Research Advances in Nanosensor for Pesticide Detection in Agricultural Products
Abstract
1. Introduction
| Categories | Representing Pesticides | Poisoning Mechanism | Harmfulness | Ref. |
|---|---|---|---|---|
| Organopsphorus | Parathion, systox, malathion, dimethoate, dichlorvos, dichlorvos | Irreversible inhibiting of AChE | Dyspnea, pulmonary edema, muscle spasms, renal dysfunction, dizziness, headache, palpitations, chest tightness, bradycardia | [9] |
| Carbamate | Carbaryl, metolcarb, aldicarb, carbofuran | Similar to organopsphorus, but with higher reversibility | Dizziness, fatigue, blurred vision, nausea and vomiting, abdominal pain, excessive sweating, muscle fiber tremors, breathing difficulties, coma, liver and kidney function damage | [10] |
| Neonictinoids | Imidacloprid, thiamethoxam, imidacloprid, fipronil | Continuously activating nicotinic ACh receptors | Nausea, vomiting, headache, dizziness, insomnia, anxiety, consciousness disorders | [11] |
| Pyrethroides | Permethrin, deltamethrin, fenvalerate, cyfluthrin | Induce neurotoxicity by interfering with sodium channels and GABA receptors | Skin rashes, nausea, vomiting, abdominal pain, diarrhea, headache, dizziness, anxiety, confusion, cough, endocrine disorders | [12] |
| Organchlorine | Hexachlorocyclohexane, indene octachloride, toxaphene, indene heptachloride | Inhibiting GABA receptors, generating ROS, disrupting inositol metabolism and endocrine system | Skin rashes, nausea, vomiting, abdominal pain, diarrhea, headache, dizziness, anxiety, confusion, cough, endocrine disorders | [13] |
2. Common Recognition Elements for Pesticide Detection
2.1. Enzyme
2.2. Antibody
2.3. Aptamer
2.4. Molecularly Imprinted Polymer (MIP)
3. Transducers of Nanosensors for Pesticide Detection
3.1. Electrochemical Biosensors
3.1.1. Potentiometric Biosensor
3.1.2. Amperometric Biosensors
3.1.3. Impedance Biosensor
3.2. Optical Biosensor
3.2.1. Fluorescent Biosensor
3.2.2. Surface Plasmon Resonance (SPR) Biosensor
3.2.3. Surface-Enhanced Raman Scattering (SERS) Biosensor
3.2.4. Other Optical Nanosensors
3.3. Other Biosensors
3.3.1. Terahertz Spectral Biosensor
3.3.2. Lateral Flow Assay (LFA)
4. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Eddleston, M. Poisoning by pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides classification and its impact on environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.; Patrick-Iwuanyanwu, K. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–42. [Google Scholar]
- Rapini, R.; Marrazza, G. Biosensor potential in pesticide monitoring. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.F.; Liu, Y.; Yang, G.J.; Deng, Y.; He, N.Y. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; El-Aty, A.M.A.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.M.; Gao, S.; She, Y.X.; Jin, F.; Wang, J. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef] [PubMed]
- Kamanyire, R.; Karalliedde, L. Organophosphate toxicity and occupational exposure. Occup. Med. 2004, 54, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bansal, O. Health impacts of the carbamate and dithiocarbamate pesticides: A review. Int. J. Sci. Res. Publ. 2022, 12, 366. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S.Y. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.-M.; Ndiaye, D.; Sabaté, J.-P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Envir. Health 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Mirres, A.; Silva, B.; Tessaro, L.; Galvan, D.; Andrade, J.C.D.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent advances in nanomaterial-based biosensors for pesticide detection in foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2010, 396, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Zhai, J.; Liu, L.H.; Shang, Z.Y.; Zhang, X.; Huang, H.; Shen, B.X.; Chen, G.X. Recent developments on nanomaterial probes for detection of pesticide residues: A review. Anal. Chim. Acta 2022, 1215, 339974. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the potential of nanosensors: A brief overview. Int. Sens. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Hu, T.; Xu, J.; Ye, Y.; Han, Y.; Li, X.; Wang, Z.; Sun, D.; Zhou, Y.; Ni, Z. Visual detection of mixed organophosphorous pesticide using QD-AChE aerogel based microfluidic arrays sensor. Biosens. Bioelectron. 2019, 136, 112–117. [Google Scholar]
- Arsawiset, S.; Sansenya, S.; Teepoo, S. Nanozymes paper−based analytical device for the detection of organophosphate pesticides in fruits and vegetables. Anal. Chim. Acta 2023, 1267, 341377. [Google Scholar] [CrossRef] [PubMed]
- Song, G.C.; Zhang, J.J.; Huang, H.X.; Wang, X.; He, X.Y.; Luo, Y.B.; Li, J.C.; Huang, K.L.; Cheng, N. Single-atom Ce-N-C nanozyme bioactive paper with a 3D-printed platform for rapid detection of organophosphorus and carbamate pesticide residues. Food Chem. 2022, 387, 132896. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; An, L.; Li, M.; Liu, H.; Jin, Z.; Ma, H.; Ma, J.G.; Zhou, J.; Duan, R.; Zhang, D. A self-assembled 3D nanoflowers based nano-ELISA platform for the sensitive detection of pyridaben. Food Chem. 2024, 445, 138756. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Yang, X.; Xie, D.H.; Wu, Y.Z.; Wen, W.G. A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen printed carbon electrode. Sensors 2010, 10, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, M.I.; Barrientos, K.; Arango, J.P.; Cano, J.B.; Peñuela, G.A. Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots–graphene oxide quenching test for analytical and commercial organophosphate pesticide detection. Front. Env. Sci. 2022, 10, 825112. [Google Scholar] [CrossRef]
- Chandra, S.; Bano, D.; Sahoo, K.; Kumar, D.; Kumar, V.; Yadav, P.K.; Hasan, S.H. Synthesis of fluorescent carbon quantum dots from Jatropha fruits and their application in fluorometric sensor for the detection of chlorpyrifos. Microchem. J. 2021, 172, 106953. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Qin, X.; Yuan, C.H.; Shi, R.; Wang, Y.L. Double responsive analysis of carbaryl pesticide based on carbon quantum dots and Au nanoparticles. Dye. Pigment. 2020, 181, 108529. [Google Scholar] [CrossRef]
- Jain, U.; Saxena, K.; Hooda, V.; Balayan, S.; Singh, A.P.; Tikadar, M.; Chauhan, N. Emerging vistas on pesticides detection based on electrochemical biosensors-An update. Food Chem. 2022, 371, 131126. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Tesla, Y.; Naumi, F. Construction of an enzymatic biosensor for chlorpyrifos pesticide detection via acetylcholinesterase inhibition on oxidative boron-doped diamond electrode. Environ. Mater. 2024, 2, 12–28. [Google Scholar] [CrossRef]
- Dhull, V. A Nafion/AChE-cSWCNT/MWCNT/Au-based amperometric biosensor for the determination of organophosphorous compounds. Environ. Technol. 2020, 103, 4200–4224. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.P.; Feng, Z.J.; Shen, G.N.; Xiu, Y.; Zhou, Y.K.; Niu, X.D.; Wang, H.S. Acetylcholinesterase Biosensor Based On Mesoporous Hollow Carbon Spheres/Core-Shell Magnetic Nanoparticles-Modified Electrode for the Detection of Organophosphorus Pesticides. Sensors 2018, 18, 4429. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Huang, C.X.; Zhang, D.D.; Shang, Y.H.; Sun, G.W.; Peng, D.P.; Chen, Y.P.; Wang, Y.L. Broad-spectrum portable magnetic relaxation switching immunosensor with gold-functionalized magnetic nanoprobes for the sensitive detection of multiple pyrethroids. J. hazard. Mater. 2023, 451, 131141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wang, G.C.; Li, C.P.; Zhou, Q.; Wang, M.; Yang, L. A novel acetylcholinesterase biosensor based on carboxylic graphene coated with silver nanoparticles for pesticide detection. Mater. Sci. Eng. C 2014, 35, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tao, L.; Song, D.D.; Li, Y.; Gao, F.M. Bimetallic Pd@ Au nanorods based ultrasensitive acetylcholinesterase biosensor for determination of organophosphate pesticides. Sensor. Actua B Chem. 2018, 255, 2575–2581. [Google Scholar] [CrossRef]
- Mashuni, M.; Ritonga, H.; Jahiding, M.; Rubak, B.; Hamid, F.H. Highly sensitive detection of carbaryl pesticides using potentiometric biosensor with nanocomposite Ag/r-graphene oxide/chitosan immobilized acetylcholinesterase enzyme. Chemosensors 2022, 10, 138. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Aiyer, R.; Karve, M. A novel inhibition based biosensor using urease nanoconjugate entrapped biocomposite membrane for potentiometric glyphosate detection. Int. J. Biol. Macromol. 2018, 108, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.R.; Mazzaracchio, V.; Duranti, L.; Gullo, L.; Brannetti, S.; Peyravian, M.; Kiani, M.A.; Arduini, F. Nanopaper Integrated Smart Device: An Opto-Electrochemical Biosensor for Real-Time Dual On-Field Detection of Organophosphorus Pesticides. ACS Sens. 2024, 9, 6542–6552. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Chen, J.Y.; Sun, M.; Gong, C.C.; Shen, Y.; Song, Y.G.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Huang, T.; Wang, Z.N.; Yang, H.; Geng, X.T.; Xu, G.L.; Samalo, M.; Sakinati, M.; Huo, D.Q.; Hou, C.J. 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2019, 279, 95–101. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, X.T.; Qiu, S.; Zhang, Q.Q.; Tang, W.; Liu, H.T.; Guo, Y.L.; Ma, Y.Q.; Guo, X.J.; Liu, Y.Q. A flexible acetylcholinesterase-modified graphene for chiral pesticide sensor. J. Am. Chem. Soc. 2019, 141, 14643–14649. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, E.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Biosensors 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, G.Y.; Jia, H.Y.; Cui, X.Y.; Wang, Y.S.; Li, D.Y.; Zheng, W.J.; She, Y.X.; Xu, D.H.; Huang, X.D.; et al. A sensitive bio-barcode immunoassay based on bimetallic Au@ Pt nanozyme for detection of organophosphate pesticides in various agro-products. Food Chem. 2021, 362, 130118. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.P.; Mercader, J.V.; Abad-Fuentes, A.; Checa-Orrego, B.I.; Costa-García, A.; de la Escosura-Muñiz, A. Escosura-Muiz. Direct competitive immunosensor for Imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 2020, 209, 120465. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhat, G.P.; Gandhi, S. MXene-rGO nanocomposite based electrochemical immunosensor for detection of endosulfan-An organochlorine pesticide. Chemosphere 2025, 370, 14377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; AEl-Aty, M.A.; Wang, S.S.; Cui, X.Y.; Zhao, J.; Lei, X.M.; Xu, L.Y.; She, Y.X.; Jin, F.; Eun, J.B.; et al. Competitive fluorescent immunosensor based on catalytic hairpin self-assembly for multiresidue detection of organophosphate pesticides in agricultural products. Food Chem. 2023, 413, 135607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.D.; Chen, C.; He, J.B.; Zhang, H.Y.; Xu, Z.X. Fluorescence assay for three organophosphorus pesticides in agricultural products based on Magnetic-Assisted fluorescence labeling aptamer probe. Food Chem. 2020, 307, 125534. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Sharma, R.K.; Wangoo, N. Development of gold nanoparticles-based aptasensor for the colorimetric detection of organophosphorus pesticide phorate. Anal. Bioanal. Chem. 2016, 408, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Dubreuil, B.; Peydecastaing, J.; Medina, G.V.; Behra, P. Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor. Sensors 2020, 20, 5942. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgnüllü, S.; Cimen, D.; Derazshamshir, A.; Bereli, N.; Yilmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Act. B-Chem. 2017, 241, 446–454. [Google Scholar]
- Gokturk, I.; Ovezova, M.; Yilmaz, G.E.; Turkmen, D.; Yilmaz, F.; Denizli, A. Synthetic Receptors Decorated on Nanoparticles for Selective and Sensitive Glyphosate Detection. Photonic Sens. 2025, 15, 250305. [Google Scholar] [CrossRef]
- Oymen, B.; Jalilzadeh, M.; Yılmaz, F.; Aşır, S.; Türkmen, D.; Denizli, A. Simple and fast pesticide nanosensors: Example of surface plasmon resonance coumaphos nanosensor. Micromachines 2023, 14, 707. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, T.; Hoque, M.E. Introduction to nanomaterials and nanomanufacturing for nanosensors. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Adam, T.; Gopinath, S.C. Nanosensors: Recent perspectives on attainments and future promise of downstream applications. Process Biochem. 2022, 117, 153–173. [Google Scholar] [CrossRef]
- Johnson, M.S.; Sajeev, S.; Nair, R.S. Role of Nanosensors in agriculture. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; IEEE: Piscataway, NJ, USA, 2021. [Google Scholar]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuat. B Chem. 2021, 345, 130344. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Alsareii, S.; Alsaiari, M.; Jalalah, M.; Harraz, F.A. Highly sensitive and selective electrochemical sensor for detecting imidacloprid pesticide using novel silver nanoparticles/mesoporous carbon/hematite ore ternary nanocomposite. Environ. J. Chem. Eng. 2022, 10, 108364. [Google Scholar] [CrossRef]
- Chen, Y.; He, K.; Sun, F.M.; Wei, D.; Li, H. Efficient electrochemical sensor based on multi-walled carbon nanotubes/gold nanocomposite for detection of dichlorvos pesticide in agricultural food. Int. J. Electrochem. Sci. 2021, 16, 210321. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El, A.; Martelet, C.; Jaffrezic-Renault, N. Amperometric enzyme biosensors: Past, present and future. IRBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Modak, N.; Friebe, V.M. Amperometric biosensors: Harnessing photosynthetic reaction centers for herbicide detection. Curr. Opin. Electroche. 2023, 42, 101414. [Google Scholar] [CrossRef]
- Zhu, H.J.; Liu, P.; Xu, L.Z.; Li, X.; Hu, P.W.; Liu, B.X.; Pan, J.M.; Yang, F.; Niu, X.H. Nanozyme-Participated Biosensing of Pesticides and Cholinesterases: A Critical Review. Biosensors 2021, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.R.; Lakshmanakumar, M.; Jayalatha, J.B.B.A.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of screen-printed electrodes: Opportunities and challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, V.; Fegatelli, G.; Moscone, D.; Arduini, F. A paper-based electrochemical device for the detection of pesticides in aerosol phase inspired by nature: A flower-like origami biosensor for precision agriculture. Biosens. Bioelectron. 2022, 205, 114119. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Wu, T.; Pan, F.; Lu, G.; Zeng, W.; Fan, W.Y.; Wu, L. An applied potential powered screen-printed electrode for sensitive EC-SERS detection of acetamiprid. Sens. Actuators B Chem. 2024, 404, 135279. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Ru, P.L.; Chen, S.M. Electrochemically synthesized Pt–MnO2 composite particles for simultaneous determination of catechol and hydroquinone. Sens. Actuators B Chem. 2012, 169, 235–242. [Google Scholar] [CrossRef]
- El-Moghazy, A.; Soliman, E.; Ibrahim, H.; Noguer, T.; Marty, J.-L.; Istamboulie, G. Ultra-sensitive biosensor based on genetically engineered acetylcholinesterase immobilized in poly (vinyl alcohol)/Fe–Ni alloy nanocomposite for phosmet detection in olive oil. Food Chem. 2016, 203, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Tan, R.N.; Cai, Z.W.; Zhao, H.; Chang, G.; He, Y.B. A novel electrochemical sensor via Zr-based metal organic framework-graphene for pesticide detection. J. Mater. Sci. 2021, 56, 19060–19074. [Google Scholar] [CrossRef]
- Zhao, W.P.; Guo, Y.M.; Zhao, Q.X.; Sun, J.F.; Wang, Z.Q.; Sun, X. The fabrication of an impedance immunosensor based on interdigitated array microelectrodes and normalized impedance changes for chlorpyrifos residue detection. Int. J. Electrochem. Sci. 2020, 15, 293–303. [Google Scholar] [CrossRef]
- Kunpatee, K.; Kaewdorn, K.; Duangtong, J.; Chaiyo, S.; Chailapakul, O.; Kalcher, K.; Kerr, M.; Samphao, A. A new disposable electrochemical sensor for the individual and simultaneous determination of carbamate pesticides using a nanocomposite modified screen-printed electrode. Microchem. J. 2022, 177, 107318. [Google Scholar] [CrossRef]
- Lu, X.; Tao, L.; Li, Y.S.; Huang, H.M.; Gao, F.M. A highly sensitive electrochemical platform based on the bimetallic Pd@ Au nanowires network for organophosphorus pesticides detection. Sens. Actuators B Chem. 2019, 284, 103–109. [Google Scholar] [CrossRef]
- Gai, K.; Qi, H.L.; Zhu, X.L.; Wang, M.Y. Preparation of Ag-Fe3O4 nanoparticles sensor and application in detection of methomyl. EDP Sci. 2019, 118, 01002. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.S. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, Z.; Mousavi, M.M.; Mahmoudpour, M. Surface functionalized gold nanoclusters based on optical biosensors for detecting pesticide residues in agricultural foods: A critical review. Microchem. J. 2024, 207, 111988. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, S.G.; Jiang, W.Q.; Zhao, Q.; Gao, W.L.; Shi, X.B. Detection of Organophosphorus Pesticides Using Silver-Coated Gold Nanoparticles. ACS Appl. Nano Mater. 2022, 5, 16402–16412. [Google Scholar] [CrossRef]
- Liao, Y.; Cui, X.Y.; Chen, G.; Wang, Y.H.; Qin, G.X.; Li, M.J.; Zhang, X.Y.; Zhang, Y.D.; Zhang, C.; Du, P.F.; et al. Simple and sensitive detection of triazophos pesticide by using quantum dots nanobeads based on immunoassay. Food Agric. Immunol. 2019, 30, 522–532. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, X.B.; Chen, X.X.; Cao, X.Z.; Zeng, W.B. A novel upconversion luminescence turn-on nanosensor for ratiometric detection of organophosphorus pesticides. RSC Adv. 2016, 6, 46317–46324. [Google Scholar] [CrossRef]
- Luo, Q.J.; Lai, J.H.; Qiu, P.; Wang, X.L. An ultrasensitive fluorescent sensor for organophosphorus pesticides detection based on RB-Ag/Au bimetallic nanoparticles. Sens. Actuators B Chem. 2018, 263, S0925400518303794. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.H.; Zhang, D.W.; Liu, H.L.; Sun, B.G. Peptide nanodots-bridged metal-organic framework (PNMOF): Intelligently design a cascade amplification platform for smartphone-facilitated mobile fluorescence imaging detection of pyrethroids. Chem. Eng. J. 2023, 468, 143690. [Google Scholar] [CrossRef]
- Sinha, N.; Ray, S. Application of Carbon Quantum Dots Derived from Waste Tea for the Detection of Pesticides in Tea: A Novel Biosensor Approach. ACS Omega 2024, 9, 50201–50213. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, J.; Luo, M.; Yu, H.; Chen, X.; Deng, J.G.; Hou, X.T.; Hao, E.; Wei, J.C.; Li, P. A ratiometric fluorescent sensing system for the selective and ultrasensitive detection of pesticide residues via the synergetic effects of copper nanoclusters and carbon quantum dots. Food Chem. 2022, 379, 132139. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Yun, S.N.; Liu, Y.J.; Yu, R.J.; Tu, Q.; Wang, J.Y.; Yuan, M.S. A highly selective and sensitive CdS fluorescent quantum dot for the simultaneous detection of multiple pesticides. Analyst 2022, 147, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.R.; Li, L.F.; Lin, D.; Jiang, C.G. Ultrasensitive and on-site detection of carbaryl pesticides via dual-mode nanosensors utilizing portable devices. ACS Sustain. Chem. Eng. 2023, 11, 4998–5006. [Google Scholar] [CrossRef]
- Bera, M.K.; Behera, L.; Mohapatra, S. A fluorescence turn-down-up detection of Cu2+ and pesticide quinalphos using carbon quantum dot integrated UiO-66-NH2. Colloids Surf. A 2021, 624, 126792. [Google Scholar] [CrossRef]
- Nethaji, P.; Revathi, P.; Kumar, P.S.; Logesh, M.; Rajabathar, J.R.; Al-Lohedan, H.; Arokiyaraj, S.; Rangasamy, G. Fluorescence enhancing and quenching signal based on new approach for selective detection of multiple organochlorine pesticides using blue emissive-carbon dot. Environ. Pollut. 2024, 345, 123418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Yuan, X.Y.; Han, L.X.; Liu, H.L.; Sun, B.G. A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens. Bioelectron. 2021, 191, 113460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Surface plasmon resonance based fiber–optic nanosensor for the pesticide fenitrothion utilizing Ta2O5 nanostructures sequestered onto a reduced graphene oxide matrix. Microchim. Acta 2020, 187, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Jiang, X.; Gao, J.; Kumari, D.; Biswas, S.; Sur, U.K.; Rao, Q.; Raha, P.; Mukherjee, S. Raman Spectroscopy-Based Chemometrics for Pesticide Residue Detection: Current Approaches and Future Challenges. ACS Agric. Sci. Technol. 2024, 4, 389–404. [Google Scholar] [CrossRef]
- Wang, K.Q.; Li, Z.L.; Li, J.J.; Lin, H. Raman spectroscopic techniques for nondestructive analysis of agri-foods: A state-of-the-art review. Trends Food Sci. Technol. 2021, 118, 490–504. [Google Scholar] [CrossRef]
- Afroozeh, A. A Review of Developed Surface-Enhanced Raman Spectroscopy (SERS)-Based Sensors for the Detection of Common Hazardous Substances in the Agricultural Industry. Plasmonics 2024, 20, 63–81. [Google Scholar] [CrossRef]
- Lovera, P. Surface Enhanced Raman Spectroscopy: Applications in Agriculture and Food Safety. Photonics 2021, 8, 568. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Chen, W.W.; Tang, Z.X.; Li, C.; Xiao, K.Y.; Jin, S.Z.; Ni, D.J.; Yu, Z. Rapid field trace detection of pesticide residue in food based on surface-enhanced Raman spectroscopy. Microchim. Acta 2021, 188, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Ma, P.; Chen, H.; Chang, M.; Lu, P.; Chen, N.; Yuan, Y.B.; Chen, N.; Zhang, X.D. Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy. Foods 2022, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Zhao, Y.; Liu, L.; Qiao, Y.X.; Yang, C.J.; Wang, X.T.; Li, Q.; Li, Y. Visual whole-process monitoring of pesticide residues: An environmental perspective using surface-enhanced Raman spectroscopy with dynamic borohydride-reduced silver nanoparticles. J. Hazard. Mater. 2024, 465, 133338. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.X.; Han, E.; Yin, L.M.; Xu, Q.; Zou, C.X.; Bai, J.W.; Wu, W.; Cai, J.R. Simultaneous detection of mixed pesticide residues based on portable Raman spectrometer and Au@ Ag nanoparticles SERS substrate. Food Control 2023, 153, 109951. [Google Scholar] [CrossRef]
- Xu, S.L.; Li, M.; Li, X.; Jiang, Y.H.; Yu, L.L.; Zhao, Y.; Wen, L.Y.; Xue, Q.W. Engineering an Ag/Au bimetallic nanoparticle-based acetylcholinesterase SERS biosensor for in situ sensitive detection of organophosphorus pesticide residues in food. Anal. Bioanal. Chem. 2023, 415, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, Q.Q.; Xu, L.L.; Jiao, A.X.; Tian, Y.; Liu, X.D.; Li, S.; Li, H.S.; Chen, M.; Chen, F. Blue laser-induced photochemical synthesis of CuAg nanoalloys on h-BN supports with enhanced SERS activity for trace-detection of residual pesticides on tomatoes. J. Alloys Compd. 2020, 825, 153996. [Google Scholar] [CrossRef]
- Ma, Y.D.; Wang, Y.H.; Luo, Y.; Duan, H.Z.; Li, D.; Xu, H.; Essy, K.F. Rapid and sensitive on-site detection of pesticide residues in fruits and vegetables using screen-printed paper-based SERS swabs. Anal. Methods 2018, 10, 1039. [Google Scholar] [CrossRef]
- Huang, Y.T.; Luo, Y.; Zhang, X.Q.; Li, S.L.; Liu, M. Regenerable AgNPs-CdSNWs/Nanofilm as SERS substrates for sensitive detection of carbamate pesticides. Food Chem. 2025, 472, 142919. [Google Scholar] [CrossRef] [PubMed]
- Liou, P.; Nayigiziki, F.X.; Kong, F.B.; Mustapha, A.; Lin, M.S. Cellulose nanofibers coated with silver nanoparticles as a SERS platform for detection of pesticides in apples. Carbohyd. Polym. 2017, 157, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.F.; Yang, Y.; Cao, Y.Q.; Huang, Z.R. Facile solvothermal synthesis of Ag/Fe3O4 nanocomposites and their SERS applications in on-line monitoring of pesticide contaminated water. RSC Adv. 2015, 5, 102610–102618. [Google Scholar] [CrossRef]
- Wei, W.; Yixuan, D.; Liangmiao, Z.; Yong, Y.; Yanfeng, G. Improving SERS Hot Spots for On-Site Pesticide Detection by Combining Silver Nanoparticles with Nanowires. J. Mater. Chem. C 2018, 6, 8793–8803. [Google Scholar] [CrossRef]
- Tang, J.S.; Chen, W.W.; Ju, H.X. Rapid detection of pesticide residues using a silver nanoparticles coated glass bead as nonplanar substrate for SERS sensing. Sens. Actuators B Chem. 2019, 287, 576–583. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.; Hassan, M.; Zhu, J.J.; Ahmad, W.; Li, H.H.; Chen, Q.S. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300–126307. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.L.; Gao, X.N.; Liu, X.; Shen, Y.H.; Xie, A.J. In-situ preparation of Ferrero® chocolate-like Cu2O@ Ag microsphere as SERS substrate for detection of thiram. J. Mater. Res. Technol. 2021, 11, 857–865. [Google Scholar] [CrossRef]
- Faghiri, F.; Hajjami, M.; Ghorbani, F. Development of a sensing system based on coupling magnetic solid phase extraction and colorimetric detection for determination of organophosphorus pesticides in fruit extract and environmental sample. Sens. Actuators B Chem. 2021, 343, 130157. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Li, W.H.; Yu, H.L. Silver nanoparticle-based chemiluminescent sensor array for pesticide discrimination. J. Agric. Food Chem. 2015, 63, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Monisha; Patel, S.; Thakur, S.S.; Shankar, R. Food safety monitoring of the pesticide phenthoate using a smartphone-assisted paper-based sensor with bimetallic Cu@Ag core-shell nanoparticles. Lab. Chip 2020, 20, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Kamyabi, M.A.; Moharramnezhad, M. A novel cathodic electrochemiluminescent sensor based on CuS/carbon quantum dots/g-C3N4 nanosheets and boron nitride quantum dots for the sensitive detection of organophosphate pesticide. Microchem. J. 2022, 179, 107421. [Google Scholar] [CrossRef]
- Hidayah, A.N.; Triyono, D.; Herbani, Y.; Saleh, R. Liquid surface-enhanced Raman spectroscopy (SERS) sensor-based Au-Ag colloidal nanoparticles for easy and rapid detection of deltamethrin pesticide in brewed tea. Crystals 2021, 12, 24. [Google Scholar] [CrossRef]
- Navaratna, N.; Tan, Y.J.; Kumar, A.; Gupta, M.; Singh, R. On-chip topological THz biosensors. Appl. Phys. Lett. 2023, 123, 033705–033720. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhao, R.; Cong, M.Y.; Qiu, J.F. Developments of terahertz metasurface biosensors: A literature review. Nanotechnol. Rev. 2024, 13, 20230182. [Google Scholar] [CrossRef]
- Xu, W.D.; Huang, Y.X.; Zhou, R.Y.; Wang, Q.; Yin, J.F.; Kono, J.; Ping, J.F.; Xie, L.J.; Ying, Y.B. Metamaterial-Free Flexible Graphene-Enabled Terahertz Sensors for Pesticide Detection at Bio-Interface. ACS Appl. Mater. Interfaces 2020, 12, 44281–44287. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.T.; Xiao, M.Y.; Cen, W.Y. Graphene-Based Metamaterial Sensor for Pesticide Trace Detection. Biosensors 2023, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Z.J.; Zhang, X.; Zhang, X.J.; Zhu, Y.Q.; Chen, S.G.; Hu, H. Excitation of surface plasmon resonance on multiwalled carbon nanotube metasurfaces for pesticide sensors. ACS Appl. Mater. Interfaces 2020, 12, 52082–52088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Zheng, S.; Zhang, C.J.; Wang, W.Q.; Wang, Q.; Li, Z.G.; Wang, S.; Zhang, L.; Liu, Y. Introduction of multilayered quantum dot nanobeads into competitive lateral flow assays for ultrasensitive and quantitative monitoring of pesticides in complex samples. Mikrochim. Acta 2023, 190, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.X.; Dong, H.W.; Wang, H.F.; Huang, J.C.; Li, D.H.; Li, Z.T.; Li, Z.P.; Li, P.S.; Zhang, P.W.; Zhao, M.X.; et al. Multifunctional nanoenzyme lateral flow immunoassay strip for rapid and ultrasensitive detection of carbofuran in vegetables. J. Hazard. Mater. 2024, 477, 135296. [Google Scholar] [CrossRef] [PubMed]





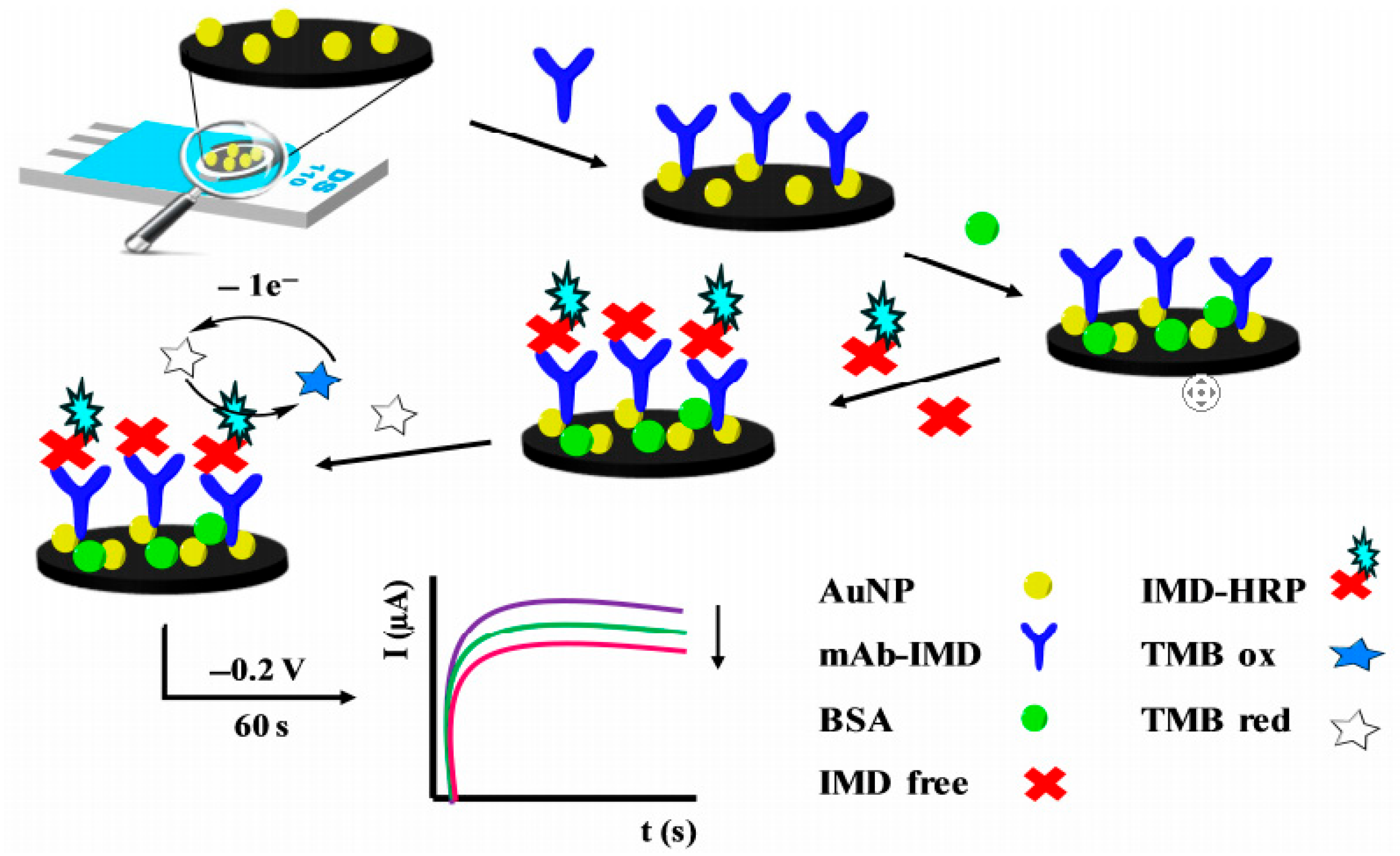











| Enzyme Types | Detection Methods | Target Pesticides | Ref. |
|---|---|---|---|
| AChE | Fluorimetry | OPs (chlorpyrifos) | [20,25,26] |
| AChE | Fluorimetry | Carbamate (carbaryl) | [27] |
| AChE | Colorimetry | OPs | [21,22] |
| AChE | SERS | OPs | [28] |
| AChE | Amperometric | OPs (chlorpyrifos) | [29,30] |
| AChE | DPV | OPs (chlorpyrifos) | [31,32,33,34] |
| AChE | Potentiometric | Carbamate (carbaryl) | [35] |
| Urease | Potentiometric | OPs (glyphosate) | [36] |
| Butyrylcholinesterase | Photoelectrochemical | OPs | [37] |
| AChE | CV | Carbamate pesticides | [38] |
| AChE | SWV | OPs (malathion) | [39] |
| AChE | Relative resistance change | Methamidophos | [40] |
| Detection Method | Nanomaterial | Targeted Analyst | Stability | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| DPV | MnO2-GNPs | Carbaryl; fenobucarb; carbosulfan | / | Carbaryl, 1–30 μM; Fenobucarb, 5–80 μM; Carbosulfan, 50–400 μM | Carbaryl, 0.30 μM; Fenobucarb, 1.40 μM; Carbosulfan, 15.15 μM | Rice and rice-field water | [72] |
| Potentiometric | Urease/Au NPs | Glyphosate | 180 days | 0.5–50 ppm | 0.5 ppm | Tap waters | [36] |
| Amperometric | Nafion/AChE-cSWCNT/MWCNT/AuNPs-Au | Methyl parathion; monocrotophos; chlorpyrifos; endosulfan | 2 months | 0.1–130 µM | Methyl parathion, 1.9 nM; monocrotophos, 2.3 nM; chlorpyrifos, 2.2 nM; endosulfan, 2.5 nM | Cabbage, Onion, Spinach | [30] |
| Amperometric | Ag NPs–CGR–NF/GCE | Chlorpyrifos and carbaryl | 30 days | Chlorpyrifos, 1.0 × 10−13–1 × 10−8 M; carbaryl, 1.0 × 10−12–1 × 10−8 M | Chlorpyrifos, 5.3 × 10−14 M; carbaryl, 5.45 × 10−13 M | Tap water, lake water sample | [33] |
| DPV | AChE-Chit/Pd@Au NWsN/GCE | Malathion | / | 0.1 pM–100 nM | 0.037 pM | Tap water | [73] |
| CV | PVA-AWP/Fe–Ni NP/AChE | Phosme | >30 days | 1 × 10−10–5 × 10−9 M | 0.1 nM | Olive oil | [69] |
| CV | Nano Ag-nano Fe3O4 | Methomyl | / | 2.97 × 10−5–3.47 × 10−4 mol/L | 2.08 × 10−5 mol/L | Vegetable | [74] |
| CV and DPV | Au@MWCNTs/GCE | Dichlorvos | / | 1–120 μM | 5 nM | Leaf lettuce | [60] |
| SWV | Zr-BDC-rGO | Methyl parathion | 28 days | 0.001–3.0 μg/mL | 0.5 ng/mL | Chinese cabbage | [70] |
| SWV | AChE-CS/3DG-CuO NFs/GCE | Malathion | 20 days | 3 pM–46.67 nM | 0.92 pM | River and lake water | [39] |
| DPV | MXene-rGO/Ed-Ab/FTO | Endosulfan | 21 days | 0.1–1 ppt | 0.497 ppt | Water, leaf and root extracts | [45] |
| CV and DPV | SPCE|CNTs/ZrO2/PB/Nf|GMP-AChE | Organophosphorus | 1.0 × 10−3–10 ng/mL | 5.6 × 10−4 ng/mL | Chinese cabbage | [24] | |
| CV | AuNPs/MPS/Au | Carbamate | 28 days | 0.003–2.00 μM | 1 nM | Fruits | [38] |
| DPV | AChE/MHCS/GCE; AChE/Fe3O4@MHCS/GCE | Malathion | 30 days | 0.01–600 ppb | 0.0182 ppb | Pear | [31] |
| Detection Method | Nanomaterial | Targeted Analyst | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| Fluorimetry | 6-carboxy-fluorescein labeling aptamer MNPs | Trichlorfon, glyphosate, and malathion | 0.0001 –10 mg/L | Trichlorfon, 72.20 ng/L; glyphosate, 88.80 ng/L; malathion 195.37 ng/L | Lettuce, carrot | [47] |
| SERS | Ag NPs coated glass bead | Chlorpyrifos, imidacloprid | Chlorpyrifos, 50–600 ng/mL; Imidacloprid, 50–700 ng/mL | Chlorpyrifos (10 ng/mL); imidacloprid (50 ng/mL) | Apple | [106] |
| SERS | AuNPs deposited in mesoporous silica film | 2,4-D, pymetrozine, and thiamethoxam | 2,4-D, 10−2–10−3 ng/mL; pymetrozine, 0.1–1000 ng/mL; thiamethoxam, 0.1–1000 ng/mL | 2,4-D (0.79 pg/mL), pymetrozine (1.04 pg/mL); thiamethoxam (1.21 pg/mL) | Tap water, apple, and milk | [107] |
| SERS | Cellulose nanofibers coated with AgNPs | Thiabendazole | 1–100 ppm | 5 ppm | Apple | [103] |
| Fluorimetry | Rhodamine B-modified Ag@Au NPs | Organophosphorus pesticides | 0.0033–0.28 ng/mL | 0.0018 ng/mL | Lake water, tomato, and apple | [80] |
| SERS | Au@Ag nanoparticles | Acetamiprid, thiram | Macetamiprid, 5–100 μM; thiram, 0.5–10 μM | Acetamiprid (1.22 μM), thiram (0.076 μM) | Apple juice | [98] |
| SERS | 4-mercaptophenylboronic acid modified Ag/Au bimetallic nanoprobes | Parathion-methyl | 5 × 10−9–5 × 10−4 M | 1.7 nM | Apple | [99] |
| SERS | Au-Ag colloid nanoparticles | Deltamethrin | 0.01–500 ppm | 0.01 ppm | Tea | [113] |
| Colorimetry | Citrate capped Cu@Ag core–shell nanoparticles | Phenthoate | 50–1500 μg/L | 15 μg/L | Water and food | [111] |
| SERS | Hexagonal boron nitride coated with highly dense and monodisperse Cu-Ag nanoalloys | Thiram and tricyclazole | Thiram, 10−4–10−9 M; tricyclazole, 10−5–10−9 M | Thiram (1 pM) and tricyclazole (1 nM) | Tomato | [100] |
| SERS | Ferrero® chocolate-like Cu2O@Ag microspheres | Thiram | 1.2 × 10−7–2.4 × 10−6 M | 1.2 × 10−7 M | Apple | [108] |
| SERS | SPE modified by dropping Ag NPs | Acetamiprids | 0.1–1000 μM | 0.04 μM | Brassica chinensis L. | [67] |
| Fluorimetry | CDs/AChE/GO | Chlorpyrifos | 1–25 ppb | 0.14 ppb | Tap water | [25] |
| Fluorimetry | CDs-AChE aerosol | Paraoxon; parathion; dichlorvos; deltamethrin | 10−5–10−12 M | Paraoxon, 1.2 pM; parathion, 0.94 pM; dichlorvos. 11.7 pM; deltamethrin, 0.38 pM | Apple | [20] |
| Fluorimetry | AuNP/Ab/ssDNAs; MNP-OVA; Au@Pt/ssDNAs | Parathion, triazophos, and chlorpyrifos | 0.01–31.62 ng/mL | Parathion, 9.88 ng/kg; triazophos, 3.91 ng/kg; chlorpyrifos, 1.47 ng/kg | Cabbage, apple, pear, and rice | [43] |
| Colorimetry | Fe3O4/GO | Malathion | 0.001–5 mg/L | 0.014 mg/L | Apple and river water | [109] |
| Fluorimetry | CQDs | viz., quinalphos 25 EC; thiamethoxam 25 WG; propargite 57 EC | 0.2–5000 ng/mL | viz., quinalphos 25 EC, 0.2 ng/mL; thiamethoxam 25 WG, 1 ng/m; propargite 57 EC, 10 ng/mL | Tea | [82] |
| Fluorimetry | CdSe/ZnS/QD/Ab | Triazophos | 10–25 μg/L | 0.508 ng/L | Apple, pear, cucumber, and rice | [78] |
| Fluorimetry and Colorimetry | B, N-doped CQDs | Carbaryl | 0.20–150 μg/L | 0.06 μg/L | Lake water and tap water | [27] |
| Fluorimetry | OPCD@UiO-66-NH2 | Quinalphos | 0–16 μM | 0.3 nM | Tomato and rice | [86] |
| Fluorimetry | Red-emission carbon dots | Pyrethroid | 1–120 μg/L | 0.89 μg/L | Tea and grapes | [88] |
| SPR | Poly nanofilms composed of N-methacryloyl-L-cysteine methyl ester, ethylene glycol dimethacrylate, 2-hydroxyethy methacrylate | Coumaphos | 0.1–250 ppb | 0.001 ppb | Honey | [53] |
| SERS | Silver colloid | Chlorpyrifos; 2,4-D | 0.001–1000 mg/L | Chlorpyrifos, 1.28 × 10−9 M; 2,4-D, 2.47 × 10−10 M | Apple | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Yue, X.; Li, J.; Zhao, F.; Yu, X.; Yang, K. Research Advances in Nanosensor for Pesticide Detection in Agricultural Products. Nanomaterials 2025, 15, 1132. https://doi.org/10.3390/nano15141132
Feng L, Yue X, Li J, Zhao F, Yu X, Yang K. Research Advances in Nanosensor for Pesticide Detection in Agricultural Products. Nanomaterials. 2025; 15(14):1132. https://doi.org/10.3390/nano15141132
Chicago/Turabian StyleFeng, Li, Xiaofei Yue, Junhao Li, Fangyao Zhao, Xiaoping Yu, and Ke Yang. 2025. "Research Advances in Nanosensor for Pesticide Detection in Agricultural Products" Nanomaterials 15, no. 14: 1132. https://doi.org/10.3390/nano15141132
APA StyleFeng, L., Yue, X., Li, J., Zhao, F., Yu, X., & Yang, K. (2025). Research Advances in Nanosensor for Pesticide Detection in Agricultural Products. Nanomaterials, 15(14), 1132. https://doi.org/10.3390/nano15141132







