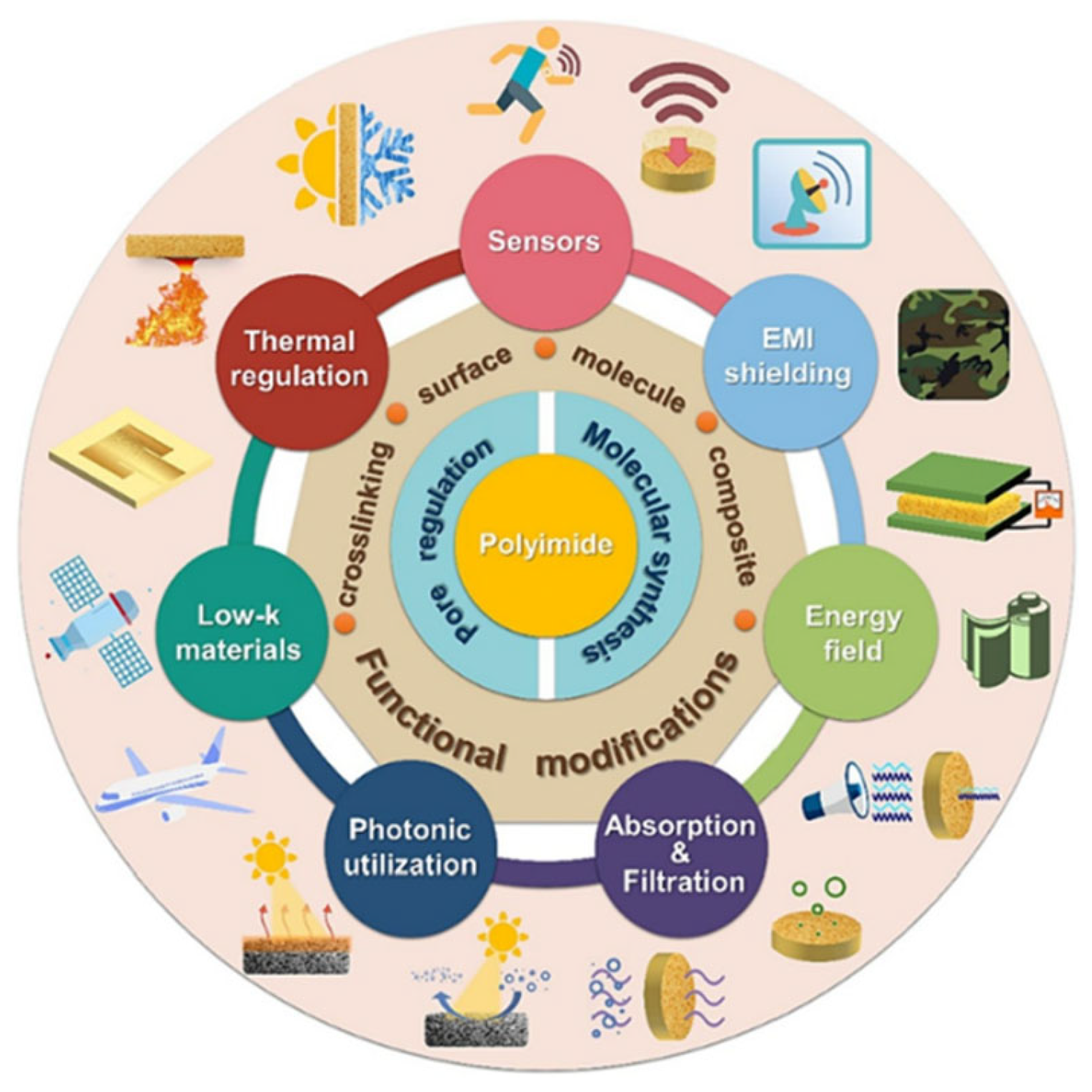
Multifunctional Polyimide for Packaging and Thermal Management of Electronics: Design, Synthesis, Molecular Structure, and Composite Engineering
Abstract
1. Introduction
2. Fabrication and Surface Modification Strategies
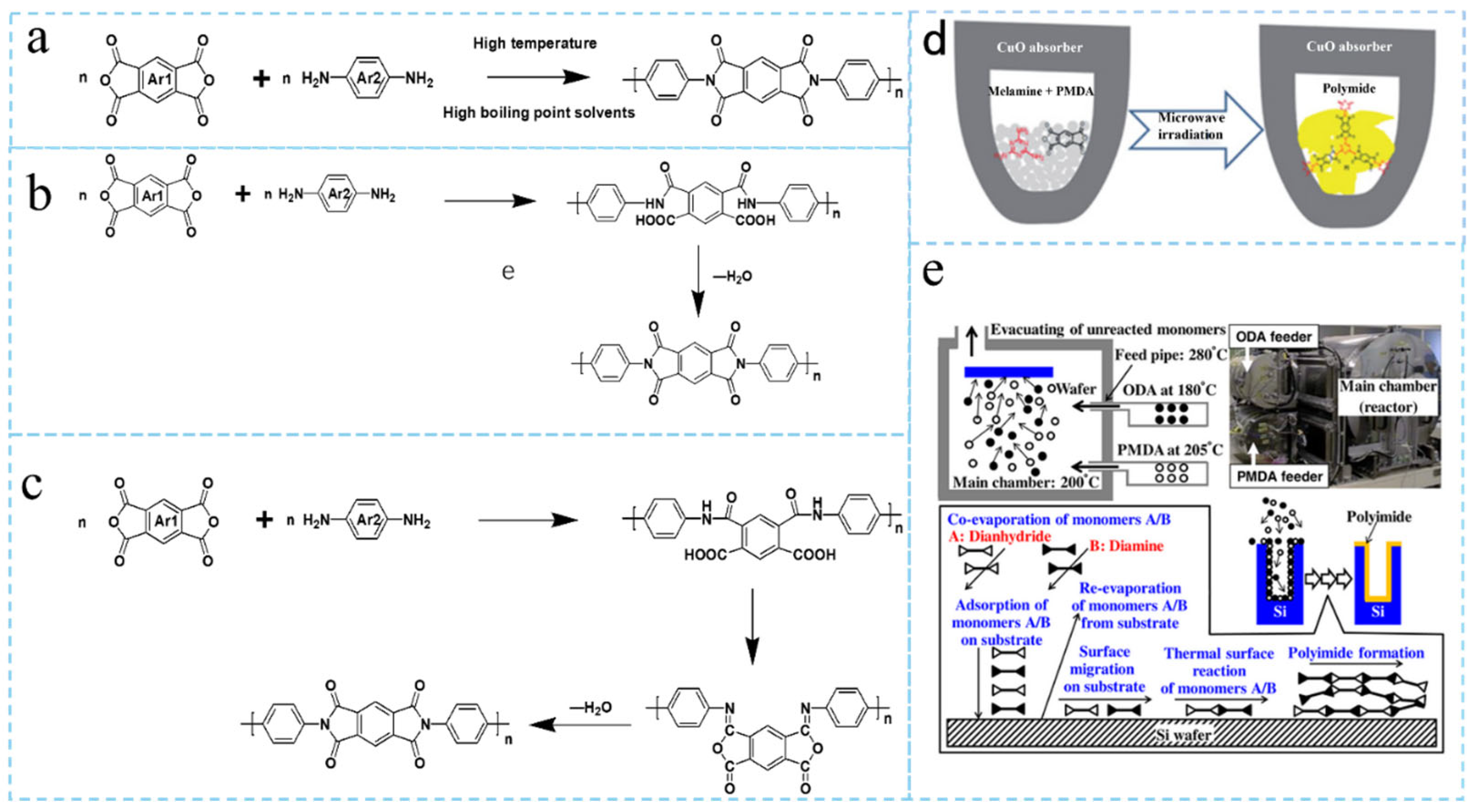
2.1. Polyimide Synthesis Methods
2.2. Surface Modification
2.2.1. Hydrophobicity Enhancement
2.2.2. Chemical Resistance Improvement
2.2.3. Friction Improvement
2.3. Fabrication of Porous Polyimide
3. Functional Polyimides and Composites
3.1. Molecular Structure Design
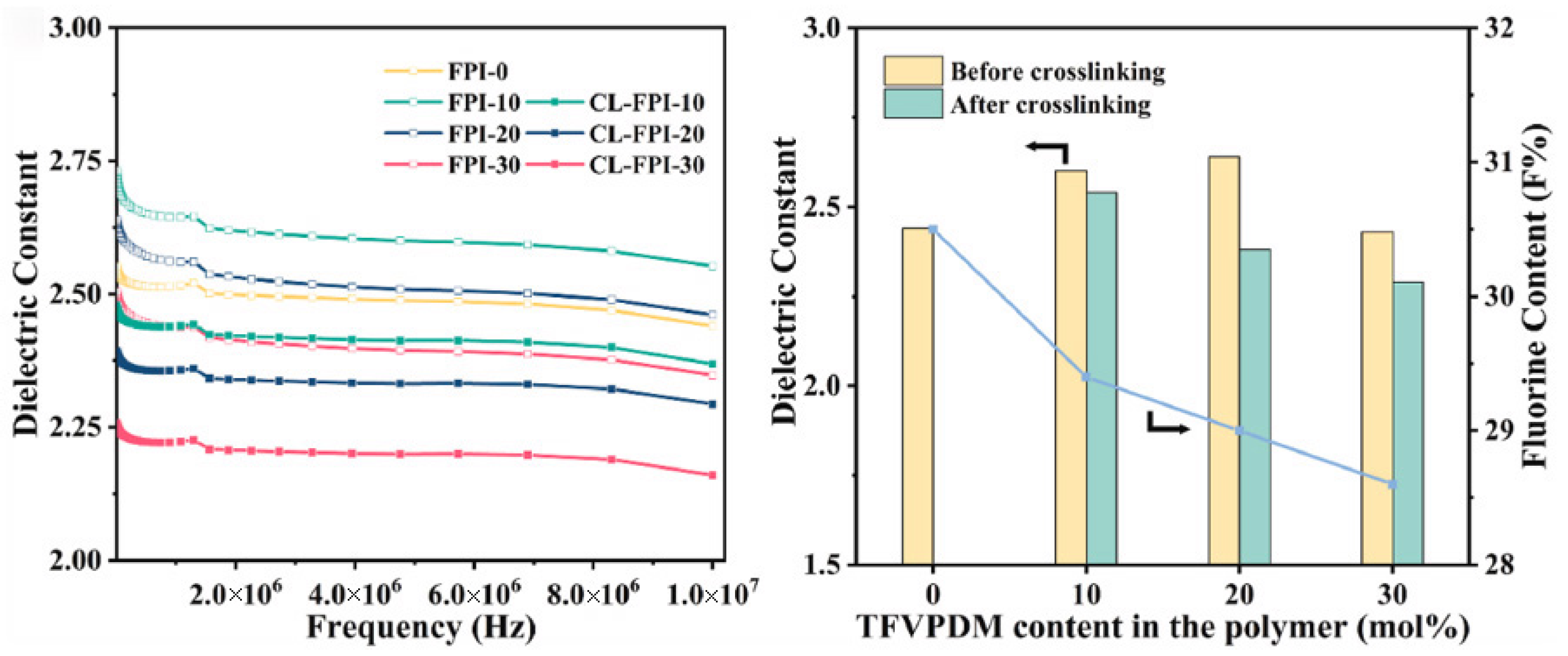
3.1.1. Low Dielectric Constant Polyimide
3.1.2. Thermal Stable Polyimide
3.1.3. Structure–Property Relationship of Polyimide
3.2. Composite Engineering
3.2.1. Thermal Conductive Polyimide Composites

3.2.2. Mechanical Robust Polyimide Composites
3.2.3. EMI-Shielding Polyimide Composites
3.2.4. Comparison of Property Enhancement by Different Fillers
4. Electronic Packaging and Thermal Management Application
4.1. Flexible Electronic Packaging
4.2. EMI Shielding of Electronics
4.3. Thermal Management Application
4.3.1. Thermal Insulation
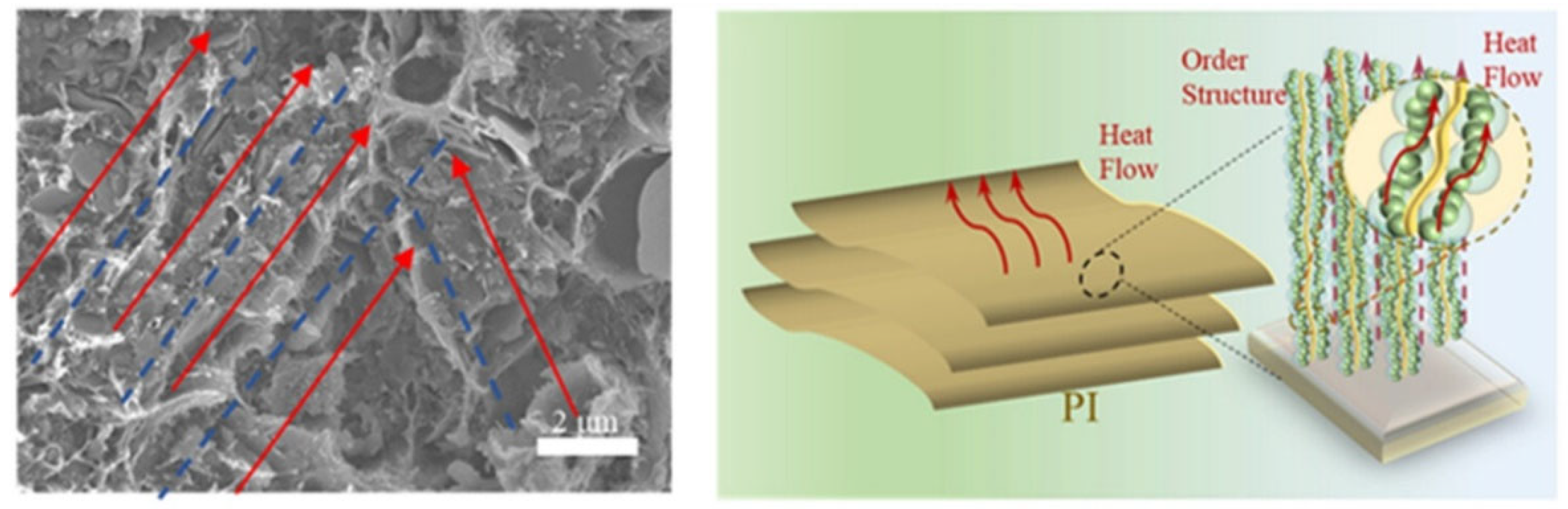
4.3.2. Thermal Conduction
5. Summary and Outlook
Funding
Conflicts of Interest
References
- Ghosh, M. Polyimides Fundamentals and Applications, 1st ed.; CRC Press: Milton, MA, USA, 1996; Volume 36. [Google Scholar]
- Yu, S.; Huang, M.; Hao, R.; He, S.; Liu, H.; Liu, W.; Zhu, C. Recent advances in thermally conductive polymer composites. High Perform. Polym. 2022, 34, 1081–1101. [Google Scholar] [CrossRef]
- Yang, S.-Y. Advanced Polyimide Materials: Synthesis, Characterization, and Applications, 1st ed.; Elsevier: Chantilly, The Netherlands, 2018. [Google Scholar]
- Bogert, M.T.; Renshaw, R.R. 4-amino-0-phthalic acid and some of its derivatives. J. Am. Chem. Soc. 1908, 30, 1135–1144. [Google Scholar] [CrossRef]
- Hu, R.; Chen, Y.; Zhang, C.; Jiang, S.; Hou, H.; Duan, G. Porous monoliths from polyimide: Synthesis, modifications and applications. Prog. Mater. Sci. 2024, 144, 101284. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, W.; Wei, J.-a.; Guo, D.; Zhang, Y.; Yu, Q.; Wang, C.; Wang, L. Synthesis of high-performance colorless polyimides with asymmetric diamine: Application in flexible electronic devices. ACS Appl. Mater. Interfaces 2024, 16, 48005–48015. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Tak, T.-M. Synthesis and characterization of polyimide derivatives prepared by different one-step methods. J. Appl. Polym. Sci. 1996, 61, 371–382. [Google Scholar] [CrossRef]
- Fang, B.; Pan, K.; Meng, Q.; Cao, B. Preparation and properties of polyimide solvent-resistant nanofiltration membrane obtained by a two-step method. Polym. Int. 2012, 61, 111–117. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Jia, Z.; Qin, J.; Gu, Y. Effect of chemical structure and preparation process on the aggregation structure and properties of polyimide film. J. Appl. Polym. Sci. 2013, 127, 4581–4587. [Google Scholar] [CrossRef]
- Lin, L.; Ye, P.; Cao, C.; Jin, Q.; Xu, G.-S.; Shen, Y.-H.; Yuan, Y.-P. Rapid microwave-assisted green production of a crystalline polyimide for enhanced visible-light-induced photocatalytic hydrogen production. J. Mater. Chem. A 2015, 3, 10205–10208. [Google Scholar] [CrossRef]
- Naganuma, T.; Naito, K.; Yang, J.-M. High-temperature vapor deposition polymerization polyimide coating for elimination of surface nano-flaws in high-strength carbon fiber. Carbon 2011, 49, 3881–3890. [Google Scholar] [CrossRef]
- Fukushima, T.; Murugesan, M.; Bea, J.C.; Hashimoto, H.; Kino, H.; Tanaka, T.; Koyanagi, M. On-wafer thermomechanical characterization of a thin film polyimide formed by vapor deposition polymerization for through-silicon via applications: Comparison to plasma-enhanced chemical vapor deposition SiO2. J. Polym. Sci. 2020, 58, 2248–2258. [Google Scholar] [CrossRef]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D.; et al. Robust, lightweight, hydrophobic, and fire-retarded polyimide/MXene aerogels for effective oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhao, Y.; Zhang, X. Superhydrophobic polyimide aerogels via conformal coating strategy with excellent underwater performances. J. Appl. Polym. Sci. 2020, 137, 48849. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Song, P.; You, B.; Sun, G. Double-cross-linking strategy for preparing flexible, robust, and multifunctional polyimide aerogel. Chem. Eng. J. 2020, 381, 122784. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. Flexible, fatigue resistant, and heat-insulated nanofiber-assembled polyimide aerogels with multifunctionality. Polym. Test. 2020, 81, 106246. [Google Scholar] [CrossRef]
- Chang, K.-C.; Lu, H.-I.; Peng, C.-W.; Lai, M.-C.; Hsu, S.-C.; Hsu, M.-H.; Tsai, Y.-K.; Chang, C.-H.; Hung, W.-I.; Wei, Y.; et al. Nanocasting technique to prepare lotus-leaf-like superhydrophobic electroactive polyimide as advanced anticorrosive coatings. ACS Appl. Mater. Interfaces 2013, 5, 1460–1467. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Shao, Z.; Gao, W.; Fan, W.; Liu, T.; Bai, H. Multifunctional polyimide aerogel textile inspired by polar bear hair for thermoregulation in extreme environments. Chem. Eng. J. 2020, 390, 124623. [Google Scholar] [CrossRef]
- Pei, X.; Zhai, W.; Zheng, W. Preparation and characterization of highly cross-linked polyimide aerogels based on polyimide containing trimethoxysilane side groups. Langmuir 2014, 30, 13375–13383. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, W.; Wang, Z. Facile synthesis of soluble, self-crosslinkable and crystalline polyimides with ultrahigh thermal/chemical resistance. Polymer 2023, 268, 125717. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application. Chem. Eng. J. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Li, T.; Tian, J.; Huang, T.; Huang, Z.; Wang, H.; Lu, R.; Cong, P. Tribological behaviors of fluorinated polyimides at different temperatures. J. Macromol. Sci. Part B 2011, 50, 860–870. [Google Scholar] [CrossRef]
- Tian, J.; Wang, H.; Huang, Z.; Lu, R.; Cong, P.; Liu, X.; Li, T. Investigation on tribological properties of fluorinated polyimide. J. Macromol. Sci. Part B 2010, 49, 791–801. [Google Scholar] [CrossRef]
- Li, S.; Zhong, M.; Zou, Y.; Xu, M.; Liu, X.; Xing, X.; Zhang, H.; Jiang, Y.; Qiu, C.; Qin, W.; et al. Fabrication of micron-structured heatable graphene hydrophobic surfaces for deicing and anti-Icing by laser direct writing. Coatings 2023, 13, 1559. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X. A review of tribological properties of polyimide composites in various lubrication environments under atmospheric conditions. Mater. Today Commun. 2025, 42, 111473. [Google Scholar] [CrossRef]
- Roy, A.; Mu, L.; Shi, Y. Tribological properties of polyimide coating filled with carbon nanotube at elevated temperatures. Polym. Compos. 2020, 41, 2652–2661. [Google Scholar] [CrossRef]
- Bijwe, J.; Logani, C.M.; Tewari, U.S. Influence of fillers and fibre reinforcement on abrasive wear resistance of some polymeric composites. Wear 1990, 138, 77–92. [Google Scholar] [CrossRef]
- Liu, S.; Dong, C.; Yuan, C.; Bai, X.; Tian, Y.; Zhang, G. A new polyimide matrix composite to improve friction-induced chatter performance through reducing fluctuation in friction force. Compos. Part B Eng. 2021, 217, 108887. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Luo, N.; Liu, Y.; Feng, Y.; Chen, S.; Wang, D. Controlling the triboelectric properties and tribological behavior of polyimide materials via plasma treatment. Nano Energy 2022, 102, 107691. [Google Scholar] [CrossRef]
- Li, M.; Gan, F.; Dong, J.; Fang, Y.; Zhao, X.; Zhang, Q. Facile preparation of continuous and porous polyimide aerogel fbers for mltifunctional aplications. ACS Appl. Mater. Interfaces 2021, 13, 10416–10427. [Google Scholar] [CrossRef]
- Xu, W.; Long, J.; Liu, J.; Luo, H.; Duan, H.; Zhang, Y.; Li, J.; Qi, X.; Chu, L. A novel porous polyimide membrane with ultrahigh chemical stability for application in vanadium redox flow battery. Chem. Eng. J. 2022, 428, 131203. [Google Scholar] [CrossRef]
- Zhuo, L.; Cai, Y.; Shen, D.; Gou, P.; Wang, M.; Hu, G.; Xie, F. Anti-oxidation polyimide-based hybrid foams assembled with bilayer coatings for efficient electromagnetic interference shielding. Chem. Eng. J. 2023, 451, 138808. [Google Scholar] [CrossRef]
- Doan, H.N.; Tagami, S.; Vo, P.P.; Negoro, M.; Sakai, W.; Tsutsumi, N.; Kanamori, K.; Kinashi, K. Scalable fabrication of cross-linked porous centrifugally spun polyimide fibers for thermal insulation application. Eur. Polym. J. 2022, 169, 111123. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Wang, Q. Controllable porous fluorinated polyimide thin films for ultralow dielectric constant interlayer dielectric applications. J. Macromol. Sci. Part A 2017, 54, 311–315. [Google Scholar] [CrossRef]
- Li, K.; Yang, L.; He, L.; Du, J.; Li, X. Low dielectric polyimide microsphere/polyimide composite films based on porous polyimide microsphere. Polym. Eng. Sci. 2024, 64, 5166–5175. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Qin, Y.; Dong, P.; Yao, W.; Matz, J.; Ajayan, P.M.; Shen, J.; Ye, M. Super-elasticity at 4 K of covalently crosslinked polyimide aerogels with negative poisson’s ratio. Nat. Commun. 2021, 12, 4092. [Google Scholar] [CrossRef]
- Qiao, S.; Yan, J.; Wang, Z.; Wang, Y.; Yu, J.; Hu, Z. Tough and lightweight polyimide/cellulose nanofiber aerogels with hierarchical porous structures as an efficient air purifier. Sep. Purif. Technol. 2023, 325, 124668. [Google Scholar] [CrossRef]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.-S.; Xia, H.; Gong, S. Poly(vinyl alcohol)/cellulose nanofibril hybrid aerogels with an aligned microtubular porous structure and their composites with polydimethylsiloxane. ACS Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef]
- Wan, J.; Song, J.; Yang, Z.; Kirsch, D.; Jia, C.; Xu, R.; Dai, J.; Zhu, M.; Xu, L.; Chen, C.; et al. Highly anisotropic conductors. Adv. Mater. 2017, 29, 1703331. [Google Scholar] [CrossRef]
- Zeng, Z.; Jin, H.; Chen, M.; Li, W.; Zhou, L.; Zhang, Z. Lightweight and anisotropic porous MWCNT/WPU composites for ultrahigh performance electromagnetic interference shielding. Adv. Funct. Mater. 2016, 26, 303–310. [Google Scholar] [CrossRef]
- Chen, S.; Wu, P.; Yu, Z.; Zou, H.; Liu, P. Fabrication and properties of anisotropic polyimide aerogels with aligned tube-like pore structure. J. Appl. Polym. Sci. 2020, 137, 48769. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Ji, J.; Chang, X.; Xu, Q.; Liu, X.; Qin, J. A facile method to fabricate the polyimide aerogels with controllable microstructure by freeze-drying. Mater. Lett. 2020, 267, 127558. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Q.; Zhu, J.; Li, J.; Cai, Z.; Chen, L.; Gong, S. Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv. 2016, 6, 96518–96526. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Yang, N.; Zhou, L.-C.; Ma, Z.-Y.; Zhu, Y.-B.; Lu, Y.-Y.; Qin, B.; Xing, W.-Y.; Ma, T.; Li, S.-C.; et al. Bioinspired polymeric woods. Sci. Adv. 2018, 4, eaat7223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lv, J.-b.; Tan, W.; He, X.; Chen, M.-h.; Zeng, K.; Hu, J.-h.; Yang, G. Ultrasound-assisted freeze-drying process for polyimide aerogels. Chem. Eng. J. 2022, 450, 138344. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, D.; Tong, F.; Lu, X.; Lu, Q. Low dielectric constant polyimide hybrid films prepared by in situ blow-balloon method. ACS Appl. Polym. Mater. 2019, 1, 2189–2196. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Kim, S.-Y.; Lee, S.; Kim, Y.; Lee, J.; Kim, J.; Jung, Y.C.; Kwon, J.; Han, H. Fabrication of highly flexible electromagnetic interference shielding polyimide carbon black composite using hot-pressing method. Compos. Part B Eng. 2021, 221, 109010. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Y.; Xie, J.; Zhang, W.; Wu, Z.; He, Z. Sandwich-type porous polyimide film with improved dielectric, water resistance and mechanical properties. J. Mater. Sci. 2019, 54, 5952–5960. [Google Scholar] [CrossRef]
- Carter, K.R.; DiPietro, R.A.; Sanchez, M.I.; Swanson, S.A. Nanoporous polyimides derived from highly fluorinated polyimide/poly(propylene oxide) copolymers. Chem. Mater. 2001, 13, 213–221. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Yan, S.; Shu, X.; Yuan, Y.; Huang, H.; Zhao, J. Overall improvement in dielectric and mechanical properties of porous graphene fluoroxide/polyimide nanocomposite films via bubble-stretching approach. Mater. Des. 2017, 117, 150–156. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Carter, K.R.; Richter, R.; Miller, R.D.; Russell, T.P.; Flores, V.; Meccereyes, D.; Dubois, P.; Jérôme, R. Polyimide nanofoams from aliphatic polyester-based copolymers. Chem. Mater. 1998, 10, 39–49. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Sun, G.; Zhou, S.; Liu, J.; Han, S. Effects of structural design including cellular structure precision controlling and sharp holes introducing on sound absorption behavior of polyimide foam. Polym. Test. 2020, 84, 106393. [Google Scholar] [CrossRef]
- He, P.; Li, J.; Zhang, B.; Huang, L.; Zhong, Y.; Li, S.; Fan, W.; Liu, F. Mechanically robust porous polyimide films for piezoelectric sensing at extreme condition. Compos. Sci. Technol. 2025, 261, 111006. [Google Scholar] [CrossRef]
- Nguyen, T.; Wang, X. Multifunctional composite membrane based on a highly porous polyimide matrix for direct methanol fuel cells. J. Power Sources 2010, 195, 1024–1030. [Google Scholar] [CrossRef]
- Woo, K.T.; Lee, J.; Dong, G.; Kim, J.S.; Do, Y.S.; Hung, W.-S.; Lee, K.-R.; Barbieri, G.; Drioli, E.; Lee, Y.M. Fabrication of thermally rearranged (TR) polybenzoxazole hollow fiber membranes with superior CO2/N2 separation performance. J. Membr. Sci. 2015, 490, 129–138. [Google Scholar] [CrossRef]
- Wen, G.; Cai, W.; Lv, S.; Hu, T.; Yang, J.; Ji, Z.; Li, H.; Wang, S.; Zhao, Z.; Fu, X.; et al. A microfluid-on-microfluid phase separation strategy fabricates high-throughout porous polyimide separator for lithium-ion batteries. Polymer 2024, 303, 127102. [Google Scholar] [CrossRef]
- Cai, R.; You, Y.; Wu, P.; Liu, Q.; Zhu, Y.; Zhang, S. Preparation of open-cell rigid polyimide foam via nonaqueous high internal phase emulsion-templating technique. ACS Appl. Polym. Mater. 2023, 5, 7795–7804. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Dastageer, M.A. Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Interface Sci. 2021, 297, 102525. [Google Scholar] [CrossRef]
- Farrell, E.; Jana, S.C. Surfactant-free oil-in-oil emulsion-templating of polyimide aerogel foams. Int. Polym. Process. 2022, 37, 427–441. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Cai, R.; Zhu, Y.; Zhang, S. Flexible polyimide foam fabricated by emulsion-templating technique toward sound absorption and heat insulation. J. Polym. Sci. 2025, 63, 2880–2890. [Google Scholar] [CrossRef]
- Teo, N.; Gu, Z.; Jana, S.C. Polyimide-based aerogel foams, via emulsion-templating. Polymer 2018, 157, 95–102. [Google Scholar] [CrossRef]
- Song, I.-H.; Kim, D.-M.; Choi, J.-Y.; Jin, S.-W.; Nam, K.-N.; Park, H.-J.; Chung, C.-M. Polyimide-based polyHIPEs prepared via pickering high internal phase emulsions. Polymers 2019, 11, 1499. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.; Zou, H.; Liu, P. Preparation of porous polyimide/in-situ reduced graphene oxide composite films for electromagnetic interference shielding. Polym. Adv. Technol. 2017, 28, 233–242. [Google Scholar] [CrossRef]
- Yu, Z.; Qu, X.; Zhou, M.; Mia, M.H.; Hou, L.; Wang, Y.; Xu, J.; Huang, L.; Zhang, L.; Zeng, Q.; et al. Integrated core-shell structured polyimide thermoelectric fiber with latent light-driven damage detection and self-driven temperature sensing performance. Chem. Eng. J. 2025, 515, 163622. [Google Scholar] [CrossRef]
- Pei, F.; Jia, H.; Xu, S.; Zhang, M.; Qu, Y. Preparation of superhydrophilic polyimide fibrous membranes by electrostatic spinning fabrication for the efficient separation of oil-in-water emulsions. Sep. Purif. Technol. 2023, 322, 124342. [Google Scholar] [CrossRef]
- Song, C.; Rutledge, G.C. Electrospun polyimide fiber membranes for separation of oil-in-water emulsions. Sep. Purif. Technol. 2021, 270, 118825. [Google Scholar] [CrossRef]
- Ji, J.; Deng, C.; Liu, X.; Qin, J. Fabrication of porous polyimide hollow microspheres through O/W/O multiple emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124537. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Kim, D.-M.; Song, I.-H.; Choi, J.-Y.; Jin, S.-W.; Kim, B.-J.; Jeong, J.-W.; Jang, C.-E.; Chu, K.; Chung, C.-M. An oligoimide particle as a pickering emulsion stabilizer. Polymers 2018, 10, 1071. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Hwang, J.; Choi, J.H.; So, Y.; Park, S.; Ko, M.J.; Won, J.C.; Suk, J.; Wu, M.; et al. Highly macroporous polyimide with chemical versatility prepared from poly(amic acid) salt-stabilized high internal phase emulsion template. ACS Omega 2024, 9, 15222–15231. [Google Scholar] [CrossRef]
- Choi, J.; Yang, K.; Bae, H.-S.; Phiri, I.; Ahn, H.J.; Won, J.C.; Lee, Y.M.; Kim, Y.H.; Ryou, M.-H. Highly stable porous polyimide sponge as a separator for lithium-metal secondary batteries. Nanomaterials 2020, 10, 1976. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Dai, J.; Wang, S.; Zhao, X.; Wang, D.; Chen, C. Synthesis and gas separation properties of aromatic polyimides containing noncoplanar rigid sites. ACS Appl. Polym. Mater. 2022, 4, 6265–6275. [Google Scholar] [CrossRef]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in aromatic polyimide films for electronic applications: Preparation, structure and properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Tan, W.; Liang, B.; Chen, M.; Xiao, H.; He, X.; Yang, W.; Hu, J.; Zeng, K.; Yang, G. Rigid-flexible mediated Co-polyimide enabling stable silicon anode in lithium-ion batteries. Chem. Eng. J. 2024, 496, 153822. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Lai, X.; Lv, X.; Li, J.; Qiu, S.; Zhang, G.; Sun, R. A comprehensive study on the effect of molecular chain flexibility on the low-temperature curing ability of polyimides. J. Mater. Chem. C 2023, 12, 177–186. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, N.; Shen, K.; Song, N.; Shi, K.; Zhu, S.; Zhang, Y.; Guan, S. From a flexible hyperbranched polyimide to a microporous polyimide network: Microporous architecture and carbon dioxide adsorption. Polymer 2017, 115, 176–183. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wang, Y.; Zhang, C.; Teng, C. Methods and strategies to decrease the dielectric properties of polyimide films: A review. J. Sol-Gel Sci. Technol. 2023, 108, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Sun, G.; Zhou, Y.; Liu, G.; Wang, J.; Han, S. Progress in low dielectric polyimide film—A review. Prog. Org. Coat. 2022, 172, 107103. [Google Scholar] [CrossRef]
- Peng, W.-F.; Lei, H.-Y.; Zhang, X.-X.; Qiu, L.-H.; Huang, M.-J. Fluorine Substitution Effect on the Material Properties in Transparent Aromatic Polyimides. Chin. J. Polym. Sci. 2022, 40, 781–788. [Google Scholar] [CrossRef]
- Yang, S.Y.; Ge, Z.Y.; Yin, D.X.; Liu, J.G.; Li, Y.F.; Fan, L. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3-trifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4143–4152. [Google Scholar] [CrossRef]
- Qian, Z.G.; Ge, Z.Y.; Li, Z.X.; He, M.H.; Liu, J.G.; Pang, Z.Z.; Fan, L.; Yang, S.Y. Synthesis and characterization of new inherent photoimageable polyimides based on fluorinated tetramethyl-substituted diphenylmethanediamines. Polymer 2002, 43, 6057–6063. [Google Scholar] [CrossRef]
- Tao, L.; Yang, H.; Liu, J.; Fan, L.; Yang, S. Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer 2009, 50, 6009–6018. [Google Scholar] [CrossRef]
- Park, S.-J.; Cho, K.-S.; Kim, S.-H. A study on dielectric characteristics of fluorinated polyimide thin film. J. Colloid Interface Sci. 2004, 272, 384–390. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, H.-S.; Jin, F.-L. Influence of fluorination on surface and dielectric characteristics of polyimide thin film. J. Colloid Interface Sci. 2005, 282, 238–240. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Zhao, J. Simultaneously improving the optical, dielectric, and solubility properties of fluorene-based polyimide with silyl ether side groups. ACS Omega 2022, 7, 11939–11945. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, R.; Wang, C.; Chang, H.; Chen, S.; Wang, L.; Cui, X.; Liu, Y.; Li, J.; Yu, G.; et al. Preparation and properties of low-dielectric polyimide films containing tert-butyl. Polymers 2024, 16, 984. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, K.-S.; Choi, H.-D.; Choi, S.-H.; Kwon, S.-J.; Kim, I.-D.; Lim, J.; Hong, J.-M. Porous polyimide membranes prepared by wet phase inversion for use in low dielectric applications. Int. J. Mol. Sci. 2013, 14, 8698–8707. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Wang, T.; Huang, C.; Ji, F.; Shan, L.; Zhang, G.; Sun, R.; Wong, C.p. Fluorinated graphene/polyimide nanocomposites for advanced electronic packaging applications. J. Appl. Polym. Sci. 2021, 138, 49801. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, S.; Liu, N.; Wei, K.; Li, L.; Zheng, S. Organic–inorganic polyimide nanocomposites containing a tetrafunctional polyhedral oligomeric silsesquioxane amine: Synthesis, morphology and thermomechanical properties. Polym. Int. 2018, 67, 301–312. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, P.; Wang, Y.; Zhang, F.; Li, F.; Tu, G. Soluble sulfoxide biphenyl polyimide film with transmittance exceeding 90. Polymer 2022, 254, 125050. [Google Scholar] [CrossRef]
- Song, G.; Chen, C.; Wang, X.; Yao, J. Synthesis and properties of polyimides derived from 2,2′-dichloro-4,4′,5,5′-biphenyltetracarboxylic dianhydride. Polymer 2019, 183, 121862. [Google Scholar] [CrossRef]
- Luo, J.-R.; Liu, Y.-D.; Liu, H.; Chen, W.-P.; Cui, T.-T.; Xiao, L.; Min, Y. Synthesis and characterization of polyimides with naphthalene ring structure introduced in the main chain. Materials 2022, 15, 8014. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Liu, C.; Sun, M.; Zhang, X.; Li, J.; Xue, G. Effect on the thermal resistance and thermal decomposition properties of thermally cross-linkable polyimide films obtained from a reactive acetylene. React. Funct. Polym. 2021, 167, 104994. [Google Scholar] [CrossRef]
- Heming, L.; Panpeng, W.; Yongqi, W.; Qiushi, Z.; Xinming, W.; Weiguo, G.; Lin, T.; Ke, M.; Zhizhi, H.; Wei, C. High-frequency 5G substrate: Low dielectric biphenyl polyimide with low CTE and high thermal stability. Mater. Today Adv. 2024, 23, 100514. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, B.; Huang, L.; Chen, K.; Fan, W.; Liang, J. Fluorine polyimide nanofibrous composites with outstanding piezoelectric property for human motion monitoring. Chem. Eng. J. 2024, 502, 157320. [Google Scholar] [CrossRef]
- Guo, K.; Zhan, J.; Qi, S.; Tian, G.; Wu, D. Preparation and characterization of fluorine-containing benzimidazole polyimide films with a micro-branched crosslink structure. Eur. Polym. J. 2024, 205, 112723. [Google Scholar] [CrossRef]
- Hu, A.J.; Hao, J.Y.; He, T.; Yang, S.Y. Synthesis and characterization of high-temperature fluorine-containing PMR polyimides. Macromolecules 1999, 32, 8046–8051. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Li, X.; Dong, J.; Xu, Q.; Xu, W.; Zhang, Q. Fabrication of shape memory polyimides with exploration of their activation mechanisms. Chem. Eng. J. 2023, 477, 146776. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. Microporous polyimides with functional groups for the adsorption of carbon dioxide and organic vapors. J. Mater. Chem. A 2016, 4, 11453–11461. [Google Scholar] [CrossRef]
- Da Conceição, T.F.; Felisberti, M.I. The influence of rigid and flexible monomers on the physical-chemical properties of polyimides. J. Appl. Polym. Sci. 2014, 131, 40351. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Watson, K.A.; Smith, J.G.; Connell, J.W.; Yokota, R. Polyimides from 2,3,3′,4′-biphenyltetracarboxylic dianhydride and aromatic diamines. Polymer 2002, 43, 5077–5093. [Google Scholar] [CrossRef]
- Qin, Z.; Lv, S.; Wu, Y.; Wang, C.; Zhang, S.; Ban, S.; Yin, D. Synthesis and properties of soluble polyimides containing tert-butyl, ether linkages, and triphenylmethane units. High Perform. Polym. 2020, 32, 924–932. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, J.; Li, X.; Jian, J.; Jiang, L.; Yin, C.; Xi, Y.; Huang, K.; Su, L.; Zhou, L. High comprehensive properties of colorless transparent polyimide films derived from fluorine-containing and ether-containing dianhydride. RSC Adv. 2024, 14, 32613–32623. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Fan, D.; Jin, M.; Wang, J.; Liu, J.; Li, Q. Enhanced heat dissipation in graphite-silver-polyimide structure for electronic cooling. Appl. Therm. Eng. 2020, 168, 114676. [Google Scholar] [CrossRef]
- Xing, S.; Pan, Z.; Wu, X.; Chen, H.; Lv, X.; Li, P.; Liu, J.; Zhai, J. Enhancement of thermal stability and energy storage capability of flexible Ag nanodot/polyimide nanocomposite films via in situ synthesis. J. Mater. Chem. C 2020, 8, 12607–12614. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Liu, F. High-performance polyimide nanocomposites with polydopamine-coated copper nanoparticles and nanowires for electronic applications. Mater. Lett. 2019, 237, 19–21. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; Ruan, K.; Kong, J.; Dong, M.; Zhang, J.; Gu, J.; Guo, Z. Reduced graphene oxide heterostructured silver nanoparticles significantly enhanced thermal conductivities in hot-pressed electrospun polyimide nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 25465–25473. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally stable silver nanowire-polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Zhu, Y.; Hui, D.; Qiu, Y. Mechanical, electrical and thermal properties of aligned carbon nanotube/polyimide composites. Compos. Part B Eng. 2014, 56, 408–412. [Google Scholar] [CrossRef]
- Li, C.X.; Kalfon-Cohen, E.; Lee, J.; Furtado, C.; Patel, P.; Kopp, R.; Hank, T.J.; Magato, J.; Kinsella, M.; Kessler, S.S.; et al. Interlaminar reinforcement of carbon fiber reinforced polyimide composites using vertically aligned carbon nanotubes. Compos. Part B Eng. 2025, 292, 112098. [Google Scholar] [CrossRef]
- Liu, C.; Song, J.; Zhao, G.; Yin, Y.; Ding, Q. Improving thermal conductivity and tribological performance of polyimide by filling Cu, CNT, and graphene. Micromachines 2023, 14, 616. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Ruan, K.; Zhang, H.; Gu, J. Highly thermally conductive carbon nanotubes pillared exfoliated graphite/polyimide composites. npj Flex. Electron. 2021, 5, 16. [Google Scholar] [CrossRef]
- Ma, X.; Peng, C.; Zhao, L.; Huang, A.; Wei, M.; Yuan, C.; Xu, Y.; Zeng, B.; Chen, G.; Luo, W.; et al. Preparation of highly thermally conductive, flexible and transparent AlOOH/polyimide composite film with high mechanical strength and low coefficient of thermal expansion. Compos. Part B Eng. 2024, 281, 111558. [Google Scholar] [CrossRef]
- Park, O.-K.; Owuor, P.S.; Jaques, Y.M.; Galvao, D.S.; Kim, N.H.; Lee, J.H.; Tiwary, C.S.; Ajayan, P.M. Hexagonal boron nitride-carbon nanotube hybrid network structure for enhanced thermal, mechanical and electrical properties of polyimide nanocomposites. Compos. Sci. Technol. 2020, 188, 107977. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Z.; Yu, J.; Dai, D.; Dai, W.; Du, S.; Li, C.; Jiang, N.; Zhan, Z.; Lin, C.-T. Graphene woven fabric-reinforced polyimide films with enhanced and anisotropic thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2016, 87, 290–296. [Google Scholar] [CrossRef]
- Lan, H.; Wu, B.; Yan, Y.; Xia, R.; Qian, J. Enhanced in-plane thermal conductivity of polyimide-based composites via in situ interfacial modification of graphene. Nanoscale 2023, 15, 4114–4122. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H.; Cheng, K.; Qiu, H.; Yang, J. Modified graphene/polyimide composite films with strongly enhanced thermal conductivity. Nanoscale 2019, 11, 8219–8225. [Google Scholar] [CrossRef]
- Xu, J.-c.; Dai, S.-c.; Li, H.-l.; Yang, J.-h. Molecular dynamics simulation of the thermal conductivity of graphitized graphene/polyimide films. New Carbon Mater. 2018, 33, 213–220. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, Z.X.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Highly thermally conductive flexible copper clad laminates based on sea-island structured boron nitride/polyimide composites. Compos. Sci. Technol. 2022, 230, 109087. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Pan, D.; Yang, G.; Su, F.; Ji, Y.; Liu, C.; Shen, C. Constructing dual thermal conductive networks in electrospun polyimide membranes with highly thermally conductivity but electrical insulation properties. Adv. Compos. Hybrid Mater. 2021, 4, 1102–1112. [Google Scholar] [CrossRef]
- Ding, D.; Wang, H.; Wu, Z.; Chen, Y.; Zhang, Q. Highly thermally conductive polyimide composites via constructing 3D networks. Macromol. Rapid Commun. 2019, 40, 1800805. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, H.; Ruan, K.; Zhang, Y.; Gu, J. Hierarchically multifunctional polyimide composite films with strongly enhanced thermal conductivity. Nano-Micro Lett. 2022, 14, 26. [Google Scholar] [CrossRef]
- Ou, X.; Chen, S.; Lu, X.; Lu, Q. Enhancement of thermal conductivity and dimensional stability of polyimide/boron nitride films through mechanochemistry. Compos. Commun. 2021, 23, 100549. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Su, B.; Shi, L.; Li, F.; Mu, J.; Che, H.; Zhang, Z.; Zhang, X.; et al. Preparation and performance study of ionic liquid @ boron nitride modified polyimide proton exchange membrane. Polym. Compos. 2023, 44, 5527–5538. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon dot biopolymer-based flexible functional films for antioxidant and food monitoring applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Althawab, S.A.; Alzahrani, A.; Alohali, B.M.; Alsulami, T. Flexible N-doped MXene quantum dot–biopolymer films with antibacterial and antioxidant functions for active food packaging. Colloids Surf. A Physicochem. Eng. Asp. 2025, 722, 137291. [Google Scholar] [CrossRef]
- Mani, D.; Qurratulain, R.; Anand, S.; Kim, S.-R. Recent advances in structural designs and fabrication of flexible polymer composite films with high thermal conductivity and electromagnetic interference shielding performance. J. Mater. Chem. C 2025, 13, 889–8933. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Ding, Y.; Zhao, S.; Ma, Z.; Zhang, H.; He, X. Multifunctional carbon fiber@NiCo/polyimide films with outstanding electromagnetic interference shielding performance. Chem. Eng. J. 2022, 427, 131937. [Google Scholar] [CrossRef]
- Weng, L.; Wang, T.; Ju, P.-H.; Liu, L.-Z. Preparation and properties of polyimide/Fe3O4 composite foams. Pigm. Resin Technol. 2018, 47, 173–179. [Google Scholar] [CrossRef]
- Zou, K.; Li, N.; Pan, X.; Shi, J.; Pu, J.; Yan, D.-X.; Wang, H. Flexible Ag/BaFe12O19 microfiber/polyimide composite film with high electromagnetic interference shielding and low reflection. Sci. China Mater. 2023, 66, 4825–4833. [Google Scholar] [CrossRef]
- Zou, K.; Sun, H.; Li, X.; Yi, S.; Li, J.; Zhou, Z.; Wang, H.; Yan, D.-X. Extreme environment-bearable polyimide film with a three-dimensional Ag microfiber conductive network for ultrahigh electromagnetic interference shielding. Sci. China Mater. 2023, 66, 1578–1586. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Zhan, M. A comparative study of structure and electromagnetic interference shielding performance for silver nanostructure hybrid polyimide foams. RSC Adv. 2015, 5, 65283–65296. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Han, M.; Zhang, Y.; Yu, J. Vertical graphene nanosheet/polyimide composite films for electromagnetic interference shielding. ACS Appl. Nano Mater. 2021, 4, 7461–7470. [Google Scholar] [CrossRef]
- Li, X.; Xu, T.; Cao, W.; Wang, M.; Chen, F.; Jin, L.; Song, N.; Sun, S.; Ding, P. Graphene/carbon fiber network constructed by co-carbonization strategy for functional integrated polyimide composites with enhanced electromagnetic shielding and thermal conductive properties. Chem. Eng. J. 2023, 464, 142595. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, H.; Li, J.; Yu, H.; Tang, Z.; Tian, G.; Wang, Q.; Wang, T. Design of polyimide/carbon nanotube@Ag@polyimide/graphene composite aerogel for infrared stealth and electromagnetic interference protection. Compos. Part A Appl. Sci. Manuf. 2024, 186, 108371. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Nie, Z.; Feng, Y.; Qi, S.; Wang, R. Flexible multilayer MXene/polyimide composite film with excellent electromagnetic interference shielding and photothermal conversion performance. J. Alloys Compd. 2024, 990, 174399. [Google Scholar] [CrossRef]
- Tan, B.; Guo, D.; Tao, Z.; Chen, Z.; Lv, Z.; Wu, G.; Lin, Y. Ultrathin and flexible polyimide/Ti3C2TX MXene composite films for electromagnetic interference shielding with harsh environment tolerance. J. Mater. Chem. C 2024, 12, 276–285. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Qin, Y.; Fang, Y.; Liu, G.; Wang, Z.; Matz, J.; Dong, P.; Shen, J.; Ye, M. Hierarchically porous polyimide/Ti3C2Tx film with stable electromagnetic interference shielding after resisting harsh conditions. Sci. Adv. 2021, 7, 1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, Q.; Liu, Y.; Wang, C.; Wang, Q.; Wei, H.; Liu, L.; Li, J.; Dong, H. Rational design and fabrication of lightweight porous polyimide composites containing polyaniline modified graphene oxide and multiwalled carbon nanotube hybrid fillers for heat-resistant electromagnetic interference shielding. Polymer 2021, 224, 123742. [Google Scholar] [CrossRef]
- Yang, F.; Yao, J.; Jin, L.; Huyan, W.; Zhou, J.; Yao, Z.; Liu, P.; Tao, X. Multifunctional Ti3C2TX MXene/aramid nanofiber/Polyimide aerogels with efficient thermal insulation and tunable electromagnetic wave absorption performance under thermal environment. Compos. Part B Eng. 2022, 243, 110161. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Peng, Q.; Zheng, H.; Xue, F.; Li, P.; Xu, Z.; He, X. Flame-retardant MXene/polyimide film with outstanding thermal and mechanical properties based on the secondary orientation strategy. Nanoscale Adv 2021, 3, 5683–5693. [Google Scholar] [CrossRef]
- He, X.; Wang, Y. Highly thermally conductive polyimide composite films with excellent thermal and electrical insulating properties. Ind. Eng. Chem. Res 2020, 59, 1925–1933. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ding, X.; Li, Y.; Zhang, P.; Shu, M.; Zhang, Q.; Gong, Y.; Zheng, K.; Wu, B.; et al. Enhanced thermal conductivity of carbon nitride-doped graphene/polyimide composite film via a “deciduous-like” strategy. Compos. Sci. Technol. 2021, 205, 108693. [Google Scholar] [CrossRef]
- Jose, M.C.; Radha, S.; Sreeja, B.S.; Gulam Nabi Alsath, M.; Kumar, P. Compact dual-band millimeter-wave antenna for 5G WLAN. Int. J. Microw. Wireless Technol. 2022, 14, 981–988. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Lee, C.-Y.; Hung, W.-J.; Chen, G.-S.; Fang, J.-S. Comparison of various low dielectric constant materials. Thin Solid Films 2018, 660, 871–878. [Google Scholar] [CrossRef]
- Wu, G.; Qi, S.; Tian, G.; Wu, D. Preparation and characterization of low CTE thermoplastic copolyimide resins based on the structural design of block sequence. Eur. Polym. J. 2023, 195, 112249. [Google Scholar] [CrossRef]
- Khim, S.; Hwang, Y.C.; Choi, J.; Park, H.; Nam, K.-H. Temperature-invariant large broadband polyimide dielectrics with multimodal porous networks. ACS Appl. Polym. Mater. 2023, 5, 4159–4169. [Google Scholar] [CrossRef]
- Fan, H.; Xie, T.; Wang, C.; Zhang, Y.; Pan, S.; Li, J.; Zhang, Y.; Guan, S.; Yao, H. Low-dielectric polyimide constructed by integrated strategy containing main-chain and crosslinking network engineering. Polymer 2023, 279, 126035. [Google Scholar] [CrossRef]
- Tan, X.; Liu, T.-H.; Zhou, W.; Yuan, Q.; Ying, J.; Yan, Q.; Lv, L.; Chen, L.; Wang, X.; Du, S.; et al. Enhanced electromagnetic shielding and thermal conductive properties of polyolefin composites with a Ti3C2Tx MXene/graphene framework connected by a hydrogen-bonded interface. ACS Nano 2022, 16, 9254–9266. [Google Scholar] [CrossRef]
- Tan, M.; Chen, D.; Cheng, Y.; Sun, H.; Chen, G.; Dong, S.; Zhao, G.; Sun, B.; Wu, S.; Zhang, W.; et al. Anisotropically oriented carbon films with dual-function of efficient heat dissipation and excellent electromagnetic interference shielding performances. Adv. Funct. Mater. 2022, 32, 2202057. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Wang, X.-Y.; Li, X.-M.; Liao, S.-Y.; Lin, Z.-Q.; Hu, Y.-G.; Zhao, T.; Zeng, X.-L.; Li, C.-H.; Yu, S.-H.; et al. Ultrathin densified carbon nanotube film with “metal-like” conductivity, superior mechanical strength, and ultrahigh electromagnetic interference shielding effectiveness. ACS Nano 2020, 14, 14134–14145. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, Z.; Wang, Z.; Liu, L.; Zhu, M.; Lei, W.; Song, P. Scalable, robust, low-cost, and highly thermally conductive anisotropic nanocomposite films for safe and efficient thermal management. Adv. Funct. Mater. 2022, 32, 2110782. [Google Scholar] [CrossRef]
- Gong, S.; Sheng, X.; Li, X.; Sheng, M.; Wu, H.; Lu, X.; Qu, J. A multifunctional flexible composite film with excellent multi-source driven thermal management, electromagnetic interference shielding, and fire safety performance, inspired by a “brick–mortar” sandwich structure. Adv. Funct. Mater. 2022, 32, 2200570. [Google Scholar] [CrossRef]
- Wu, T.; Liang, L.; Bai, Y.; Mei, X.; Jiao, J.; Ma, Y.; Wang, G.; Zhang, S. Fabrication of corrosion-resistant and heat-resistant multi-walled carbon nanotubes/poly ether ether ketone/thermoplastic polyimide electromagnetic shielding foams based on double percolation structure. Carbon 2023, 215, 118423. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhan, M.-S.; Wang, K. Preparation and characterization of electromagnetic interference shielding polyimide foam. J. Appl. Polym. Sci. 2013, 127, 4129–4137. [Google Scholar] [CrossRef]
- Cheng, K.; Li, H.; Zhu, M.; Qiu, H.; Yang, J. In situ polymerization of graphene-polyaniline@polyimide composite films with high EMI shielding and electrical properties. RSC Adv. 2020, 10, 2368–2377. [Google Scholar] [CrossRef]
- Yu, Z.; Dai, T.; Yuan, S.; Zou, H.; Liu, P. Electromagnetic interference shielding performance of anisotropic polyimide/graphene composite aerogels. ACS Appl. Mater. Interfaces 2020, 12, 30990–31001. [Google Scholar] [CrossRef]
- Ma, S.; Jia, T.; Wang, C.; Xu, H.; Zhou, H.; Zhao, X.; Chen, C.; Wang, D.; Liu, C.; Qu, C. Anisotropic MWCNT/polyimide aerogels with multifunctional EMI shielding and strain sensing capabilities. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107208. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Wang, Z.; Ma, P.; Dong, W.; Hou, H.; Fan, W.; Liu, T. Mechanically strong and thermally insulating polyimide aerogels by homogeneity reinforcement of electrospun nanofibers. Compos. Part B Eng. 2020, 182, 107624. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, Z.; Chen, Y.; Tong, S.; Ge, M.; Zhao, N.; Xu, J. Superelastic and ultralight polyimide aerogels as thermal insulators and particulate air filters. J. Mater. Chem. A 2018, 6, 828–832. [Google Scholar] [CrossRef]
- Xue, T.; Zhu, C.; Feng, X.; Wali, Q.; Fan, W.; Liu, T. Polyimide aerogel fibers with controllable porous microstructure for super-thermal insulation under extreme environments. Adv. Fiber Mater. 2022, 4, 1118–1128. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Y.-P.; Ding, B.; Zhang, Z.-H.; Huang, Z.-Q. Thermal management and structural parameters optimization of MCM-BGA 3D package model. Int. J. Therm. Sci. 2020, 147, 106120. [Google Scholar] [CrossRef]
- Dong, X.; Wan, B.; Huang, L.; Zhao, Q.; Yao, R.; Gao, J.; Ding, C.; Wang, X.; Dang, Z.M.; Chen, G.; et al. Coordination of ultralow permittivity and higher thermal conductivity of polyimide induced by unique interfacial self-assembly behavior. Adv. Funct. Mater. 2025, 35, 2417843. [Google Scholar] [CrossRef]
- Song, Q.; Wang, M.; Lai, Y.; Wang, Q.; Yin, X.; Hou, L. Preparation and properties of branch-leaf-like polyimide composite films with high thermal conductivity and low dielectric loss. J. Appl. Polym. Sci. 2023, 140, e54520. [Google Scholar] [CrossRef]
- Shi, T.; Zheng, Z.; Liu, H.; Wu, D.; Wang, X. Flexible and foldable composite films based on polyimide/phosphorene hybrid aerogel and phase change material for infrared stealth and thermal camouflage. Compos. Sci. Technol. 2022, 217, 109127. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]









| Samples | DMF | DMAC | DMSO | NMP | Aecetone |
|---|---|---|---|---|---|
| FPI | + | + | + | + | + |
| CNFPI | ++ | ++ | ++ | ++ | - |
| CNOPI | ++ | ++ | ++ | ++ | - |
| CCNFPI | - - | - - | - - | - - | - - |
| CCNOPI | - - | - - | - - | - - | - - |
| Filler | Loading (wt%) | Theral Conductivity (W/m·K) | SE (dB/mm) | Ref. |
|---|---|---|---|---|
| Ag/rGO | 15% (Ag:rGO = 1:4) | 2.12 | [107] | |
| CuNPs-CuNWs | 10% | 4.13 | [106] | |
| AlOOH nanowires | 10% | 3.568 | [113] | |
| Ag/BaFe12O19 | 15–20% | 5 | 989 | [130] |
| CF@NiCo | 41% | 80.6 | [128] | |
| CNT/Ag | CNT: 0.1% Ag: 12.4% | 0.72 | 598 | [135] |
| Vertical graphene nanosheets | 20% | 2.1 | 207.7 | [133] |
| Graphene/carbon fiber | 20% | 1.65 | 36.5 | [134] |
| Ti3C2Tₓ | 60% | 2466.7 | [137] | |
| M@GNS | 15% | 16.10 | [116] | |
| h-BN | 30% | 2.56 | [119] | |
| Mxene | 40% | 5.12 | [141] | |
| GO/BN | GO: 1% BN: 20% | 11.203 | [142] | |
| rGO@CN | 10% | 6.08 | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Fu, X.; Chen, Z.; Zhai, Z.; Miu, H.; Tao, P. Multifunctional Polyimide for Packaging and Thermal Management of Electronics: Design, Synthesis, Molecular Structure, and Composite Engineering. Nanomaterials 2025, 15, 1148. https://doi.org/10.3390/nano15151148
Chen X, Fu X, Chen Z, Zhai Z, Miu H, Tao P. Multifunctional Polyimide for Packaging and Thermal Management of Electronics: Design, Synthesis, Molecular Structure, and Composite Engineering. Nanomaterials. 2025; 15(15):1148. https://doi.org/10.3390/nano15151148
Chicago/Turabian StyleChen, Xi, Xin Fu, Zhansheng Chen, Zaiteng Zhai, Hongkang Miu, and Peng Tao. 2025. "Multifunctional Polyimide for Packaging and Thermal Management of Electronics: Design, Synthesis, Molecular Structure, and Composite Engineering" Nanomaterials 15, no. 15: 1148. https://doi.org/10.3390/nano15151148
APA StyleChen, X., Fu, X., Chen, Z., Zhai, Z., Miu, H., & Tao, P. (2025). Multifunctional Polyimide for Packaging and Thermal Management of Electronics: Design, Synthesis, Molecular Structure, and Composite Engineering. Nanomaterials, 15(15), 1148. https://doi.org/10.3390/nano15151148








