Nanocarriers for Combination Therapy in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review
Abstract
1. Introduction
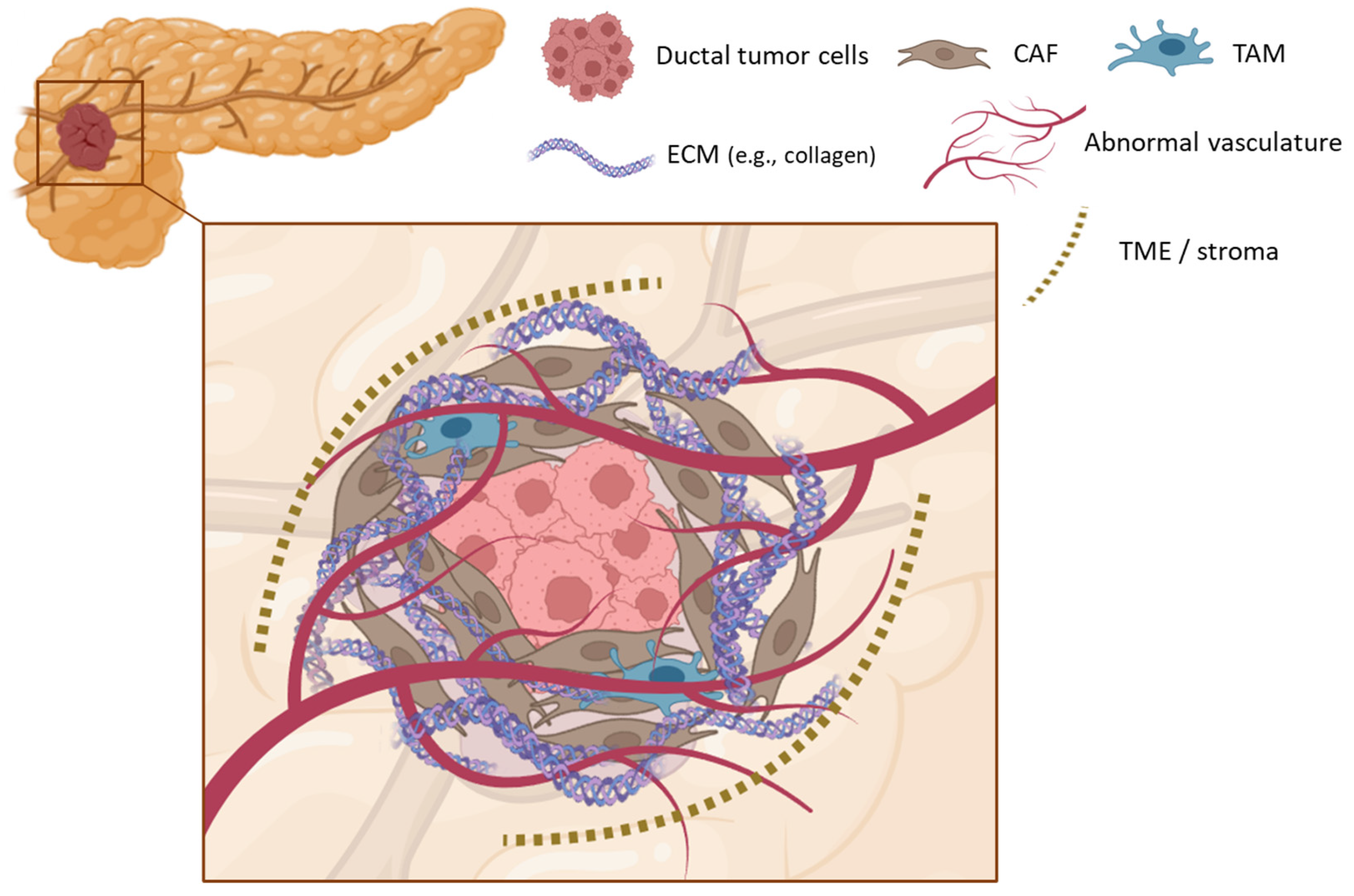
2. Pancreatic Ductal Adenocarcinoma
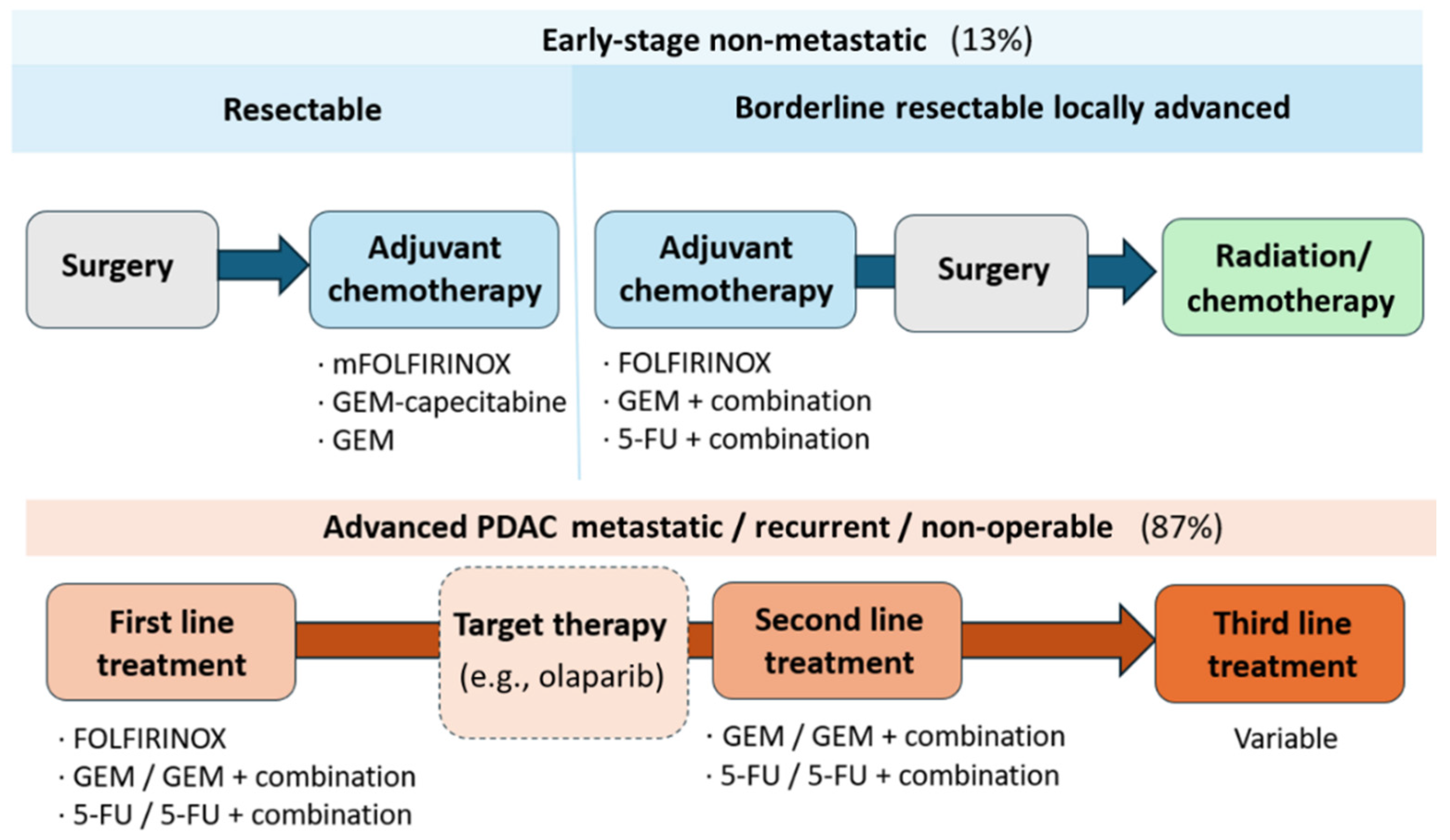
3. Conventional Treatments in PDAC
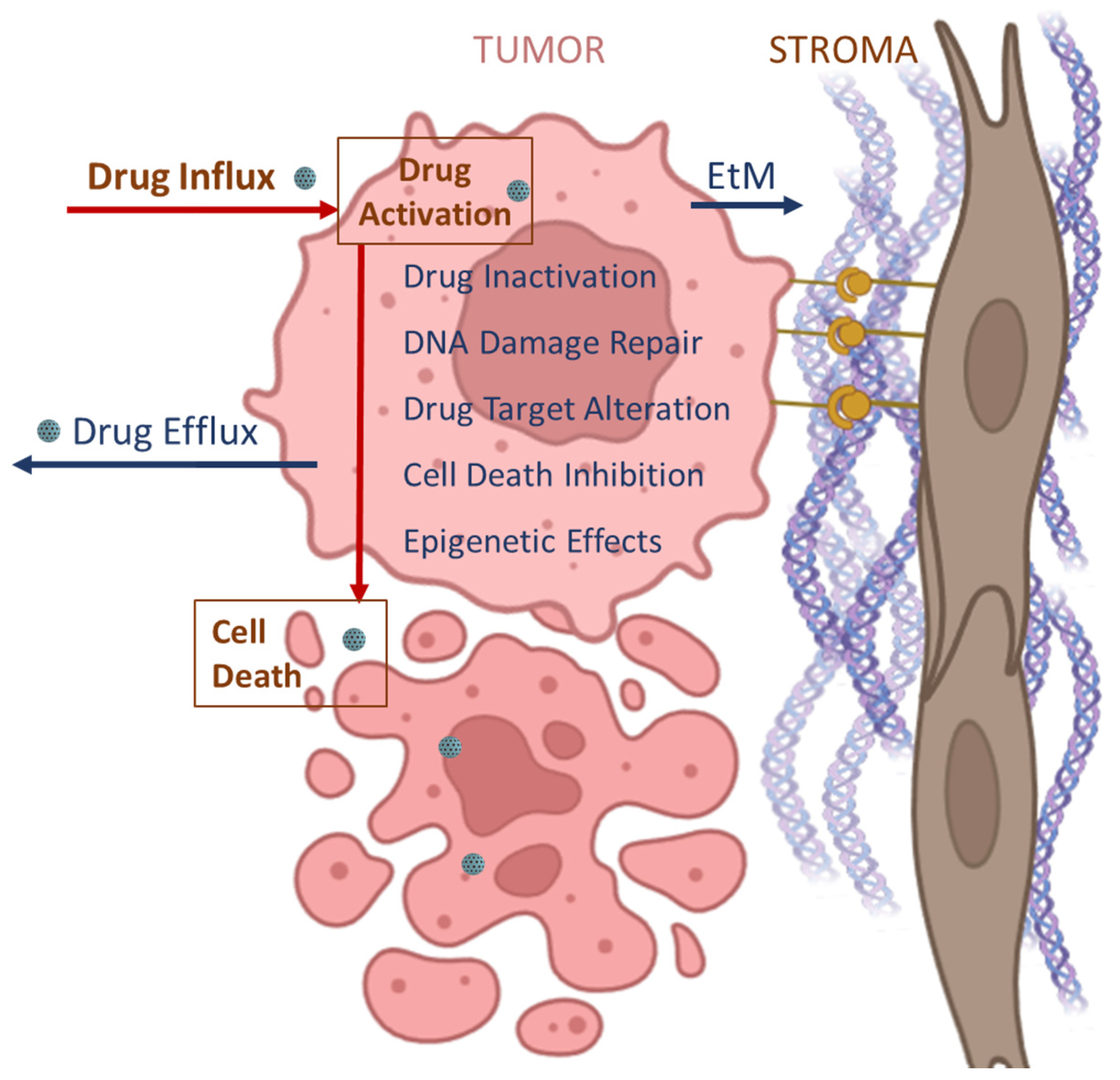
3.1. Limitations of Conventional Treatments
3.2. Evolution of Chemotherapy in PDAC Treatment
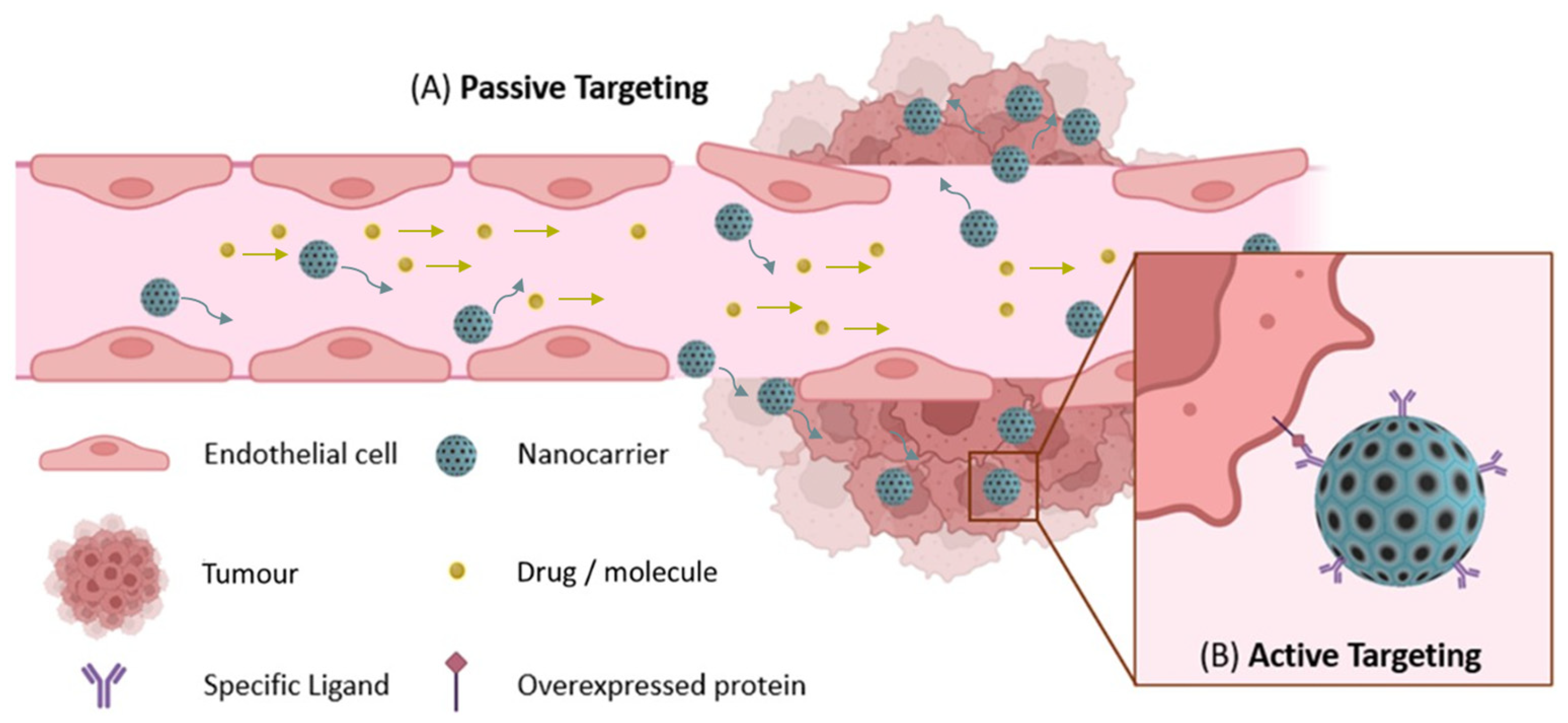
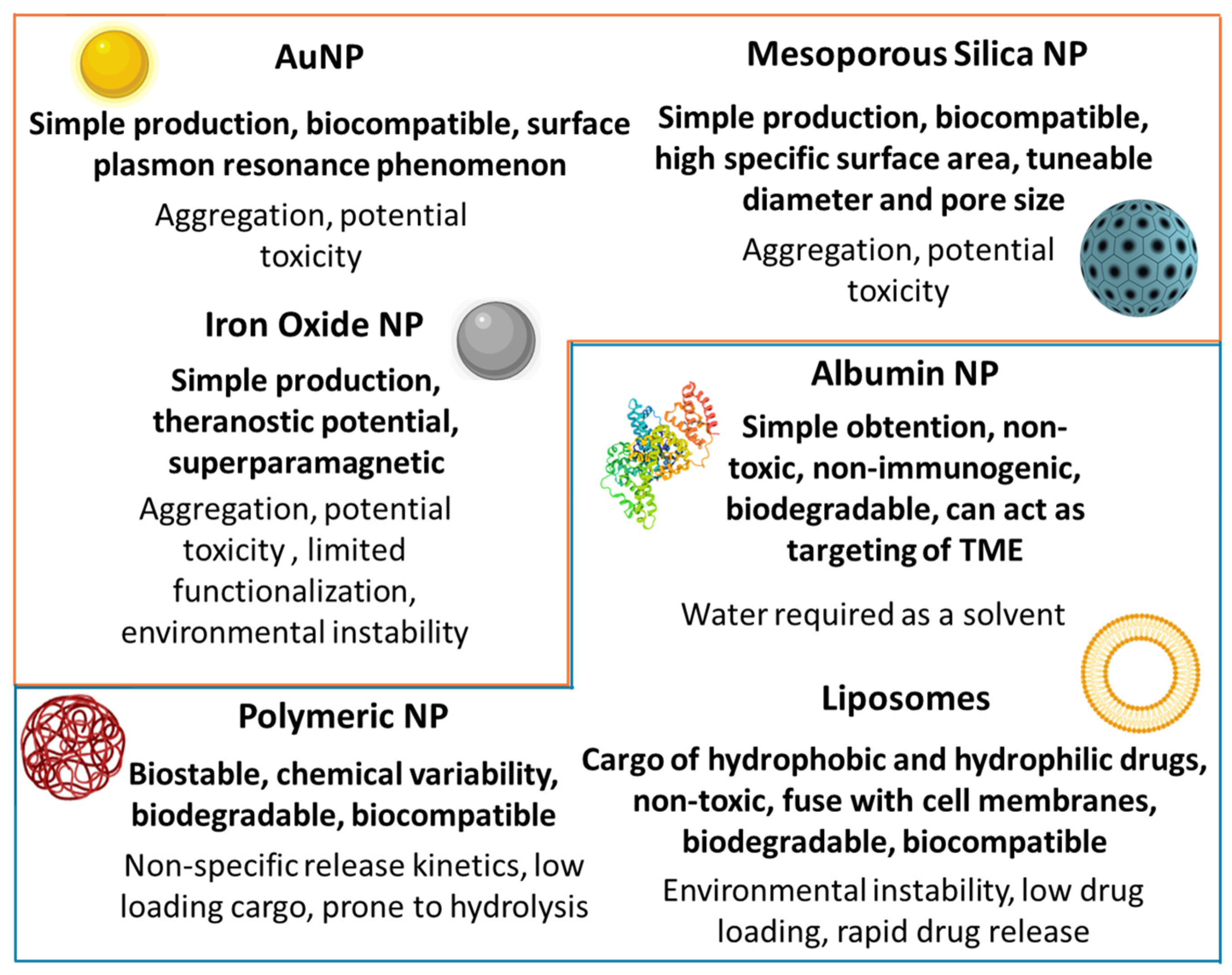
4. Nanomedicines as Emergent Treatment for Cancer
5. Emerging Nanosystems in PDAC Therapy
5.1. Monotherapy Nanocarriers for PDAC Treatment
| Drug | Formulation | Nanocarrier | Size (nm) | Characteristics | Ref. |
|---|---|---|---|---|---|
| PTX paclitaxel | nab-PTX | Albumin NP | 130 RS | TME Targeting | [83] |
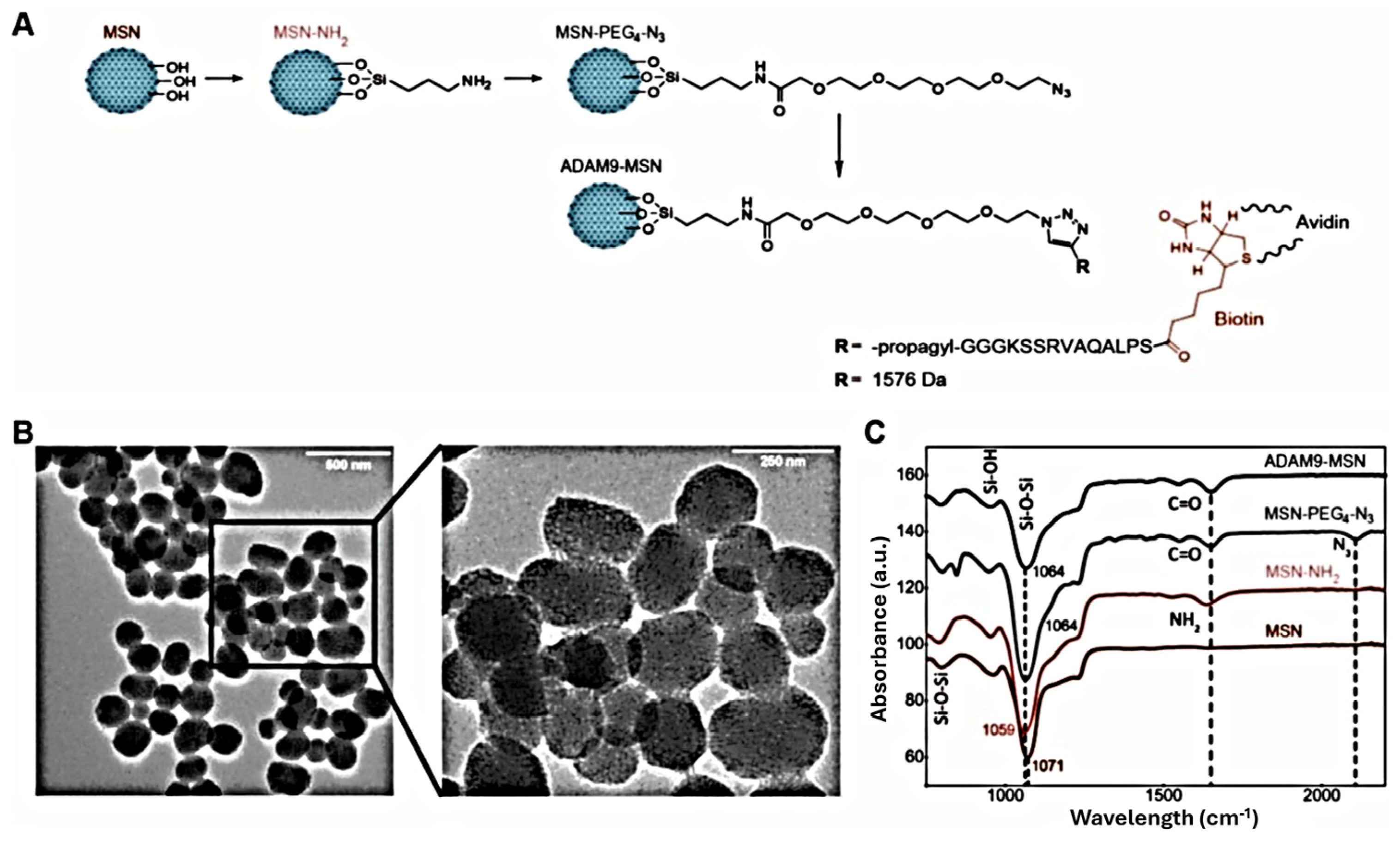
| PTX@MSN-responsive-ADAM9–biotyn–avidin | MSN | 189 HS | Avidin-capping Protease-responsive linker | [80] | |
| PTX@MSN-responsive-CAPN2–biotyn–avidin | MSN | 235 HS | Avidin-capping Protease-responsive linker | [81] | |
| PLGA-PTX | Polymeric NP | 160 HS | pH-sensitive polymeric coating | [79] | |
| PTX@MSN | MSN | 100 RS | - | [82] | |
| IRI irinotecan | nal-IRI/MM-398 | Liposomal NP | 111 RS | - | [84] |
| Liposome conjugated to MSN | Hybrid lipid MSN | 80 RS 100–150 HS | Lipid bilayer | [93] | |
| Liposome conjugated to MSN with Au core | Hybrid lipid MSN | 130 HS | Lipid bilayer | [94] | |
| GEM gemcitabine | RGD peptide-conjugated magnetic MSN | Magnetic modified MSN | 50 RS | Tumor-targeting | [89] |
| Liposome–exosome fusion | Liposome | <200 HS | Tumor-targeting Increased uptake | [87] | |
| Liposome PEGylation-ligand | Liposome | <100 RS | Tumor-targeting | [102] | |
| Flow Focusing® | Polymeric NP | 655 HS | High payload Narrow size distribution | [72] | |
| MSN | MSN | 42–64 RS | Pore-expanded | [99] | |
| HSA-GEM/IR780 | Albumin | <10 HS | Cleavable peptide cathepsin B Imaging with IR780 | [101] | |
| Curcumin | Curcumin@PEGylated MSN-Transferrin | MSN | 120 RS/167 HS | PEGylation Tumor-targeting | [108] |
| Liposomal curcumin | Liposome | - | pH-responsive | [106] | |
| NanoCurc™ | Polymeric NP | 50 RS | pH-responsive | [107] | |
| Methylene Blue | Gold-NP attached organically MSN | Au-MSN hybrid NP | 30/55/80 RS | PDT | [90] |
| CisPt cisplatin | Iron oxide NP covered silica shell -CisPt | Magnetic- MSN | 54 RS | pH-responsive | [91] |
| Liposome conjugated to MSN | Hybrid lipid MSN | 82 RS 137 HS | pH-responsive | [95] | |
| FdUMP 5-FU metabolite | Aptamer (CCK-B)-PEG-FdUMP-CPNs | Calcium phosphosilicate NPs (CPN) | <100 RS | Tumor-targeting pH-sensitive | [112] |
| CPT camptothecin | CPT@MSN–Folate modification | MSN | 100–150 RS | Tumor-targeting | [109] |
| Dendrimers–CPT Charge switchable | Polymeric NP | 26 RS | ROS-responsive linker Increased uptake | [111] | |
| aptamer/cell-penetrating peptide–camptothecin prodrug NPs | camptothecin prodrug NPs | 131 HS | Tumor-targeting | [113] | |
| DOX doxorubicin | DOX liposome Charge switchable | Liposome | 65 HS | GSH-responsive Increased uptake | [92] |
| Fluorinated amphiphilic dendrimer | Micelle | 10 RS | Self-assembly micelles | [114] | |
| Ce6 chlorin e6 | Polyphosphoester-based nanocarrier | Polymeric NP | 40 RS | pH-responsive evasion immune clearance | [115] |
| Benzoporphyrin derivative | Eutectic gallium–indium NPs, gallium oxide shell Conjugated to hyaluronic acid | Gallium-indium NPs | 25–65 RS | PDT | [116] |
| IRT80 | Encapsulated fluorocarbon chains and IRT80 | Hollow MSN | <200 RS | Oxygen delivery, SDT | [117] |
| Rose bengal | Sulfur hexafluoride PEG–biotin–avidin | Lipid microbubbles | 1–2 μm | Oxygen delivery, SDT | [118] |
5.2. Combination Therapy Nanocarriers for PDAC Treatment

| Drugs | Therapy | Nanocarrier | Composition | Size (nm) | Features | Ref. |
|---|---|---|---|---|---|---|
| GEM/PTX | Chemo | Polymeric NP | Tri-block co-polymer tumor-targeted peptide | 159 HS | pH-responsive tumor-targeted Inhibition GEM deactivation | [104] |
| Chemo | Hybrid lipid polymeric NPs | Lipid-bilayer PGLA | 70 HS | pH-sensitive Drug conjugate Inhibition GEM deactivation | [124] | |
| Chemo | Hybrid lipid MSNs | Lipid bilayer | 65 RS | pH-responsive | [96] | |
| GEM/CisPt | Chemo | Antibody (TAB004)-GEM-CisPt-MSN | PEI/PEG | 150–200 HS | Redox-responsive Tumor-targeting | [100] |
| GEM/iron oxide NPs | Chemo, Thermal | Polymeric NPs | PLGA HER-2 Antibody | 534 HS | Tumor-targeting | [73] |
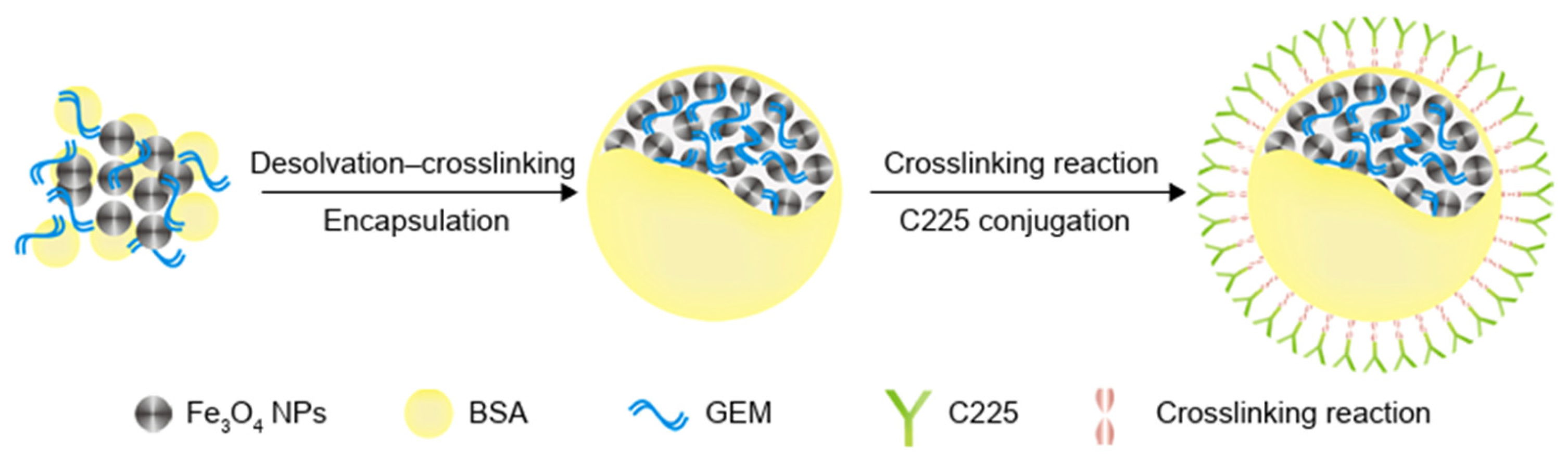
| GEM/Iron oxide NPs/Cetuximab | Thermal, Chemo, Targeted | Magnetic Albumin NP | Iron oxide NPs Cetuximab | 200 HS | Tumor-targeting Imaging | [125] |
| GEM/Au-NP | Chemo, PDT, Thermal | Gold-nanoshell-coated MSN | Transferrin Gold-nanoshell | 100–150 RS | Tumor-targeting | [120] |
| GEM/Ferulic acid | Chemo, Antioxidant | MSN | anti-GPC1 antibodies | 206 HS | Tumor-targeting Low cargo | [98] |
| GEM/ONC201 | Chemo, Targeted | Liposomes | ONC201 (MUC1 peptide) | 113 HS | Tumor-targeting Apoptosis upregulation | [103] |
| Oxaliplatin/Indoximod | Chemo, Immune | Hybrid lipid MSN | Lipid bilayer | 83 RS | Immunoactivator | [126] |
| Bortezomib/IR-820 | Chemo, PDT, thermal | Hybrid lipid MSN | Ciclosporin A | 160 | Increased uptake | [121] |
| Zn-Pc/Cetuximab | PDT, Targeted | MSN | PEGylated Cetuximab | 303 HS 79 RS | Tumor-targeting | [74] [75] |
| CPT/DOX | Chemo | MSN | Quantum dot | 150–200 RS | pH-responsive | [110] |
| DOX/hydroxychloroquine | Chemo | Mesoporous silica nanorods | - | 180 × 60 RS | Autophagy inhibition Macropinocytosis selectivity | [127] |
| DOX/iron oxide NP | Chemo, Thermal | Magnetic-MSN | Shell of MSN | 55 RS 106 HS | Thermal-sensitive caps | [128] |
5.3. Nanocarriers for Combined Anticancer and Stromal Therapies in PDAC Treatment
| Nanocarrier | Modifications | Tumor Therapy | Stromal Approach | Size (nm) | Features | Ref. |
|---|---|---|---|---|---|---|
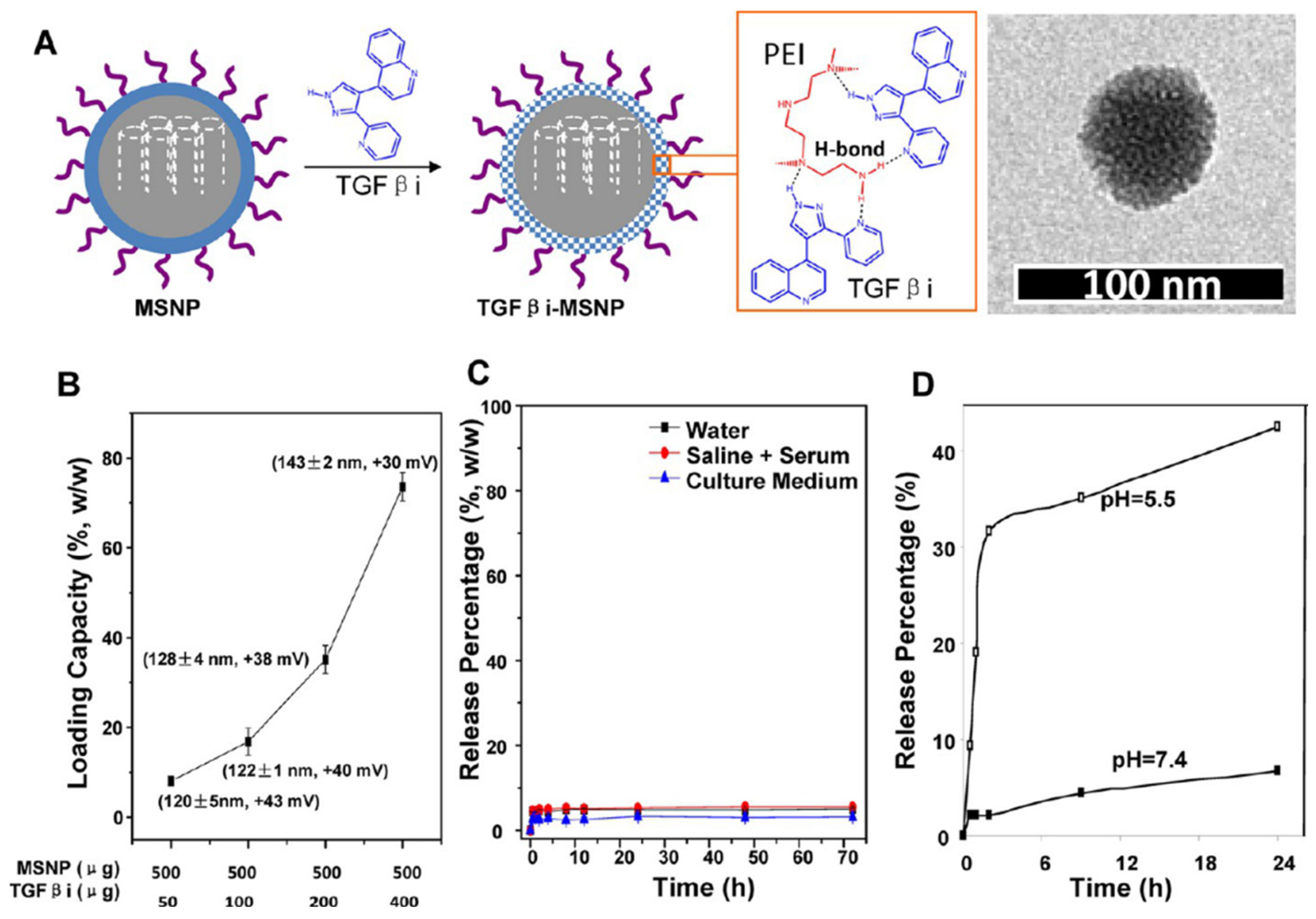
| Hybrid liposome-MSN | Co-polymer coating of MSN: PEG/PEI | GEM | TGF-β inhibitor | 143→50 RS | pH-responsive Low loading | [97] |
| MSN | DOX@MSN-S-nitrosothiol/PEG | DOX | S-nitrosothiol | 107 HS | Collagen depletion | [134] |
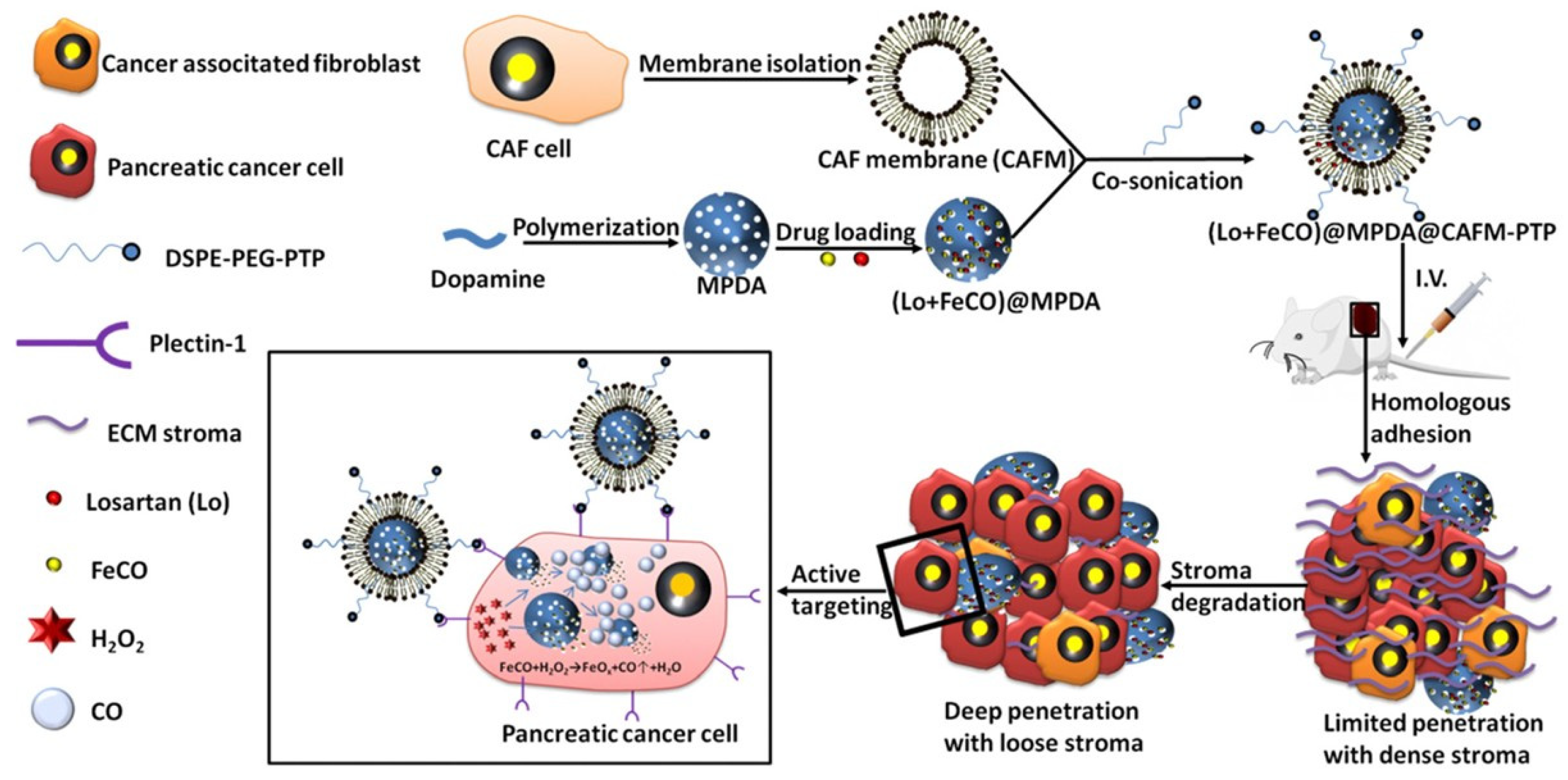
| Mesoporous polydopamine | CAF membrane, PTP | Iron carbonyl | Losartan | 126 nm | ECM degradation | [135] |
| Lipid NPs | aPD-L1 (antibody), perfluoropentane | DTX | Pulsed ultrasound stimulation | 95 nm | Antifibrotic, recover hypoxia | [136] |
| HMON | Hollow organically MSN | GEM | pirfenidone | 115→33 RS | pH/GSH-responsive | [137] |
| Liposome-modified Polymeric NP | Small NP: PEG-PGLA | PTX | TGF-β inhibitor Shrinkable | 155→40 RS | pH-responsive | [138] |
| Polymeric NP | Chloroquine phosphate | GEM | Shrinkable | 125→30 HS | pH-responsive Inhibition autophagy | [105] |
| Dendrigraft to PP micelle (DGL/DOX@PP) | DOX | Shrinkable | 100→30 RS | MMP-responsive | [139] | |
| dendrigraft poly-l-lysine to PP micelle | GEM | 18β-glycyrrhetinic acid Shrinkable | 151→30 RS | MMP-responsive | [28] | |
| PLGA polymer | GEM | Simvastatin | 258 RS | Mitigation of stroma pH-sensitive | [77] | |
| Iron oxide NP | pH low insertion peptides: pHLIP | GEM | Metformin | 23 HS 10 RS | Stromal depletion pH-responsive | [130] |
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garima, M.; Sumitra, N.; Pramod, K.S. Cancer: An Overview. Acad. J. Cancer Res. 2015, 8, 1–9. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent Advances in Cancer Therapy: An Overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef]
- Haeberle, L.; Esposito, I. Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019, 4, 50. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.; Fonseca, L.; Macedo, A.S.; Duarte, S.O.D.; Fonte, P. Advances in Pancreatic Cancer Treatment by Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 2363. [Google Scholar] [CrossRef]
- Imperial, R.; Mosalem, O.; Majeed, U.; Tran, N.H.; Borad, M.J.; Babiker, H. Second-Line Treatment of Pancreatic Adenocarcinoma: Shedding Light on New Opportunities and Key Talking Points from Clinical Trials. Clin. Exp. Gastroenterol. 2024, 17, 121–134. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, G.; Jacobs, S.; Toledo, B.; Borea, R.; Russo, G.; Pepe, F.; Serrano, M.J.; Calabrò, V.; Troncone, G.; Giovannoni, R.; et al. The potential application of stroma modulation in targeting tumor cells: Focus on pancreatic cancer and breast cancer models. Semin. Cancer Biol. 2025, 113, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Jäger, D.; Büchler, M.W.; Strobel, O.; Hackert, T.; Palmer, D.H.; Neoptolemos, J.P. Chemotherapy for Pancreatic Cancer. Presse Medicale 2019, 48, e159–e174. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Yabuuchi, S.; Pai, S.G.; Tong, Z.; Hou, S.; Bateman, S.; Pierce, D.W.; Heise, C.; Von Hoff, D.D.; Maitra, A.; et al. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br. J. Cancer 2016, 115, 442–453. [Google Scholar] [CrossRef]
- Carnevale, J.; Ko, A.H. MM-398 (Nanoliposomal Irinotecan): Emergence of a Novel Therapy for the Treatment of Advanced Pancreatic Cancer. Futur. Oncol. 2016, 12, 453–464. [Google Scholar] [CrossRef]
- Nagarajan, Y.; Chandrasekaran, N.; Deepa Parvathi, V. Functionalized Nanomaterials In Pancreatic Cancer Theranostics and Molecular Imaging. ChemistryOpen 2025, 14, e202400232. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Li, Y.; Chen, Z.; Shi, T.; Tong, Q.; Zou, X.; Ju, L.; Pan, J.; Zhuang, R.; et al. Extensive Review of Nanomedicine Strategies Targeting the Tumor Microenvironment in PDAC. Int. J. Nanomed. 2025, 20, 3379–3406. [Google Scholar] [CrossRef]
- Cade, J.E.; Hanison, J. The Pancreas. Anaesth. Intensive Care Med. 2017, 18, 527–531. [Google Scholar] [CrossRef]
- Valente, R.; Coppola, A.; Scandavini, C.M.; Halimi, A.; Magnusson, A.; Lauro, A.; Sotirova, I.; Arnelo, U.; Franklin, O. Interactions between the Exocrine and the Endocrine Pancreas. J. Clin. Med. 2024, 13, 1179. [Google Scholar] [CrossRef]
- Pour, P.M.; Pandey, K.K.; Batra, S.K. What is the origin of pancreatic adenocarcinoma? Mol. Cancer 2003, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.C.; Kimmelman, A.C.; Hezel, A.F.; DePinho, R.A. Stromal Biology of Pancreatic Cancer. J. Cell. Biochem. 2007, 101, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.; Motoo, Y.; Iovanna, J.L. Epithelial-to-Mesenchymal Transition in Pancreatic Adenocarcinoma. Sci. World J. 2010, 10, 1947–1957. [Google Scholar] [CrossRef]
- Beuran, M.; Negoi, I.; Paun, S.; Ion, A.D.; Bleotu, C.; Negoi, R.I.; Hostiuc, S. The Epithelial to Mesenchymal Transition in Pancreatic Cancer: A Systematic Review. Pancreatology 2015, 15, 217–225. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Cun, X.; Chen, J.; Li, M.; He, X.; Tang, X.; Guo, R.; Deng, M.; Li, M.; Zhang, Z.; He, Q. Tumor-Associated Fibroblast-Targeted Regulation and Deep Tumor Delivery of Chemotherapeutic Drugs with a Multifunctional Size-Switchable Nanoparticle. ACS Appl. Mater. Interfaces 2019, 11, 39545–39559. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic Cancer Stroma: An Update on Therapeutic Targeting Strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiang, J.; Qiu, N.; Wang, Y.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Shen, Y. Tumor Abnormality-Oriented Nanomedicine Design. Chem. Rev. 2023, 123, 10920–10989. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The Pancreatic Stellate Cell: A Star on the Rise in Pancreatic Diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Pereira, B.A.; Vennin, C.; Papanicolaou, M.; Chambers, C.R.; Herrmann, D.; Morton, J.P.; Cox, T.R.; Timpson, P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 2019, 5, 724–741. [Google Scholar] [CrossRef]
- von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The Role of Stromal Cancer-Associated Fibroblasts in Pancreatic Cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In Search of Definitions: Cancer-associated Fibroblasts and Their Markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Foster, D.; Chinta, M.; Titan, A.; Longaker, M. Pancreatic Cancer Associated Fibroblasts (CAF): Under-Explored Target for Pancreatic Cancer Treatment. Cancers 2020, 12, 1347. [Google Scholar] [CrossRef]
- Carapuça, E.F.; Gemenetzidis, E.; Feig, C.; Bapiro, T.E.; Williams, M.D.; Wilson, A.S.; Delvecchio, F.R.; Arumugam, P.; Grose, R.P.; Lemoine, N.R.; et al. Anti-Stromal Treatment Together With Chemotherapy Targets Multiple Signalling Pathways in Pancreatic Adenocarcinoma. J. Pathol. 2016, 239, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Q.; Liao, Q. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming. Front. Cell. Dev. Biol. 2020, 8, 607209. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Therapeutic Opportunities and Clinical Challenges. Cancers 2021, 13, 2860. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining Normoxia, Physoxia and Hypoxia in Tumours—Implications for Treatment Response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Davidson, S.M.; Jonas, O.; Keibler, M.A.; Hou, H.W.; Luengo, A.; Mayers, J.R.; Wyckoff, J.; Del Rosario, A.M.; Whitman, M.; Chin, C.R.; et al. Direct Evidence for Cancer-Cell-Autonomous Extracellular Protein Catabolism in Pancreatic Tumors. Nat. Med. 2016, 23, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Hu, X.; Xia, F.; Lee, J.; Li, F.; Lu, X.; Zhuo, X.; Nie, G.; Ling, D. Tailor-Made Nanomaterials for Diagnosis and Therapy of Pancreatic Ductal Adenocarcinoma. Adv. Sci. 2021, 8, 2002545. [Google Scholar] [CrossRef]
- Longley, D.; Johnston, P. Molecular Mechanisms of Drug Resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the War on Cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Andersson, R.; Aho, U.; Nilsson, B.I.; Peters, G.J.; Pastor-Anglada, M.; Rasch, W.; Sandvold, M.L. Gemcitabine Chemoresistance in Pancreatic Cancer: Molecular Mechanisms and Potential Solutions. Scand. J. Gastroenterol. 2009, 44, 782–786. [Google Scholar] [CrossRef]
- Torphy, R.J.; Fujiwara, Y.; Schulick, R.D. Pancreatic Cancer Treatment: Better, but a Long Way to Go. Surg. Today 2020, 50, 1117–1125. [Google Scholar] [CrossRef]
- Singh, R.R.; O′Reilly, E.M. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef]
- Casolino, R.; Biankin, A.V. Treatment of Pancreatic Cancer in 2022. Camb. Prism. 2023, 1, e14. [Google Scholar] [CrossRef]
- Hoyer, K.; Hablesreiter, R.; Inoue, Y.; Yoshida, K.; Briest, F.; Christen, F.; Kakiuchi, N.; Yoshizato, T.; Shiozawa, Y.; Shiraishi, Y.; et al. A Genetically Defined Signature of Responsiveness to Erlotinib in Early-Stage Pancreatic Cancer Patients: Results From the CONKO-005 Trial. EBioMedicine 2021, 66, 103327. [Google Scholar] [CrossRef]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A Review of Preclinical and Clinical Studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Siddiqui, N.S.; Godara, A.; Byrne, M.M.; Saif, M.W. Capecitabine for the Treatment of Pancreatic Cancer. Expert. Opin. Pharmacother. 2019, 20, 399–409. [Google Scholar] [CrossRef]
- Yardley, D.A. Nab-Paclitaxel Mechanisms of Action and Delivery. J. Control. Release 2013, 170, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Faller, B.A.; Burtness, B. Treatment of Pancreatic Cancer with Epidermal Growth Factor Receptor-Targeted Therapy. Biologics 2009, 3, 419–428. [Google Scholar] [PubMed][Green Version]
- Keraliya, R.A.; Patel, C.; Patel, P.; Keraliya, V.; Soni, T.G.; Patel, R.C.; Patel, M.M. Osmotic Drug Delivery System as a Part of Modified Release Dosage Form. ISRN Pharm. 2012, 2012, 528079. [Google Scholar] [CrossRef]
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent Advances in Long-Acting Drug Delivery Systems for Anticancer Drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef]
- Escalera-Anzola, S.; Rosado, M.; Yang, Y.; Parra-Sanchez, D.; Pedro-Liberal, C.S.; Acedo, P. Breakthroughs in nanoparticle-based strategies for pancreatic cancer therapy. Biochem. Pharmacol. 2025, 232, 116685. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles′ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Miao, L.; Lin, C.M.; Huang, L. Stromal Barriers and Strategies for the Delivery of Nanomedicine to Desmoplastic Tumors. J. Control. Release 2015, 219, 192–204. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. Methods Mol. Biol. 2018, 1800, 347–365. [Google Scholar] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.; Luger, T.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine-Challenge and Perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N.; et al. Application of Mesoporous Silica Nanoparticles as Drug Delivery Carriers for Chemotherapeutic Agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef]
- Feng, W.; Zong, M.; Wan, L.; Yu, X.; Yu, W. pH/redox Sequentially Responsive Nanoparticles With Size Shrinkage Properties Achieve Deep Tumor Penetration and Reversal of Multidrug Resistance. Biomater. Sci. 2020, 8, 4767–4778. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, J.; Li, Y.; Shi, H.; Gong, Y.; Li, R.; Huo, Q.; Ma, T.; Liu, Y. Size Shrinkable Drug Delivery Nanosystems and Priming the Tumor Microenvironment for Deep Intratumoral Penetration of Nanoparticles. J. Control. Release 2018, 277, 35–47. [Google Scholar] [CrossRef]
- Martín-Banderas, L.; Sáez-Fernández, E.; Holgado, M.Á.; Durán-Lobato, M.M.; Prados, J.C.; Melguizo, C.; Arias, J.L. Biocompatible Gemcitabine-Based Nanomedicine Engineered by Flow Focusing® for Efficient Antitumor Activity. Int. J. Pharm. 2013, 443, 103–109. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chellappan, D.R.; Bhavsar, D.V.; Ranganathan, R.; Sivanantham, B.; Subramanian, A.; Sharma, U.; Jagannathan, N.R.; Krishnan, U.M.; Sethuraman, S. Multi-Functional Nanoparticles as Theranostic Agents for the Treatment & Imaging of Pancreatic Cancer. Acta Biomater. 2017, 49, 422–433. [Google Scholar] [CrossRef]
- Er, Ö.; Colak, S.G.; Ocakoglu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, M.L.; Yurt, F.; Nonell, S. Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules 2018, 23, 2749. [Google Scholar] [CrossRef]
- Er, O.; Tuncel, A.; Ocakoglu, K.; Ince, M.; Kolatan, E.H.; Yilmaz, O.; Aktaş, S.; Yurt, F. Radiolabeling, in Vitro Cell Uptake, and in Vivo Photodynamic Therapy Potential of Targeted Mesoporous Silica Nanoparticles Containing Zinc Phthalocyanine. Mol. Pharm. 2020, 17, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Y.; Chen, S.; Liu, Y.; Wang, S.; Tian, Y.; Wang, C.; Teng, Z.; Lu, G. Sequential Therapy for Pancreatic Cancer by Losartan- And Gemcitabine-Loaded Magnetic Mesoporous Spheres. RSC Adv. 2019, 9, 19690–19698. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Aamir Mirza, M.; Anwer, M.K.; Thakur, P.S.; Alshahrani, S.M.; Alshetaili, A.S.; Telegaonkar, S.; Panda, A.K.; Iqbal, Z. Co-Delivery of Gemcitabine and Simvastatin Through PLGA Polymeric Nanoparticles for the Treatment of Pancreatic Cancer: In-Vitro Characterization, Cellular Uptake, and Pharmacokinetic Studies. Drug Dev. Ind. Pharm. 2019, 45, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Slapak, E.J.; el Mandili, M.; Bijlsma, M.F.; Spek, C.A. Mesoporous Silica Nanoparticle-Based Drug Delivery Systems for the Treatment of Pancreatic Cancer: A Systematic Literature Overview. Pharmaceutics 2022, 14, 390. [Google Scholar] [CrossRef]
- Massey, A.E.; Sikander, M.; Chauhan, N.; Kumari, S.; Setua, S.; Shetty, A.B.; Mandil, H.; Kashyap, V.K.; Khan, S.; Jaggi, M.; et al. Next-Generation Paclitaxel-Nanoparticle Formulation for Pancreatic Cancer Treatment. Nanomedicine 2019, 20, 102027. [Google Scholar] [CrossRef]
- Slapak, E.J.; Kong, L.; el Mandili, M.; Nieuwland, R.; Kros, A.; Bijlsma, M.F.; Spek, C.A. ADAM9-Responsive Mesoporous Silica Nanoparticles for Targeted Drug Delivery in Pancreatic Cancer. Cancers 2021, 13, 3321. [Google Scholar] [CrossRef]
- Slapak, E.J.; el Mandili, M.; Ten Brink, M.S.; Kros, A.; Bijlsma, M.F.; Spek, C.A. CAPN2-responsive Mesoporous Silica Nanoparticles: A Promising Nanocarrier for Targeted Therapy of Pancreatic Cancer. Cancer Lett. 2024, 590, 216845. [Google Scholar] [CrossRef]
- Fu, Q.; Hargrove, D.; Lu, X. Improving Paclitaxel Pharmacokinetics by Using Tumor-Specific Mesoporous Silica Nanoparticles With Intraperitoneal Delivery. Nanomedicine 2016, 12, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.R.; Dahut, W.L.; Scripture, C.D.; Jones, J.; Aragon-Ching, J.B.; Desai, N.; Hawkins, M.J.; Sparreboom, A.; Figg, W.D. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin. Cancer Res. 2008, 14, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Adiseshaiah, P.P.; Crist, R.M.; Hook, S.S.; McNeil, S.E. Nanomedicine Strategies to Overcome the Pathophysiological Barriers of Pancreatic Cancer. Nat. Rev. Clin. Oncol. 2016, 13, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhu, H.; Qin, Y.; Zhang, R.; Wang, L.; Zhang, E.; Zhou, X.; Meng, R. GP60 and SPARC as Albumin Receptors: Key Targeted Sites for the Delivery of Antitumor Drugs. Front. Pharmacol. 2024, 15, 1329636. [Google Scholar] [CrossRef]
- Raza, F.; Evans, L.; Motallebi, M.; Zafar, H.; Pereira-Silva, M.; Saleem, K.; Peixoto, D.; Rahdar, A.; Sharifi, E.; Veiga, F.; et al. Liposome-Based Diagnostic and Therapeutic Applications for Pancreatic Cancer. Acta Biomater. 2023, 157, 1–23. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Liu, H.; Kim, S.; Lee, Y.-K.; Kim, Y.-C. Hybrid Nanoparticles of Extracellular Vesicles and Gemcitabine Prodrug-Loaded Liposomes with Enhanced Targeting Ability for Effective PDAC Treatment. ACS Appl. Bio Mater. 2024, 7, 6025–6033. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in Mesoporous Silica Nanoparticles for Targeted Stimuli-Responsive Drug Delivery. Expert. Opin. Drug Deliv. 2014, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kim, D.-H.; Guo, Y.; Teng, Z.; Li, Y.; Zheng, L.; Zhang, Z.; Larson, A.C.; Lu, G. A c(RGDfE) conjugated multi-functional nanomedicine delivery system for targeted pancreatic cancer therapy. J. Mater. Chem. B 2015, 3, 1049–1058. [Google Scholar] [CrossRef]
- Roy, I. Gold Nanoparticle-Enhanced Photodynamic Therapy from Photosensitiser-Entrapped Ormosil Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 6942–6948. [Google Scholar]
- Ferreira, L.; Martel, F.; Silva, C.; Santos, T.M.; Daniel-da-Silva, A.L. Nanostructured Functionalized Magnetic Platforms for the Sustained Delivery of Cisplatin: Synthesis, Characterization and in Vitro Cytotoxicity Evaluation. J. Inorg. Biochem. 2020, 213, 111258. [Google Scholar] [CrossRef]
- Wang, G.; Wu, B.; Li, Q.; Chen, S.; Jin, X.; Liu, Y.; Zhou, Z.; Shen, Y.; Huang, P. Active Transportation of Liposome Enhances Tumor Accumulation, Penetration, and Therapeutic Efficacy. Small 2020, 16, 2004172. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.-P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-Penetrating Peptide Enhances Transcytosis of Silicasome-Based Chemotherapy for Pancreatic Cancer. J. Clin. Investig. 2017, 127, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Chang, C.H.; Liao, Y.; Lodico, J.J.; Tang, I.; Zheng, E.; Qiu, W.; Lin, M.; Wang, X.; et al. Development of Facile and Versatile Platinum Drug Delivering Silicasome Nanocarriers for Efficient Pancreatic Cancer Chemo-Immunotherapy. Small 2021, 17, 2005993. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, Y.; Dong, J.; Xue, M.; Lin, Y.-S.; Ji, Z.; Mai, W.X.; Zhang, H.; Chang, C.H.; Brinker, C.J.; et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 2013, 7, 10048–10065. [Google Scholar] [CrossRef]
- Estevão, B.M.; Comparetti, E.J.; Rissi, N.C.; Zucolotto, V. Anti-Gpc1-Modified Mesoporous Silica Nanoparticles as Nanocarriers for Combination Therapy and Targeting of PANC-1 Cells. Mater. Adv. 2021, 2, 5224–5235. [Google Scholar] [CrossRef]
- Saini, K.; Prabhuraj, R.S.; Bandyopadhyaya, R.; Bandyopadhyaya, R. Development of Mesoporous Silica Nanoparticles of Tunable Pore Diameter for Superior Gemcitabine Drug Delivery in Pancreatic Cancer Cells. J. Nanosci. Nanotechnol. 2020, 20, 3084–3096. [Google Scholar] [CrossRef]
- Tarannum, M.; Hossain, M.A.; Holmes, B.; Yan, S.; Mukherjee, P.; Vivero-Escoto, J.L. Advanced Nanoengineering Approach for Target-Specific, Spatiotemporal, and Ratiometric Delivery of Gemcitabine-Cisplatin Combination for Improved Therapeutic Outcome in Pancreatic Cancer. Small 2021, 18, 2104449. [Google Scholar] [CrossRef]
- Han, H.; Wang, J.; Chen, T.; Yin, L.; Jin, Q.; Ji, J. Enzyme-sensitive gemcitabine conjugated albumin nanoparticles as a versatile theranostic nanoplatform for pancreatic cancer treatment. J. Colloid Interface Sci. 2017, 507, 217–224. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.; Chen, X.; Wan, J.; Wang, Y.; Li, T.; Wang, H. Self-Assembled Gemcitabine Prodrug Nanoparticles Show Enhanced Efficacy against Patient-Derived Pancreatic Ductal Adenocarcinoma. ACS Appl. Mater. Interfaces 2020, 12, 3327–3340. [Google Scholar] [CrossRef]
- Shrestha, P.; Ghanwatkar, Y.; Mahto, S.; Pramanik, N.; Mahato, R.I. Gemcitabine–Lipid Conjugate and ONC201 Combination Therapy Effectively Treats Orthotopic Pancreatic Tumor-Bearing Mice. ACS Appl. Mater. Interfaces 2024, 16, 29686–29698. [Google Scholar] [CrossRef] [PubMed]
- Nakka, N.M.R.; Rachamala, H.K.; Angom, R.S.; Indla, N.R.; Dutta, S.K.; Wang, E.; Bhattacharya, S.; Sesha Sainath, A.V.; Babiker, H.; Pal, K.; et al. Dual Drug-Loaded Tumor-Targeted Polymeric Nanoparticles for Enhancing Therapeutic Response in Pancreatic Ductal Adenocarcinoma. Mater. Today Bio 2024, 28, 101199. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, Y.; He, M.; Deng, M.; Guo, R.; Sheng, Q.; Wang, X.; Ren, K.; Li, T.; He, X.; et al. Co-Delivery of Autophagy Inhibitor and Gemcitabine Using a pH-activatable Core-Shell Nanobomb Inhibits Pancreatic Cancer Progression and Metastasis. Theranostics 2021, 11, 8692–8705. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-Encapsulated Curcumin: In Vitro and in Vivo Effects on Proliferation, Apoptosis, Signaling, and Angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Bisht, S.; Mizuma, M.; Feldmann, G.; Ottenhof, N.A.; Hong, S.-M.; Pramanik, D.; Chenna, V.; Karikari, C.; Sharma, R.; Goggins, M.G.; et al. Systemic Administration of Polymeric Nanoparticle-Encapsulated Curcumin (NanoCurc) Blocks Tumor Growth and Metastases in Preclinical Models of Pancreatic Cancer. Mol. Cancer Ther. 2010, 9, 2255–2264. [Google Scholar] [CrossRef]
- Prabhuraj, R.S.; Mal, A.; Valvi, S.K.; Srivastava, R.; De, A.; Bandyopadhyaya, R. Noninvasive Preclinical Evaluation of Targeted Nanoparticles for the Delivery of Curcumin in Treating Pancreatic Cancer. ACS Appl. Bio Mater. 2020, 3, 4643–4654. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In Vivo Tumor Suppression Efficacy of Mesoporous Silica Nanoparticles-Based Drug-Delivery System: Enhanced Efficacy by Folate Modification. Nanomedicine 2012, 8, 212–220. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Wang, A.; Zhao, J.; Qi, W.; Guo, Y.; Zhu, G. Responsive Delivery of Drug Cocktail via Mesoporous Silica Nanolamps. J. Colloid. Interface Sci. 2014, 434, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Zhao, Z.; Li, Q.; Wu, Y.; Yan, S.; Shen, Y.; Huang, P. Enzyme-Triggered Transcytosis of Dendrimer-Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 2020, 14, 4890–4904. [Google Scholar] [CrossRef] [PubMed]
- Loc, W.S.; Linton, S.S.; Wilczynski, Z.R.; Matters, G.L.; McGovern, C.O.; Abraham, T.; Fox, T.; Gigliotti, C.M.; Tang, X.; Tabakovic, A.; et al. Effective Encapsulation and Biological Activity of Phosphorylated Chemotherapeutics in Calcium Phosphosilicate Nanoparticles for the Treatment of Pancreatic Cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Liu, L.; Zhang, Y.; Lu, Y.; Zhang, Y.; Chen, Q.; Ruan, C.; Guo, Q.; Li, C.; et al. Sequentially Triggered Nanoparticles with Tumor Penetration and Intelligent Drug Release for Pancreatic Cancer Therapy. Adv. Sci. 2018, 5, 1701070. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wei, T.; Gayet, O.; Loncle, C.; Borge, L.; Dusetti, N.; Ma, X.; Marson, D.; Laurini, E.; et al. Dendrimeric nanosystem consistently circumvents heterogeneous drug response and resistance in pancreatic cancer. Exploration 2021, 1, 21–34. [Google Scholar] [CrossRef]
- Ding, F.; Li, H.-J.; Wang, J.-X.; Tao, W.; Zhu, Y.-H.; Yu, Y.; Yang, X.-Z. Chlorin e6-Encapsulated Polyphosphoester Based Nanocarriers with Viscous Flow Core for Effective Treatment of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2015, 7, 18856–18865. [Google Scholar] [CrossRef]
- Hafiz, S.S.; Xavierselvan, M.; Gokalp, S.; Labadini, D.; Barros, S.; Duong, J.; Foster, M.; Mallidi, S. Eutectic Gallium-Indium Nanoparticles for Photodynamic Therapy of Pancreatic Cancer. ACS Appl. Nano Mater. 2022, 5, 6125–6139. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Liu, Y.; Zhang, W.; Li, H.; Luo, T.; Zhang, K.; Zhao, Y.; Liu, J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 2017, 11, 12849–12862. [Google Scholar] [CrossRef]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen Carrying Microbubbles for Enhanced Sonodynamic Therapy of Hypoxic Tumours. J. Control. Release 2015, 203, 51–56. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Zhao, R.; Han, X.; Li, Y.; Wang, H.; Ji, T.; Zhao, Y.; Nie, G. Photothermal Effect Enhanced Cascade-Targeting Strategy for Improved Pancreatic Cancer Therapy by Gold Nanoshell@Mesoporous Silica Nanorod. ACS Nano 2017, 11, 8103–8113. [Google Scholar] [CrossRef]
- Thapa, R.K.; Nguyen, H.T.; Gautam, M.; Shrestha, A.; Lee, E.S.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Hydrophobic binding peptide-conjugated hybrid lipid-mesoporous silica nanoparticles for effective chemo-photothermal therapy of pancreatic cancer. Drug Deliv. 2017, 24, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Lacy, J.; Portales, F.; Sobrero, A.; Pazo-Cid, R.; Manzano Mozo, J.L.; Kim, E.J.; Dowden, S.; Zakari, A.; Borg, C.; et al. Nab-Paclitaxel Plus Gemcitabine in Patients With Locally Advanced Pancreatic Cancer (LAPACT): A Multicentre, Open-Label Phase 2 Study. Lancet Gastroenterol. Hepatol. 2020, 5, 285–294. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.-M.J.; Zhang, L. Combinatorial drug conjugation enables nanoparticle dual-drug delivery. Small 2010, 6, 1442–1448. [Google Scholar] [CrossRef]
- Wang, L.; An, Y.; Yuan, C.; Zhang, H.; Liang, C.; Ding, F.; Gao, Q.; Zhang, D. GEM-loaded Magnetic Albumin Nanospheres Modified With Cetuximab for Simultaneous Targeting, Magnetic Resonance Imaging, and Double-Targeted Thermochemotherapy of Pancreatic Cancer Cells. Int. J. Nanomed. 2015, 10, 2507–2519. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.-P.; Salazar, F.; Sun, B.; Jiang, W.; Chang, C.H.; Jiang, J.; Wang, X.; Wu, A.M.; et al. Nano-Enabled Pancreas Cancer Immunotherapy Using Immunogenic Cell Death and Reversing Immunosuppression. Nat. Commun. 2017, 8, 1811. [Google Scholar] [CrossRef]
- Cong, V.T.; Tilley, R.D.; Sharbeen, G.; Phillips, P.A.; Gaus, K.; Gooding, J.J. How to Exploit Different Endocytosis Pathways to Allow Selective Delivery of Anticancer Drugs to Cancer Cells Over Healthy Cells. Chem. Sci. 2021, 12, 15407–15417. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Zink, J.I. Spatial, Temporal, and Dose Control of Drug Delivery using Noninvasive Magnetic Stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef]
- Ghaferi, M.; Koohi Moftakhari Esfahani, M.; Raza, A.; Al Harthi, S.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Mesoporous silica nanoparticles: Synthesis methods and their therapeutic use-recent advances. J. Drug Target. 2021, 29, 131–154. [Google Scholar] [CrossRef]
- Han, H.; Hou, Y.; Chen, X.; Zhang, P.; Kang, M.; Jin, Q.; Ji, J.; Gao, M. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef]
- Ruan, S.; Cao, X.; Cun, X.; Hu, G.; Zhou, Y.; Zhang, Y.; Lu, L.; He, Q.; Gao, H. Matrix Metalloproteinase-Sensitive Size-Shrinkable Nanoparticles for Deep Tumor Penetration and pH Triggered Doxorubicin Release. Biomaterials 2015, 60, 100–110. [Google Scholar] [CrossRef]
- Gu, X.; Minko, T. Targeted Nanoparticle-Based Diagnostic and Treatment Options for Pancreatic Cancer. Cancers 2024, 16, 1589. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Li, L.; Zhang, P.; Guo, H.; Liu, N.; Yang, X.; Xu, F. Engineering Extracellular Matrix to Improve Drug Delivery for Cancer Therapy. Drug Discov. Today 2020, 25, 1727–1734. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.-J.; Feng, H.-Y.; Yang, S.-C.; Liu, X.-L.; Lai, X.; Lu, Q.; Lovell, J.F.; Chen, H.-Z.; Fang, C. Enhanced Drug Delivery by Nanoscale Integration of a Nitric Oxide Donor To Induce Tumor Collagen Depletion. Nano Lett. 2019, 19, 997–1008. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Cai, Z.; Yao, H.; Liu, M.; Jiang, C.; Cheng, Z. A biomimetic dual-targeting nanomedicine for pancreatic cancer therapy. J. Mater. Chem. B 2025, 13, 3716–3729. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, Q.; Lin, P.; Chen, Z.; Fan, D.; Zhuo, M.; Gan, Y.; Su, Y.; Qian, Q.; Lin, L.; et al. aPD-L1-facilitated theranostic and tumor microenvironment remodeling of pancreatic cancer via docetaxel-loaded phase-transformation nanoparticles triggered by low-intensity pulsed ultrasound. J. Nanobiotechnol. 2025, 23, 48. [Google Scholar] [CrossRef]
- Gao, F.; Wu, J.; Niu, S.; Sun, T.; Li, F.; Bai, Y.; Jin, L.; Lin, L.; Shi, Q.; Zhu, L.-M.; et al. Biodegradable, pH-Sensitive Hollow Mesoporous Organosilica Nanoparticle (HMON) with Controlled Release of Pirfenidone and Ultrasound-Target-Microbubble-Destruction (UTMD) for Pancreatic Cancer Treatment. Theranostics 2019, 9, 6002–6018. [Google Scholar] [CrossRef] [PubMed]
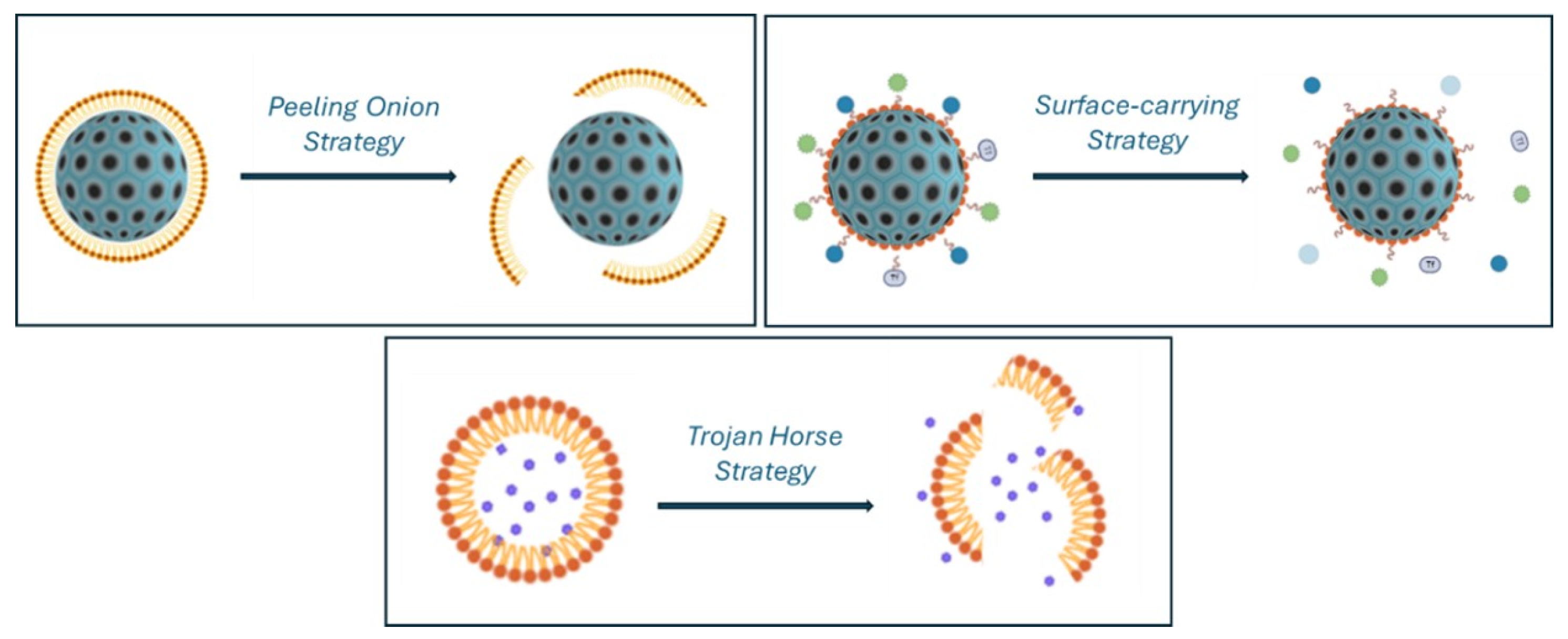
- Zhao, X.; Yang, X.; Wang, X.; Zhao, X.; Zhang, Y.; Liu, S.; Anderson, G.J.; Kim, S.-J.; Li, Y.; Nie, G. Penetration Cascade of Size Switchable Nanosystem in Desmoplastic Stroma for Improved Pancreatic Cancer Therapy. ACS Nano 2021, 15, 14149–14161. [Google Scholar] [CrossRef] [PubMed]
- Cun, X.; Li, M.; Wang, S.; Wang, Y.; Wang, J.; Lu, Z.; Yang, R.; Tang, X.; Zhang, Z.; He, Q. A Size Switchable Nanoplatform for Targeting the Tumor Microenvironment and Deep Tumor Penetration. Nanoscale 2018, 10, 9935–9948. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive Nanoscale Materials as Robust Drug Delivery Systems for Cancer Therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]


| Drug/Treatment | Mechanism of Action | Use | Therapy |
|---|---|---|---|
| Capecitabine | Oral prodrug of 5-FU; inhibits DNA synthesis by blocking thymidylate synthase. | Both | Chemotherapy |
| Erlotinib | EGFR tyrosine kinase inhibitor; blocks signaling pathways involved in cell proliferation. | Combination | Targeted therapy |
| Gemcitabine | Antimetabolite; inhibits DNA synthesis by incorporation into DNA and inhibition of ribonucleotide reductase. | Both | Chemotherapy |
| Irinotecan | Topoisomerase I inhibitor; prevents DNA unwinding, leading to DNA damage. | Combination | Chemotherapy |
| Oxaliplatin | Platinum-based drug; causes DNA crosslinking and subsequent apoptosis. | Combination | Chemotherapy |
| Paclitaxel | Binds and stabilizes microtubules, preventing cell division. | Combination | Chemotherapy |
| 5-FU | Pyrimidine analog; inhibits thymidylate synthase, blocking DNA synthesis. | Both | Chemotherapy |
| FOLFOX | Combination of 5-FU (DNA synthesis inhibition), leucovorin (enhances 5-FU effect), and oxaliplatin (DNA crosslinking). | Combination | Chemotherapy |
| FOLFIRINOX | Combination of 5-FU (DNA synthesis inhibition), leucovorin (enhances 5-FU effect), irinotecan (topoisomerase I inhibition), and oxaliplatin (DNA crosslinking). | Combination | Chemotherapy |
| mFOLFIRINOX | Modified doses of FOLFIRINOX with reduced irinotecan to reduce toxicity; same mechanisms as FOLFIRINOX. | Combination | Chemotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontón, I.; Sánchez-García, D. Nanocarriers for Combination Therapy in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Nanomaterials 2025, 15, 1139. https://doi.org/10.3390/nano15151139
Pontón I, Sánchez-García D. Nanocarriers for Combination Therapy in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Nanomaterials. 2025; 15(15):1139. https://doi.org/10.3390/nano15151139
Chicago/Turabian StylePontón, Iris, and David Sánchez-García. 2025. "Nanocarriers for Combination Therapy in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review" Nanomaterials 15, no. 15: 1139. https://doi.org/10.3390/nano15151139
APA StylePontón, I., & Sánchez-García, D. (2025). Nanocarriers for Combination Therapy in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Nanomaterials, 15(15), 1139. https://doi.org/10.3390/nano15151139








