod*” OR “gold nanoshell*” OR “AuNPs” OR “GNPs”)) | 101,410 | 14 May 2025 |
| (TITLE-ABS-KEY(“randomized controlled trial” OR “randomised controlled trial” OR “randomized” OR “randomised” OR “RCT”)) | 1,465,658 | ||
| (TITLE-ABS-KEY(“wound healing” OR “wound repair” OR “wound*” OR “tissue regeneration” OR “tissue repair” OR “skin regeneration” OR “skin repair”)) | 750,089 | ||
| (TITLE-ABS-KEY(“gold nanoparticle*” OR “gold nanorod*” OR “gold nanoshell*” OR “AuNPs” OR “GNPs”)) AND (TITLE-ABS-KEY(“randomized controlled trial” OR “randomised controlled trial” OR “randomized” OR “randomised” OR “RCT”)) AND (TITLE-ABS-KEY(“wound healing” OR “wound repair” OR “wound*” OR “tissue regeneration” OR “tissue repair” OR “skin regeneration” OR “skin repair”)) | |||
| Web of Science | TS=(“gold nanoparticle*” OR “gold nanorod*” OR “gold nanoshell*” OR “AuNPs” OR “GNPs”) | 127,319 | 14 May 2025 |
| (TS=(“randomized controlled trial” OR “randomised controlled trial” OR “randomized” OR “randomised” OR “RCT”)) | 1,153,119 | ||
| TS=(“wound healing” OR “wound repair” OR “wound*” OR “tissue regeneration” OR “tissue repair” OR “skin regeneration” OR “skin repair”) | 341,754 | ||
| (TS=(“gold nanoparticle*” OR “gold nanorod*” OR “gold nanoshell*” OR “AuNPs” OR “GNPs”)) AND (TS=(“randomized controlled trial” OR “randomised controlled trial” OR “randomized” OR “randomised” OR “RCT”)) AND (TS=(“wound healing” OR “wound repair” OR “wound*” OR “tissue regeneration” OR “tissue repair” OR “skin regeneration” OR “skin repair”)) | 5 |
References
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring skin wound healing models and the impact of natural lipids on the healing process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, P.P.; Satapathy, T.; Roy, A. A review of wound healing activity on different wound models. J. Appl. Pharm. Res. 2019, 7, 1–7. [Google Scholar]
- Nukaly, H.Y.; Ansari, S.A. An insight into the physicochemical properties of gold nanoparticles in relation to their clinical and diagnostic applications. Cureus 2023, 15, 4. [Google Scholar] [CrossRef]
- Lau, P.S.; Wong, W.T.; Chin, W.W.L.; Ko, K.R.; Wong, Y.H.; Lau, C.C. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 2017, 49, 380–386. [Google Scholar] [CrossRef]
- Sharif, S.; Asif, M.; Raza, A.; Khan, N.A.; Ahmad, A.; Nazir, A. Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing. Open Chem. 2024, 22, 1. [Google Scholar] [CrossRef]
- Batool, Z.; Farooq, M.; Yaqoob, S.; Jalil, A.; Iqbal, J.; Nazir, A. Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q. Current research on fungi in chronic wounds. Front. Mol. Biosci. 2023, 9, 1057766. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, X.; Xiao, L.; Wang, L.; Qiang, S. The role of microRNA in the inflammatory response of wound healing. Front. Immunol. 2022, 13, 852419. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.M.; Maturana, M.A.; Kayali, S.; Khouzam, A.; Efeovbokhan, N. Diabetic foot ulcer: A comprehensive review of pathophysiology and management modalities. World J. Clin. Cases 2023, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Borojeny, L.; Albatineh, A.; Dehkordi, A.; Gheshlagh, R. The incidence of pressure ulcers and its associations in different wards of the hospital: A systematic review and meta-analysis. Int. J. Prev. Med. 2020, 11, 171. [Google Scholar] [CrossRef]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global prevalence and incidence of pressure injuries in hospitalised adult patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef]
- Edsberg, L.E.; Black, J.M.; Goldberg, M.; McNichol, L.; Moore, L.; Sieggreen, M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J. Wound Ostomy Cont. Nurs. 2016, 43, 585. [Google Scholar] [CrossRef]
- Probst, S.; Gethin, G.; Roumen, R.M.H.; Chopin, L.; Claes, S.; Parnham, A.; Woo, K.Y. Prevalence and incidence of venous leg ulcers—A systematic review and meta-analysis. Int. Wound J. 2023, 20, 3906–3921. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, H.; Sun, H.; Cui, L. Effect of hyperbaric oxygen therapy on the patients with venous leg ulcer: A systematic review and meta-analysis. Asian J. Surg. 2023, 46, 4131–4137. [Google Scholar] [CrossRef]
- Cherng, J.H.; Su, Y.C.; Hsu, C.C.; Liao, Y.C.; Wang, Y.L.; Hsu, S.H. Hemostasis and anti-inflammatory abilities of AuNPs-coated chitosan dressing for burn wounds. J. Pers. Med. 2022, 12, 1089. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, X.; Zhao, J.; Li, Y.; Zheng, Y. Hydrogel-based dressings designed to facilitate wound healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Zhang, W.; Zhao, J.; Li, Y.; Zheng, Y. A novel wound dressing based on a gold nanoparticle self-assembled hydrogel to promote wound healing. Mater. Adv. 2023, 4, 2918–2925. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Wang, Y.; Xie, W.; Lin, Y.; Li, Y. Ultrasmall gold nanoparticles/carboxymethyl chitosan composite hydrogel: Tough, restorable, biocompatible antimicrobial dressing for wound healing. Appl. Mater. Today 2024, 38, 102206. [Google Scholar] [CrossRef]
- Danscher, G.; Rasmussen, S. nanoGold and µGold inhibit autoimmune inflammation: A review. Histochem. Cell Biol. 2023, 159, 225–232. [Google Scholar] [CrossRef]
- Rasmussen, S.; Frederickson, C.; Danscher, G. Inhibition of local inflammation by implanted gold: A narrative review of the history and use of gold. J. Rheumatol. 2023, 50, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Blichfeldt-Eckhardt, M.R.; Skovsen, A.P.; Nielsen, C.; Petersen, L.J.; Jørgensen, N.K. Intra-articular injection of gold micro-particles with hyaluronic acid for painful knee osteoarthritis. BMC Musculoskelet. Disord. 2024, 25, 211. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Skjoldemose, E.; Jørgensen, N.K. Intraarticular gold microparticles using hyaluronic acid as the carrier for hip osteoarthritis. A 2-year follow-up pilot study. Sci. Rep. 2024, 14, 26249. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Yang, X.; Wang, Y.; Ji, W.; Jia, G. Mussel-inspired gold nanoparticle and PLGA/L-lysine-g-graphene oxide composite scaffolds for bone defect repair. Int. J. Nanomed. 2021, 16, 6693–6718. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; El-Sayed, M.M.H.; Emam, A.N.; Abd-Rabou, A.A.; Dawood, R.M.; Oudadesse, H. Bioactive glass doped with noble metal nanoparticles for bone regeneration: In vitro kinetics and proliferative impact on human bone cell line. RSC Adv. 2021, 11, 25628–25638. [Google Scholar] [CrossRef]
- Wang, T.; Sun, M.; Zhang, X.; Liu, Z.; Liu, Y.; Zhang, Y. Multifunctional gold clusterzymes with distinct glucose depletion and macrophage reprogramming capability towards regulating the regeneration cascade. Chem. Eng. J. 2024, 482, 149068. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal nanoparticle hybrid hydrogels: The state-of-the-art of combining hard and soft materials to promote wound healing. Theranostics 2024, 14, 1534. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Su, J.; Cai, B.; Li, J.; Zhang, K. New insights into balancing wound healing and scarless skin repair. J. Tissue Eng. 2023, 14, 20417314231185848. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ji, S.F.; Fu, X.B.; Jiang, Y.F.; Sun, X.Y. Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration. Mil. Med. Res. 2024, 11, 13. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Y.; Wang, H.; Yang, L. Tumor chemo-radiotherapy with rod-shaped and spherical gold nano probes: Shape and active targeting both matter. Theranostics 2019, 9, 1893. [Google Scholar] [CrossRef]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold nanoparticles (AuNPs): A versatile material for biosensor application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Yang, W.; Huang, J.; Wu, H.; Yan, W.; Lin, Y.; Shen, M.; Shi, X. Shape effects of gold nanoparticles in photothermal cancer therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sudhakumari, B.; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C 2016, 63, 131–141. [Google Scholar] [CrossRef]
- Bruggeman, K.F.; Williams, R.J.; Nisbet, D.R. Dynamic and responsive growth factor delivery from electrospun and hydrogel tissue engineering materials. Adv. Healthc. Mater. 2018, 7, 1700836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, W.; Wang, W.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial electrospun nanofibrous materials for wound healing. Adv. Fiber Mater. 2022, 5, 107–129. [Google Scholar] [CrossRef]
- Pan, P.; Zhang, Z.; Zhang, X.; Zhang, C.; Wu, G.; Liu, X.; Chen, Y. Recent advances in multifunctional microneedle patches for wound healing and health monitoring. Adv. Nanobiomed. Res. 2023, 3, 2200126. [Google Scholar] [CrossRef]
- Priya, S.; Tomar, Y.; Desai, V.M.; Singhvi, G. Enhanced skin drug delivery using dissolving microneedles: A potential approach for the management of skin disorders. Expert Opin. Drug Deliv. 2023, 20, 721–738. [Google Scholar] [CrossRef]
- Guillot, A.J.; Martínez-Navarrete, M.; Zinchuk-Mironova, V.; Melero, A. Microneedle-assisted transdermal delivery of nanoparticles: Recent insights and prospects. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1884. [Google Scholar] [CrossRef]
- Paula, A.; Nozaki, M.; Helena, M.; Lima, Â.; Moraes, M. Sprayable bioactive dressings for skin wounds: Recent developments and future prospects. Biomed. Mater. Devices 2022, 1, 569–586. [Google Scholar] [CrossRef]
- Patel, V.N.; Chaudhari, R.; Shah, S.; Prajapati, V.D.; Sheth, N.R.; Patel, R.R. Comprehensive developmental investigation on simvastatin enriched bioactive film forming spray using the quality by design paradigm: A prospective strategy for improved wound healing. J. Drug Target. 2024, 32, 1139–1153. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Tan, S.H.; Ngo, Z.H.; Leavesley, D.; Liang, K. Recent advances in the design of three-dimensional and bioprinted scaffolds for full-thickness wound healing. Tissue Eng. Part B Rev. 2022, 28, 160–181. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Hannoodee, S.; Nasuruddin, D.N. Acute inflammatory response. Nature 2024, 206, 20. [Google Scholar] [CrossRef]
- Ryan, R.; Hill, S.; Prictor, M.; McKenzie, J. Study Quality Guide: How to Cite This Guide. 2013. Available online: https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/uploads/StudyQualityGuide_May%202013.pdf (accessed on 17 October 2024).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2024; Available online: https://training.cochrane.org/handbook (accessed on 7 December 2024).
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 1203–1208. [Google Scholar] [CrossRef]
- Wilson, E.; Ramage, F.J.; Wever, K.E.; Sena, E.S.; Macleod, M.R.; Currie, G.L. Designing, conducting, and reporting reproducible animal experiments. J. Endocrinol. 2023, 258, 1. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Kumari, P.L.; Das, R.K.; Sivakumar, A.; Patil, S.H.; Andhalkar, V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 2016, 62, 293–300. [Google Scholar] [CrossRef]
- Comune, M.; Rai, R.; Chereddy, K.K.; Pinto, S.; Chaurasia, M.; Ferreira, A.F.; Boudy, V. Antimicrobial peptide-gold nanoscale therapeutic formulation with high skin regenerative potential. J. Control. Release 2017, 262, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; He, W.; Dou, H.; Liu, Y.; Guo, L.; Xia, Y.; Yang, Y. Topical application of keratinocyte growth factor conjugated gold nanoparticles accelerate wound healing. Nanomedicine 2018, 14, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.P.H.; Reyes, L.R.; Guzmán, D.V.; Ramírez, I.C.C.; Meza, M.N.; Estrada, V.A.; Salazar, F.C. A novel gold calreticulin nanocomposite based on chitosan for wound healing in a diabetic mice model. Nanomaterials 2019, 9, 75. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Chen, C.; Li, Q.; Zhang, Y.; Wang, H.; Jiang, X. Mercaptophenylboronic acid-activated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159. [Google Scholar] [CrossRef]
- Hu, W.C.; Younis, M.R.; Zhou, Y.; Wang, C.; Xia, X.H. In situ fabrication of ultrasmall gold nanoparticles/2D MOFs hybrid as nanozyme for antibacterial therapy. Small 2020, 16, 2000553. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Abdulhussain, N.A.; Al-Mayah, A.H.; Al-Kinani, A.A.; Hadi, M.Y.; Sahib, U.I. Antibacterial activity of honey/chitosan nanofibers loaded with capsaicin and gold nanoparticles for wound dressing. Molecules 2020, 25, 4770. [Google Scholar] [CrossRef]
- Nor Azlan, A.Y.H.; Katas, H.; Mohamad Zin, N.; Fauzi, M.B. Dual action gels containing DsiRNA loaded gold nanoparticles: Augmenting diabetic wound healing by promoting angiogenesis and inhibiting infection. Eur. J. Pharm. Biopharm. 2021, 169, 78–90. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Hou, Q.; Zhong, L.; Li, Q.; Jiang, X. Breathable and stretchable dressings for accelerating healing of infected wounds. Adv. Healthc. Mater. 2022, 11, 2201053. [Google Scholar] [CrossRef]
- Du, N.; Wang, S.; Yang, Z.; He, X.; Tang, Y.; Xie, H.; Zhang, L. Upcycling of expanded polystyrene waste into multifunctional antibacterial platforms for microbial control. ACS Appl. Mater. Interfaces 2025, 17, 23656–23665. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Y.; Chen, R.; Zhang, H.; Sun, T.; Guo, W.; Tang, S. Piezoelectric dual-network tough hydrogel with on-demand thermal contraction and sonopiezoelectric effect for promoting infected-joint-skin-wound healing via FAK and AKT signaling pathways. Natl. Sci. Rev. 2025, 12, nwaf118. [Google Scholar] [CrossRef] [PubMed]
- Choodari Gharehpapagh, A.; Farahpour, M.R.; Jafarirad, S. The biological synthesis of gold/perlite nanocomposite using Urtica dioica extract and its chitosan-capped derivative for healing wounds infected with methicillin-resistant Staphylococcus aureus. Int. J. Biol. Macromol. 2021, 183, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, N.; Joshi, R.S.; Sawant, S.N.; Umekar, M.J. Synergistic effects of Woodfordia fruticosa gold nanoparticles in preventing microbial adhesion and accelerating wound healing in Wistar albino rats in vivo. Mater. Sci. Eng. C 2017, 80, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Liu, Y.; Chen, R.; Dong, H.; Zhou, J.; Yu, Y.; Liu, Z. Sericin-assisted green synthesis of gold nanoparticles as broad-spectrum antimicrobial and biofilm-disrupting agents for therapy of bacterial infection. Int. J. Nanomed. 2025, 20, 3559–3574. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.; Sharma, R.; Pradhan, D.; Patro, T.U.; Samanta, S.; Sahoo, D.; Sahoo, B. Nano CaCO3 mediated in vitro and in vivo wound healing characteristics of chitosan films without added drugs. Int. J. Biol. Macromol. 2025, 307, 142057. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Y.; Li, M.; Yang, Q.; Zhang, L.; Zhang, C.; Zhao, Y. Construction of multifunctional hydrogel containing pH-responsive gold nanozyme for bacteria-infected wound healing. Int. J. Biol. Macromol. 2024, 283, 137746. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Zhang, Z.; Ren, W.; Liu, Y.; Zhang, Y.; Wang, H. Albumin broadens the antibacterial capabilities of nonantibiotic small molecule-capped gold nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 45381–45389. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Liu, L.; Zhang, H.; Li, Y.; Lin, Z.; Yang, Y. Flexible PDMS-SERS platform for culture-free diagnosis of bacterial infections in clinical wound care. Talanta 2025, 293, 128089. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhang, Y.; Wang, Z.; Zhang, X.; Wang, C. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine 2019, 14, 2909–2923. [Google Scholar] [CrossRef]
- Yao, M.Y.; Zhang, Y.X.; Wang, R.Z.; Liu, H.; Han, J.Z.; Zhou, J.M.; Li, H.L. Effects of interleukin-4-modified gold nanozymes on the full-thickness skin defects in diabetic mice. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2023, 39, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Li, Z.; Zhao, W.; Zhang, H.; Song, Y.; Feng, X.; He, X. Sprayable hydrogel for pH-responsive nanozyme-derived bacteria-infected wound healing. ACS Appl. Mater. Interfaces 2025, 17, 5921–5932. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liu, H.; Gao, R.; Zhu, K.; Wang, C.; Zhang, X.; Yang, H. Injectable light-responsive hydrogel dressing promotes diabetic wound healing by enhancing wound angiogenesis and inhibiting inflammation. Polymers 2025, 17, 607. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.G.; Lin, L.C.; Huang, T.K.; Hsu, S.H.; Chen, P.R.; Hsu, C.Y.; Huang, C.S. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, X.; Sui, B.; Wang, J.; Liu, X.; Zhang, Z. Using hybrid MnO2-Au nanoflowers to accelerate ROS scavenging and wound healing in diabetes. Pharmaceutics 2024, 16, 1244. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Yang, Y.; Zhang, Z.; Wang, X.; Liu, Y.; Liu, X. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, X.; Song, J.; Xie, J.; Li, J.; Zhang, Y. Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano 2023, 17, 22106–22120. [Google Scholar] [CrossRef]
- Gerile, S.; Wu, X.; Kang, J.; Qi, Y.; Dong, A. Thiol-terminated N-halamine ligands to photothermal gold nanorods for synergistically combating antibiotic-resistant bacteria. Soft Matter 2024, 21, 556–560. [Google Scholar] [CrossRef]
- Jiang, M.; Nie, R.; Kang, J.; Li, P.; Dong, A. Mild phototherapy strategies for preventing pathogen infection and enhancing cell proliferation in diabetic wound. Adv. Healthc. Mater. 2025, 14, e2500862. [Google Scholar] [CrossRef]
- Salama, A.; Younes, H.; El-Melegy, N.; Elkasabgy, N.; El-Nabarawi, M.; Khattab, A. Curcumin-loaded gold nanoparticles with enhanced antibacterial efficacy and wound healing properties in diabetic rats. Int. J. Pharm. 2024, 666, 124761. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; Van Netten, J.J.; Probst, S.; Care, W. The impact of patient health and lifestyle factors on wound healing, part 2. J. Wound Manag. 2022. Available online: https://journals.cambridgemedia.com.au/application/files/7216/9691/5261/The_impact_of_patient_health_and_lifestyle_factors_on_wound_healing._Part_2.pdf (accessed on 31 July 2025).
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud. Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; Iñiguez-Palomares, R. Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res. Lett. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Alshaer, W.; Abu-Yousef, I.A.; Al-Kurdi, M.; Alqudah, D.A.; Al-Mahmoud, N. Investigating inflammatory markers in wound healing: Understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jakic, K.; Resanovic, M.; Dakic, D.; Nikolic, M.; Petrovic, A.; Vucicevic, L.; Zivkovic, L. Long-term accumulation, biological effects and toxicity of BSA-coated gold nanoparticles in the mouse liver, spleen, and kidneys. Int. J. Nanomed. 2024, 19, 4103. [Google Scholar] [CrossRef]
- Yasmin-Karim, S.; Richards, G.; Fam, A.; Ogurek, A.M.; Sridhar, S.; Makrigiorgos, G.M. Aerosol delivery of hesperetin-loaded nanoparticles and immunotherapy increases survival in a murine lung cancer model. Nanomaterials 2025, 15, 586. [Google Scholar] [CrossRef]
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The biological processes during wound healing. Regen. Med. 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The role of the extracellular matrix (ECM) in wound healing: A review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Inbathamizh, L.; Varthan, M.K.H.; Kumar, R.S.R.; Rohinth, M.; Tawfeeq Ahmed, Z.H. Nanotechnology: Ethical impacts, health issues, and safety issues. In Modern Nanotechnology: Volume 2: Green Synthesis, Sustainable Energy and Impacts; Springer Nature: Cham, Switzerland, 2023; pp. 455–477. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef] [PubMed]

| ID | Randomization | Allocation | Blinding | Assessment | Data | Reporting | Other |
|---|---|---|---|---|---|---|---|
| 1. Chao 2025 [76] | Low | Unclear | High | High | Low | Low | Low |
| 2. Gerile 2025 [82] | Low | Unclear | High | High | Low | Low | Low |
| 3. Ma 2025 [77] | Low | Unclear | High | High | Low | Low | Low |
| 4. Biswal 2025 [70] | Low | Unclear | High | High | Low | Low | Low |
| 5. Cai 2025 [69] | Low | Unclear | High | High | Low | Low | Low |
| 6. Du 2025 [65] | Low | Unclear | High | High | Low | Low | Low |
| 7. Zhou 2025 [73] | Low | Unclear | High | High | Low | Low | Low |
| 8. Jiang 2025 [83] | Low | Unclear | High | High | Low | Low | Low |
| 9. Luo 2025 [66] | Low | Unclear | High | High | Low | Low | Low |
| 10. Salama 2024 [84] | Low | Unclear | High | High | Low | Low | Low |
| 11. Peng 2024 [71] | Low | Unclear | High | High | Low | Low | Low |
| 12. Jiang 2024 [79] | Low | Unclear | High | High | Low | Low | Low |
| 13. Li 2023 [81] | Low | Unclear | High | High | Low | Low | Low |
| 14. Yao 2023 [75] | Low | Unclear | High | High | Low | Low | Low |
| 15. Chen 2023 [20] | Low | Unclear | High | High | Low | Low | Low |
| 16. Wang 2022 [64] | Low | Unclear | High | High | Low | Low | Low |
| 17. Azlan 2021 [63] | Low | Unclear | High | High | Low | Low | Low |
| 18. Gharehpapagh 2021 [67] | Low | Unclear | High | High | Low | Low | Low |
| 19. Wang 2020 [60] | Low | Unclear | High | High | Low | Low | Low |
| 20. Al-Musawi 2020 [62] | Low | Unclear | High | High | Low | Low | Low |
| 21. Hu 2020 [61] | Low | Unclear | High | High | Low | Low | Low |
| 22. Li 2019 [74] | Low | Unclear | High | High | Low | Low | Low |
| 23. Sun 2019 [72] | Low | Unclear | High | High | Low | Low | Low |
| 24. Martínez 2019 [59] | Low | Unclear | High | High | Low | Low | Low |
| 25. Xu 2018 [80] | Low | Unclear | High | High | Low | Low | Low |
| 26. Pan 2018 [58] | Low | Unclear | High | High | Low | Low | Low |
| 27. Raghuwanshi 2017 [68] | Low | Unclear | High | High | Low | Low | Low |
| 28. Comune 2017 [57] | Low | Unclear | High | High | Low | Low | Low |
| 29. Lau 2017 [7] | Low | Unclear | High | High | Low | Low | Low |
| 30. Naraginti 2016 [56] | Low | Unclear | High | High | Low | Low | Low |
| 31. Leu 2012 [78] | Low | Unclear | High | High | Low | Low | Low |
| Publication | Year | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chao [76] | 2025 | X | X | X | X | X | X | X | 7 | |||||||
| Gerile [82] | 2025 | X | X | X | X | X | X | X | 7 | |||||||
| Ma [77] | 2025 | X | X | X | X | X | X | 6 | ||||||||
| Biswal [70] | 2025 | X | X | X | X | X | X | 6 | ||||||||
| Cai [69] | 2025 | X | X | X | X | X | X | X | X | 8 | ||||||
| Du [65] | 2025 | X | X | X | X | X | X | X | 7 | |||||||
| Zhou [73] | 2025 | X | X | X | X | X | 5 | |||||||||
| Jiang [83] | 2025 | X | X | X | X | X | X | X | 7 | |||||||
| Luo [66] | 2025 | X | X | X | X | X | X | X | 7 | |||||||
| Salama [84] | 2024 | X | X | X | X | X | X | X | 7 | |||||||
| Peng [71] | 2024 | X | X | X | X | X | X | X | 7 | |||||||
| Jiang [79] | 2024 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Li [81] | 2023 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Yao [75] | 2023 | X | X | X | X | X | X | X | 7 | |||||||
| Chen [20] | 2023 | X | X | X | X | X | X | X | X | 8 | ||||||
| Wang [64] | 2022 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Azlan [63] | 2021 | X | X | X | X | X | X | X | X | 8 | ||||||
| Gharehpapagh [67] | 2021 | X | X | X | X | X | X | X | X | X | 9 | |||||
| Wang [60] | 2020 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Al-Musawi [62] | 2020 | X | X | X | X | X | X | X | 7 | |||||||
| Hu [61] | 2020 | X | X | X | X | X | X | X | 7 | |||||||
| Li [74] | 2019 | X | X | X | X | X | X | X | X | X | 9 | |||||
| Sun [72] | 2019 | X | X | X | X | X | X | X | 7 | |||||||
| Martínez [59] | 2019 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Xu [80] | 2018 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Pan [58] | 2018 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Raghuwanshi [68] | 2017 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Comune [57] | 2017 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Lau [7] | 2017 | X | X | X | X | X | X | X | X | X | 9 | |||||
| Naraginti [56] | 2016 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| Leu [78] | 2012 | X | X | X | X | X | X | X | X | X | X | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klavsen, S.; Rasmussen, S. Gold Nanoparticles for Wound Healing in Animal Models. Nanomaterials 2025, 15, 1213. https://doi.org/10.3390/nano15161213
Klavsen S, Rasmussen S. Gold Nanoparticles for Wound Healing in Animal Models. Nanomaterials. 2025; 15(16):1213. https://doi.org/10.3390/nano15161213
Chicago/Turabian StyleKlavsen, Stephen, and Sten Rasmussen. 2025. "Gold Nanoparticles for Wound Healing in Animal Models" Nanomaterials 15, no. 16: 1213. https://doi.org/10.3390/nano15161213
APA StyleKlavsen, S., & Rasmussen, S. (2025). Gold Nanoparticles for Wound Healing in Animal Models. Nanomaterials, 15(16), 1213. https://doi.org/10.3390/nano15161213






