Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
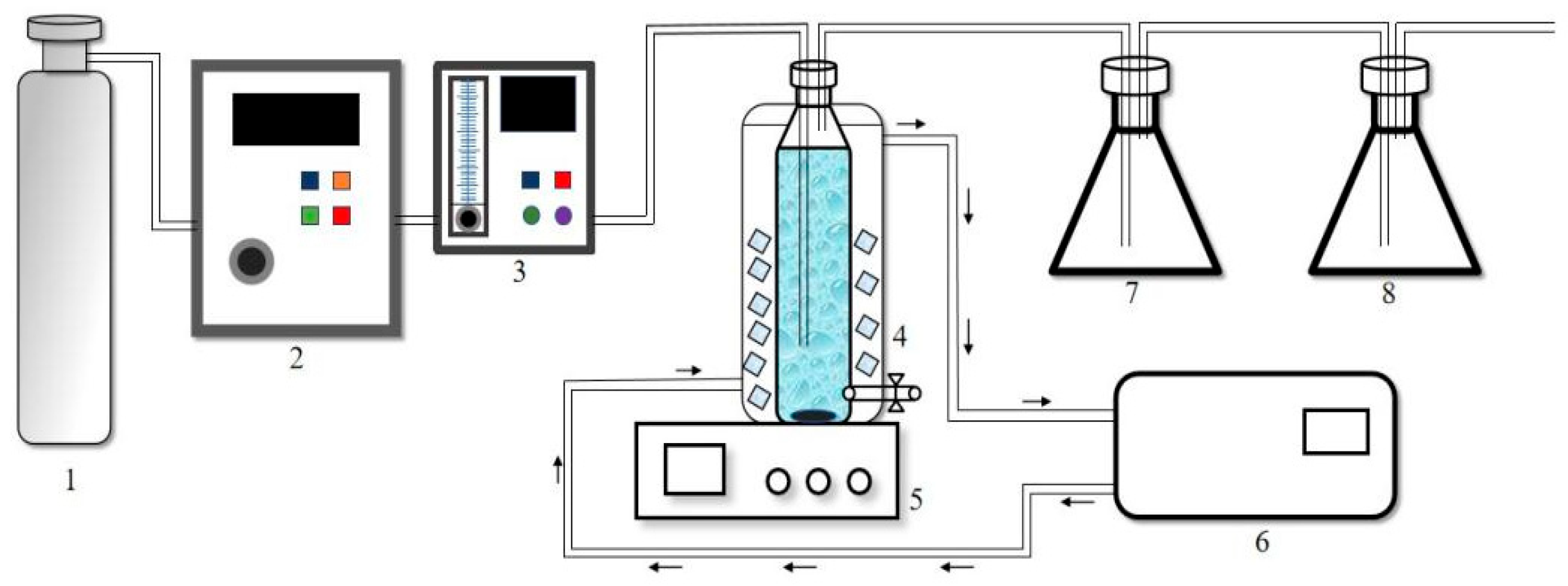
2.2. Experimental Flow
2.3. Experimental Steps
- (1)
- Before the start of the experiment, the pipeline was first connected to the tail gas treatment device, and the O3 generator was opened to the desired gear to run for 15 min to stabilize the O3 concentration in the subsequent reaction. At the same time, the water bath was preheated to 25 °C.
- (2)
- Then the target reaction solution was added to the reactor, while magnetic stirring was turned on (with the aim of eliminating the dead water zone), and the pipeline valve was switched to the reactor.
- (3)
- After the experiment started, samples were taken at 2 min intervals, 1 mL of water samples were taken each time, and 100 μL of Na2S2O3 standard solution (reaction terminator) was dropped into the sample bottle after sampling.
- (4)
- The reaction solution was poured out 30 min after the end of the experiment to avoid the release of O3 from the water into the laboratory.
2.4. Analysis and Detection Methods
3. Structural Characterization of FeMoBC Material
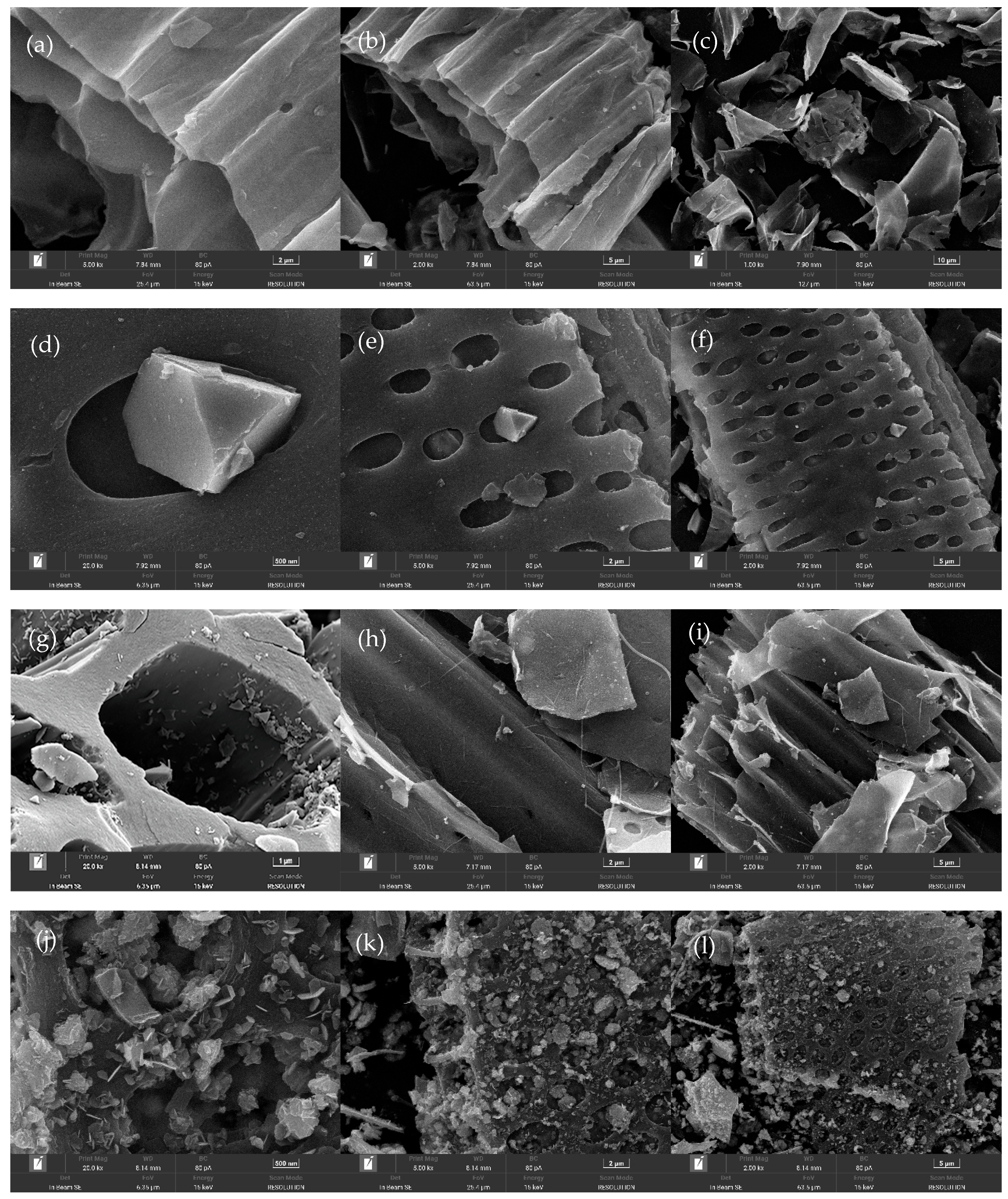
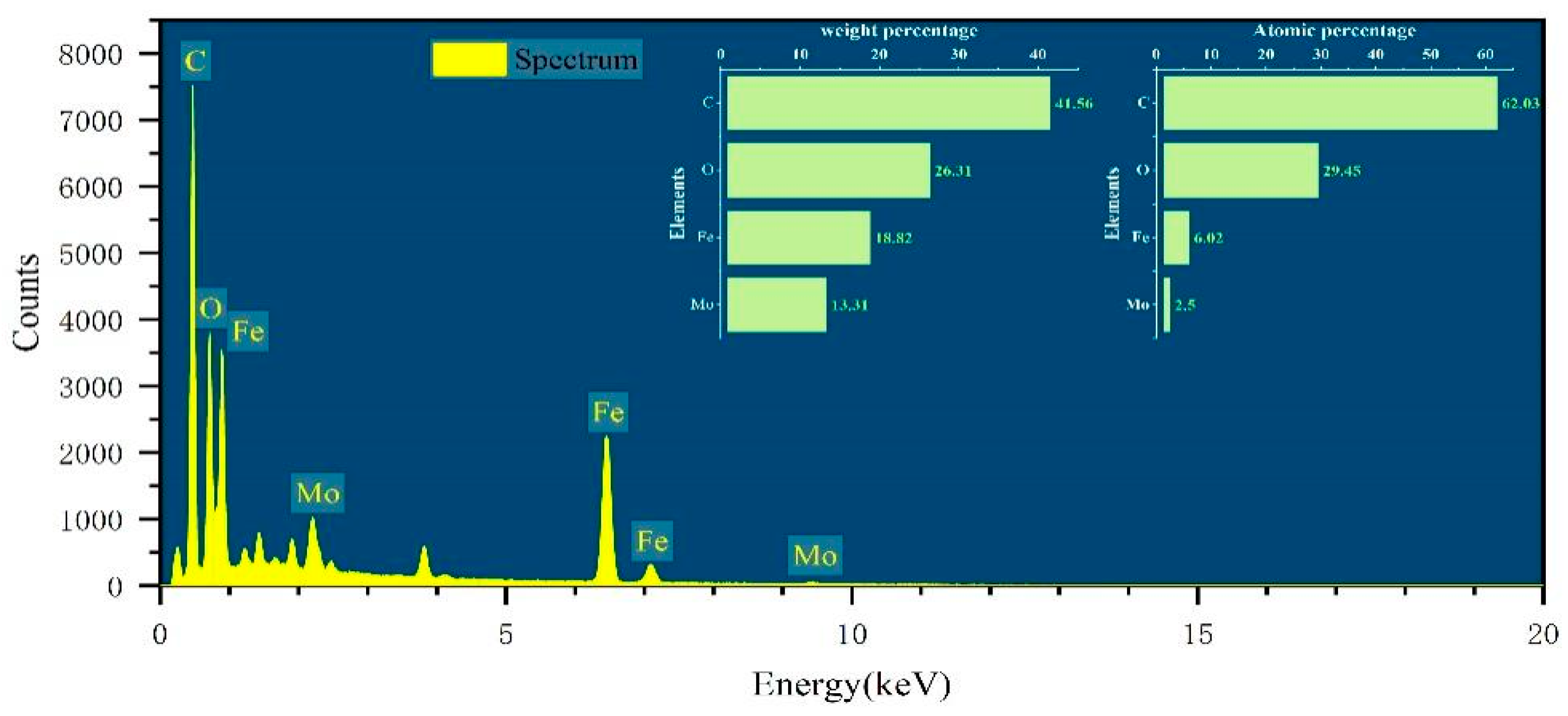
3.1. SEM–EDS Characterization Analysis
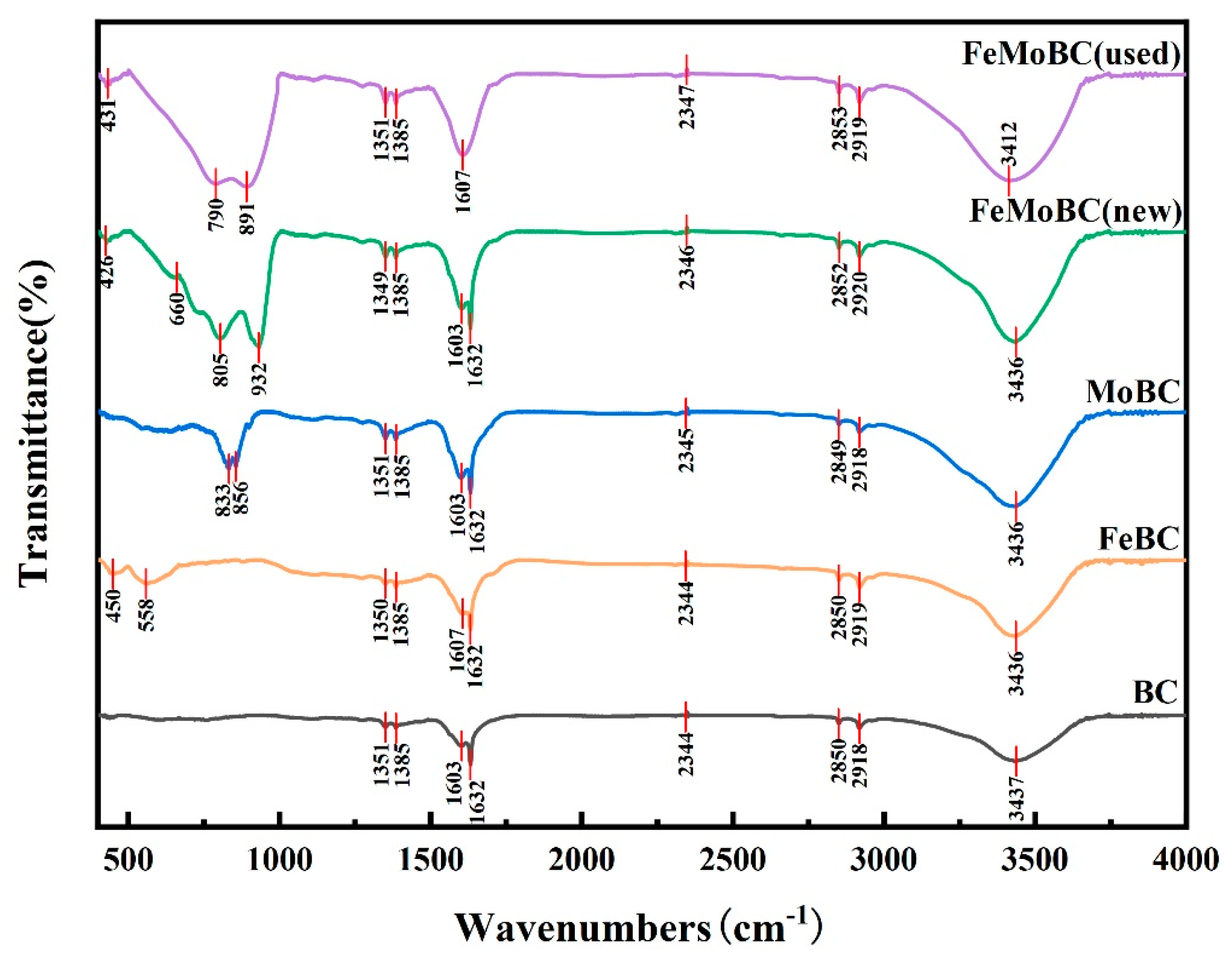
3.2. FTIR Characterization Analysis
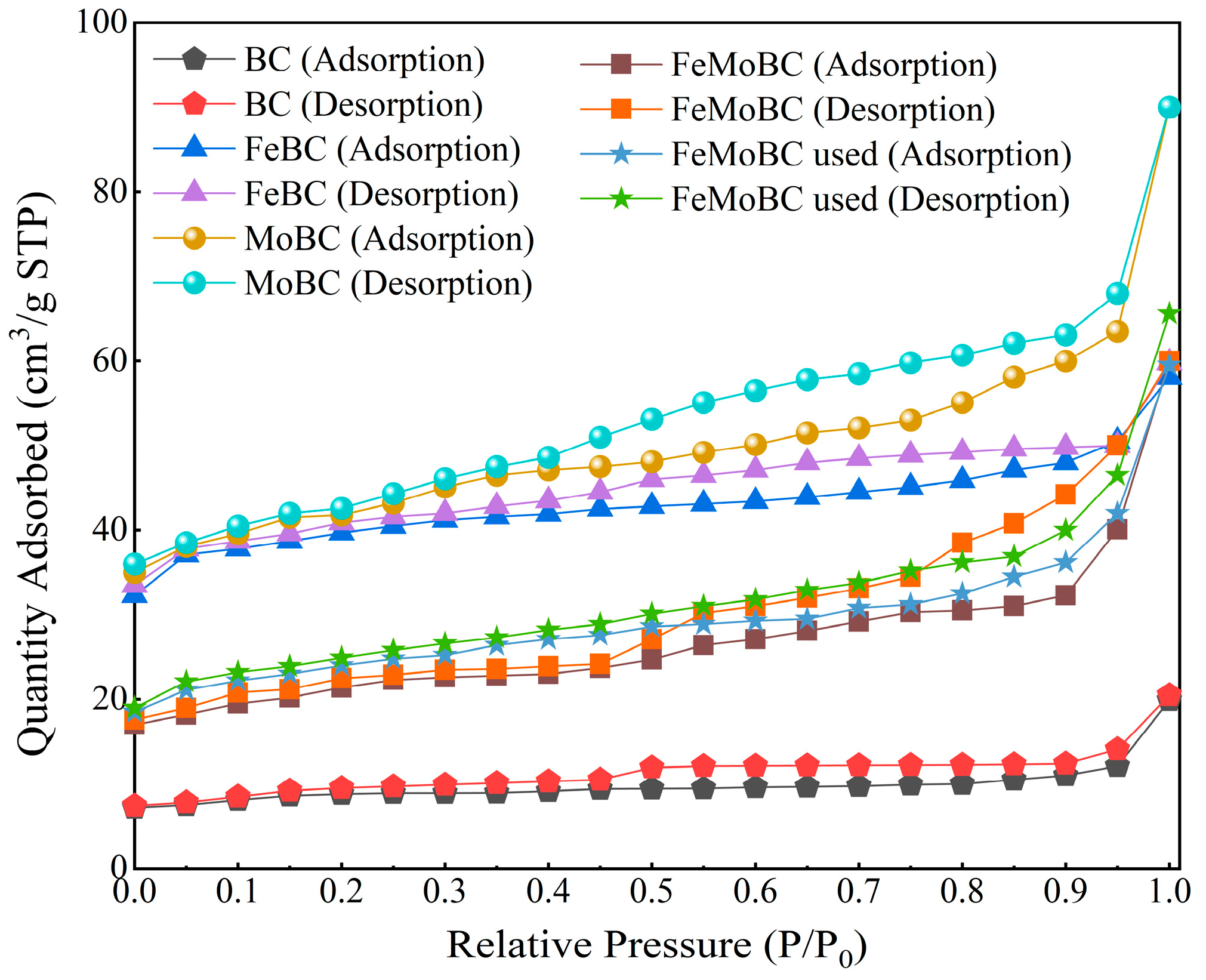
3.3. BET Characterization Analysis
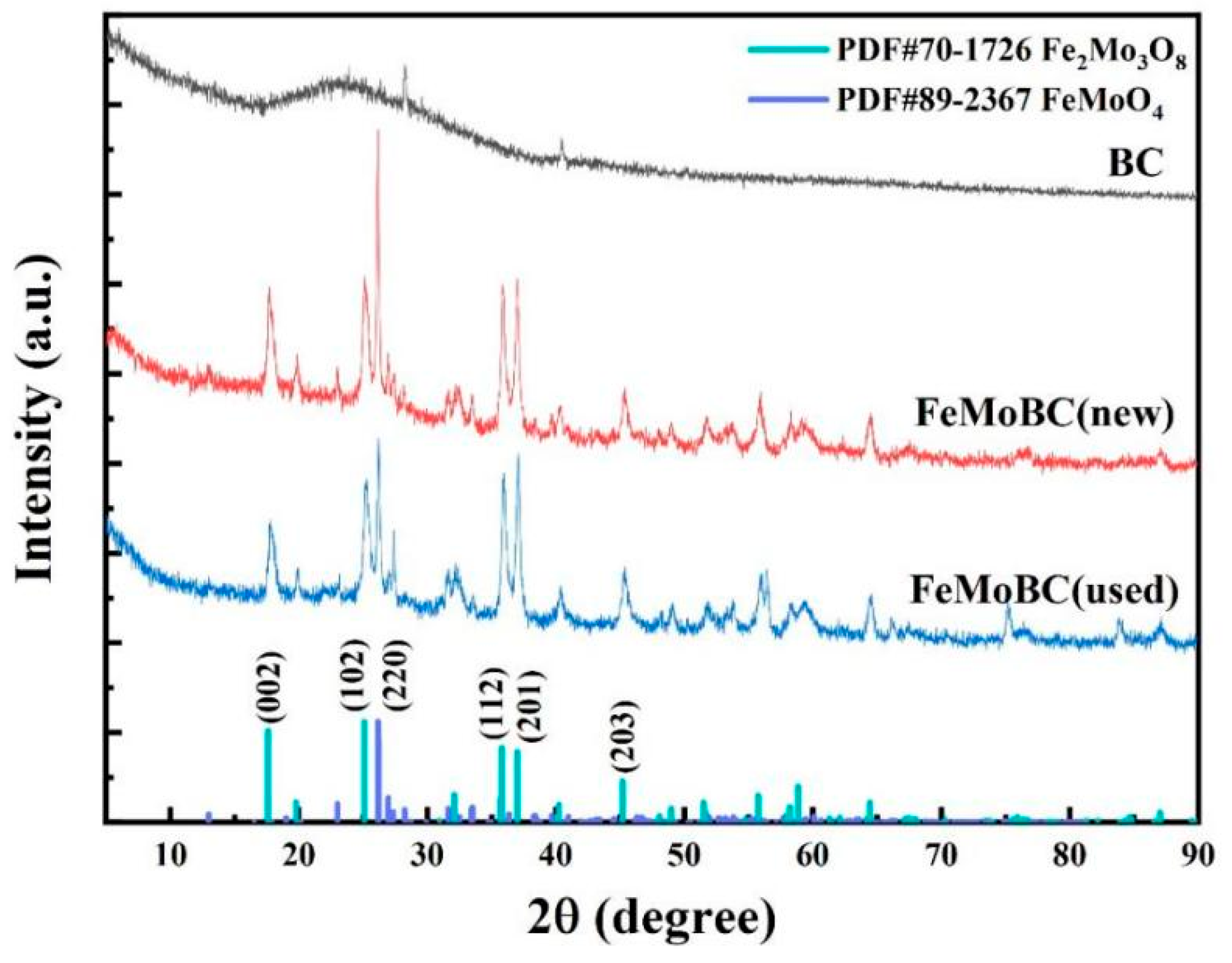
3.4. XRD Characterization Analysis
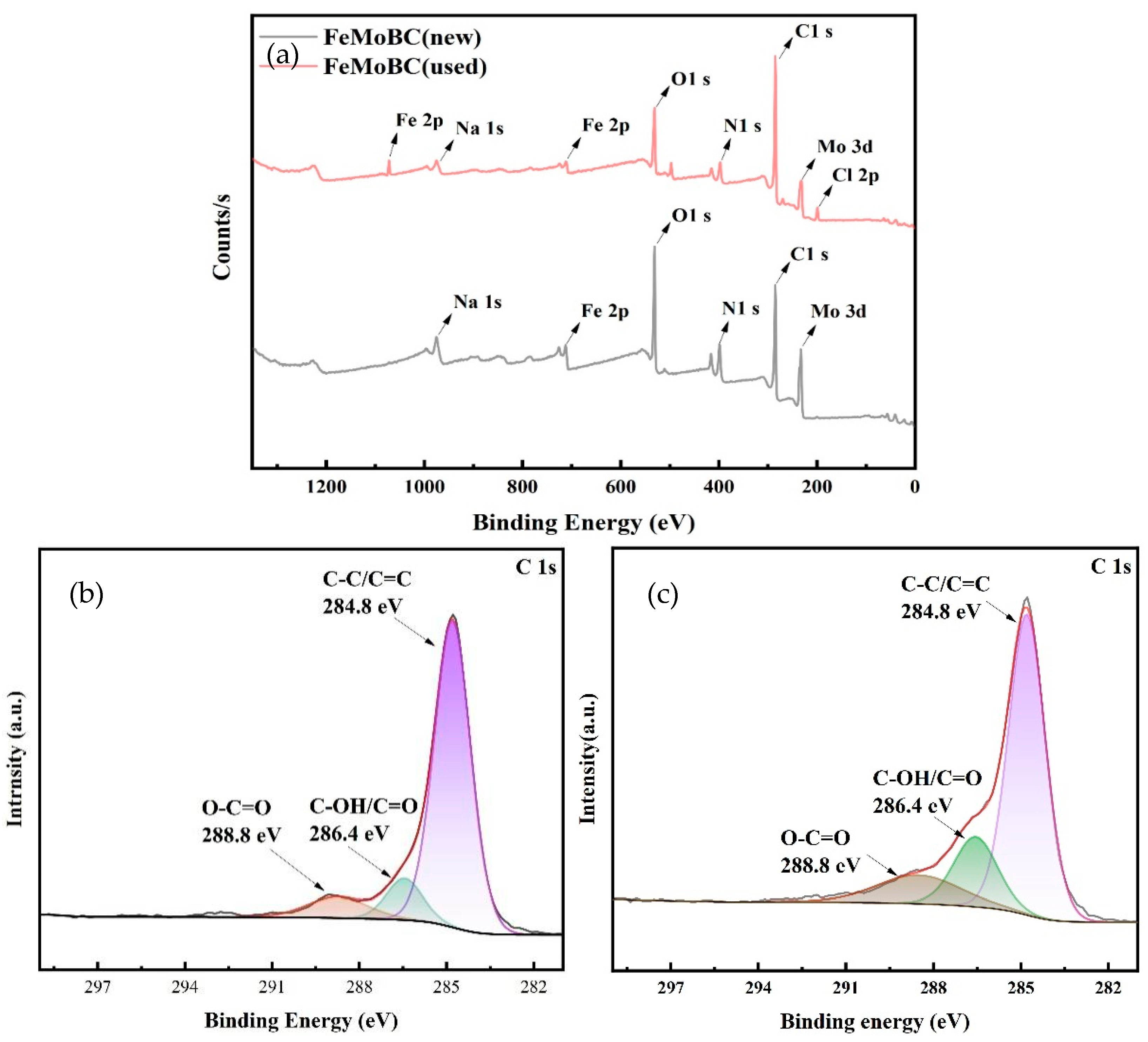
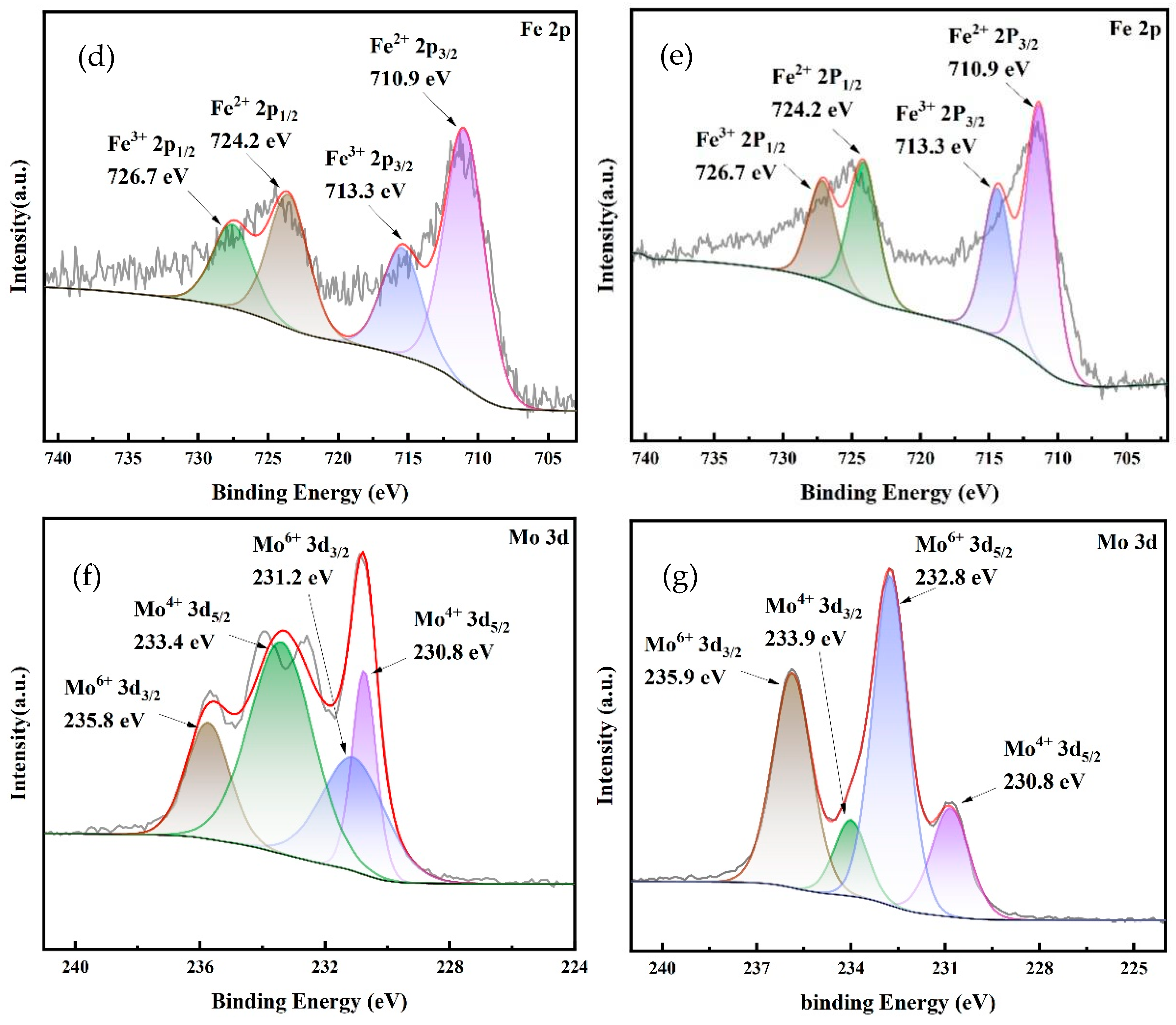
3.5. XPS Characterization
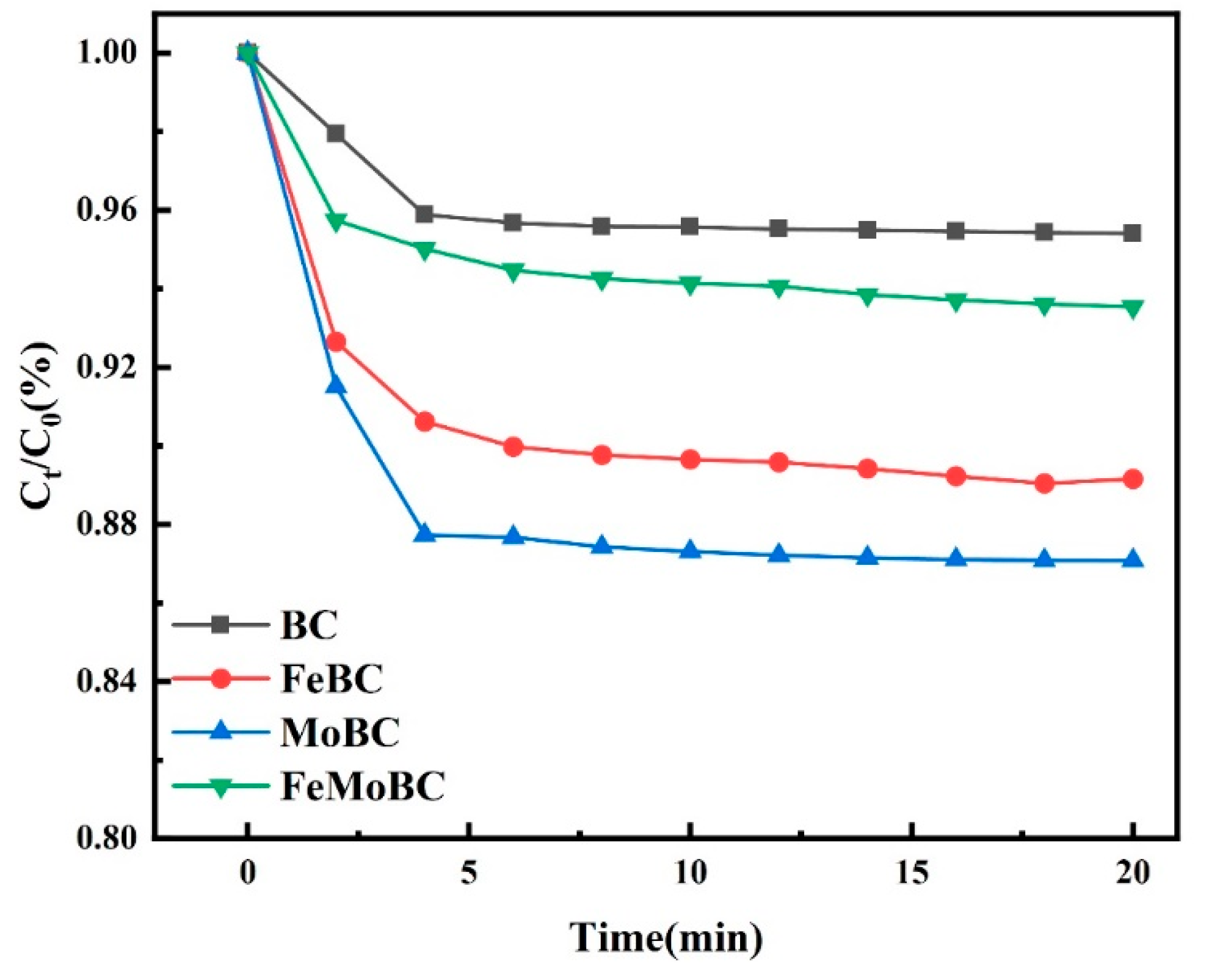
3.6. Effect of Catalyst on TC Adsorption
4. Efficacy of O3, O3/PMS, and O3/PMS/FeMoBC Processes for TC Degradation in Single-Factor Test
4.1. Effect of Gaseous O3 Concentration on Degradation Efficacy
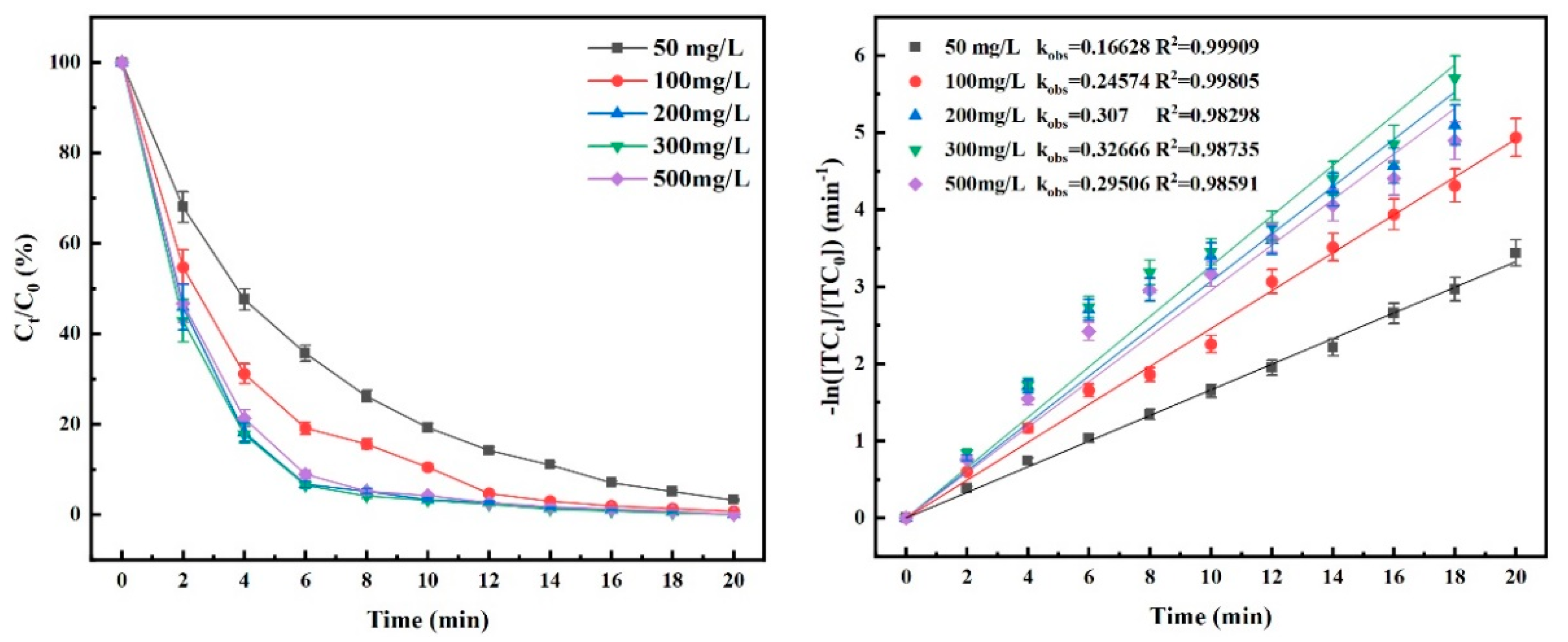
4.2. Effect of Initial TC Concentration on Degradation Efficiency
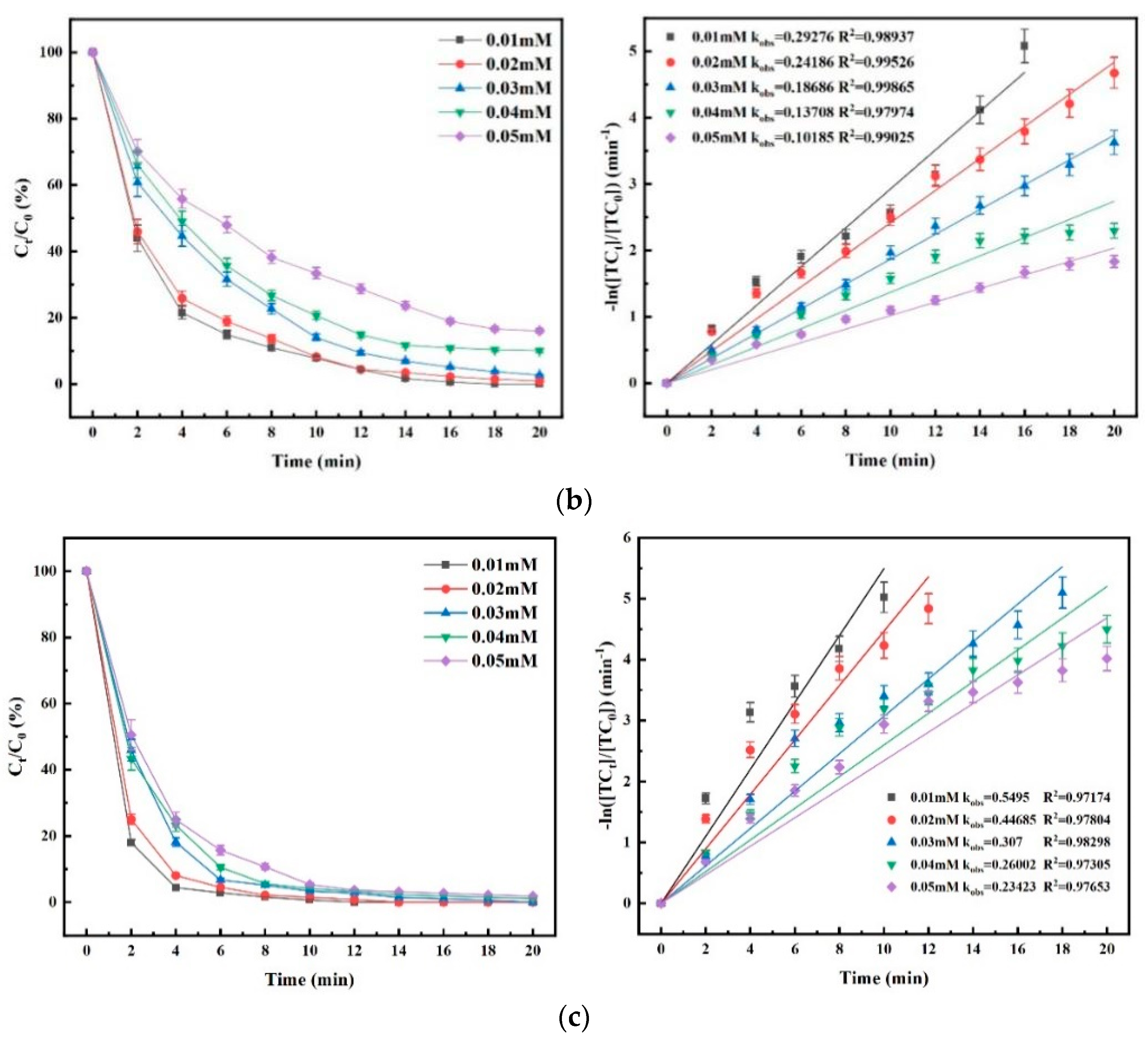
4.3. Effect of PMS Concentration on Degradation Efficiency
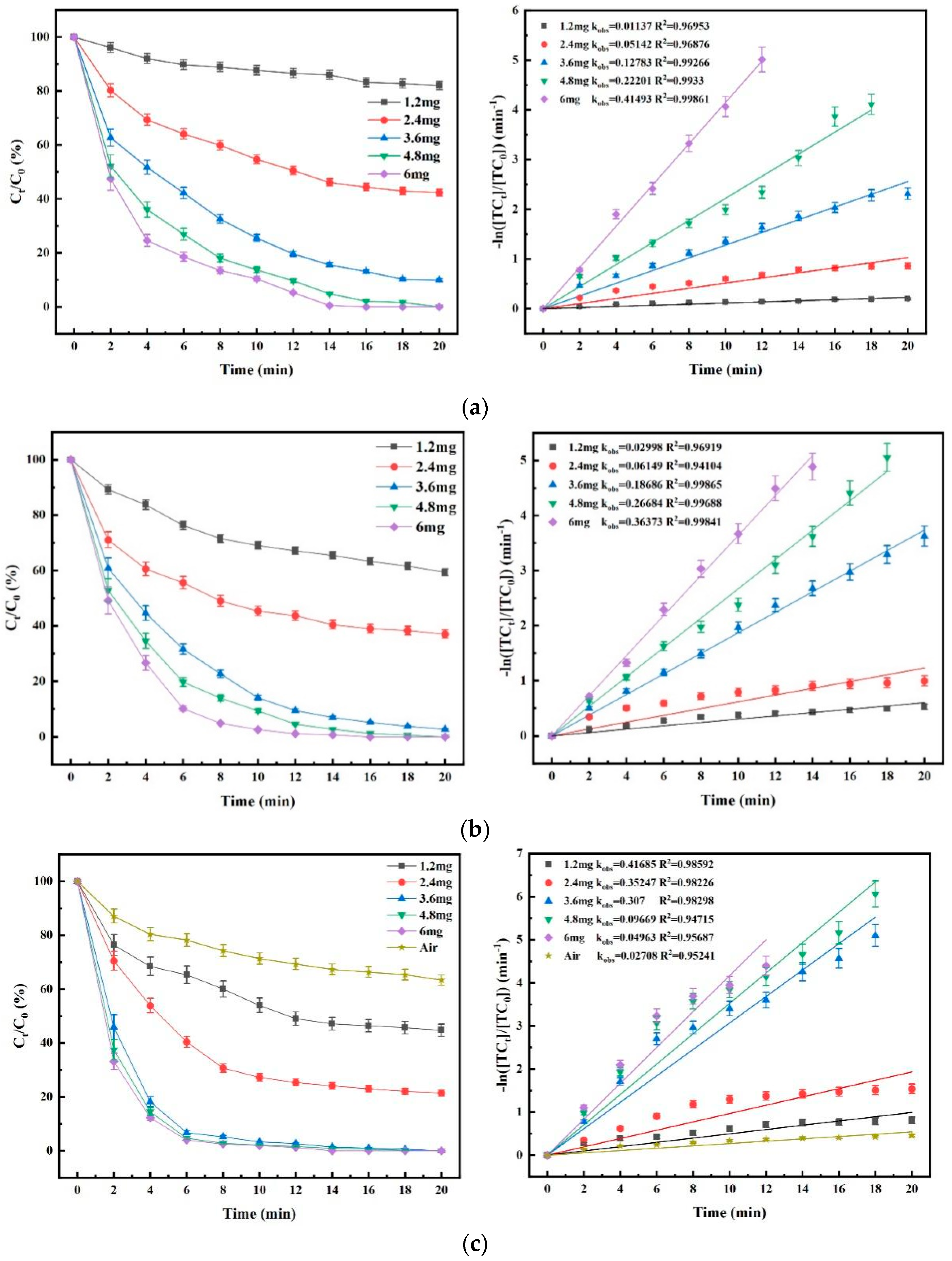
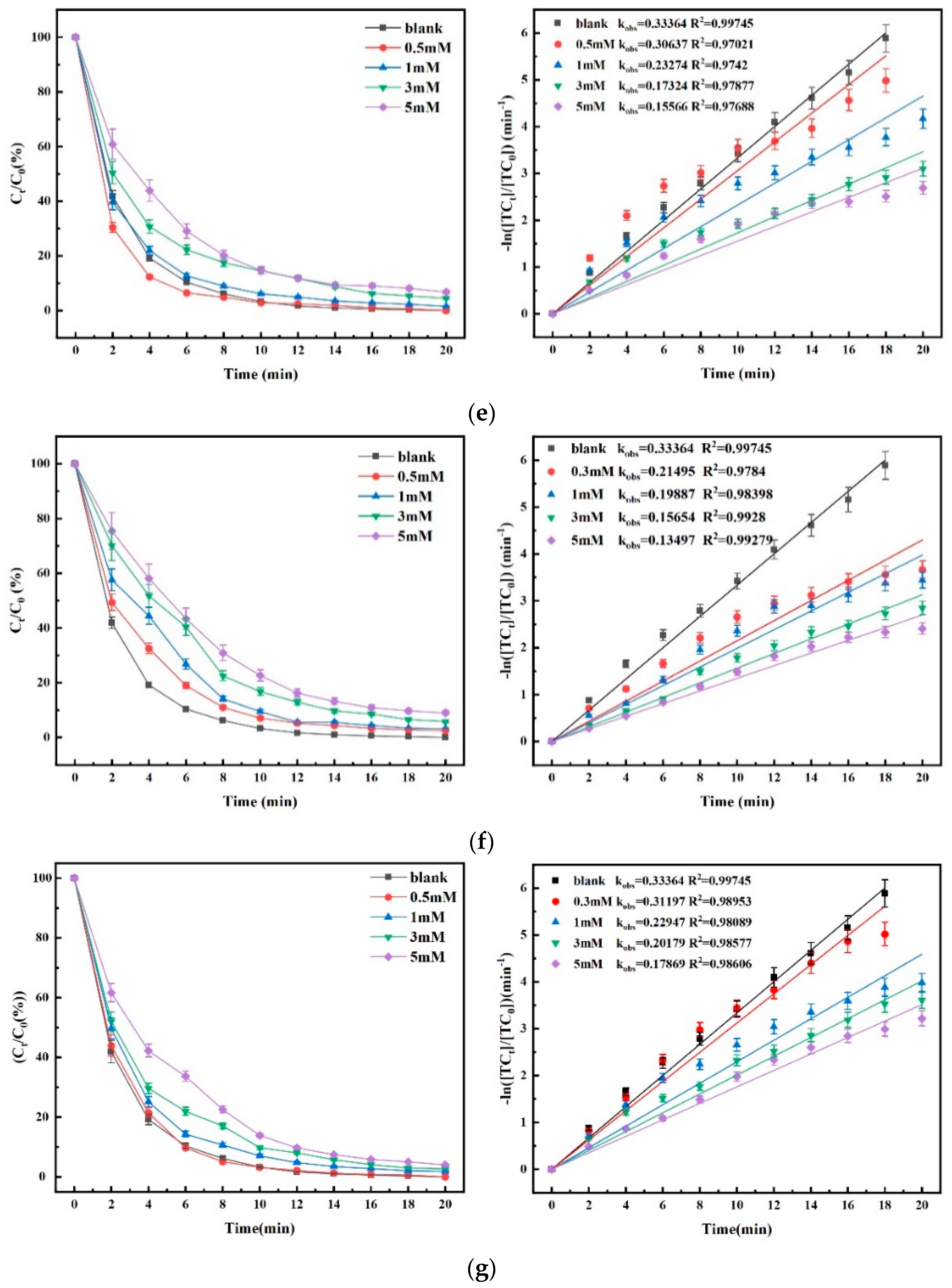
4.4. Effect of Material Dosage on Degradation Efficacy
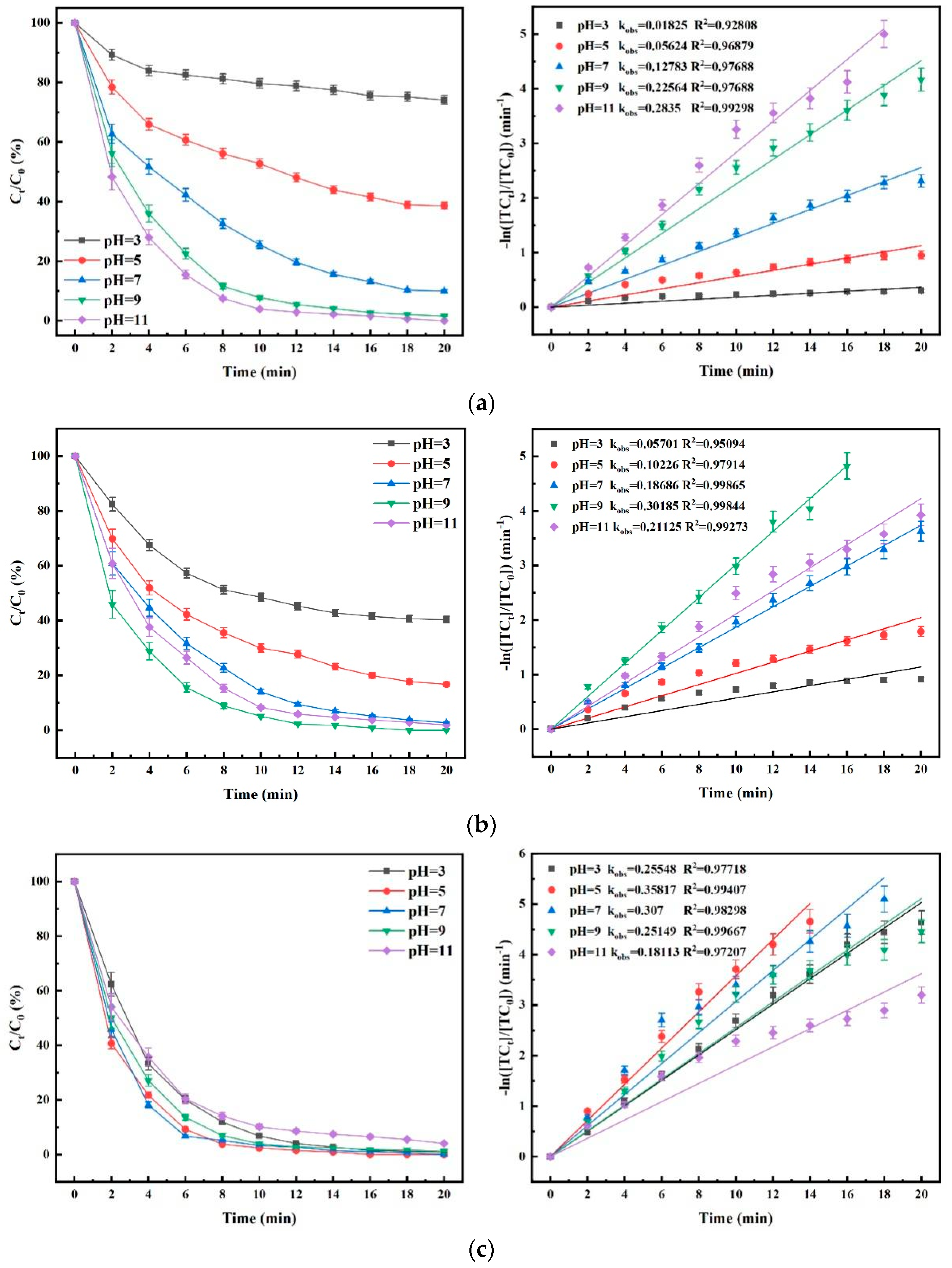
4.5. Influence of Aqueous Environment pH on TC Degradation Efficacy
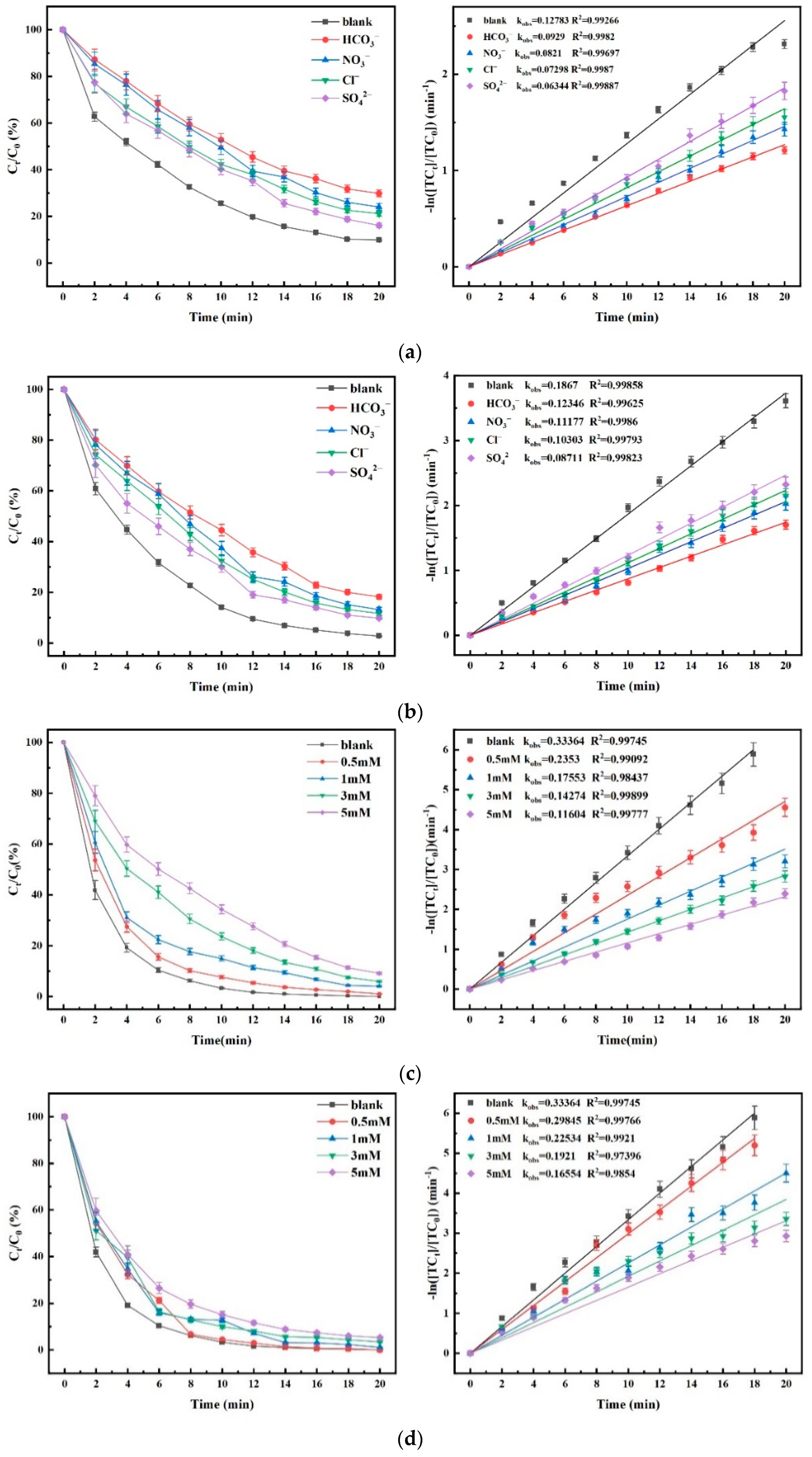
4.6. Effect of Common Inorganic Anions in Water on Degradation Efficacy
| No. | Equation of a Chemical Reaction | Reaction Rate (M−1·s−1) | Reference | |
|---|---|---|---|---|
| HCO3− | (6) | 8.6 × 106 | [33] | |
| (7) | 3.9 × 108 | [37] | ||
| NO3− | (8) | None | [35] | |
| (9) | None | [35] | ||
| (10) | None | [35] | ||
| (11) | None | [36] | ||
| (12) | None | [36] | ||
| Cl− | (13) | 4.3 × 109 | [38] | |
| (14) | 6.1 × 109 | [38] | ||
| (15) | 3.0 × 108 | [39] | ||
| (16) | 2.5 × 108 | [39] | ||
| (17) | 8.5 × 109 | [39] | ||
| (18) | 6.0 × 104 | [40] | ||
| (19) | 2.1 × 1010 | [37] | ||
| (20) | 1.0 × 109 | [40] | ||
| SO42− | (21) | None | [39] | |
| (22) | None | [39] | ||
| (23) | None | [39] |
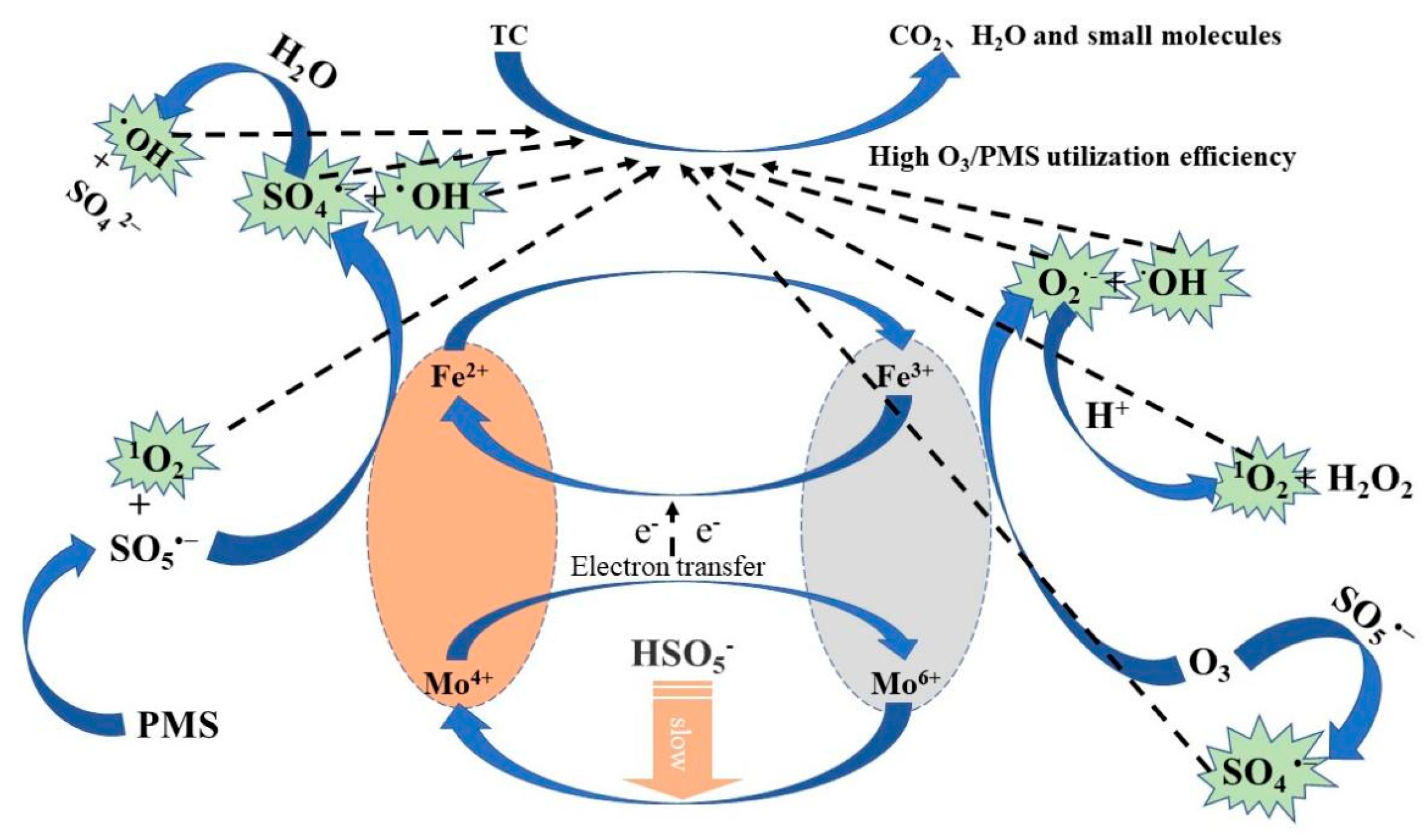
5. O3/PMS/FeMoBC Reaction Mechanism Speculation
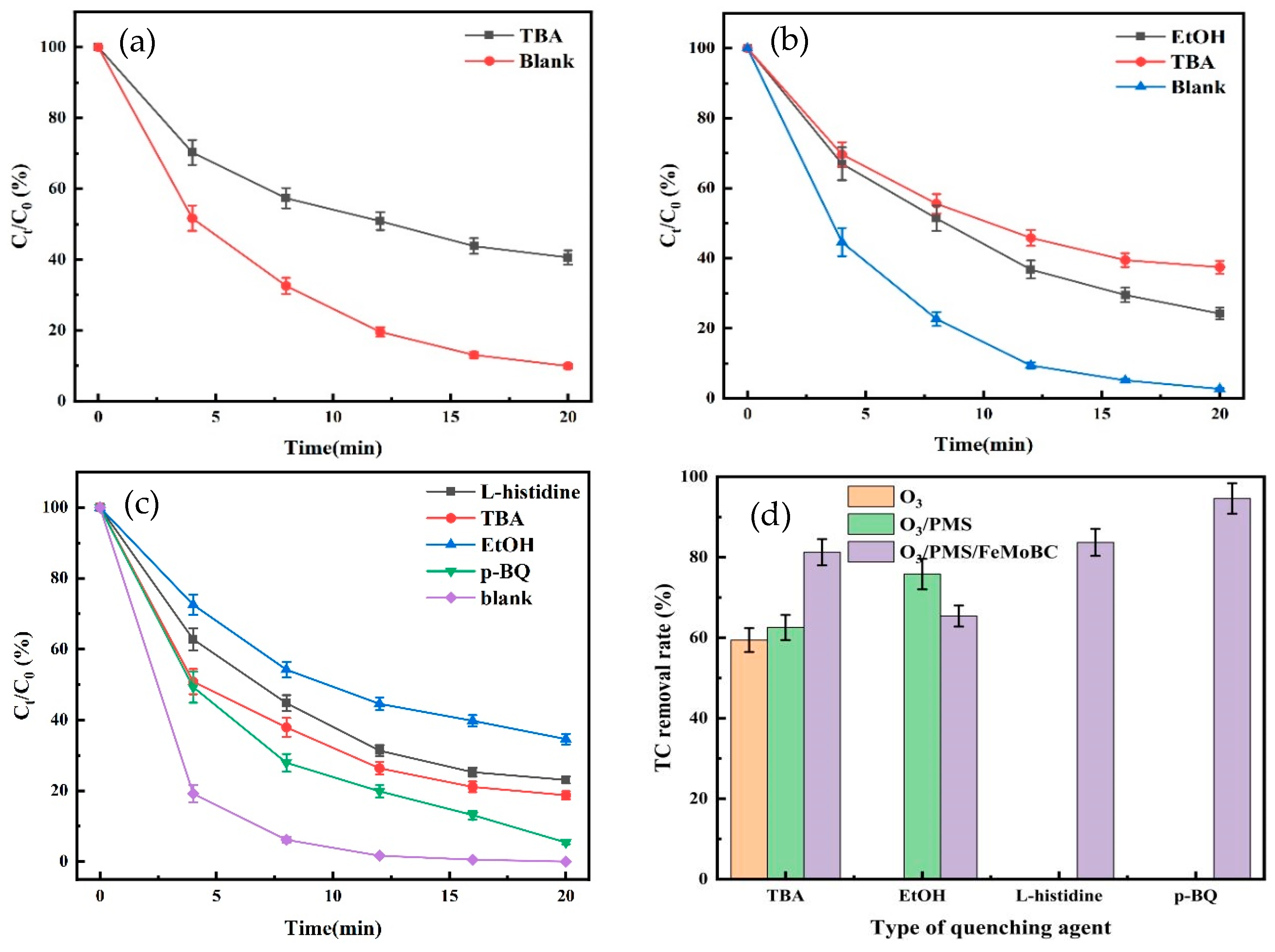
5.1. Free Radical Burst Experiments for O3, O3/PMS, and O3/PMS/FeMoBC
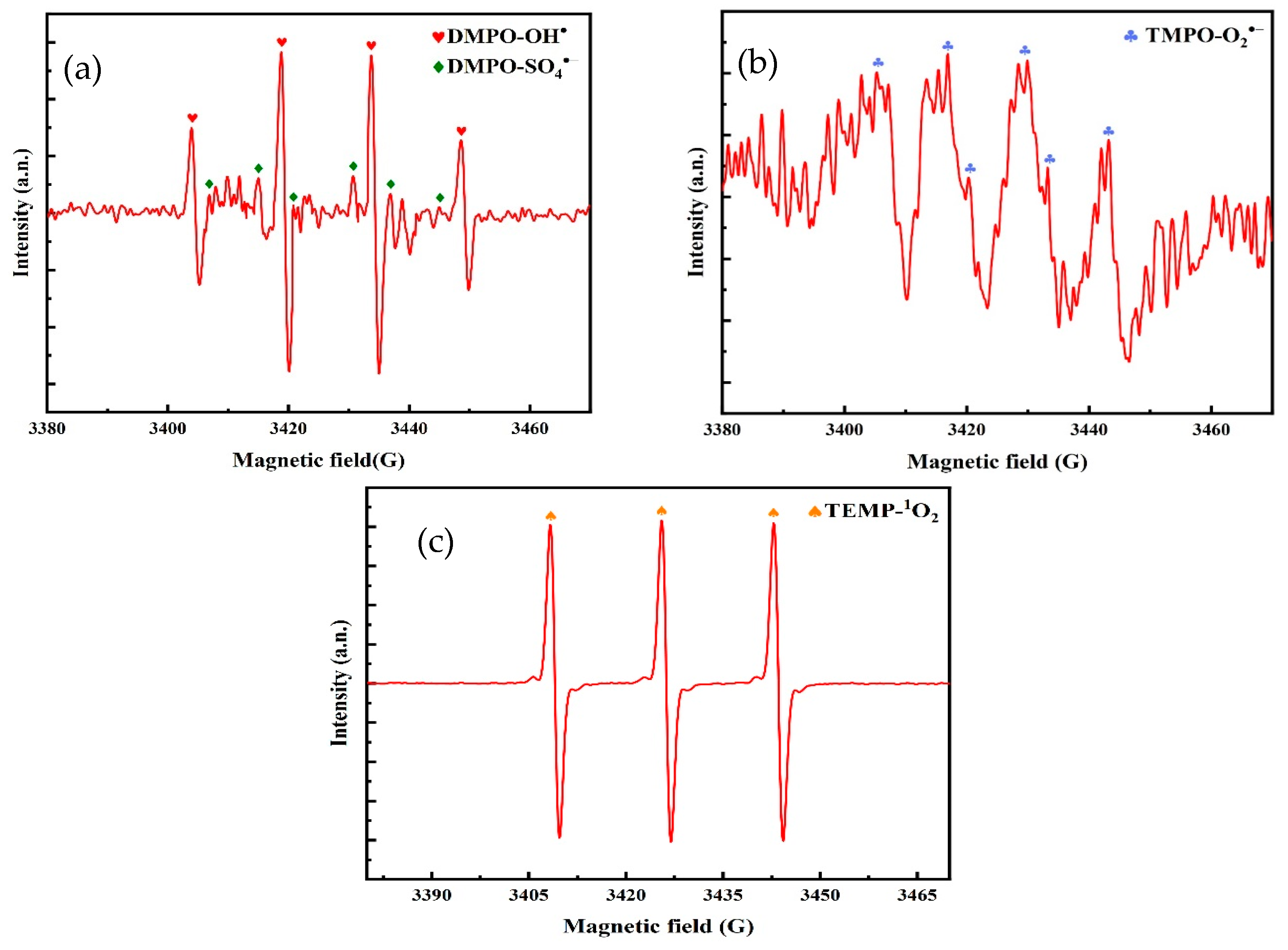
5.2. Analysis of Free Radical Species for TC Degradation by O3/PMS/FeMoBC
6. Conclusions
- (1)
- SEM–EDS, FTIR, BET, XRD, XPS, and other analysis results showed that Mo and Fe were stably loaded on the surface and pores of BC during the preparation of iron molybdate composite, forming more oxygen-containing functional groups of Mo and Fe. The main components of the composite catalytic material were Fe2Mo3O8 and FeMoO4, accounting for 26.3% and 73.7%, respectively. In addition, the catalytic reaction was mainly concentrated on the catalyst surface.
- (2)
- O3, O3/PMS, and O3/PMS/FeMoBC processes all could effectively remove TC from water, among which the O3/PMS/FeMoBC process had the best effect. The results of single factor experiments show that in the O3/PMS/FeMoBC process, the degradation rate of TC decreased with the increase in its initial concentration and increased with the increase in O3 concentration. The best pH condition is weak acidity. Too high of an oxidant concentration would reduce the degradation effect of TC, which was consistent with the trend of the other two methods. However, the O3/PMS/FeMoBC system showed stronger anti-interference ability.
- (3)
- Quenching experiments were carried out using TBA, EtOH, L–histidine, and p–BQ to quench the free radicals generated in the O3, O3/PMS, and O3/PMS/FeMoBC systems, respectively. The results showed that the O3 molecule played the main oxidizing role in the O3 system alone, and both •OH and SO4•− reactive substances were present in the O3/PMS system. For the O3/PMS/FeMoBC system, EtOH had the greatest effect on it, suggesting that •OH and SO4•− were the main reactive substances in the system. L–histidine and p–BQ decreased the degradation efficiency during the chemical actions, suggesting that 1O2 and •O2− may be generated in this system. Combined with the above free radical quenching experiments and the analysis results of EPR technique, it could be concluded that four active oxidants, •OH, SO4•−, 1O2, and •O2−, were present in the O3/PMS/FeMoBC system, and their contributions were, in descending order, •OH, 1O2, SO4•−, and •O2−.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morin–Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Huang, S.; Du, X. Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem. Eng. J. 2012, 203, 269–276. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S. Fabrication of novel magnetic MnFe2O4/bio–char composite and heterogeneous photo–Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Hoigne, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water. Water Res. 1983, 17, 173–183. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Zeng, Z.; Arowo, M.; Zou, H.; Chen, J.; Shao, L. Degradation of methyl orange by ozone in the presence of ferrous and persulfate ions in a rotating packed bed. Chemosphere 2016, 146, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Peyton, G.R. The free–radical chemistry of persulfate–based total organic carbon analyzers. Mar. Chem. 1993, 41, 91–103. [Google Scholar] [CrossRef]
- Du, W.; Liu, L.; Zhou, K.; Ma, X.; Hao, Y.; Qian, X. Black lead molybdate nanoparticles: Facile synthesis and photocatalytic properties responding to visible light. Appl. Surf. Sci. 2015, 328, 428–435. [Google Scholar] [CrossRef]
- Cleophas, T.J.M. An Alternative Analysis for Crossover Studies that Accounts for Between-Group Disparities in Drug Response. Clin. Chem. Lab. Med. 1998, 36, 981–982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, J.; Guo, X.; Zhang, T.; Zha, F. Design of micro-nano spherical β-NiOOH/FeMo04 composite with enhanced photo-Fenton performance. Appl. Catal. A Gen. 2022, 630, 118427. [Google Scholar] [CrossRef]
- GB-28232-2020; Hygienic Requirements for Ozone Disinfector. Standards Press of China: Beijing, China, 2020.
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Zhen, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, G. Study on the degradation of N-methylpyrrolidone by iron–molybdenum modified biochar activated persulfuric acid. Appl. Chem. Ind. 2024, 53, 40–44. [Google Scholar]
- Khan, Z.H.; Gao, M.; Wu, J.; Bin, R.; Mehmood, C.T.; Song, Z. Mechanism of As(III) removal properties of biochar-supported molybdenum-disulfide/iron-oxide system. Environ. Pollut. 2021, 287, 117600. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Sere, C.; Ferey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. 2008, 120, 4212–4216. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, L.; Zhang, S.; Tang, J. Nitrogen–doped biochar (N-doped BC) and iron/nitrogen co–doped biochar (Fe/N co-doped BC) for removal of refractory organic pollutants. J. Hazard. Mater. 2023, 446, 130727. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Qin, Y.; Jia, Y.; Li, Y.; Zhao, Y.; Pan, Y.; Sun, J. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: A review. Chemosphere 2021, 274, 129766. [Google Scholar]
- Manna, I.; Dutta Majumdar, J.; Ramesh, C.B.; Nayak, S.; Dahotre, N.B. Laser surface cladding of Fe-B-C, Fe-B-Si and Fe-BC-Si-Al-C on plain carbon steel. Surf. Coat. Tech. 2006, 201, 434–440. [Google Scholar] [CrossRef]
- Han, X.; Gerke, C.S.; Banerjee, S.; Zubair, M.; Jiang, J.; Bedford, N.M.; Miller, E.M.; Thoi, V.S. Strategic Design of MoO2 Nanoparticles Supported by Carbon Nanowires for Enhanced Electrocatalytic Nitrogen Reduction. ACS Energy Lett. 2020, 5, 3237–3243. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Y.; Sun, Y.; Tu, R.; Ren, Z.; Liang, K.; Jiang, E.; Ren, Y.; Xu, X. Functional groups anchoring–induced Ni/MoOx-Ov interface on rice husk char for hydrodeoxygenation of bio-guaiacol to BTX at ambient-pressure. Renew. Energy 2022, 200, 579–591. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, H.; Liang, Y.; Jiang, Z.; Wang, H.; Nian, Y. Activation of persulfate by blue algae biochar supported FeOX particles for tetracycline degradation: Performance and mechanism. Sep. Purif. Technol. 2023, 319, 124005. [Google Scholar] [CrossRef]
- Zhang, N.; Li, M.; Liu, X. Distribution and migration of antibiotic resistance genes in soil. China Environ. Sci. 2018, 38, 2609–2617. [Google Scholar]
- Yuan, Z.; Sui, M.; Yuan, B.; Li, P.; Wang, J.; Qin, J.; Xu, G. Degradation of ibuprofen using ozone combined with peroxymonosulfate. Environ. Sci.: Water Res. Technol. 2017, 3, 960–969. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Li, X.; Li, L. The relation of interface electron transfer and PMS activation by the H-bonding interaction between composite metal and MCM-48 during sulfamethazine ozonation. Chem. Eng. J. 2020, 398, 125529. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, H.; Liu, S.; Zhang, L.; Qiang, Z. Accelerated oxidation of iopamidol by ozone/peroxymonosulfate (O3/PMS) process: Kinetics, mechanism, and simultaneous reduction of iodinated disinfection by–product formation potential. Water Res. 2020, 173, 115615. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, Q.; Chen, X.; Deng, H.; Feng, W.; Lu, H. Application of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Highly Concentrated Organic Wastewater: A Review. Separations 2022, 9, 444. [Google Scholar] [CrossRef]
- Yang, N.; Cui, J.; Zhang, L.; Xiao, W.; Alshawabkeh, A.N.; Mao, X. Iron electrolysis-assisted peroxymonosulfate chemical oxidation for the remediation of chlorophenol-contaminated groundwater. J. Chem. Technol. Biotechnol. 2016, 91, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Deniere, E.; Hulle, S.V.; Langenhove, H.V.; Demeestere, K. Advanced oxidation of pharmaceuticals by the ozone-activated peroxymonosulfate process: The role of different oxidative species. J. Hazard. Mater. 2018, 360, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ji, F.; Liu, T.; Yan, P.; Guan, W.; Xu, X. Synergistic effect of bifunctional Co-TiO2 catalyst on degradation of Rhodamine B: Fenton-photo hybrid process. Chem. Eng. J. 2013, 229, 57–65. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Liao, C.H.; Kang, S.F.; Wu, F.A. Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2/UV process. Chemosphere 2001, 44, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical–based water treatment in presence of chloride: Formation of chlorate, inter–conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lei, X.; Westerhoff, P.; Zhang, X.; Yang, X. Reactivity of Chlorine Radicals (Cl• and Cl2•−) with Dissolved Organic Matter and the Formation of Chlorinated Byproducts. Environ. Sci. Technol. 2021, 55, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.J.; Doyle, A.G. Direct C(sp3)-H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals. J. Am. Chem. Soc. 2016, 138, 12719–12722. [Google Scholar] [CrossRef] [PubMed]
- Huie, R.E.; Clifton, C.L. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4−, with anions. J. Am. Chem. Soc. 1990, 94, 8561–8567. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- DAS, T.N. Reactivity and Role of SO5•− Radical in Aqueous Medium Chain Oxidation of Sulfite to Sulfate and Atmospheric Sulfuric Acid Generation. J. Phys. Chem. A. 2001, 105, 9142–9155. [Google Scholar] [CrossRef]
- Yu, X.Y.; Barker, J.R. Hydrogen Peroxide Photolysis in Acidic Aqueous Solutions Containing Chloride Ions I. Chemical Mechanism. J. Phys. Chem. A 2003, 107, 1313–1324. [Google Scholar] [CrossRef]
- Jaafarzaden, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Naumov, S.; Mark, G.; Jarocki, A.; Sonntag, C.V. The Reactions of Nitrite Ion with Ozone in Aqueous Solution—New Experimental Data and Quantum-Chemical Considerations. Ozone: Sci. Eng. 2010, 32, 430–434. [Google Scholar] [CrossRef]
- Wu, D.; Wong, D.; Di Bartolo, B. Evolution of Cl−2 in aqueous NaCl solutions. J. Photochem. 1980, 14, 303–310. [Google Scholar] [CrossRef]
- Lin, X.; Ma, Y.; Wan, J.; Wang, Y.; Li, Y. Efficient degradation of Orange G with persulfate activated by recyclable FeMoO4. Chemosphere 2019, 214, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.H.; Tu, Y.T.; Chen, D.S.; Chen, X.; Xiong, Y. Degradation of Acid Orange II at neutral pH using Fe2(MoO4)3 as a heterogeneous Fenton-like catalyst. Chem. Eng. J. 2011, 169, 31–37. [Google Scholar] [CrossRef]
- Lu, Y.S.; Wang, Z.; Xu, Y.F.; Liu, Q.; Qian, G.R. Fe2(MoO4)3 as a novel heterogeneous catalyst to activate persulfate for Rhodamine B degradation. Desalin. Water Treat. 2015, 57, 7898–7909. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, J.; Xu, J. Fe (II)-promoted activation of peroxymonosulfate bymolybdenum disulfide for effective degradation of acetaminophen. Chem. Eng. J. 2020, 381, 122718. [Google Scholar] [CrossRef]
- Yang, J.C.E.; Lin, Y.; Peng, H.H.; Yuan, B.; Dionysiou, D.D.; Huang, X.D.; Zhang, D.D.; Fu, M.L. Novel magnetic rod-like n-Fe oxycarbide toward peroxymonosulfate activation for efficient oxidation of butyl paraben: Radical oxidation versus singlet oxygenation. Appl. Catal. B–Environ. Energy 2020, 268, 118549. [Google Scholar] [CrossRef]
- Wei, C.; Song, B.; Yuan, J.; Feng, Z.; Jia, G.; Li, C. Luminescence and Raman spectroscopic studies on the damage of tryptophan, histidine and carnosine by singlet oxygen. J. Photochem. Photobiol. A Chem. 2007, 189, 39–45. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, J.; Hu, Y.; Liu, Y.; Hu, S.; Zhu, C. Photochemical reactions between 1,4–benzoquinone and O2•−. Environ. Sci. Pollut. Res. 2020, 27, 31289–31299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, W.; Zhang, C.; Sun, Y.; Wang, Y.; Gong, Z.; Zhan, M.; Fu, Y.; Liu, K. Effective removal of refractory organic contaminants from reverse osmosis concentrated leachate using PFS–nZVI/PMS/O3 process. Waste Manag. 2021, 128, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cao, J.; Xiang, Y.; Xie, R.; Suo, Z.; Ao, Z.; Yang, X.; Huang, H. Accelerated iron cycle inducing molecular oxygen activation for deep oxidation of aromatic VOCs in MoS2 co-catalytic Fe3+/PMS system. Appl. Catal. B Environ. 2022, 309, 121235. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Long, X.; Song, J.; Chen, Y.; Zhang, I.Y.; Wang, A.; Huang, R. Effective degradation of recalcitrant naphthenic acids by the Fe(III)/peroxymonosulfate oxidation enhanced by molybdenum disulfide: Degradation performance and mechanisms. J. Environ. Chem. Eng. 2024, 12, 112842. [Google Scholar] [CrossRef]
- Fu, W.; Yi, J.; Cheng, M.; Liu, Y.; Zhang, G.; Li, L.; Du, L.; Li, B.; Wang, G.; Yang, X. When bimetallic oxides and their complexes meet Fenton-like process. J. Hazard. Mater. 2022, 424, 127419. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Bimetallic Sites for Catalysis: From Binuclear Metal Sites to Bimetallic Nanoclusters and Nanoparticles. Chem. Rev. 2023, 123, 4855–4933. [Google Scholar] [CrossRef] [PubMed]



| Reagent | Chemical Formula | Pureness | Manufacturer |
|---|---|---|---|
| Tetracycline hydrochloride | C22H24N2O8•HCl | ≥99% | Shanghai McLean Biochemical Technology Co. (Shanghai, China) |
| Sodium hyposulfide | Na2S2O3 | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| Ferric chloride hexahydrate | Fecl3•6H2O2 | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| Sodium chloride | NaCl | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| Methanoic acid | HCOOH | ≥99% | Shanghai McLean Biochemical Technology Co. (Shanghai, China) |
| Sodium nitrate | NaNO3 | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| Tertiary butyl alcohol | C4H10O | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| Sodium bicarbonate | NaHCO3 | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| L–Histidine | C6H9N3O2 | ≥99% | Shanghai McLean Biochemical Technology Co. (Shanghai, China) |
| Potassium peroxymonosulfate | KHSO5 | AR | Sigma–Aldrich Ltd. (St. Louis, MO, USA) |
| Methyl alcohol | CH3OH | Superior Pure | Sigma–Aldrich Ltd. (St. Louis, MO, USA) |
| Sodium molybdate | Na2MoO4 | AR | Sinopharm Chemical Reagent Co. (Shanghai, China) |
| P–benzoquinone | C6H4O2 | ≥99% | Shanghai McLean Biochemical Technology Co. (Shanghai, China) |
| Sample | SBET (m2g−1) | Smicro (m2g−1) | Vtotal pore (cm3g−1) | Daver (nm) |
|---|---|---|---|---|
| BC | 28.8771 | 18.0232 | 0.031685 | 4.3890 |
| FeBC | 151.4387 | 110.0570 | 0.091465 | 2.4159 |
| MoBC | 160.4957 | 94.4786 | 0.139421 | 3.4748 |
| FeMoBC (new) | 80.3445 | 44.5264 | 0.094498 | 4.7046 |
| FeMoBC (used) | 82.7599 | 58.6966 | 0.102354 | 4.9470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Q.; Chen, X.; Yan, B.; Li, S.; Deng, H.; Lu, H. Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process. Nanomaterials 2025, 15, 1108. https://doi.org/10.3390/nano15141108
Li X, Li Q, Chen X, Yan B, Li S, Deng H, Lu H. Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process. Nanomaterials. 2025; 15(14):1108. https://doi.org/10.3390/nano15141108
Chicago/Turabian StyleLi, Xuemei, Qingpo Li, Xinglin Chen, Bojiao Yan, Shengnan Li, Huan Deng, and Hai Lu. 2025. "Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process" Nanomaterials 15, no. 14: 1108. https://doi.org/10.3390/nano15141108
APA StyleLi, X., Li, Q., Chen, X., Yan, B., Li, S., Deng, H., & Lu, H. (2025). Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process. Nanomaterials, 15(14), 1108. https://doi.org/10.3390/nano15141108






