Assessing Antibacterial Properties of Copper Oxide Nanomaterials on Gut-Relevant Bacteria In Vitro: A Multifaceted Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. CuO Nanomaterial Characterisation
2.2. CuO Nanomaterial Preparation
2.3. Bacterial Models and Culture Conditions
2.4. Time Kill Assay (Plate Count)
2.5. Optical Density Measurement
2.6. Diffusion Assay (Well and Disc)
2.7. Live/Dead Fluorescent Images
2.8. DCFH-DA Fluorescent Images
2.9. Statistical Analysis
3. Results
3.1. CuO NM Characterisation
3.2. Time Kill (Plate Count) Assay
3.3. OD Measurements
3.4. Diffusion Assays
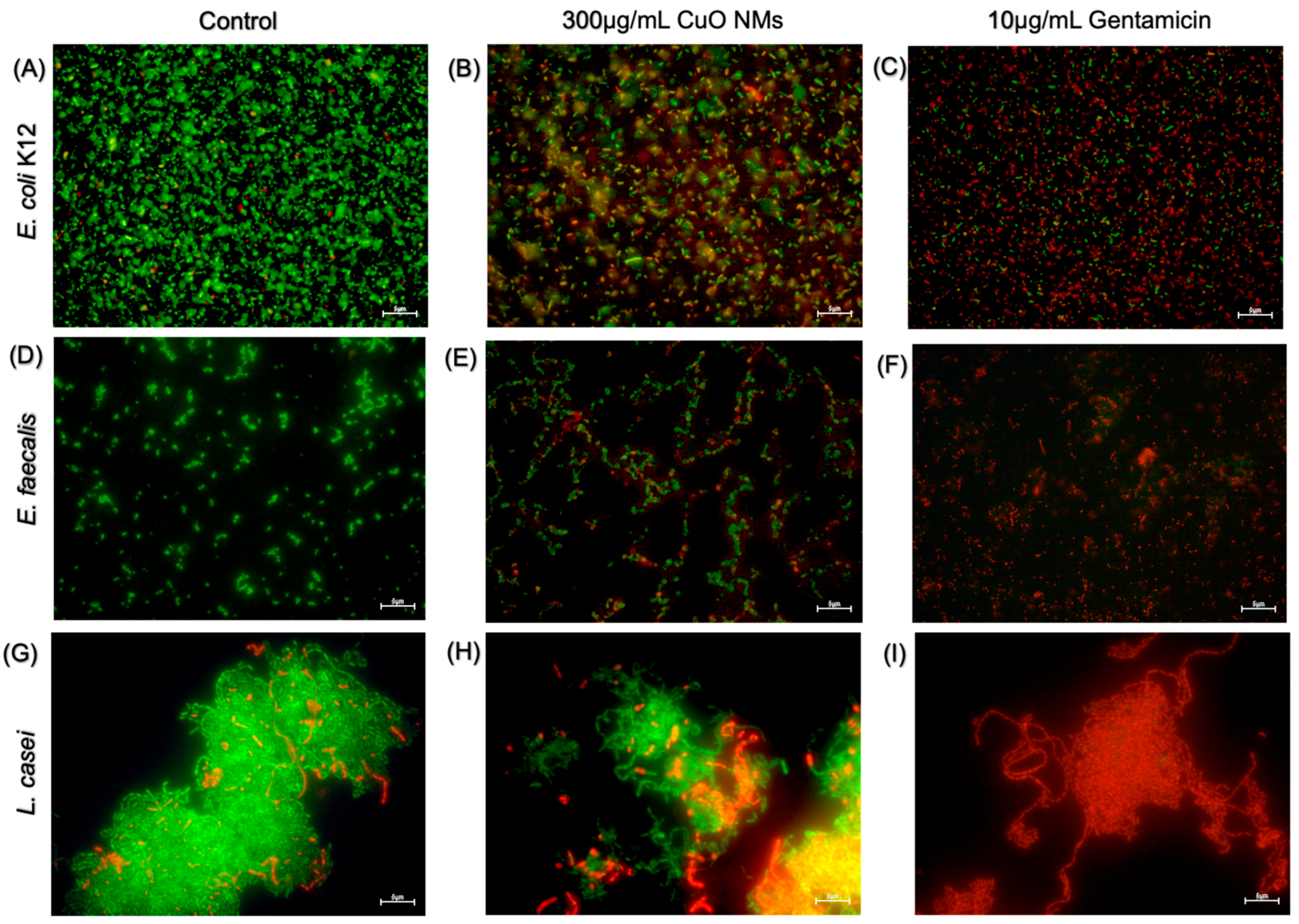
3.5. Live/Dead Fluorescent Microscopy
3.6. DCFH-DA Fluorescent Microscopy
4. Discussion
4.1. Impact of CuO NMs on Commensal Gut Bacteria
4.2. Sensitivity of In Vitro Approaches
4.3. Physiochemical Properties of CuO NMs
4.4. CuO NM-Mediated Intracellular ROS Production
4.5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Commission Recommendation of 10 June 2022 on the Definition of Nanomaterial. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H0614(01) (accessed on 11 July 2025).
- Hsiao, I.L.; Huang, Y.-J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.; Brown, D.M.; Kanase, N.; Euston, M.; Gaiser, B.K.; Robb, C.T.; Dyrynda, E.; Rossi, A.G.; Brown, E.R.; Stone, V. Mechanism of neutrophil activation and toxicity elicited by engineered nanomaterials. Toxicol. Vitr. 2015, 29, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Monsé, C.; Raulf, M.; Hagemeyer, O.; van Kampen, V.; Kendzia, B.; Gering, V.; Marek, E.-M.; Jettkant, B.; Bünger, J.; Merget, R.; et al. Airway inflammation after inhalation of nano-sized zinc oxide particles in human volunteers. BMC Pulm. Med. 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; da Silva, S.S. Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef]
- Gopinath, K.; Sathishkumar, G.; Xu, L. An Overview of the Copper Oxide Nanofillers Integrated in Food Packaging Systems. Coatings 2024, 14, 81. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; McDonald, J.A.; Petrof, E.O.; Allen-Vercoe, E.; Walker, V.K. Nanosilver-mediated change in human intestinal microbiota. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; MaädLer, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. 4), 547–551. [Google Scholar] [PubMed]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology 2019, 13, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Büttner, J.; Schneider, T.; Westermann, M.; Glei, M. Artificial Digestion of Polydisperse Copper Oxide Nanoparticles: Investigation of Effects on the Human In Vitro Intestinal Co-Culture Model Caco-2/HT29-MTX. Toxics 2022, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Tang, F.; Adam, P.-M.; Laratte, B.; Ionescu, R.E. Fate and Characterization Factors of Nanoparticles in Seventeen Subcontinental Freshwaters: A Case Study on Copper Nanoparticles. Environ. Sci. Technol. 2016, 50, 9370–9379. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Brown, D.M.; Smith, D.G.E.; Stone, V.; Johnston, H.J. Comparison of In Vitro Approaches to Assess the Antibacterial Effects of Nanomaterials. J. Funct. Biomater. 2022, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, D.L.; Shaw, B.J.; Harper, G.M.; Saoud, I.P.; Davies, S.J.; Handy, R.D.; Henry, T.B. Ingestion of metal-nanoparticle contaminated food disrupts endogenous microbiota in zebrafish (Danio rerio). Environ. Pollut. 2013, 174, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Swart, E.; Dvorak, J.; Hernádi, S.; Goodall, T.; Kille, P.; Spurgeon, D.; Svendsen, C.; Prochazkova, P. The Effects of In Vivo Exposure to Copper Oxide Nanoparticles on the Gut Microbiome, Host Immunity, and Susceptibility to a Bacterial Infection in Earthworms. Nanomaterials 2020, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Sizentsov, A.N.; Kvan, O.V.; Miroshnikova, E.P.; Gavrish, I.A.; Serdaeva, V.A.; Bykov, A.V. Assessment of biotoxicity of Cu nanoparticles with respect to probiotic strains of microorganisms and representatives of the normal flora of the intestine of broiler chickens. Environ. Sci. Pollut. Res. 2018, 25, 15765–15773. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Araya, M.; Pizarro, F.; Olivares, M. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig. Dis. Sci. 2001, 46, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.E.; Fröhlich, E. Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota. Int. J. Mol. Sci. 2016, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, M.; Wang, C.; Yong, Y.; Gu, Z. Recent advances in understanding the effects of nanomaterials on gut microbiota. Chem. Eng. J. 2022, 435, 134976. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomedicine 2007, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-H.; Lin, K.-S.; Ke, W.-J.; Hsieh, C.-T.; Chiang, C.-L.; Tzou, D.-Y.; Liu, S.-T.; Mishra, Y.K. The Antimicrobial Properties of Silver Nanoparticles in Bacillus subtilis Are Mediated by Released Ag+ Ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In Vivo and In Vitro Antimicrobial Activity of Biogenic Silver Nanoparticles against Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Kvítek, L.; Panáček, A.; Soukupova, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Nahvi, I.; Nahvi, I.; Rehman, S. Nanotechnology and Multidrug Resistance. In Nanotechnology for Infectious Diseases; Hameed, S., Rehman, S., Eds.; Springer: Singapore, 2022; pp. 305–320. [Google Scholar]
- Zhou, G.; Yu, R.; Ahmed, T.; Jiang, H.; Zhang, M.; Lv, L.; Alhumaydhi, F.A.; Allemailem, K.S.; Li, B. Biosynthesis and Characterization of Zinc Oxide Nanoparticles and Their Impact on the Composition of Gut Microbiota in Healthy and Attention-Deficit Hyperactivity Disorder Children. Front. Microbiol. 2021, 12, 700707. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- van den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.-J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Song, J.; Jang, J. Photocatalytic Antibacterial Capabilities of TiO2−Biocidal Polymer Nanocomposites Synthesized by a Surface-Initiated Photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Magrini, A.; Campagnolo, L. New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol. Appl. Pharmacol. 2016, 299, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossain, S.; Hasan, M.A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E.; et al. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem. Sci. 2015, 6, 5186–5196. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Cheloni, G.; Marti, E.; Slaveykova, V.I. Interactive effects of copper oxide nanoparticles and light to green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2016, 170, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Verdon, R.; Gillies, S.; Brown, D.M.; Fernandes, T.F.; Henry, T.B.; Rossi, A.G.; Tran, L.; Tucker, C.; Tyler, C.R.; et al. Adoption of in vitro systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanomaterials. Crit. Rev. Toxicol. 2018, 48, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Mitjans, M.; Rolim, C.M.; Vinardell, M.P. Mechanisms Underlying Cytotoxicity Induced by Engineered Nanomaterials: A Review of In Vitro Studies. Nanomaterials 2014, 4, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2014, 5, 10–1128. [Google Scholar]
- Ayaz Ahmed, K.B.; Anbazhagan, V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef]
- Gosens, I.; Cassee, F.R.; Zanella, M.; Manodori, L.; Brunelli, A.; Costa, A.L.; Bokkers, B.G.H.; de Jong, W.H.; Brown, D.; Hristozov, D.; et al. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 2016, 10, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Pantano, D.; Neubauer, N.; Navratilova, J.; Scifo, L.; Civardi, C.; Stone, V.; von der Kammer, F.; Müller, P.; Sobrido, M.S.; Angeletti, B.; et al. Transformations of Nanoenabled Copper Formulations Govern Release, Antifungal Effectiveness, and Sustainability throughout the Wood Protection Lifecycle. Environ. Sci. Technol. 2018, 52, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Ude, V.C.; Brown, D.M.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H.J. Impact of copper oxide nanomaterials on differentiated and undifferentiated Caco-2 intestinal epithelial cells; assessment of cytotoxicity, barrier integrity, cytokine production and nanomaterial penetration. Part. Fibre Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Dadi, R.; Azouani, R.; Traore, M.; Mielcarek, C.; Kanaev, A. Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109968. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; MacCormack, T.J.; Clark, R.J.; Ede, J.D.; Ortega, V.A.; Felix, L.C.; Dang, M.K.M.; Ma, G.; Fenniri, H.; Veinot, J.G.C.; et al. Widespread Nanoparticle-Assay Interference: Implications for Nanotoxicity Testing. PLoS ONE 2014, 9, e90650. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 2012, 23, 085103. [Google Scholar] [CrossRef] [PubMed]
- Yausheva, E.; Miroshnikov, S.; Sizova, E. Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environ. Sci. Pollut. Res. 2018, 25, 18109–18120. [Google Scholar] [CrossRef] [PubMed]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms to Inform Redesign Strategies to Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.; Mohanty, S.; Mallick, R.; Jacob, B.; Sonawane, A. Toxicity and antibacterial assessment of chitosan-coated silver nanoparticles on human pathogens and macrophage cells. Int. J. Nanomed. 2012, 7, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Kourmouli, A.; Valenti, M.; van Rijn, E.; Beaumont, H.J.; Kalantzi, O.I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? J. Nanopart Res. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Sanket, A.S.; Pati, S.; Kant, R.; Dwivedi, G.R. Nanoparticles: Warheads to Overcome the Resistance Mechanism of Bacterial Superbugs. In Nanotechnology for Infectious Diseases; Hameed, S., Rehman, S., Eds.; Springer: Singapore, 2022; pp. 321–344. [Google Scholar]
- Zheng, J.; Clogston, J.D.; Patri, A.K.; Dobrovolskaia, M.A.; McNeil, S.E. Sterilization of Silver Nanoparticles Using Standard Gamma Irradiation Procedure Affects Particle Integrity and Biocompatibility. J. Nanomed. Nanotechnol. 2011, 2011 (Suppl. 5), 001. [Google Scholar] [CrossRef] [PubMed]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L. Nanoscale and fine zinc oxide particles: Can in vitro assays accurately forecast lung hazards following inhalation exposures? Environ. Sci. Technol. 2009, 43, 7939–7945. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Ude, V.C. An In Vitro Investigation of Biochemical and Molecular Markers of Nanomaterial Toxicity to the Intestine; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2018; Available online: https://www.ros.hw.ac.uk/handle/10399/3967 (accessed on 11 July 2025).
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, M.; Kasemets, K.; Vodovnik, M.; Marinšek-Logar, R.; Kahru, A. Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila. Environ. Sci. Technol. 2011, 45, 6617–6624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Cho, H.; Ryu, S.-E.; Choi, M.-U. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: Highly potent and reversible oxidative inactivation by Cu2+ ion. Arch. Biochem. Biophys. 2000, 382, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; Murphy, F.; Ma-Hock, L.; Dekkers, S.; Keller, J.; Oomen, A.G.; Stone, V. An Integrated Approach to Testing and Assessment to Support Grouping and Read-Across of Nanomaterials After Inhalation Exposure. Appl. Vitr. Toxicol. 2021, 7, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, N.; Tang, H.; Zhang, C.; Chen, K.; Wang, W.; Liu, J. Effective Antibacterial Activity of Degradable Copper-Doped Phosphate-Based Glass Nanozymes. ACS Appl. Mater. Interfaces 2021, 13, 11631–11645. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, L.; Oomen, A.G.; Dekkers, S.; Moore, C.; Rocchia, W.; Murphy, F.; Johnston, H.J.; Janer, G.; Haase, A.; Stone, V.; et al. Grouping Hypotheses and an Integrated Approach to Testing and Assessment of Nanomaterials Following Oral Ingestion. Nanomaterials 2021, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef] [PubMed]





| Bacteria | CuO NM (100 µg/mL) | CuO NM (200 µg/mL) | CuO NM (300 µg/mL) |
|---|---|---|---|
| E. coli K12 | 9% | 46% | 66% |
| E. faecalis | 43% | 47% | 50% |
| L. casei | 33% | 76% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardlaw, T.A.; Masri, A.; Brown, D.M.; Johnston, H.J. Assessing Antibacterial Properties of Copper Oxide Nanomaterials on Gut-Relevant Bacteria In Vitro: A Multifaceted Approach. Nanomaterials 2025, 15, 1103. https://doi.org/10.3390/nano15141103
Wardlaw TA, Masri A, Brown DM, Johnston HJ. Assessing Antibacterial Properties of Copper Oxide Nanomaterials on Gut-Relevant Bacteria In Vitro: A Multifaceted Approach. Nanomaterials. 2025; 15(14):1103. https://doi.org/10.3390/nano15141103
Chicago/Turabian StyleWardlaw, Tia A., Abdulkader Masri, David M. Brown, and Helinor J. Johnston. 2025. "Assessing Antibacterial Properties of Copper Oxide Nanomaterials on Gut-Relevant Bacteria In Vitro: A Multifaceted Approach" Nanomaterials 15, no. 14: 1103. https://doi.org/10.3390/nano15141103
APA StyleWardlaw, T. A., Masri, A., Brown, D. M., & Johnston, H. J. (2025). Assessing Antibacterial Properties of Copper Oxide Nanomaterials on Gut-Relevant Bacteria In Vitro: A Multifaceted Approach. Nanomaterials, 15(14), 1103. https://doi.org/10.3390/nano15141103








