From Batch to Pilot: Scaling Up Arsenic Removal with an Fe-Mn-Based Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FMBO Nanocomposite Characterization
2.3. Investigated Water Matrices
2.4. Batch Adsorption Experiment
2.5. Column Adsorption Experiment
2.6. Pilot Experiment
2.7. Analytical Methods
3. Results and Discussion
3.1. FMBO Nanocomposite Chracterization
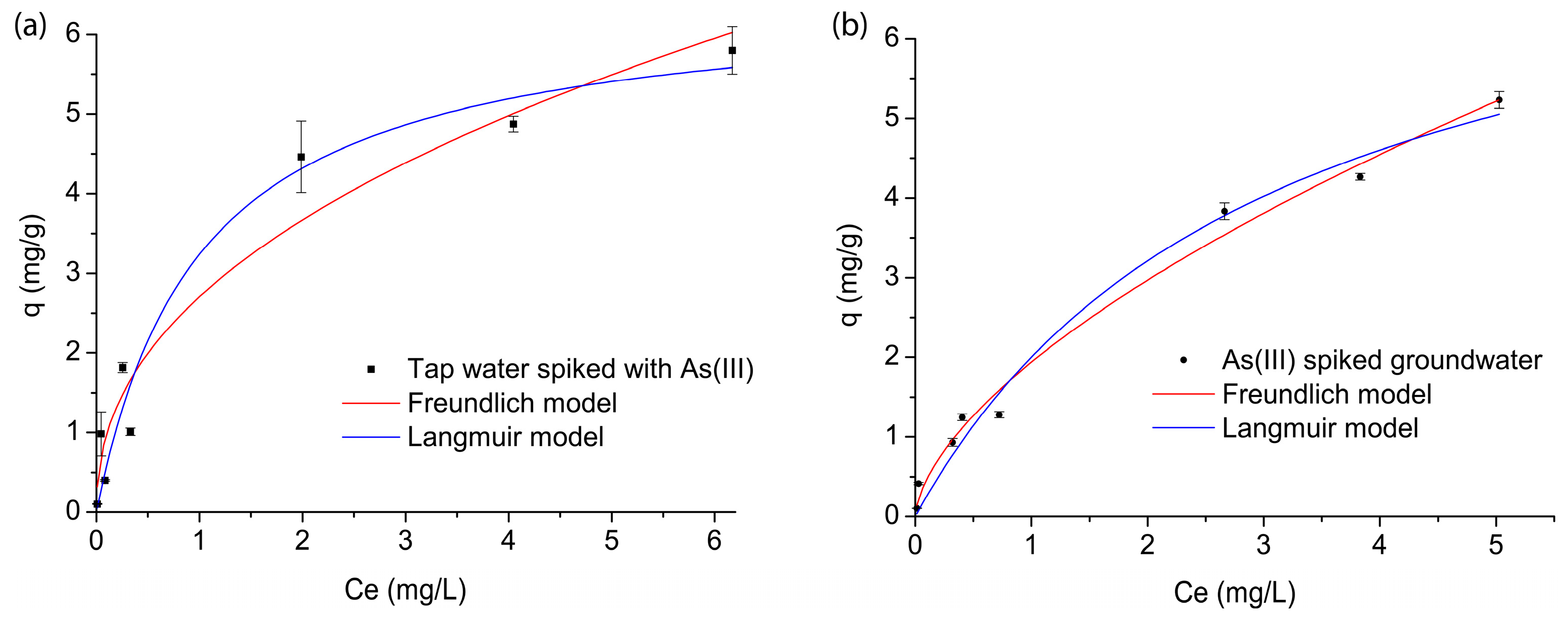
3.2. Batch Adsorption Study
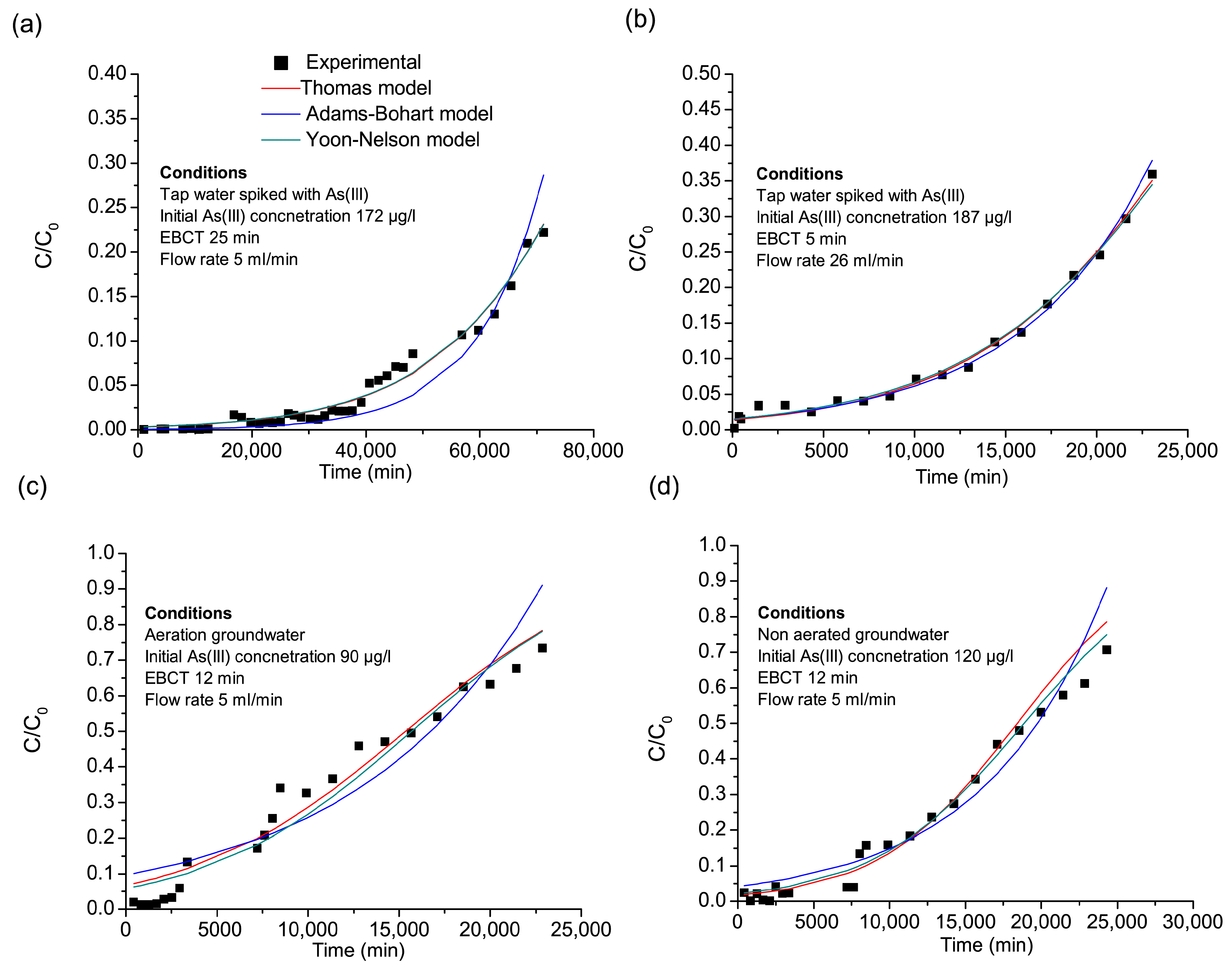
3.3. Column Adsorption Study
Modeling of Arsenic Adsorption in Fixed-Bed Columns
3.4. Pilot Study
3.4.1. Modeling of Arsenic Adsorption Under Realistic Treatment Conditions
3.4.2. Arsenic Speciation and Removal Mechanism
3.5. Comparison Between Batch Experiments and Pilot-Scale Studies
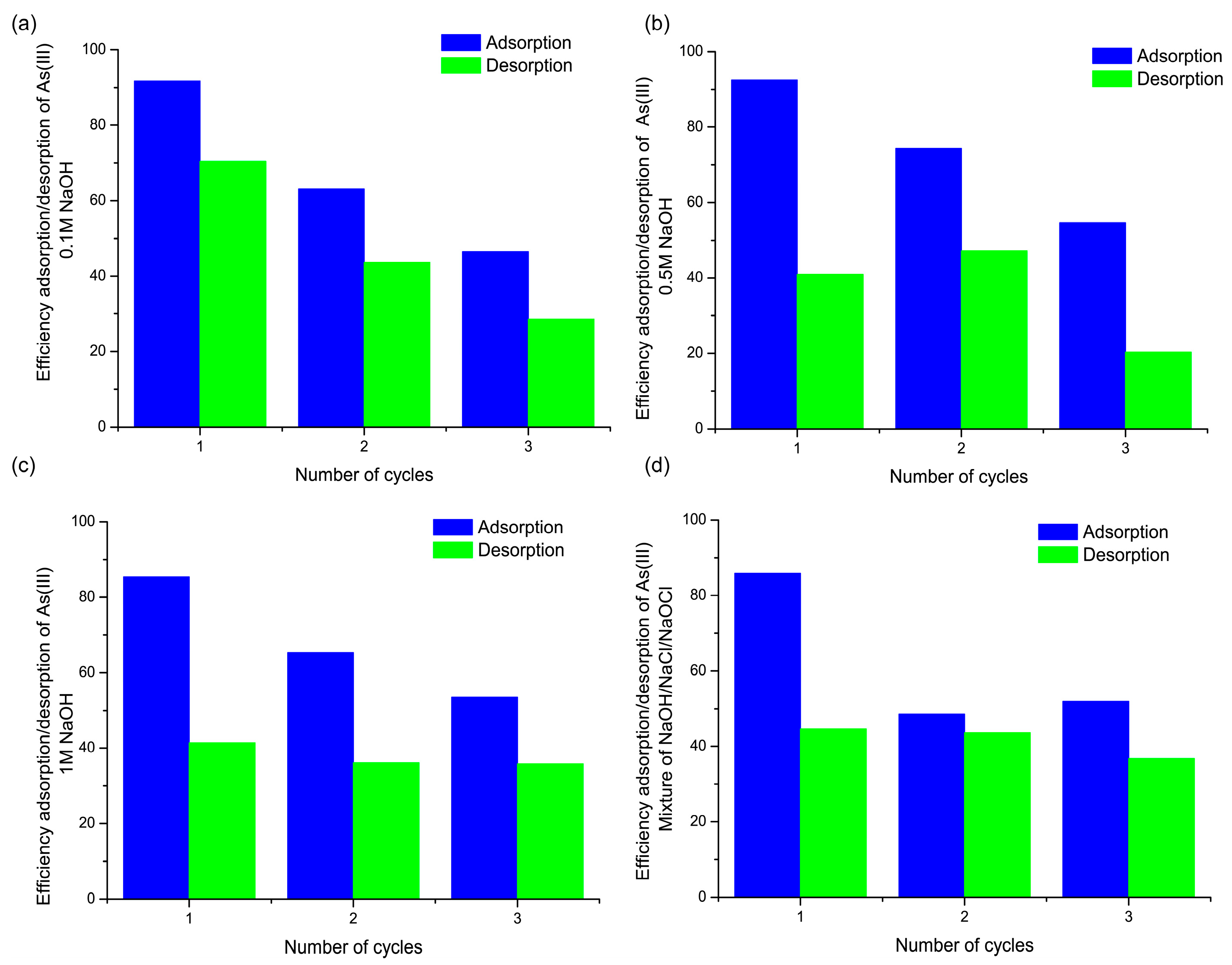
3.6. Regeneration and Reusability of the FMBO Nanocomposite
3.7. Cost Analysis and Practical Feasibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Guo, J.; Cao, W.; Lang, G.; Sun, Q.; Nan, T.; Li, X.; Ren, Y.; Li, Z. Worldwide Distribution, Health Risk, Treatment Technology, and Development Tendency of Geogenic High-Arsenic Groundwater. Water 2024, 16, 478. [Google Scholar] [CrossRef]
- Mukherjee, A.; Coomar, P.; Sarkar, S.; Johannesson, K.H.; Fryar, A.E.; Schreiber, M.E.; Ahmed, K.M.; Alam, M.A.; Bhattacharya, P.; Bundschuh, J.; et al. Arsenic and other geogenic contaminants in global groundwater. Nat. Rev. Earth Environ. 2024, 5, 312–328. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Patel, B.; Gundaliya, R.; Desai, B.; Shah, M.; Shingala, J.; Kaul, D.; Kandya, A. Groundwater arsenic contamination: Impacts on human health and agriculture, ex situ treatment techniques and alleviation. Environ. Geochem. Health 2023, 45, 1331–1358. [Google Scholar] [CrossRef] [PubMed]
- Sadee, B.A.; Zebari, S.M.S.; Galali, Y.; Saleem, M.F. A review on arsenic contamination in drinking water: Sources, health impacts, and remediation approaches. RSC Adv. 2025, 15, 2684–2703. [Google Scholar] [CrossRef] [PubMed]
- Senthil Rathi, B.; Senthil Kumar, P. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Igwegbe, C.A.; Malloum, A.; Elmakki, M.A.E.; Onyeaka, H.; Fahmy, A.H.; Aquatar, O.; Ahmadi, S.; Alameri, B.M.; Ghosh, S.; et al. Sustainable technologies for removal of arsenic from water and wastewater: A comprehensive review. J. Mol. Liq. 2025, 427, 127412. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.; Wu, R. Preparation and Evaluation of a Novel Fe–Mn Binary Oxide Adsorbent for Effective Arsenite Removal. Water Res. 2007, 41, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Mo, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. As(III) removal by a recyclable granular adsorbent through doping Fe-Mn binary oxides into graphene oxide chitosan. Int. J. Biol. Macromol. 2023, 237, 124184. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, Z.; Ding, J.; Huang, X.; Zhou, X.; Zheng, Z.; Cui, H.; Huang, C.; Xiang, L.; Huang, Y.; et al. Arsenate adsorption, desorption, and re-adsorption on Fe–Mn binary oxides: Impact of co-existing ions. Desalination Water Treat. 2024, 320, 100678. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.; Jokić Govedarica, J.; Vujić, M.; Pešić, J.; Rončević, S.; Agbaba, J. Adsorption Performance of Fe–Mn Polymer Nanocomposites for Arsenic Removal: Insights from Kinetic and Isotherm Models. Materials 2024, 17, 5089. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Jeon, E.; Yang, J.S.; Baek, K. Adsorption of As(III) and As(V) in groundwater by Fe–Mn binary oxide-impregnated granular activated carbon (IMIGAC). J. Taiwan Inst. Chem. Eng. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Jovanovic, D.; Jakovljević, B.; Rašić-Milutinović, Z.; Paunović, K.; Peković, G.; Knezević, T. Arsenic occurrence in drinking water supply systems in ten municipalities in Vojvodina Region, Serbia. Environ. Res. 2011, 111, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Nikić, J.; Agbaba, J.; Watson, M.A.; Tubić, A.; Šolić, M.; Maletić, S.; Dalmacija, B. Arsenic adsorption on Fe–Mn modified granular activated carbon (GAC–FeMn): Batch and fixed-bed column studies. J. Environ. Sci. Health A 2019, 54, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Nikić, J.; Watson, M.; Tenodi, K.Z.; Dalmacija, B.; Agbaba, J. Pilot study on arsenic removal from phosphate rich groundwater by in-line co-agulation and adsorption. J. Hazard. Mater. Adv. 2023, 10, 100280. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakrabarti, S.; Ghosh, U.D.C. Fixed-bed column performance of Mn-incorporated iron(III) oxide nanoparticle agglomerates on As(III) removal from the spiked groundwater in lab bench scale. Chem. Eng. J. 2014, 248, 18–26. [Google Scholar] [CrossRef]
- Malbenia John, M.; Benettayeb, A.; Belkacem, M.; Mitchel, C.R.; Brahim, M.H.; Benettayeb, I.; Haddou, B.; Al-Farraj, S.; Alkahtane, A.A.; Ghosh, S.; et al. An overview on the key advantages and limitations of batch and dynamic modes of biosorption of metal ions. Chemosphere 2024, 357, 142051. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Arsenic and antimony desorption in water treatment processes: Scaling up challenges with emerging adsorbents. Sci. Total Environ. 2024, 929, 172602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal mechanism of As(III) by a novel Fe-Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Nikić, J.; Watson, M.A.; Isakovski, M.K.; Tubić, A.; Šolić, M.; Kordić, B.; Agbaba, J. Synthesis, characterization and application of magnetic nanoparticles modified with Fe-Mn binary oxide for enhanced removal of As(III) and As(V). Environ. Technol. 2019, 42, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Qu, J.; Liu, H.; Liu, R.; Zhao, X. Fe-Mn binary oxide incorporated into diatomite as an adsorbent for arsenite removal: Preparation and evaluation. J. Colloid Interface Sci. 2009, 338, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, K.; Pan, B.; Zhang, S.; Lu, L.; Zhang, W. Efficient As(III) removal by macroporous anion exchanger-supported Fe-Mn binary oxide: Behavior and mechanism. Chem. Eng. J. 2012, 193–194, 131–138. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.; Dai, Y.; Wu, S.; Tang, X. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J. Environ. Manag. 2019, 245, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Wang, Y.; Hu, Q.; Olusegun, A.K. Magnetic nanoscale Fe-Mn binary oxides loaded zeolite for arsenic removal from synthetic groundwater. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 220–227. [Google Scholar] [CrossRef]
- Awasthi, V.D.; Yamamura, G.; Kobayashi, A.; Bandyopadhyaya, R. Enhanced impregnation of iron manganese binary oxide nanoparticles on nylon 6 fibre for rapid and continuous arsenic [As(III)] removal from drinking water. J. Water Process. Eng. 2024, 64, 105656. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.; Tubić, A.; Kragulj Isakovski, M.; Maletić, S.; Mohora, E.; Agbaba, J. Arsenic removal from water using a one-pot synthesized low-cost mesoporous Fe–Mn-modified biosorbent. J. Serbian Chem. Soc. 2019, 84, 327–342. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe-Mn binary oxide impregnated chitosan bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Zhu, Z.; Zhang, L.; Zhao, N.; Fang, Y.; Zhu, Y.; Liu, G. Enhanced Arsenic Removal from Aqueous Solution by Fe/Mn-C Layered Double Hydroxide Composite. Adsorpt. Sci. Technol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Roy, P.; Mondal, N.K.; Bhattacharya, S.; Das, B.; Das, K. Removal of arsenic(III) and arsenic(V) on chemically modified low-cost adsorbent: Batch and column operations. Appl. Water Sci. 2013, 3, 293–309. [Google Scholar] [CrossRef]
- Panda, A.P.; Jha, U.; Kumar, S.A.; Swain, S.K. A Simplified and Affordable Arsenic Filter to Prevent Arsenic Poisoning: Lab-Scale Study and Pilot Experiment. ACS ES&T Water 2023, 3, 4092–4102. [Google Scholar] [CrossRef]
- Pan, B.; Li, Z.; Zhang, Y.; Xu, J.; Chen, L.; Dong, H.; Zhang, W. Acid and organic resistant nano-hydrated zirconium oxide (HZO)/polystyrene hybrid adsorbent for arsenic removal from water. Chem. Eng. J. 2014, 248, 290–296. [Google Scholar] [CrossRef]
- Chang, F.; Qu, J.; Zhao, X.; Liu, W.; Wu, K. Migration of manganese and iron during the adsorption-regeneration cycles for arsenic removal. Front Environ. Sci. Eng. China 2011, 5, 512–518. [Google Scholar] [CrossRef]
- Chang, F.; Qu, J.; Liu, R.; Zhao, X.; Lei, P. Practical performance and its efficiency of arsenic removal from groundwater using Fe-Mn binary oxide. J. Environ. Sci. 2010, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dangić, A.; Dangić, J. Arsenic in the soil environment of central Balkan Peninsula, southeastern Europe: Occurrence, geochemistry, and impacts. TMCE 2007, 9, 207–236. [Google Scholar] [CrossRef]
- Vesković, J.; Bulatović, S.; Ražić, S.; Lučić, M.; Miletić, A.; Nastasović, A.; Onjia, A. Arsenic-Contaminated Groundwater of the Western Banat (Pannonian Basin): Hydrogeochemical Appraisal, Pollution Source Apportionment, and Monte Carlo Simulation of Source-Specific Health Risks. Water Environ. Res. 2024, 96, e11087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xie, X.; Pi, K.; Gong, J.; Wang, Y. Effects of arsenic–iron–dissolved organic matter interactions on arsenic mobilization: Insight from column experiments. J. Hydrol. 2023, 616, 128837. [Google Scholar] [CrossRef]
- SRPS EN 27888:2009; Water Quality—Determination of Electrical Conductivity. Institute for Standardization of Serbia: Belgrade, Serbia, 2009.
- SRPS ISO 8245:2007; Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- SRPS EN ISO 6878:2008; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- SRPS ISO 7890-3:1998; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. Institute for Standardization of Serbia: Belgrade, Serbia, 1998.
- SRPS ISO 9297/1:2007; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr Method). Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- SRPS H.Z1.184:1974; Deterination of Ammonia in Water Spectrophotometrically. Institute for Standardization of Serbia: Belgrade, Serbia, 1974.
- USEPA. Method 6020B: Inductively Coupled Plasma—Mass Spectrometry; Revision 2; United States Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- Asif, Z.; Chen, Z. Removal of arsenic from drinking water using rice husk. Appl. Water Sci. 2017, 7, 1449–1458. [Google Scholar] [CrossRef]
- Brion-Roby, R.; Gagnon, J.; Deschênes, J.S.; Chabot, B. Investigation of fixed bed adsorption column operation parameters using a chitosan material for treatment of arsenate contaminated water. J. Environ. Chem. Eng. 2018, 6, 505–511. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Apiratikul, R.; Chu, K.H. Improved fixed bed models for correlating asymmetric adsorption breakthrough curves. J. Water Process Eng. 2021, 40, 101810. [Google Scholar] [CrossRef]
- Yunnen, C.; Ye, W.; Chen, L.; Lin, G.; Jinxia, N.; Rushan, R. Continuous fixed-bed column study and adsorption modeling: Removal of arsenate and arsenite in aqueous solution by organic modified spent grains. Pol. J. Environ. Stud. 2017, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Nikić, J.; Tubić, A.; Kragulj Isakovski, M.; Šolić, M.; Dalmacija, B.; Agbaba, J. Repurposing spent filter sand from iron and manganese removal systems as an adsorbent for treating arsenic contaminated drinking water. J. Environ. Manag. 2022, 302, 114115. [Google Scholar] [CrossRef] [PubMed]
- Tiberg, C.; Sjöstedt, C.; Eriksson, A.K. Phosphate competition with arsenate on poorly crystalline iron and aluminum (hydr)oxide mixtures. Chemosphere 2020, 255, 126937. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, X.; Cao, H.; Wang, Y. Insights into the selectivity of metallic oxides for arsenic and phosphate from EXAFS and DFT calculations. Chemosphere 2023, 336, 139276. [Google Scholar] [CrossRef] [PubMed]
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268B, 115668. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.T.; Steiger, B.G.K.; Wilson, L.D. A Route to Selective Arsenate Adsorption in Phosphate Solutions via Ternary Metal Biopolymer Composites. Appl. Sci. 2024, 14, 7577. [Google Scholar] [CrossRef]
- Nwokonkwo, O.; Muhich, C. Mechanistic Insights into the Selectivity for Arsenic over Phosphate Adsorption by Fe3+-Cross-Linked Chitosan Using DFT. J. Phys. Chem. B 2024, 128, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Pincus, L.N.; Petrović, P.V.; Gonzalez, I.S.; Stavitski, E.; Fishman, Z.S.; Anastas, P.T.; Zimmerman, J.B. Selective adsorption of arsenic over phosphate by transition metal cross-linked chitosan. Chem. Eng. J. 2021, 412, 128582. [Google Scholar] [CrossRef] [PubMed]
- Vujić, M.; Nikić, J.; Vijatovic Petrovic, M.; Pejin, Đ.; Watson, M.; Rončević, S.; Agbaba, J. End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water. Polymers 2025, 17, 1353. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Saidulu, D.; Ghosal, P.S.; Mukherjee, A.; Gupta, A.K. A review on the management of arsenic-laden spent adsorbent: Insights of global practices, process criticality, and sustainable solutions. Environ. Technol. Innov. 2022, 27, 102500. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Tomasi, I.T.; Boaventura, R.A.R.; Botelho, C.M.S.; Pintor, A.M.A. Environmental impact and cost analysis of arsenic removal from water using iron-based adsorbents: Comparison between natural and commercial materials. J. Environ. Chem. Eng. 2025, 13, 115044. [Google Scholar] [CrossRef]




| Fe–Mn-Based Adsorbents | Initial As Concentration (mg/L) | Water Matrix | pH | Adsorption Capacity in Batch System (mg/g) | References | |
|---|---|---|---|---|---|---|
| As(III) | As(V) | |||||
| Fe–Mn binary oxide | 1–200 | Synthetic | 5 | 69.8 | 133 | [21] |
| Magnetite coated with FMBO | 0.2–50 | Synthetic | 7.0 | 55.9 | 54.1 | [22] |
| Diatomite coated with Fe–Mn binary oxide | 0.05–20 | Synthetic | 7.0 | 1.68 | - | [23] |
| Macroporous anion exchanger-supported Fe–Mn binary oxide | 1–50 | Synthetic water | 7.0 | 44.9 | 13.17 | [24] |
| Starch-FMBO | 0–300 | Synthetic | 161 | - | [25] | |
| Gelatin-FMBO | 0–300 | 141 | - | [25] | ||
| CMC-FMBO | 0–300 | 104 | - | [25] | ||
| Nanoscale Fe–Mn binary oxides loaded on zeolite (NIMZ) | 2–100 | Synthetic | 7.0 | 47 | 49 | [26] |
| Iron–manganese binary oxide nanoparticles on nylon 6 fiber | 1–100 | Synthetic | 7.0 | 134 | - | [27] |
| Biochar coated with FMBO | 0.01–10 | Synthetic | 7.0 | 14.4 | 12.2 | [9] |
| GAC-FeMn | 0.1–1 | Synthetic | 7.0 | 2.30 | 2.87 | [16] |
| Chitosan-FMBO | 0–24 | Synthetic | 7.0 | 54.2 | - | [28] |
| Chitosan-FeMn | 0.1–1 | Synthetic | 7.0 | 3.91 | 3.89 | [29] |
| PET-FMBO | 0.1–10 | Synthetic | 7.0 | 8.74 | 13.3 | [13] |
| PE-FMBO | 0.1–10 | Synthetic | 7.0 | 5.29 | 5.37 | [13] |
| Graphene oxide chitosan-coated FMBO (Fe/Mn GOCS) | 5–300 | Synthetic | 7.62 | 109 | - | [11] |
| Fe/Mn-C-layered double-hydroxide composite | 5–100 | Synthetic | - | 41.9 | 33.6 | [30] |
| FMBO nanocomposite | 0.1–10 | Synthetic | 7.0 | 6.2 | - | This study |
| Parameter | Water | ||
|---|---|---|---|
| Groundwater | Aerated Groundwater | Spiked Tap Water | |
| pH | 7.73 ± 0.05 | 7.81 ± 0.05 | 7.51 ± 0.04 |
| Conductivity (µS/cm) | 514 ± 43 | 466 ± 12 | 490 ± 22 |
| Turbidity (NTU) | 1.55 ± 0.29 | 0.70 ± 0.05 | 0.31 ± 0.07 |
| TOC (mg/L) | 1.38 ± 0.10 | 1.53 ± 0.05 | 1.460 ± 0.05 |
| Arsenic (μg/L) | 115 ± 6.4 | 90.0 ± 18.7 | 172 ± 20 |
| Iron (μg/L) | 395 ± 28.1 | 5.36 ± 3.55 | 26.04 ± 2.20 |
| Manganese (μg/L) | 54 ± 4.67 | 6.35 ± 8.52 | 3.22 ± 4.10 |
| Ammonium (mg N/L) | 0.44 ± 0.09 | 0.228 ± 0.12 | 0.454 ± 0.01 |
| Nitrate (mg N/L) | 1.57 ± 0.14 | 0.67 ± 0.11 | 0.05 ± 0.01 |
| Orthophosphate (mg PO4/L) | 0.323 ± 0.11 | 0.075 ± 0.02 | 0.024 ± 0.01 |
| Chloride (mg Cl/L) | 3.77 ± 3.19 | 0.673 ± 0.19 | 27.25 ± 0.01 |
| Model | Equation | Parameters |
|---|---|---|
| Freundlich | qₑ—Adsorbed amount at equilibrium (mg/g), Cₑ—Equilibrium concentration (mg/L), KF—Freundlich adsorption constant [(mg/g)(L/mg)^(1/n)], nF—Freundlich exponent | |
| Langmuir | qe—Adsorbed amount at equilibrium (mg/g), Cₑ—Equilibrium concentration (mg/L), qmax—Maximum adsorption capacity (mg/g), KL—Langmuir constant (L/mg) |
| Models | Equation | Parameters |
|---|---|---|
| Thomas | ct: Concentration at time (mg/L), c0: Initial concentration (mg/L), kTh: Thomas rate constant (L/min·mg), q0: Maximum adsorption capacity (mg/g), m: Mass of adsorbent (g), Q: Flow rate (L/min), t: Time (min) | |
| Adams–Bohart | ct: Concentration at time (mg/L), c0: Initial concentration (mg/L), kAB: Adams–Bohart rate constant (L/mg·min), N0: Saturated adsorption capacity (mg/L), L: Packed bed length (cm), t: Time (min), u: Linear velocity (cm/min) | |
| Yoon–Nelson | ct: Concentration at time (mg/L), c0: Initial concentration (mg/L), kYN: Yoon–Nelson rate constant (L/min), τ: Time required for 50% breakthrough (min), t: Time (min) |
| Filter Media Volume (L) | Mass of Media (kg) | Bed Depth (m) | Filtration Rate (m/h) | EBCT (min) | Flow Rate Q (L/h) | |
|---|---|---|---|---|---|---|
| Pilot A | 3.5 | 1.6 | 0.11 | 1.25 | 5.12 | 40 |
| Pilot B | 5.5 | 2.5 | 0.17 | 0.62 | 16.5 | 22 |
| Freundlich Model | Langmuir Model | |||||
|---|---|---|---|---|---|---|
| Matrix Type | nF | KF (mg/g)/(mg/L)n | R2 | qmax (mg/g) | KL (L/mg) | R2 |
| As(III) spiked tap water | 0.640 | 2.70 | 0.9459 | 6.25 | 0.992 | 0.9577 |
| As(III) spiked groundwater | 0.420 | 1.79 | 0.9349 | 4.63 | 0.343 | 0.9452 |
| Fe–Mn-Based Adsorbent | Length (cm) | Diameter (cm) | Mass of Adsorbent (g) | Bed Depth (cm) | Flow Rate (mL/min) | EBCT (min) | Water Matrix | BV Before MAC Breakthrough | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Graphene oxide chitosan-coated FMBO (Fe/Mn GOCS) | 3 | 1.6 | 7.67 | - | 1.5 | - | Synthetic water matrix 10 or 50 mg/L As(III) pH 7 | 40 and 3 | [11] |
| Macroporous anion exchanger-supported Fe–Mn binary oxide | 13.0 | 1.2 | - | - | - | 3 | Simulated water As(III) 100 µg/L; Nitrate 150 mg/L; Carbonate 200 mg/L; Chloride 300 mg/L; Sulfate 300 mg/L; pH 8.10 | 2300 | [24] |
| Chitosan coated with Fe–Mn binary | 32 | 1.9 | 30 | 25 | - | 10 | Simulated groundwater As(III)/As(V) 233 μg/L; Nitrate 5 mg/L, Carbonate: 159 mg/L; Silicate 12 mg/L; Phosphate 0.13 mg/L; pH 7.3 | 500 and 3200 for As(V) and As(III) | [29] |
| GAC-FMBO | 80 | 1.7 | - | 30 | 6 | 12 | Groundwater As(III): 120 µg/L; Conduct. 678 mS/cm; DOC 2.00 mg/L; Alkalinity 7.68 mmol/L; Chloride 19.7 mg/L; Carbonate 118 mg/L; Sulfate 15.7 mg SO4/L; Phosphate 1.33 mg/L; Fe 35.4 µg/L Mn 21.5 µg/L; pH 8.22 | 83 | [16] |
| HZO@D201 nanocomposite | 13 | 1.2 | 5 mL | - | - | 3 | Simulated groundwater As(III) 0.1 mg/L; Magnesium 5 mg/L; Sulfate 50 mg/L; Calcium 15 mg/L; Silicate 5 mg/L; Chloride 40 mg/L; Nitrate 8 mg/L; Carbonate 150 mg/L; pH: 8.2 | 600 | [33] |
| FMBO-diatomite | 40 | 3 | - | - | 34 | 5 | Spiked DI water with As(III) | 4500 | [34] |
| FMBO-diatomite | 40 | 3 | 58 | 24 | 17 | 10 | Anaerobic groundwater; Astot 0.0477 mg/L; As(III) 0.03 mg/L; Turbidity 0.7 NTU; Conductivity 530 mS/cm; TOC 2.84 mg/L; 305 mg/L; Magnesium 16 mg/L; Calcium 28 mg/L; Chloride 40 mg/L; Nitrate 9.8 mg/L; Phosphor 1.21 mg/L; Mn 0.15.1 mg/L; Fe 0.257 mg/L; Nitrogen 66 mg/L; pH: 7.4 | 7000 BV after 15 regenerations | [35] |
| FMBO-impregnated nylon 6 fiber (IMBNP-nylon 6) | 4 | 1 | - | - | - | 0.65 | Spiked RO water with As(III) 0.1 and 0.038 mg/L; Magnesium 5 mg/L; Sulfate 50 mg/L; Calcium 15 mg/L; Silicate 5 mg/ L; Chloride 40 mg/L; Nitrate 8 mg/L; Carbonate 150 mg/L; pH: 8.2 | 5200 and 21,000 | [27] |
| FMBO nanocomposite | 30 | 2 | 28 | 20 | 5.2 | 12 | Raw groundwater Astot 0.115 mg/L; Orthophosphate 0.323 mg/L; Chloride 3.77 mg/L; Nitrate 1.57 mg/L; Mn 0.054 mg/L; Fe 0.395 mg/L pH: 7.73 | 587 | This work |
| 60 | 2 | 60 | 42 | 5.2 | 25 | Spiked tap water with As(III) Astot 0.172 mg/L; Orthophosphate 0.024 mg/L; Chloride 27.25 mg/L; Nitrate 0.05 mg/L; Mn 0.003 mg/L; Fe 0.026 mg/L pH: 7.51 | 1750 |
| Thomas Constant | Adams–Bohart | Yoon–Nelson | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qt (mg/g) | KTh (L/mg min) | R2 | No (mg/L) | KAB (L/mg min) | R2 | kYN (/min) | τ (min) | R2 | |
| Column I | 1.02 | 1.34 | 0.0003765 | 0.9744 | 588 | 0.000489 | 0.9124 | 0.0000641 | 90,014 | 0.9915 |
| Column II | 1.42 | 1.71 | 0.000845 | 0.9920 | 863 | 0.000747 | 0.9909 | 0.000153 | 27,248 | 0.9915 |
| Column III | 0.238 | 0.252 | 0.00191 | 0.9824 | 122 | 0.00109 | 0.8754 | 0.000177 | 15,690 | 0.9497 |
| Column IV | 0.343 | 0.405 | 0.00183 | 0.9760 | 174 | 0.00104 | 0.9384 | 0.000202 | 18,871 | 0.9733 |
| Thomas Constant | Adams–Bohart | Yoon–Nelson | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qt (mg/g) | KTb (L/mg min) | R2 | No (mg/L) | KAB (L/mg min) | R2 | KYN (min−1) | τ (min) | R2 | ||
| Pilot A | Arsenic | 0.337 | 0.551 | 0.00206 | 0.9497 | 327 | 0.00171 | 0.9589 | 0.000218 | 12,197 | 0.9802 |
| Phosphate | 0.811 | 0.926 | 0.00118 | 0.9726 | 792 | 0.000448 | 0.8598 | 0.000445 | 6091 | 0.9635 | |
| Pilot B | Arsenic | 0.243 | 0.417 | 0.00139 | 0.9485 | 216 | 0.000924 | 0.9502 | 0.000181 | 23,463 | 0.9234 |
| Phosphate | 0.731 | 0.789 | 0.000196 | 0.9864 | 770 | 0.000111 | 0.8818 | 0.0000667 | 15,719 | 0.9881 | |
| Scale | Mass Adsorbent | qexp (mg/g) | q (Langmuir or Thomas Model) (mg/g) | Breakthrough Point |
|---|---|---|---|---|
| Batch experiments | 20 mg | 4.71 | ||
| Column III | 28 g | 0.238 | 0.252 | 587 |
| Column IV | 28 g | 0.343 | 0.405 | 365 |
| Pilot A | 1.6 kg | 0.337 | 0.551 | 100 |
| Pilot B | 2.5 kg | 0.243 | 0.417 | 475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikić, J.; Jokić Govedarica, J.; Watson, M.; Pejin, Đ.; Tubić, A.; Agbaba, J. From Batch to Pilot: Scaling Up Arsenic Removal with an Fe-Mn-Based Nanocomposite. Nanomaterials 2025, 15, 1104. https://doi.org/10.3390/nano15141104
Nikić J, Jokić Govedarica J, Watson M, Pejin Đ, Tubić A, Agbaba J. From Batch to Pilot: Scaling Up Arsenic Removal with an Fe-Mn-Based Nanocomposite. Nanomaterials. 2025; 15(14):1104. https://doi.org/10.3390/nano15141104
Chicago/Turabian StyleNikić, Jasmina, Jovana Jokić Govedarica, Malcolm Watson, Đorđe Pejin, Aleksandra Tubić, and Jasmina Agbaba. 2025. "From Batch to Pilot: Scaling Up Arsenic Removal with an Fe-Mn-Based Nanocomposite" Nanomaterials 15, no. 14: 1104. https://doi.org/10.3390/nano15141104
APA StyleNikić, J., Jokić Govedarica, J., Watson, M., Pejin, Đ., Tubić, A., & Agbaba, J. (2025). From Batch to Pilot: Scaling Up Arsenic Removal with an Fe-Mn-Based Nanocomposite. Nanomaterials, 15(14), 1104. https://doi.org/10.3390/nano15141104










