Synergistic Enhancement of LiNO3-NaNO3-KNO3-NaNO2 Thermophysical Properties Through Dual Nano-Additives: SiO2 and MgO
Abstract
1. Introduction
2. Experiments
2.1. Sample Preparation
2.2. Measurement
2.2.1. Heat Storage Performance
2.2.2. Heat Transfer Properties
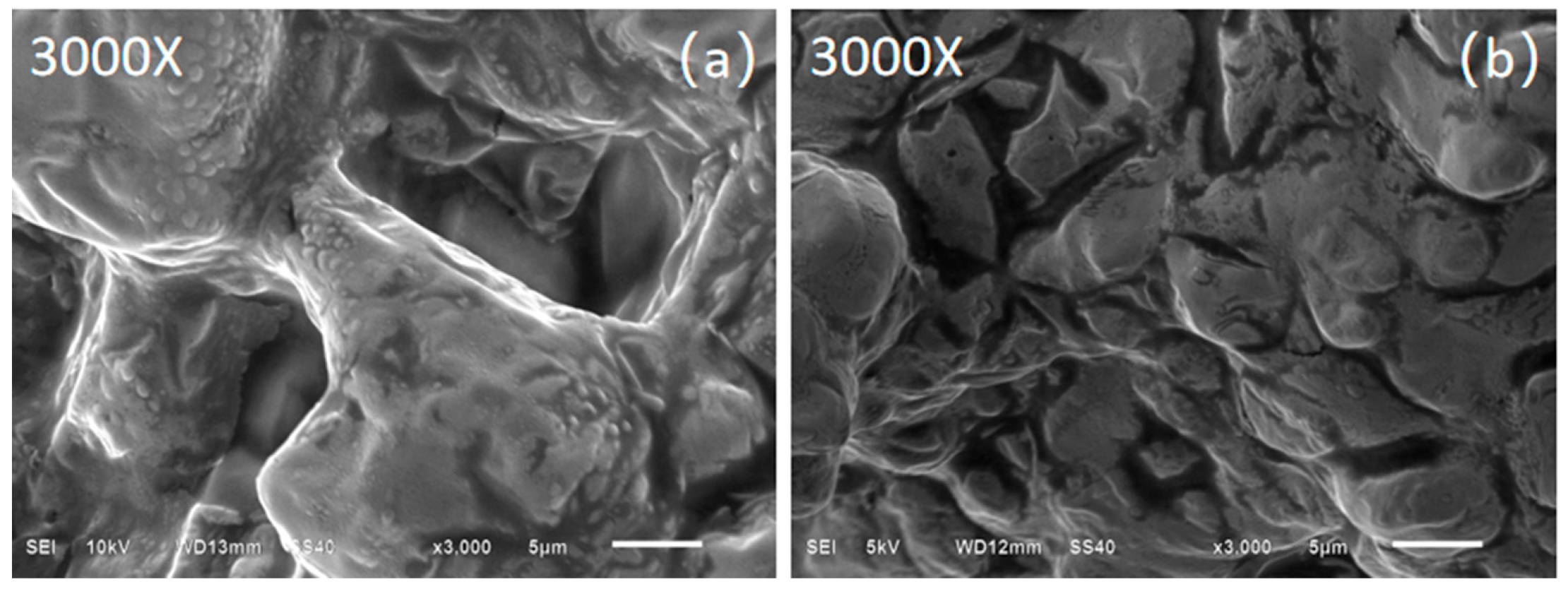
2.2.3. Microstructure
3. Results and Discussion
3.1. The Nanoparticles
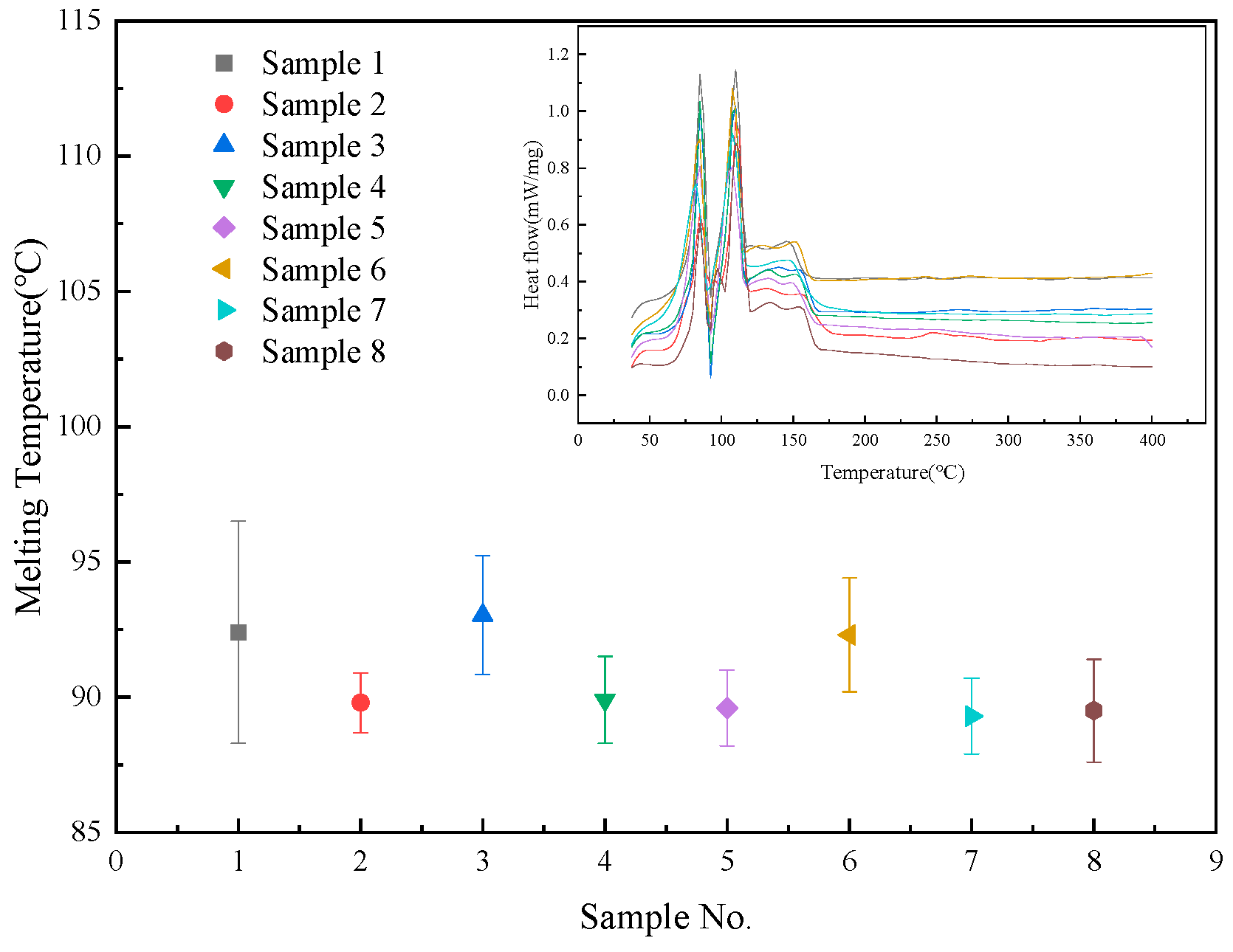
3.2. Melting Point
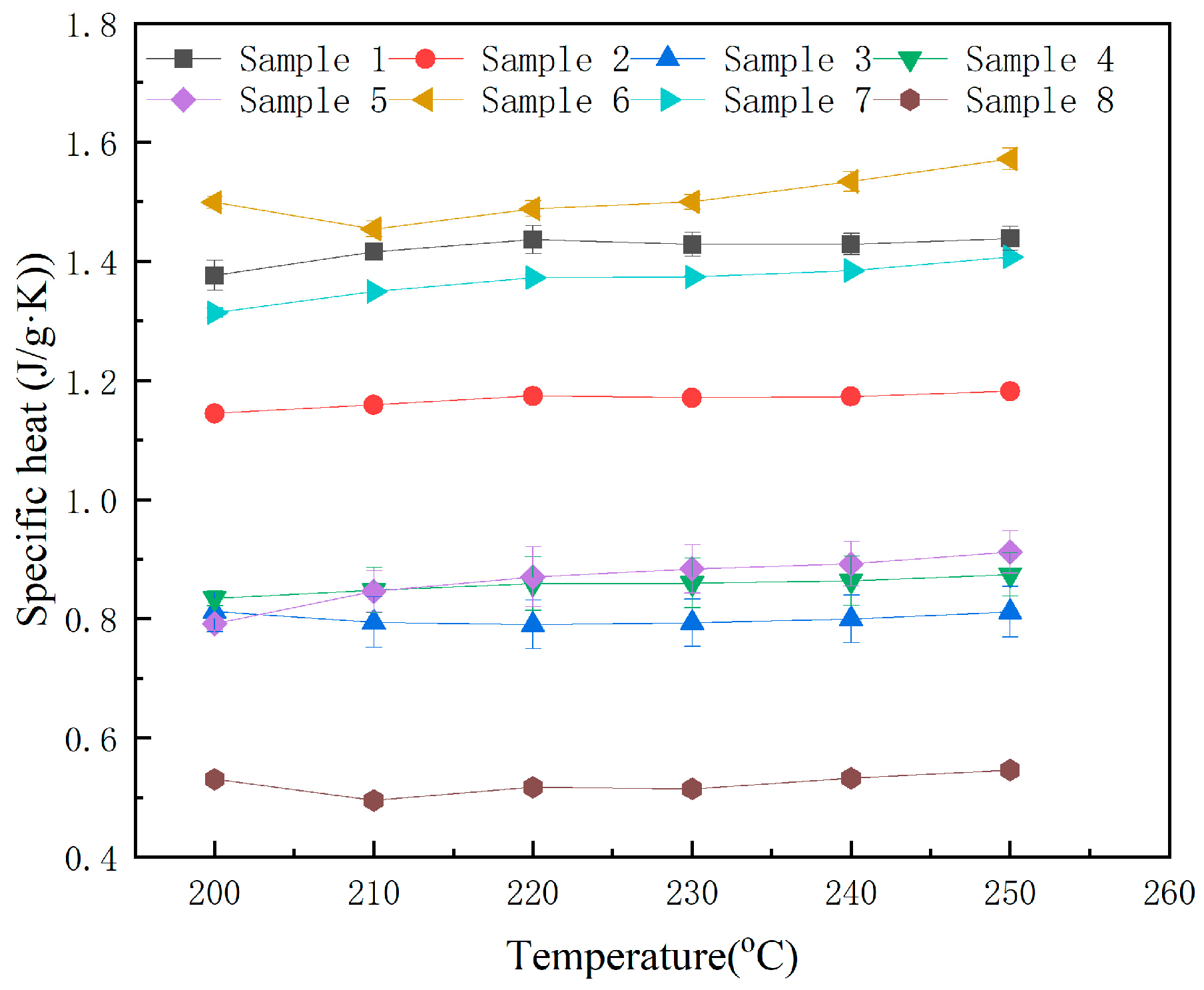
3.3. Specific Heat Capacity
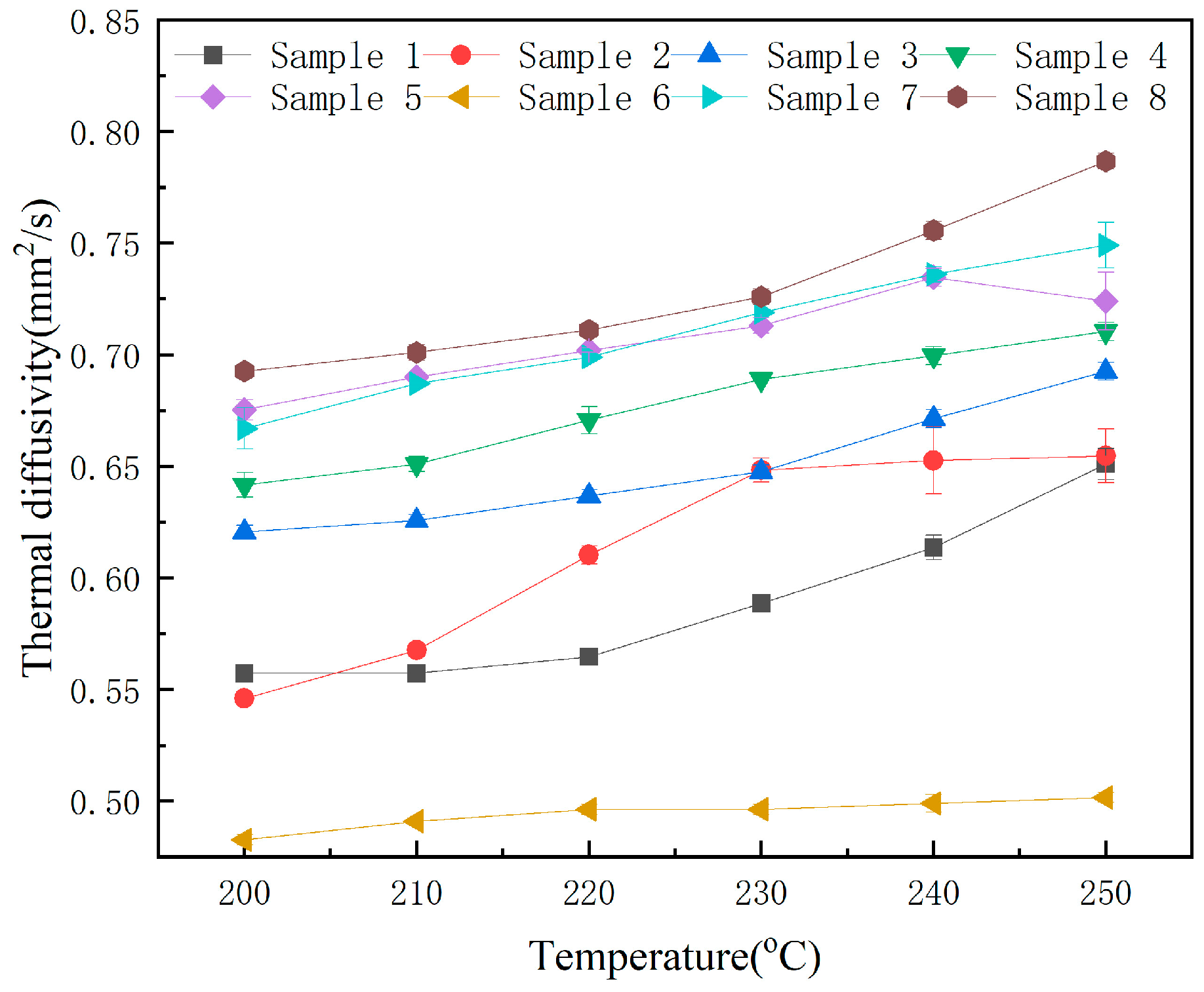
3.4. Heat Transfer Characters
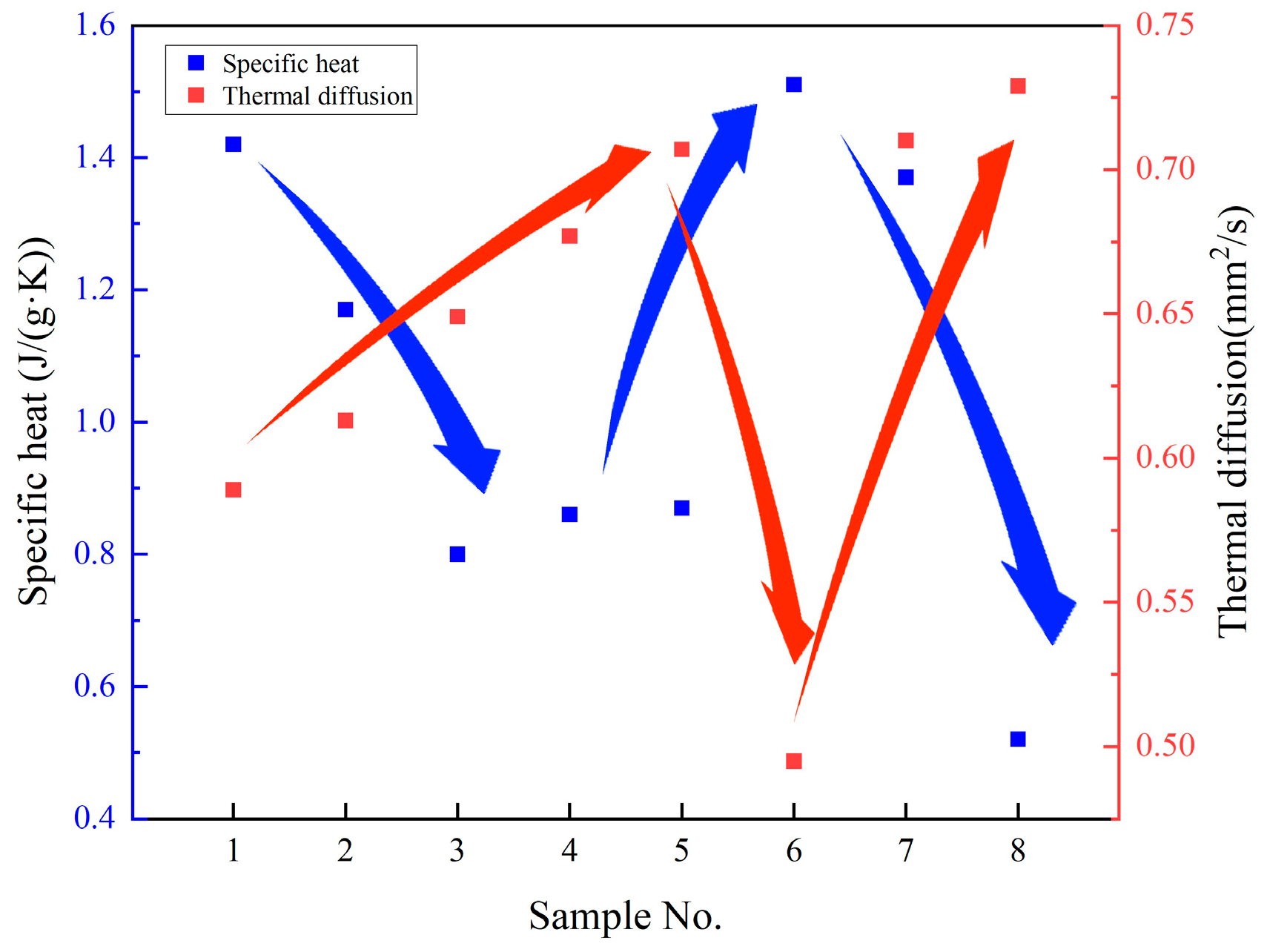
3.5. Discussion of the Relationship Between Specific Heat and Thermal Diffusivity
4. Conclusions
- (1)
- The specific heat capacity measurement results of MNs exhibit significant nanoparticle-dependent variations. A 6% enhancement in molten-state specific heat capacity (1.51 J/(g·K)) is achieved at the optimal binary doping concentration of 0.7 wt.% SiO2 and 0.3 wt.% MgO, compared to 1.42 J/(g·K) for the base salt.
- (2)
- The results obtained indicate that the combined doping of SiO2 and MgO in an appropriate ratio can enhance the thermal conductivity of the molten salt. The base salt exhibits an average thermal diffusion of 0.589 mm2/S. However, with the incorporation of 1.0 wt.% SiO2, this value increases significantly to 0.729 mm2/S, achieving a 24% enhancement compared to the pristine material.
- (3)
- The dispersion of nanoparticles within molten salts introduces interfacial structures and/or semi-solid boundary layers. While these structures enhance the specific heat capacity, they simultaneously impede the thermal transport efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.-H.; Li, H.-H.; Zhang, L.-H.; Liu, Z. Phase Change Energy Storage Material Suitable for Solar Heating System. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012044. [Google Scholar] [CrossRef]
- Muhammad Review of PCMS and heat transfer enhancement methods applied in parabolic trough solar plants thermal storage systems. Niger. J. Technol. 2018, 37, 96. [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Wei, Q.-S. Physical Insights on Nanoparticle-Induced Enhancement of Thermal Conduction and Heat Storage Properties of Molten Salts. Master’s Thesis, North China Electric Power University, Beijing, China, 2023. [Google Scholar]
- Yu, Q.; Lu, Y.; Zhang, X.; Yang, Y.; Zhang, C.; Wu, Y. Comprehensive thermal properties of molten salt nanocomposite materials base on mixed nitrate salts with SiO2/TiO2 nanoparticles for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 203, 111215. [Google Scholar] [CrossRef]
- Wei, X.; Chen, D.; Zhang, X.; Wang, W.; Lu, J.; Ding, J.; Liu, S. Experimental and Simulation Study on Enhancing Thermal Energy Storage and Heat Transfer Performance of Ternary Chloride Eutectic Salt by Doping MgO Nanoparticles. J. Therm. Sci. 2024, 33, 2221–2234. [Google Scholar] [CrossRef]
- Suraparaju, S.K.; Aljaerani, H.A.; Samykano, M.; Kadirgama, K.; Noor, M.M.; Natarajan, S.K. Thermal properties analysis and thermal cycling of HITEC molten salt with h-BN nanoparticles for CSP thermal energy storage applications. Environ. Sci. Pollut. Res. Int. 2024, 31, 50166–50178. [Google Scholar] [CrossRef]
- Jeong, S.; Jo, B. Understanding mechanism of enhanced specific heat of single molten salt-based nanofluids: Comparison with acid-modified salt. J. Mol. Liq. 2021, 336, 116561. [Google Scholar] [CrossRef]
- Akanda, M.-A.-M.; Shin, D. A synthesis parameter of molten salt nanofluids for solar thermal energy storage applications. J. Energy Storage 2023, 60, 106608. [Google Scholar] [CrossRef]
- Divakar, M.; Wang, T.; Reddy, G.-R. Thermodynamic modeling of eutectic point in the LiNO3-NaNO3-KNO3-NaNO2 quaternary system. Sol. Energy Mater. Sol. Cells 2013, 118, 18–21. [Google Scholar]
- Zhang, X.; Lu, Y.; Yu, Q.; Wu, Y. Effect of Microwave Method on Thermal Properties of Nano-Quaternary Mixed Nitrate. Acta Energiae Solaris Sinica 2021, 42, 153–159. [Google Scholar]
- Feng, L.; Zhao, W.; Zheng, J.; Frisco, S.; Song, P.; Li, X. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mater. Sol. Cells 2011, 95, 3550–3556. [Google Scholar] [CrossRef]
- Oh, S.H.; Kauffmann, Y.; Scheu, C.; Kaplan, W.D.; RühLe, M. Ordered Liquid Aluminum at the Interface with Sapphire. Science 2005, 310, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Alexiadis, A.; Ding, Y. Simulation Study of Anomalous Thermal Properties of Molten Nitrate Salt. Powder Technol. 2017, 314, 660–664. [Google Scholar] [CrossRef]
- Li, Y.; Tan, W.W.; Wang, C.G.; Zhu, Q.Z. Research on the effect of adding NaCl on the performance of KNO3–NaNO3 binary molten salt. J. Therm. Anal. Calorim. 2022, 148, 733–739. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Sun, M.; Wu, Y.; Xu, P.; Qian, X.; Li, C.; Ding, Y.; Ma, C. Enhanced thermal energy storage of nitrate salts by silica nanoparticles for concentrating solar power. Int. J. Energy Res. 2020, 45, 5248–5262. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-T.; Zhang, L.-D.; Wang, X.; Ma, C.-F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Wang, L.; Tan, Z.; Meng, S.; Liang, D.; Li, G. Enhancement of Molar Heat Capacity of Nanostructured Al2O3. J. Nanoparticle Res. 2001, 3, 483–487. [Google Scholar] [CrossRef]
- Lasfargues, M.; Bell, A.; Ding, Y. In situ production of titanium dioxide nanoparticles in molten salt phase for thermal energy storage and heat-transfer fluid applications. J. Nanoparticle Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Wen, H.; Lin, S.; Zhao, C.; Wang, E. Nanoparticle surface charge-enhanced heat capacity in molten salt phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 243, 111795. [Google Scholar] [CrossRef]
- Tian, B.; Zhu, C.; Gu, M.; Xu, M.; He, W. Thermophysical properties enhancement of KNO3–NaNO3–NaNO2 mixed with SiO2/MgO nanoparticles. J. Sci. Adv. Mater. Devices 2025, 10, 100849. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Tian, B.; Gu, M.; Gong, L. Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles. Materials 2024, 17, 4611. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.L.; Hsiao, P.Y.; Hung, S.W.; Chieng, C.C.; Liu, M.S.; Lu, M.C. Enhanced Thermal Conductivity of Nanofluids Diagnosis by Molecular Dynamics Simulations. J. Nanosci. Nanotechnol. 2008, 8, 3710–3718. [Google Scholar] [CrossRef]

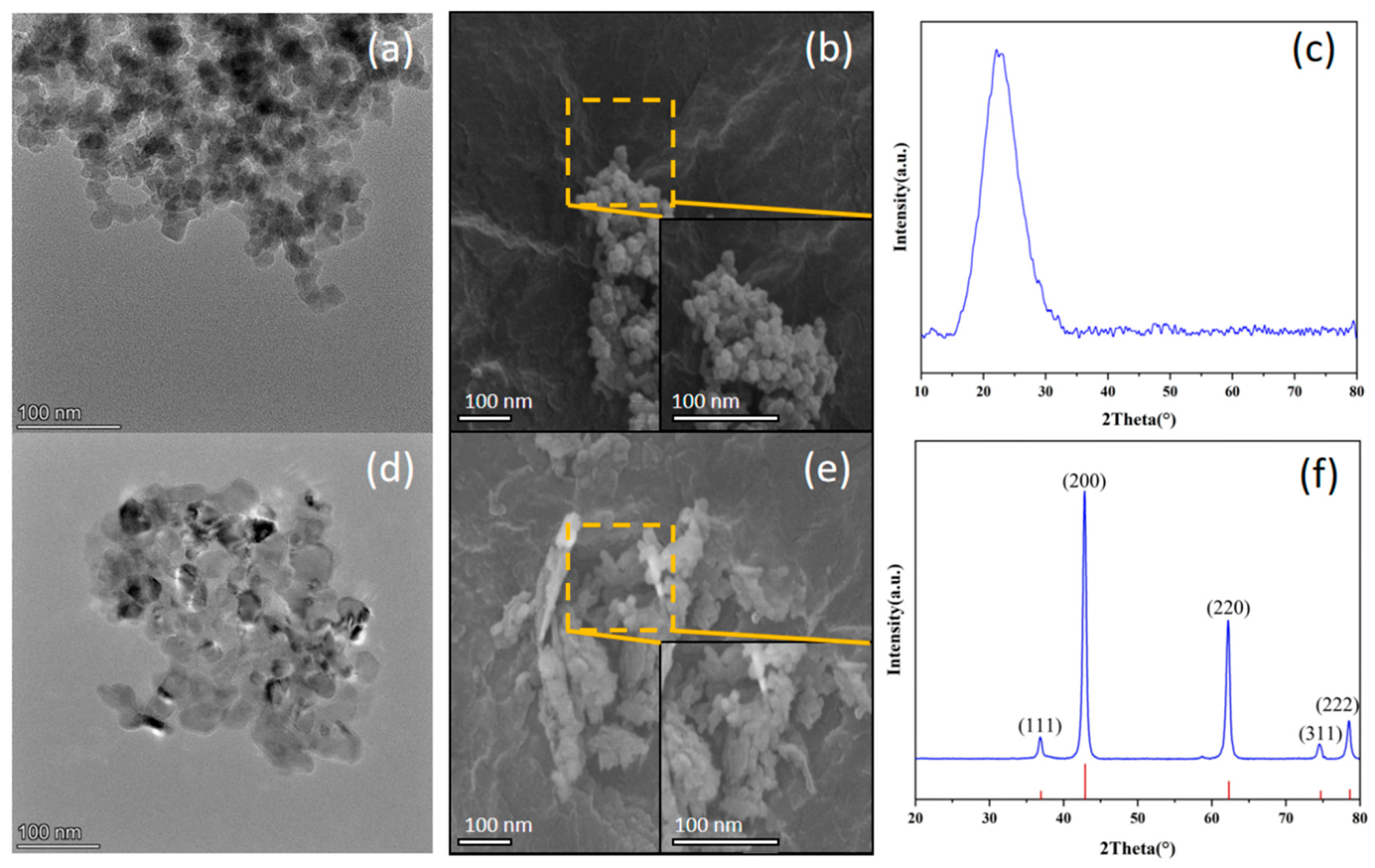
| Characteristics | Parameters | |
|---|---|---|
| Nano-SiO2 | Nano-MgO | |
| Size (nm) | About 30 | About 50 |
| Specific surface area (m2/g) | 2.65 | 3.58 |
| Density (g/cm3) | 143 | 50 |
| Thermal conductivity (W/(m·K)) | 8.3 | 36 |
| Specific heat (J/(g·K)) | 0.99 | 0.92 |
| Sample No. | LiNO3-NaNO3-KNO3-NaNO2 | SiO2 | MgO |
|---|---|---|---|
| 1 | 1 | 0 | 0 |
| 2 | 0.99 | 0 | 0.01 |
| 3 | 0.99 | 0.001 | 0.009 |
| 4 | 0.99 | 0.003 | 0.007 |
| 5 | 0.99 | 0.005 | 0.005 |
| 6 | 0.99 | 0.007 | 0.003 |
| 7 | 0.99 | 0.009 | 0.001 |
| 8 | 0.99 | 0.01 | 0 |
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Mass (mg) | 5.61 | 5.33 | 9.67 | 5.06 | 5.36 | 5.28 | 5.05 | 5.36 |
| Sample No. | 200 °C | 210 °C | 220 °C | 230 °C | 240 °C | 250 °C |
|---|---|---|---|---|---|---|
| 1 | 1.38 (±0.03) | 1.42 (±0.01) | 1.44 (±0.02) | 1.43 (±0.02) | 1.43 (±0.02) | 1.44 (±0.02) |
| 2 | 1.14 (±0.01) | 1.16 (±0.00) | 1.17 (±0.00) | 1.17 (±0.00) | 1.17 (±0.00) | 1.18 (±0.01) |
| 3 | 0.81 (±0.03) | 0.79 (±0.04) | 0.79 (±0.04) | 0.79 (±0.04) | 0.80 (±0.04) | 0.81 (±0.04) |
| 4 | 0.83 (±0.01) | 0.85 (±0.04) | 0.86 (±0.05) | 0.86 (±0.04) | 0.86 (±0.04) | 0.87 (±0.04) |
| 5 | 0.79 (±0.01) | 0.85 (±0.03) | 0.87 (±0.05) | 0.88 (±0.04) | 0.89 (±0.04) | 0.91 (±0.04) |
| 6 | 1.45 (±0.01) | 1.45 (±0.01) | 1.49 (±0.01) | 1.50 (±0.01) | 1.53 (±0.02) | 1.57 (±0.02) |
| 7 | 1.31 (±0.01) | 1.35 (±0.00) | 1.37 (±0.00) | 1.37 (±0.00) | 1.38 (±0.00) | 1.41 (±0.00) |
| 8 | 0.53 (±0.01) | 0.50 (±0.01) | 0.52 (±0.00) | 0.51 (±0.01) | 0.53 (±0.00) | 0.55 (±0.00) |
| Sample No. | 200 °C | 210 °C | 220 °C | 230 °C | 240 °C | 250 °C |
|---|---|---|---|---|---|---|
| 1 | 0.557 (±0.003) | 0.557 (±0.003) | 0.565 (±0.003) | 0.589 (±0.003) | 0.614 (±0.006) | 0.651 (±0.007) |
| 2 | 0.546 (±0.000) | 0.568 (±0.003) | 0.610 (±0.004) | 0.648 (±0.006) | 0.653 (±0.015) | 0.655 (±0.012) |
| 3 | 0.621 (±0.003) | 0.626 (±0.003) | 0.637 (±0.003) | 0.648 (±0.003) | 0.671 (±0.004) | 0.693 (±0.004) |
| 4 | 0.642 (±0.006) | 0.651 (±0.003) | 0.671 (±0.006) | 0.689 (±0.000) | 0.700 (±0.004) | 0.710 (±0.004) |
| 5 | 0.675 (±0.005) | 0.690 (±0.00) | 0.702 (±0.001) | 0.713 (±0.003) | 0.735 (±0.004) | 0.724 (±0.013) |
| 6 | 0.483 (±0.002) | 0.491 (±0.000) | 0.496 (±0.002) | 0.496 (±0.002) | 0.499 (±0.004) | 0.502 (±0.002) |
| 7 | 0.667 (±0.001) | 0.687 (±0.000) | 0.699 (±0.000) | 0.719 (±0.000) | 0.736 (±0.003) | 0.749 (±0.010) |
| 8 | 0.693 (±0.000) | 0.701 (±0.003) | 0.711 (±0.000) | 0.726 (±0.003) | 0.756 (±0.004) | 0.787 (±0.004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; He, W.; Gu, M.; Zhang, D.; Tian, B. Synergistic Enhancement of LiNO3-NaNO3-KNO3-NaNO2 Thermophysical Properties Through Dual Nano-Additives: SiO2 and MgO. Nanomaterials 2025, 15, 1094. https://doi.org/10.3390/nano15141094
Zhu C, He W, Gu M, Zhang D, Tian B. Synergistic Enhancement of LiNO3-NaNO3-KNO3-NaNO2 Thermophysical Properties Through Dual Nano-Additives: SiO2 and MgO. Nanomaterials. 2025; 15(14):1094. https://doi.org/10.3390/nano15141094
Chicago/Turabian StyleZhu, Chuang, Wenxuan He, Manting Gu, Dan Zhang, and Baiyuan Tian. 2025. "Synergistic Enhancement of LiNO3-NaNO3-KNO3-NaNO2 Thermophysical Properties Through Dual Nano-Additives: SiO2 and MgO" Nanomaterials 15, no. 14: 1094. https://doi.org/10.3390/nano15141094
APA StyleZhu, C., He, W., Gu, M., Zhang, D., & Tian, B. (2025). Synergistic Enhancement of LiNO3-NaNO3-KNO3-NaNO2 Thermophysical Properties Through Dual Nano-Additives: SiO2 and MgO. Nanomaterials, 15(14), 1094. https://doi.org/10.3390/nano15141094





