Nanomaterials for Persistent Organic Pollutants Decontamination in Water: Mechanisms, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Properties and Characteristics of POPs Affecting Their Removal
2.1. Chemical and Physical Properties
2.2. Environmental Fate and Transport
2.3. Challenges in Removing POPs from Water
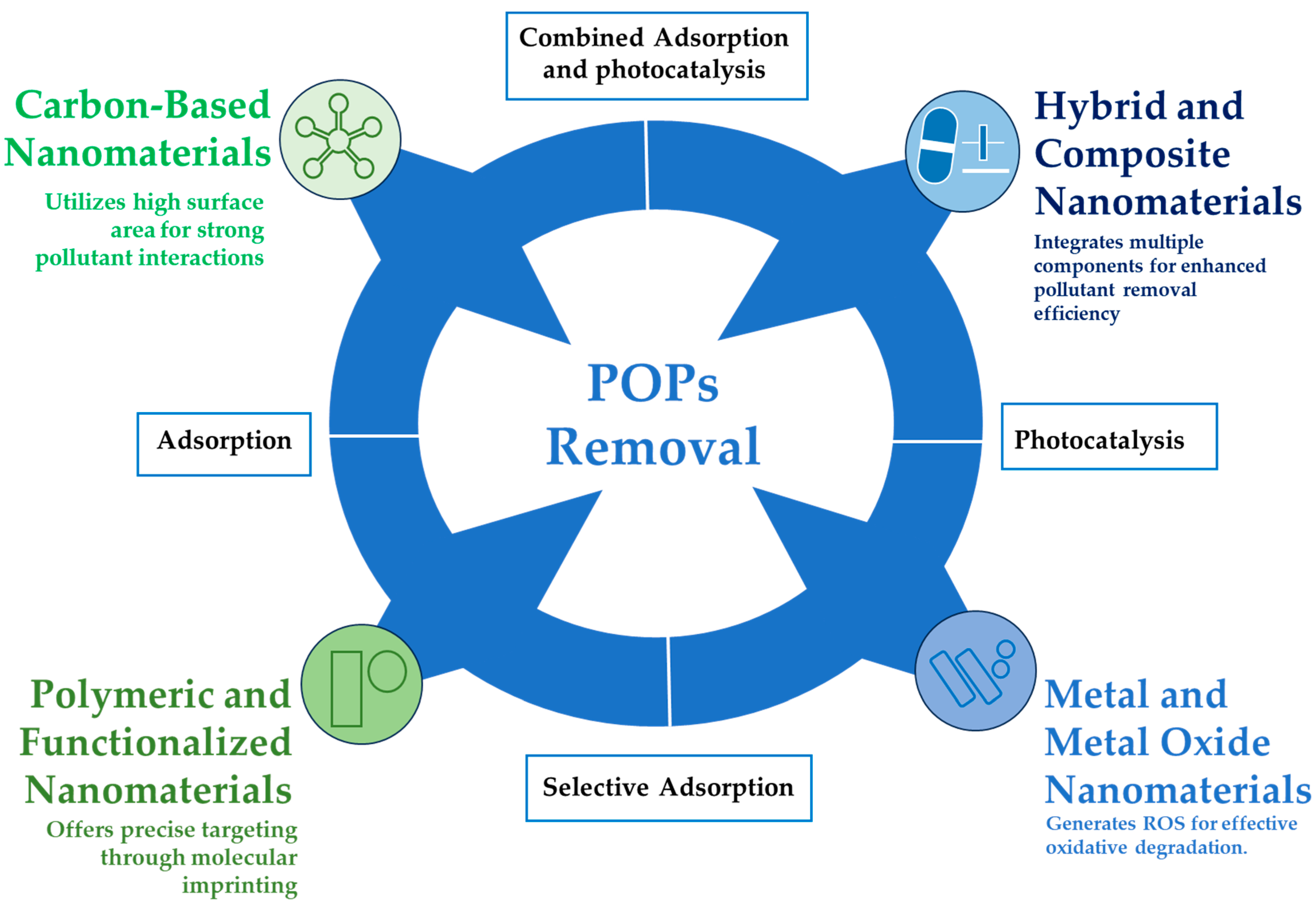
3. Nanomaterials Used for POPs Decontamination
3.1. Carbon-Based Nanomaterials
3.2. Metal and Metal Oxide Nanoparticles
3.3. Polymeric and Functionalized Nanomaterials
3.4. Hybrid and Composite Nanomaterials
4. Mechanisms of POP Removal Using Nanomaterials
4.1. Adsorption Mechanisms
4.2. Photocatalytic Degradation
4.3. Reductive Dechlorination and Redox Reactions
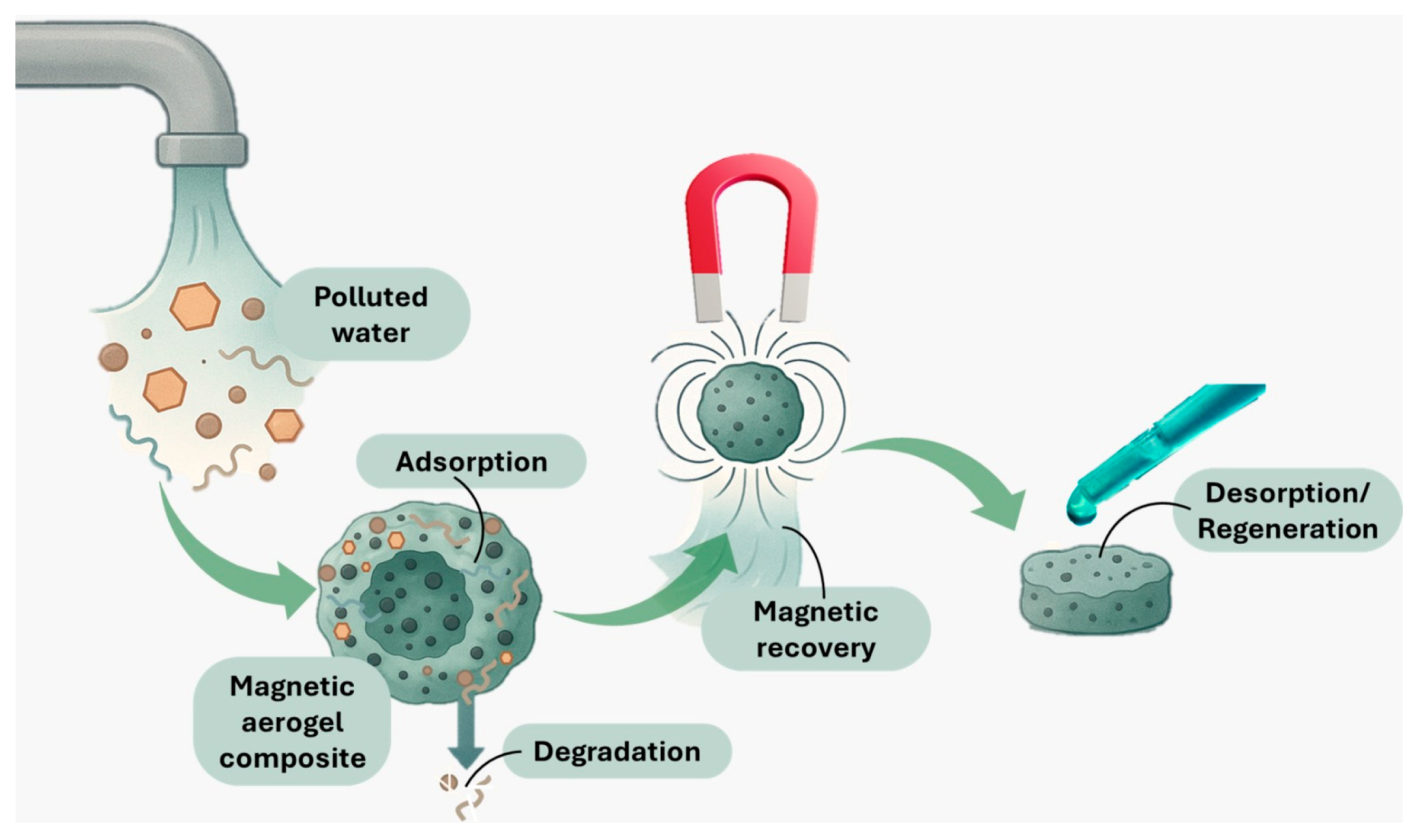
4.4. Magnetic Separation and Recovery
4.5. Hybrid and Synergistic Removal Mechanisms
5. Challenges and Limitations of Nanomaterial-Based POP Decontamination
5.1. Nanomaterial Stability and Reusability
5.2. Formation of Toxic Byproducts
5.3. Environmental Fate and Potential Toxicity of Nanomaterials
5.4. Scalability and Economic Feasibility
5.5. Regulatory and Safety Concerns
6. Case Studies and Real-World Applications
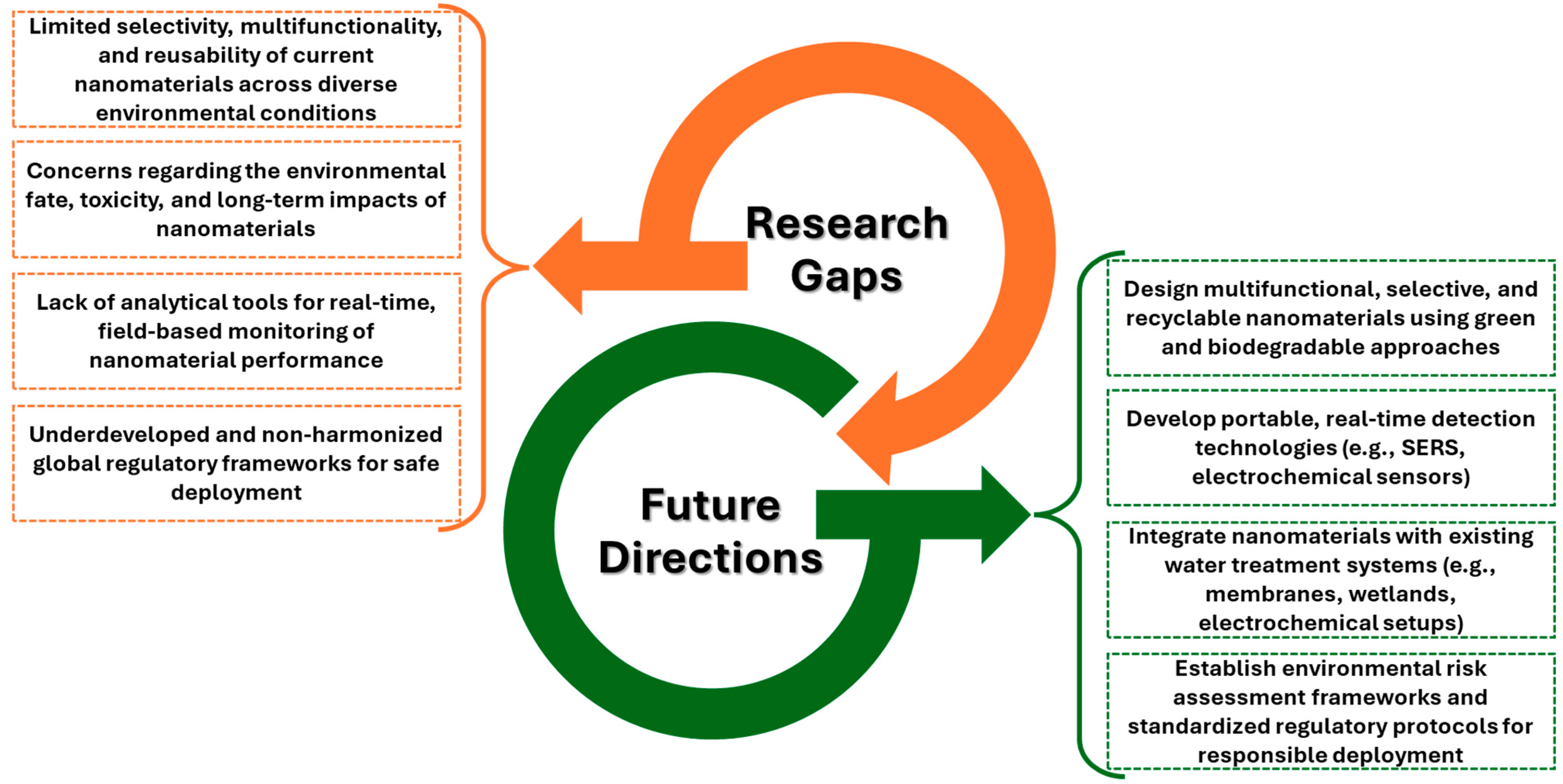
7. Future Directions and Research Gaps
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matei, M.; Zaharia, R.; Petrescu, S.-I.; Radu-Rusu, C.G.; Simeanu, D.; Mierliță, D.; Pop, I.M. Persistent Organic Pollutants (POPs): A Review Focused on Occurrence and Incidence in Animal Feed and Cow Milk. Agriculture 2023, 13, 873. [Google Scholar] [CrossRef]
- Rauseo, J.; Spataro, F.; Patrolecco, L. Persistent and Emerging Organic Contaminants in Natural Environments. Water 2025, 17, 436. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Lewińska, K.; Mammadov, E.; Karczewska, A.; Smreczak, B.; Medyńska-Juraszek, A. Residues of Persistent Organic Pollutants (POPs) in Agricultural Soils Adjacent to Historical Sources of Their Storage and Distribution—The Case Study of Azerbaijan. Molecules 2020, 25, 1815. [Google Scholar] [CrossRef] [PubMed]
- Ochs, C.; Garrison, K.; Saxena, P.; Romme, K.; Sarkar, A. Contamination of aquatic ecosystems by persistent organic pollutants (POPs) originating from landfills in Canada and the United States: A rapid scoping review. Sci. Total Environ. 2024, 924, 171490. [Google Scholar] [CrossRef] [PubMed]
- Souaf, B.; Methneni, N.; Toumi, D.; Albergamo, A.; Beltifa, A.; Turco, V.L.; Hassani, R.; Fathallah, S.; Mansour, H.B.; Di Bella, G. Persistent organic pollutants in abiotic and biotic matrices from an anthropized area: Investigation of their occurrence and associated health risks. Mar. Pollut. Bull. 2025, 218, 118071. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Ahmed, S.; Muhammad Zaigham Abbas Naqvi, S.; Awais, M.; Zhang, Y.; Zhang, H.; Raghavan, V.; Zang, Y.; Zhao, G.; Hu, J. Agricultural Non-Point Source Pollution: Comprehensive Analysis of Sources and Assessment Methods. Agriculture 2025, 15, 531. [Google Scholar] [CrossRef]
- Gaftonianu, S.A.; Chifiriuc, C.; Andronescu, E.; Surdu, A.; Burdusel, A.C.; Trusca, R. Functionalized Magnetite and Cerium Oxide Nanostructures: Morphostructural Properties and Biomedical Applicability. Rev. Romana Mater. 2024, 54, 198–204. [Google Scholar]
- Abd Rahim, N.N.; Peng, P.W.; Shahrir, N.F.; Wan Mahiyuddin, W.R.; Sayed Mohamed Zain, S.M.; Ismail, R. Characteristics, Distribution, and Sources of Atmospheric Microplastics in Southeast Asia: A Scoping Review. Atmosphere 2025, 16, 515. [Google Scholar] [CrossRef]
- Ferri, E.N.; Bolelli, L. Wastewater Remediation Treatments Aimed at Water Reuse: Recent Outcomes from Pilot- and Full-Scale Tests. Appl. Sci. 2025, 15, 2448. [Google Scholar] [CrossRef]
- Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.-u.; Ahn, H.-J.; et al. Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review. Nanomaterials 2022, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pandit, P.P.; Chopade, R.L.; Nagar, V.; Aseri, V.; Singh, A.; Awasthi, K.K.; Awasthi, G.; Sankhla, M.S. Eradication of microplastics in wastewater treatment: Overview. Biointerface Res. Appl. Chem. 2023, 13, 223. [Google Scholar]
- Sowmya, M.; Puttaswamy, A.A.; Shivabasappa, H.; Prasad, N.; Geetha, N.; Girija, S.; Venkatachalam, P. Biogenic Synthesis of Alternanthera Sessilis Titanium Dioxide Nanoparticles (AS@ TiO2NP’s): A Potential Contender against Perilous Pathogens and Catalytic Degradation of Organic Dyes. Biointerface Res. Appl. Chem. 2023, 13, 462. [Google Scholar]
- Pandey, P.; Khan, F.; Agarwal, S.; Singh, M. Nano adsorbents in wastewater treatment: A new paradigm in wastewater management. Lett. Appl. Nanobiosci. 2022, 12, 125. [Google Scholar]
- Paun, I.; Covaliu-Mierla, C.I.; Vasile, E. TiO2 Nanomaterial Used as Photocatalyst for Degradation of Benzalkonium Chlorides (C14-Bac and C16-Bac) from Wastewater. Rev. Romana Mater. 2023, 53, 124–129. [Google Scholar]
- Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kirankumar, V.S.; Kumar, M.; Arulvel, R.; Suresh, S. Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application. Water 2023, 15, 3003. [Google Scholar] [CrossRef]
- Visan, A.; Ciurcan, I.; Paraschiv, G.; Biris, S.S.; Pirvu, F.; Covaliu-mierla, C.I. Application of Tio2 for The Removal of Benzyl Dimethyl Dodecyl Ammonium Chloride (Ddbac) from Wastewater. Rev. Romana Mater. 2024, 54, 113–117. [Google Scholar]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent advances in the elimination of persistent organic pollutants by photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Alwera, S.; Talismanov, V.S.; Alwera, V.; Domyati, D. Synthesis and characterization of Sn-doped CeO2-Fe2O3 nanocomposite and application in photocatalytic degradation of Sudan I. Biointerface Res. Appl. Chem. 2023, 13, 179. [Google Scholar]
- Singh, N.A.; Narang, J.; Garg, D.; Jain, V.; Payasi, D.; Suleman, S.; Swami, R.K. Nanoparticles synthesis via microorganisms and their prospective applications in agriculture. Plant Nano Biol. 2023, 5, 100047. [Google Scholar] [CrossRef]
- Yadav, D.; Das, S.; Dhillayan, D.; Yadav, S.; Bhukal, S. Environmentally benign nanotechnology: Transforming elements into nano-agrochemicals for sustainable farming. Discov. Agric. 2025, 3, 67. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Aravind kumar, J.; Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent organic pollutants in water resources: Fate, occurrence, characterization and risk analysis. Sci. Total Environ. 2022, 831, 154808. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Z. Multi-cascade physiologically based kinetic (PBK) matrix model: Simulating chemical bioaccumulation across food webs. Environ. Int. 2025, 198, 109376. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Pereira, M.G.; Tyler, C.R.; Ormerod, S.J. Biological Traits and the Transfer of Persistent Organic Pollutants through River Food Webs. Environ. Sci. Technol. 2019, 53, 13246–13256. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, X.; Wang, C.; Gong, P.; Wang, X.; Yao, T. Biomagnification of persistent organic pollutants along a high-altitude aquatic food chain in the Tibetan Plateau: Processes and mechanisms. Environ. Pollut. 2017, 220, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Nakajima, R.; Tsuchiya, M.; Chiba, S.; Fujikura, K. Interdecadal distribution of persistent organic pollutants in deep-sea chemosynthetic bivalves. Front. Mar. Sci. 2021, 8, 751848. [Google Scholar] [CrossRef]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The sources of chemical contaminants in food and their health implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Alsaedi, W.H.; Zikry, M.M. A collective study on the fabrication of nano-materials for water treatment. J. Umm Al-Qura Univ. Appl. Sci. 2025. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, V.I.; Vedenyapina, M.D.; Kurmysheva, A.Y.; Weichgrebe, D.; Nair, R.R.; Nguyen, N.P.; Kustov, L.M. Modern Carbon–Based Materials for Adsorptive Removal of Organic and Inorganic Pollutants from Water and Wastewater. Molecules 2021, 26, 6628. [Google Scholar] [CrossRef] [PubMed]
- Phouthavong, V.; Yan, R.; Nijpanich, S.; Hagio, T.; Ichino, R.; Kong, L.; Li, L. Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials 2022, 15, 1053. [Google Scholar] [CrossRef] [PubMed]
- Grămadă, A.M.; Stoica, A.-E.; Niculescu, A.-G.; Bîrcă, A.C.; Vasile, B.Ș.; Holban, A.M.; Mihaiescu, T.; Șerban, A.I.; Ciceu, A.; Balta, C.; et al. Zinc Oxide-Loaded Recycled PET Nanofibers for Applications in Healthcare and Biomedical Devices. Polymers 2025, 17, 45. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Wang, F.; Luo, H.; Liang, D.; Wang, J.; Huang, J.; Yu, C.; Jin, L.; Sun, D. Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review. Toxics 2023, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Tse Sum Bui, B.; Haupt, K. Molecularly imprinted polymers: Synthetic receptors in bioanalysis. Anal. Bioanal. Chem. 2010, 398, 2481–2492. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Ma, Z.; Zhang, R.; He, Y.; Fan, X.; Lei, X.; Xiao, Y.; Zhang, M.; Sun, D. The Application of Nano Zero-Valent Iron in Synergy with White Rot Fungi in Environmental Pollution Control. Toxics 2024, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Romero, P.; Pokhriyal, A.; Rueda-García, D.; Bengoa, L.N.; González-Gil, R.M. Hybrid Materials: A Metareview. Chem. Mater. 2024, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Godja, N.-C.; Munteanu, F.-D. Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications. Biosensors 2024, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Halder, A.; Aggarwal, P.; Govind Rao, V. Plasmonic chemistry for sustainable ammonia production. Commun. Mater. 2024, 5, 69. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Chen, M. Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications. Catalysts 2023, 13, 940. [Google Scholar] [CrossRef]
- Rokni, L.; Rezaei, M.; Rafieizonooz, M.; Khankhajeh, E.; Mohammadi, A.A.; Rezania, S. Effect of Persistent Organic Pollutants on Human Health in South Korea: A Review of the Reported Diseases. Sustainability 2023, 15, 10851. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative Adsorbents for Pollutant Removal: Exploring the Latest Research and Applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.A.A.; El-Zahhar, A.A.; El-Desouky, M.G.; El-Bindary, A. Superior adsorption and removal of doxorubicin from aqueous solution using activated carbon via thermally treated green adsorbent: Isothermal, kinetic, and thermodynamic studies. Environ. Technol. 2024, 45, 1969–1988. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Cheng, S.; Wang, X.; Chen, M.; Wei, X.; Ma, G.; Yu, H. The adsorption of p-hydroxybenzoic acid on graphene oxide under different pH and in-situ desorption in direct current electric field. J. Mol. Liq. 2024, 415, 126369. [Google Scholar] [CrossRef]
- Dong, Y.; Laaksonen, A.; Gao, Q.; Ji, X. Molecular Mechanistic Insights into the Ionic-Strength-Controlled Interfacial Behavior of Proteins on a TiO2 Surface. Langmuir 2021, 37, 11499–11507. [Google Scholar] [CrossRef] [PubMed]
- Kamal, E.M.; Mohamed, A.E.-A.; Hamdy, G.; El-Sabbagh, I.A.E.-A. Highly efficient capture of Th (IV) from aqueous solutions using GO/TiO2 nanocomposite. Egypt. J. Chem. 2021, 64, 1353–1362. [Google Scholar]
- Luong, H.V.T.; Le, T.P.; Le, T.L.T.; Dang, H.G.; Tran, T.B.Q. A graphene oxide based composite granule for methylene blue separation from aqueous solution: Adsorption, kinetics and thermodynamic studies. Heliyon 2024, 10, e28648. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chang, H.; Mao, T.; Teng, Y. Planarity effect of polychlorinated biphenyls adsorption by graphene nanomaterials: The influence of graphene characteristics, solution pH and temperature. Chem. Eng. J. 2019, 362, 160–168. [Google Scholar] [CrossRef]
- Arham, Z.; Ramli, A.K.; Natsir, M.; Nurdin, M. Photoelectrode properties based on TiO2 photocatalyst doped non-metallic agent as initial model for detection and degradation of pesticide pollutants. Biointerface Res. Appl. Chem. 2023, 3, 4. [Google Scholar]
- Feliczak-Guzik, A. Nanomaterials as Photocatalysts—Synthesis and Their Potential Applications. Materials 2023, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Settimi, C.; Zingaretti, D.; Sanna, S.; Verginelli, I.; Luisetto, I.; Tebano, A.; Baciocchi, R. Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors. Sustainability 2022, 14, 7760. [Google Scholar] [CrossRef]
- Rani, J.; Goyal, T.; Kaur, A.; Ganesan, S.; Sharma, A.K.; Chauhan, A.S.; Kumar, S.; Kaushal, S. Bimetallic nanoparticles as pioneering eco-friendly catalysts for remediation of pharmaceuticals and personal care products (PPCPs). Nanoscale Adv. 2025, 7, 3160–3188. [Google Scholar] [CrossRef] [PubMed]
- Valiyeva, G.G.; Bavasso, I.; Di Palma, L.; Hajiyeva, S.R.; Ramazanov, M.A.; Hajiyeva, F.V. Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water. Nanomaterials 2019, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Chernavskiy, P.A.; Novakova, A.A.; Pankina, G.V.; Pankratov, D.A.; Panfilov, S.I.; Petrovskaya, G.A. Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel. Magnetochemistry 2023, 9, 228. [Google Scholar] [CrossRef]
- Grouli, A.; Bachra, Y.; Damiri, F.; Pandit, V.U.; Berrada, M. Removal of Pollutants from Wastewater Using Fe-Doped Hydroxyapatite Encapsulated with Alginate. Biointerface Res. Appl. Chem. 2023, 13, 438. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Mihaiescu, B.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Magnetic Composites for Water Decontamination. Polymers 2024, 16, 709. [Google Scholar] [CrossRef] [PubMed]
- Keshta, B.E.; Gemeay, A.H.; Kumar Sinha, D.; Elsharkawy, S.; Hassan, F.; Rai, N.; Arora, C. State of the art on the magnetic iron oxide Nanoparticles: Synthesis, Functionalization, and applications in wastewater treatment. Results Chem. 2024, 7, 101388. [Google Scholar] [CrossRef]
- Saud, A.; Gupta, S.; Allal, A.; Preud’homme, H.; Shomar, B.; Zaidi, S.J. Progress in the Sustainable Development of Biobased (Nano)materials for Application in Water Treatment Technologies. ACS Omega 2024, 9, 29088–29113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, T.; Shen, J.; Meng, Y.; Tong, S.; Guan, Q.; Xia, X. Core-Shell Structured Magnetic Carboxymethyl Cellulose-Based Hydrogel Nanosorbents for Effective Adsorption of Methylene Blue from Aqueous Solution. Polymers 2021, 13, 3054. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.; Khatun, M.H.; Mostafa, M.G. Green-synthesized magnetite-maghemite nanocomposites for the removal of lead and cadmium from water: Competitive adsorption behavior and mechanism studies. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.T.; Saed, A.; Mahmoud, A.; Badr, M.; Garas, S.S.; Yahya, S.; Hamad, K.H. Preparation of magnetite nanoparticles and their application in the removal of methylene blue dye from wastewater. Sci. Rep. 2024, 14, 20100. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Abdel-Hady, N.A.; Badawy, M.I.; Attia, M.S.; Gad-Allah, T.A. Magnetic self-doped TiO2−x/Fe3O4@g-C solar-driven photocatalytic composite for water decontamination. RSC Adv. 2024, 14, 33666–33680. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.D.; Chau, J.H.; Lai, C.W.; Khe, C.S.; Sharma, G.; Kumar, A.; Siengchin, S.; Sanjay, M.R. GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials 2022, 12, 3536. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Darajeh, N.; Rupani, P.F.; Mojiri, A.; Kamyab, H.; Taghavijeloudar, M. Recent Advances in the Adsorption of Different Pollutants from Wastewater Using Carbon-Based and Metal-Oxide Nanoparticles. Appl. Sci. 2024, 14, 11492. [Google Scholar] [CrossRef]
- Shah, N.; Rehan, T.; Li, X.; Tetik, H.; Yang, G.; Zhao, K.; Lin, D. Magnetic aerogel: An advanced material of high importance. RSC Adv. 2021, 11, 7187–7204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mangla, D.; Shehnaz; Chaudhry, S.A. Recent advances in magnetic composites as adsorbents for wastewater remediation. J. Environ. Manag. 2022, 306, 114483. [Google Scholar] [CrossRef] [PubMed]
- Ganesamoorthy, R.; Vadivel, V.K.; Kumar, R.; Kushwaha, O.S.; Mamane, H. Aerogels for water treatment: A review. J. Clean. Prod. 2021, 329, 129713. [Google Scholar] [CrossRef]
- Bober, P.; Minisy, I.M.; Acharya, U.; Pfleger, J.; Babayan, V.; Kazantseva, N.; Hodan, J.; Stejskal, J. Conducting polymer composite aerogel with magnetic properties for organic dye removal. Synth. Met. 2020, 260, 116266. [Google Scholar] [CrossRef]
- Lei, C.; Wen, F.; Chen, J.; Chen, W.; Huang, Y.; Wang, B. Mussel-inspired synthesis of magnetic carboxymethyl chitosan aerogel for removal cationic and anionic dyes from aqueous solution. Polymer 2021, 213, 123316. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, Y.; Wang, Z.; Lu, X.; Xia, H. Ultralight NiCo@rGO aerogel microspheres with magnetic response for oil/water separation. Chem. Eng. J. 2022, 430, 132894. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Qin, L.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. A novel robust adsorbent for efficient oil/water separation: Magnetic carbon nanospheres/graphene composite aerogel. J. Hazard. Mater. 2020, 392, 122499. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Mihaiescu, B.; Bîrcă, A.C.; Moroșan, A.; Munteanu, O.M.; Vasile, B.Ș.; Hadibarata, T.; Istrati, D.; Mihaiescu, D.E.; Grumezescu, A.M. Fabrication and Advanced Imaging Characterization of Magnetic Aerogel-Based Thin Films for Water Decontamination. Gels 2024, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Mendke, T.; Srilakshmi, C.; Thirunavukkarasu, T.; Das, P.P. Studies on Photocatalytic Degradation of Phenol Red over CaFe2O5 and CaFe2O5-TiO2 nanocomposite. Biointerface Res. Appl. Chem. 2024, 14, 77. [Google Scholar]
- He, Y.; Li, S.; Yuan, J.; Cheng, J.; Dou, J.; Yang, X.; Xu, J. A systematic understanding of microbial reductive dechlorination towards an improved “one health” soil bioremediation: A review and perspective. Sci. China Technol. Sci. 2024, 67, 3009–3031. [Google Scholar] [CrossRef]
- Natrayan, L.; Kalam, S.A.; Sheela, S.; Paramasivam, P.; Shanmugam, K. Bio-synthesis of nano-zero-valent iron using barberry leaf extract: Classification and utilization in the processing of methylene blue-polluted water. Discov. Appl. Sci. 2024, 6, 662. [Google Scholar] [CrossRef]
- Chauke, N.M.; Ngqalakwezi, A.; Raphulu, M. Transformative advancements in visible-light-activated titanium dioxide for industrial wastewater remediation. Int. J. Environ. Sci. Technol. 2025, 22, 8521–8552. [Google Scholar] [CrossRef]
- Dhanapal, A.R.; Thiruvengadam, M.; Vairavanathan, J.; Venkidasamy, B.; Easwaran, M.; Ghorbanpour, M. Nanotechnology Approaches for the Remediation of Agricultural Polluted Soils. ACS Omega 2024, 9, 13522–13533. [Google Scholar] [CrossRef] [PubMed]
- Hamdany, A.H.; Satyanaga, A.; Zhang, D.; Kim, Y.; Kim, J.R. Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review. Appl. Sci. 2022, 12, 8741. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Qian, H.; Chen, L.; Wu, B.; Yang, X.; Zou, H.; Hu, Y.; Chen, F.; Liao, B.; et al. Study on the Anti-Photocorrosion Mechanism of Novel Self-Assembled Spherical Cu2O/FePO4 Z-Scheme Heterojunctions. Reactions 2025, 6, 24. [Google Scholar] [CrossRef]
- Mateescu, C.; Lungulescu, E.-M.; Nicula, N.-O. Effectiveness of Biological Approaches for Removing Persistent Organic Pollutants from Wastewater: A Mini-Review. Microorganisms 2024, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, B.; Ma, W.; Chen, C.; Zhao, J. Photocatalytic Degradation of Aromatic Pollutants: A Pivotal Role of Conduction Band Electron in Distribution of Hydroxylated Intermediates. Environ. Sci. Technol. 2012, 46, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Sobczyński, A.; Duczmal, Ł.; Zmudziński, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Mol. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
- Brumovský, M.; Oborná, J.; Micić, V.; Malina, O.e.; Kašlík, J.; Tunega, D.; Kolos, M.; Hofmann, T.; Karlický, F.e.; Filip, J. Iron nitride nanoparticles for enhanced reductive dechlorination of trichloroethylene. Environ. Sci. Technol. 2022, 56, 4425–4436. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V. Nanomaterials for groundwater remediation. In Environmental Nanotechnology; Wiesner, M.R., Bottero, J.-Y., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2007. [Google Scholar]
- Peeters, K.; Lespes, G.; Zuliani, T.; Ščančar, J.; Milačič, R. The fate of iron nanoparticles in environmental waters treated with nanoscale zero-valent iron, FeONPs and Fe3O4NPs. Water Res. 2016, 94, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef] [PubMed]
- Boros, B.-V.; Ostafe, V. Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials 2020, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Hasnain, A.; Wang, J.; Zheng, H.; Naqvi, S.A.; Prasad, R.; Rehman, A.U.; Sohail, M.A.; Hassan, M.Z.; Farhan, M.; et al. Current Progress and Open Challenges for Combined Toxic Effects of Manufactured Nano-Sized Objects (MNO’s) on Soil Biota and Microbial Community. Coatings 2023, 13, 212. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Maharramov, A.M.; Hasanova, U.A.; Suleymanova, I.A.; Osmanova, G.E.; Hajiyeva, N.E. The engineered nanoparticles in food chain: Potential toxicity and effects. SN Appl. Sci. 2019, 1, 1362. [Google Scholar] [CrossRef]
- Sun, C.; Hu, K.; Mu, D.; Wang, Z.; Yu, X. The Widespread Use of Nanomaterials: The Effects on the Function and Diversity of Environmental Microbial Communities. Microorganisms 2022, 10, 2080. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Harper, B.J.; Harper, S.L. Comparative dissolution, uptake, and toxicity of zinc oxide particles in individual aquatic species and mixed populations. Environ. Toxicol. Chem. 2019, 38, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, U.T.; Velidandi, A. An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability 2025, 17, 1250. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Olowe, O.M.; Ayilara, M.S.; Fasusi, O.A.; Omotayo, O.P.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Biosynthesis of nanoparticles using microorganisms: A focus on endophytic fungi. Heliyon 2024, 10, e39636. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Chávez-Hernández, J.A.; Velarde-Salcedo, A.J.; Navarro-Tovar, G.; Gonzalez, C. Safe nanomaterials: From their use, application, and disposal to regulations. Nanoscale Adv. 2024, 6, 1583–1610. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Jansson, M.; Boholm, Å. Expert stakeholders’ perception of nanotechnology: Risk, benefit, knowledge, and regulation. J. Nanopart. Res. 2019, 21, 57. [Google Scholar] [CrossRef]
- Isigonis, P.; Afantitis, A.; Antunes, D.; Bartonova, A.; Beitollahi, A.; Bohmer, N.; Bouman, E.; Chaudhry, Q.; Cimpan, M.R.; Cimpan, E. Risk governance of emerging technologies demonstrated in terms of its applicability to nanomaterials. Small 2020, 16, 2003303. [Google Scholar] [CrossRef] [PubMed]
- Schwirn, K.; Voelker, D.; Galert, W.; Quik, J.; Tietjen, L. Environmental Risk Assessment of Nanomaterials in the Light of New Obligations Under the REACH Regulation: Which Challenges Remain and How to Approach Them? Integr. Environ. Assess. Manag. 2020, 16, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.B.; Fernández-Cruz, M.L.; Hund-Rinke, K.; Scott-Fordsmand, J.J. Environmental hazard testing of nanobiomaterials. Environ. Sci. Eur. 2020, 32, 101. [Google Scholar] [CrossRef]
- Gross, J.; Sayle, S.; Karow, A.R.; Bakowsky, U.; Garidel, P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: Influence of experimental and data evaluation parameters. Eur. J. Pharm. Biopharm. 2016, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.R.; Lowry, G.V.; Jones, K.L.; Hochella, J.M.F.; Di Giulio, R.T.; Casman, E.; Bernhardt, E.S. Decreasing Uncertainties in Assessing Environmental Exposure, Risk, and Ecological Implications of Nanomaterials. Environ. Sci. Technol. 2009, 43, 6458–6462. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.U.M.; Hanafiah, M.M.; Woon, K.S. A Content Review of Life Cycle Assessment of Nanomaterials: Current Practices, Challenges, and Future Prospects. Nanomaterials 2021, 11, 3324. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiang, L.; Leung, K.S.-Y.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G. Emerging contaminants: A one health perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef] [PubMed]
- Ismael, A.M.; El-Shazly, A.N.; Gaber, S.E.; Rashad, M.M.; Kamel, A.H.; Hassan, S.S.M. Novel TiO2/GO/CuFe2O4 nanocomposite: A magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020, 10, 34806–34814. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, J.X.; Md. Jamil, S.N.; Syukri, F.; Koyama, M.; Nourouzi Mobarekeh, M. Comparison between Conventional Treatment Processes and Advanced Oxidation Processes in Treating Slaughterhouse Wastewater: A Review. Water 2022, 14, 3778. [Google Scholar] [CrossRef]
- Tripathy, J.; Mishra, A.; Pandey, M.; Thakur, R.R.; Chand, S.; Rout, P.R.; Shahid, M.K. Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water 2024, 16, 1481. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. VIEW 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Alfeeli, B.; Al-Rawashdeh, M.m.; Bumajdad, A.; Al Lawati, H.; Abdelgawad, M.; Baccar, Z.M.; Salem, I.B.; Benaskar, F. A review of nanotechnology development in the Arab World. Nanotechnol. Rev. 2013, 2, 359–377. [Google Scholar] [CrossRef]
- Alarifi, S.S.; El-Sorogy, A.S.; Al-Kahtany, K.; Alotaibi, M. Contamination and Environmental Risk Assessment of Potentially Toxic Elements in Soils of Palm Farms in Northwest Riyadh, Saudi Arabia. Sustainability 2022, 14, 15402. [Google Scholar] [CrossRef]
- Chen, H.; Qian, L. Performance of field demonstration nanoscale zero-valent iron in groundwater remediation: A review. Sci. Total Environ. 2024, 912, 169268. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Ghoshal, S.; Tufenkji, N. Deposition of Carboxymethylcellulose-Coated Zero-Valent Iron Nanoparticles onto Silica: Roles of Solution Chemistry and Organic Molecules. Langmuir 2010, 26, 12832–12840. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ruan, X.; Zhao, D.; Fan, X.; Feng, T. Enhanced Adsorption of 2,4-Dichlorophenol by Nanoscale Zero-Valent Iron Loaded on Bentonite and Modified with a Cationic Surfactant. Ind. Eng. Chem. Res. 2017, 56, 191–197. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, Y.; Lan, X.; Yang, Y.; Wu, X.; Du, L. Comprehensive assessment of harmful heavy metals in contaminated soil in order to score pollution level. Sci. Rep. 2022, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Narimani, R.; Murgulet, I.; Murgulet, D. Emerging Contaminants in Groundwater: Challenges, Management, and Policy Perspectives. In Emerging Pollutants: Protecting Water Quality for the Health of People and the Environment; Zandaryaa, S., Fares, A., Eckstein, G., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 189–226. [Google Scholar]
- Sekar, M.S.; Shanmugam, G.; Kalimuthu, G.; Sundaram, K.B. Nano-Bioremediation for Environmental Pollutions—A Review. Lett. Appl. NanoBioScience 2023, 12, 175. [Google Scholar]
- Williams, W.A.; Aravamudhan, S. Micro-Nanoparticle Characterization: Establishing Underpinnings for Proper Identification and Nanotechnology-Enabled Remediation. Polymers 2024, 16, 2837. [Google Scholar] [CrossRef] [PubMed]
- Rontani, J.-F. Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples. Molecules 2022, 27, 1629. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ye, Q.; Wang, M.; Sun, F.; Chen, Q.; Yu, X.; Wang, Y.; Liang, P. Research Progress in Small-Molecule Detection Using Aptamer-Based SERS Techniques. Biosensors 2025, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Kant, K.; Abalde-Cela, S. Surface-Enhanced Raman Scattering Spectroscopy and Microfluidics: Towards Ultrasensitive Label-Free Sensing. Biosensors 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Adsorption Capacity of POPs (PCBs/OCPs) | |
|---|---|---|
| GO [45,46,47] | CNTs [48,49,50] | |
| pH | GO contains abundant oxygen-containing functional groups (carbonyl, hydroxyl, and epoxy), which ionize depending on pH, affecting surface charge and hydrophilicity. Adsorption capacity for PCBs and OCPs often decreases at high pH due to increased negative charges causing repulsion or reduced hydrophobic interaction. Adsorption capacity decreases by up to 30–50% when pH shifts from pH 4 to pH 9. | CNTs are generally hydrophobic with limited surface functional groups, so adsorption of hydrophobic pollutants like PCBs and OCPs is less sensitive to pH changes. Slight decreases in adsorption capacity at extreme pH due to surface charge alterations and possible aggregation. Adsorption capacity changes <15% across pH 3–9 for PCBs/OCPs. |
| Temperature | Adsorption of PCBs and OCPs may be more sensitive to temperature due to involvement of hydrogen bonding and electrostatic interactions. Adsorption capacity often decreases with increasing temperature, but sometimes shows slight increases if diffusion is rate-limiting. Adsorption capacity change varies widely ±10–20% via improved kinetics and thermodynamic favorability. | Adsorption is generally exothermic; increasing temperature typically decreases adsorption capacity. Adsorption capacity may decline by 10–30% when the temperature increases from 20 to 40 °C. |
| Ionic strength | Higher ionic strength screens electrostatic repulsion between negatively charged GO species, potentially increasing adsorption capacity. At very high ionic strengths, the aggregation of GO may reduce the available surface area. Adsorption may increase up to 30% at moderate ionic strength (0.05–0.1 M), but decline beyond this due to aggregation effects. | Increasing ionic strength (e.g., NaCl concentration) can enhance adsorption slightly via salting-out effects that reduce pollutant solubility, promoting partitioning onto CNTs. Electrostatic screening is minimal due to CNTs’ low surface charge. Adsorption capacity may increase by ~10–20% with ionic strength from 0 to 0.1 M NaCl. |
| Mechanism | Description | Key Features | Comparative Efficiency | Refs. |
|---|---|---|---|---|
| Adsorption | Physical or chemical capture of POPs on nanomaterial surfaces via surface interactions. Nanomaterials such as GO, CNTs, and activated carbon provide abundant active sites and tunable functionalities. | High surface area; π–π stacking, hydrogen bonding, and electrostatic attraction; enhanced selectivity via functional groups; performance influenced by pH, temperature, and ionic strength. | ~60–95% depending on material type, surface area, and conditions | [15,31,36,44] |
| Photocatalytic Degradation | Semiconductor nanomaterials (e.g., TiO2 and ZnO) absorb UV or visible light to generate electron-hole pairs, which initiate redox reactions and produce ROS that degrade POPs. | ROS generation (•OH, O2−•, and 1O2); capable of mineralization to CO2 and H2O; effective for a broad range of POPs; limited by light penetration and electron-hole recombination. | ~70–99% under optimized light intensity and catalyst loading | [18,34,52,53,77] |
| Reductive Dechlorination/Redox Reactions | Electron transfer reactions, particularly from nZVI or bimetallic nanoparticles, reduce halogenated POPs by replacing chlorine with hydrogen atoms, breaking C–Cl bonds. | Effective for halogenated POPs (PCBs and dioxins); rapid dechlorination via nZVI or Fe/Pd and Fe/Ni systems; enhances biodegradability; applicable in anaerobic or reducing environments. | ~65–98% depending on POP type and nanoparticle composition | [35,56,78,79] |
| Magnetic Separation and Recovery | Using magnetic nanomaterials (Fe3O4 and γ-Fe2O3) allows rapid separation and recovery of adsorbents or catalysts from treated water via external magnetic fields. | Easy separation post-treatment; integration with catalytic/adsorptive materials (GO and TiO2); reusability after regeneration; reduces material loss and operational costs; performance can decline with repeated cycles. | Removal remains ~70–90% initially, may decline after reuse cycles | [32,57,65] |
| Hybrid and Synergistic Mechanisms | Multifunctional nanocomposites (GO–TiO2–Fe3O4) integrate multiple mechanisms (adsorption, photocatalysis, redox) in one platform for synergistic pollutant removal. | Combines advantages of individual mechanisms; higher degradation efficiency; rapid kinetics; magnetic separability; tailored for broad pH, pollutant types, and complex matrices; supports continuous degradation with reduced regeneration frequency; scalable and robust for field applications. | Up to 99% removal; enhanced synergy under combined treatment | [15,31,53,65,68,80] |
| Challenge | Description | Refs. |
|---|---|---|
| Nanomaterial Stability and Reusability | Structural degradation, surface fouling, aggregation, and photo corrosion reduce nanomaterial performance over time. nZVI is prone to passivation and agglomeration. Enhancing material robustness and recovery methods is essential for long-term use. | [35,38,79,81,82,83] |
| Formation of Toxic Byproducts | Incomplete mineralization can lead to hazardous degradation intermediates, including hydroxylated or chlorinated byproducts. Photocatalysis and reductive reactions often leave residual toxicity. Comprehensive degradation pathway studies and ecotoxicological assessments are needed. | [42,78,84,91] |
| Environmental Fate and Nanotoxicity | Nanoparticles may accumulate in aquatic organisms, alter microbial communities, and pose risks to ecosystems and human health. Understanding their transport, transformation, and long-term toxicity is critical. Green synthesis and lifecycle assessments can mitigate environmental impact. | [92,93,96] |
| Scalability and Economic Feasibility | High production costs, complex synthesis methods, and difficulty integrating into the existing infrastructure limit scalability. Green and low-cost synthesis approaches, as well as pilot-scale demonstrations, are needed to ensure economic viability. | [1,10,39,97,98] |
| Regulatory and Safety Concerns | Lack of standardized regulations, monitoring tools, and toxicity testing protocols hinders responsible deployment. The current risk assessments often overlook nanoparticle-specific behaviors. Harmonized international standards are essential for commercialization and public acceptance. | [97,105,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristanti, R.A.; Hadibarata, T.; Niculescu, A.-G.; Mihaiescu, D.E.; Grumezescu, A.M. Nanomaterials for Persistent Organic Pollutants Decontamination in Water: Mechanisms, Challenges, and Future Perspectives. Nanomaterials 2025, 15, 1133. https://doi.org/10.3390/nano15141133
Kristanti RA, Hadibarata T, Niculescu A-G, Mihaiescu DE, Grumezescu AM. Nanomaterials for Persistent Organic Pollutants Decontamination in Water: Mechanisms, Challenges, and Future Perspectives. Nanomaterials. 2025; 15(14):1133. https://doi.org/10.3390/nano15141133
Chicago/Turabian StyleKristanti, Risky Ayu, Tony Hadibarata, Adelina-Gabriela Niculescu, Dan Eduard Mihaiescu, and Alexandru Mihai Grumezescu. 2025. "Nanomaterials for Persistent Organic Pollutants Decontamination in Water: Mechanisms, Challenges, and Future Perspectives" Nanomaterials 15, no. 14: 1133. https://doi.org/10.3390/nano15141133
APA StyleKristanti, R. A., Hadibarata, T., Niculescu, A.-G., Mihaiescu, D. E., & Grumezescu, A. M. (2025). Nanomaterials for Persistent Organic Pollutants Decontamination in Water: Mechanisms, Challenges, and Future Perspectives. Nanomaterials, 15(14), 1133. https://doi.org/10.3390/nano15141133











