High-Sensitivity, Low Detection Limit, and Fast Ammonia Detection of Ag-NiFe2O4 Nanocomposite and DFT Study
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ag-NiFe2O4 Nanocomposites
3. Results and Discussion
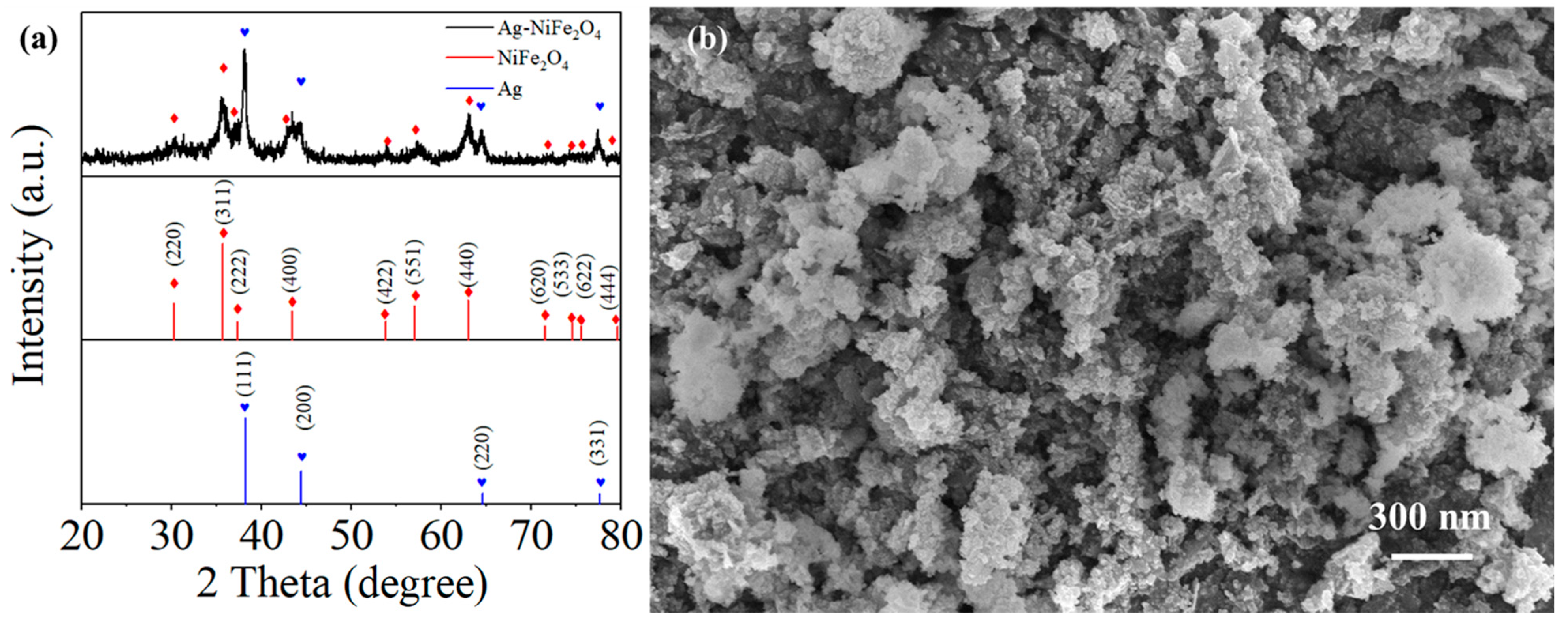
3.1. Characterization of the Prepared Material
3.2. NH3 Sensitive Properties
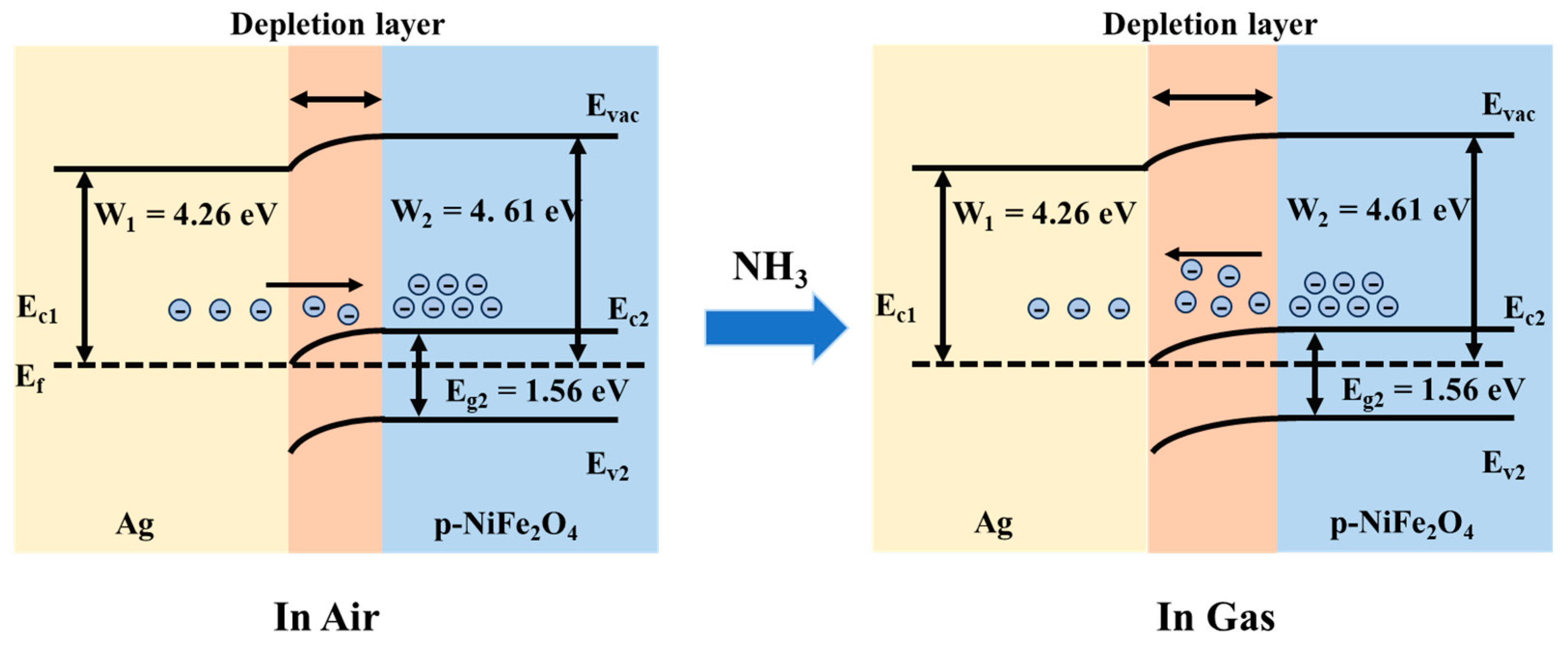
3.3. NH3 Sensitive Mechanism
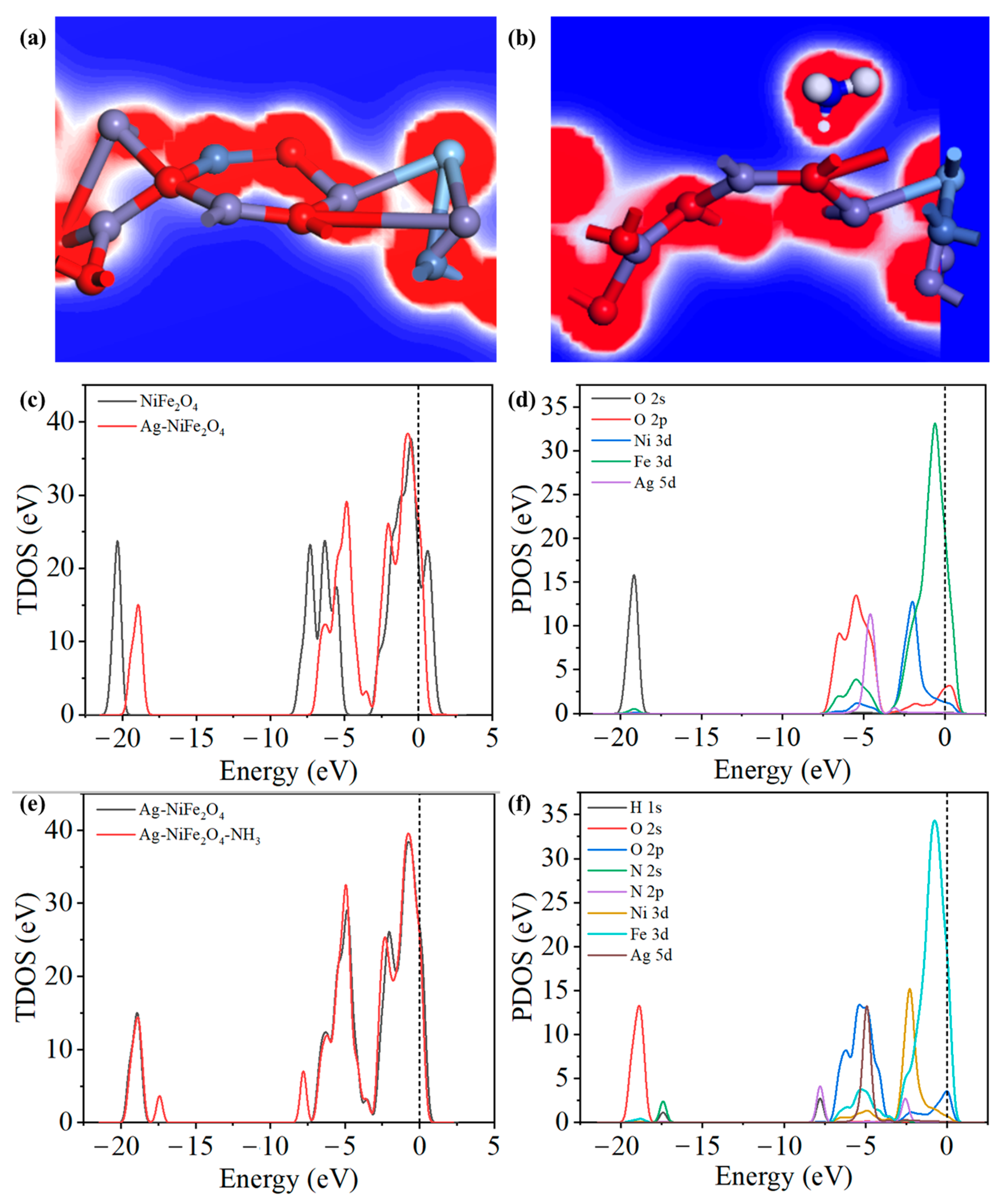
3.4. DFT Calculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, J.F.; Liu, X.N.; Sun, H.Y.; He, Y.; Qiao, S.D.; Zhang, C.; Zhao, W.J.; Ma, Y.F. Deep learning based light-induced thermoelastic spectroscopy signal separation for hybrid gas sensing. Sens. Actuators B Chem. 2025, 440, 137918. [Google Scholar] [CrossRef]
- Lv, W.; Yang, J.H.; Xu, Q.D.; Mehrez, J.A.A.; Shi, J.; Quan, W.J.; Luo, H.Y.; Zeng, M.; Hu, N.T.; Wang, T.; et al. Wide-range and high-accuracy wireless sensor with self-humidity compensation for real-time ammonia monitoring. Nat. Commun. 2024, 15, 6936. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Hou, L.X.; Cui, X.P.; Zhan, P.X.; He, P.; Dai, C.Y.; Li, R.A.; Dong, J.C.; Zou, Y.; Liu, G.M.; et al. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals. Nat. Commun. 2024, 15, 3618. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Nam, G.B.; Ryu, J.E.; Eom, T.H.; Kim, S.J.; Suh, J.M.; Lee, S.; Choi, S.; Moon, C.W.; Park, S.J.; Lee, S.M.; et al. Real-time tunable gas sensing platform based on SnO2 nanoparticles activated by blue micro-light-emitting diodes. Nano-Micro Lett. 2024, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Mulimani, P.; Bhat, M.P.; Patil, P.; Aralekallu, S.; Kapavarapu, R.; Yu, J.; Kurkuri, M.; Kalkhambkar, R.G. Colorimetric devices for naked-eye detection of Fe3+ and Cu2+: Optical properties, DFT calculations, and molecular docking studies. J. Water Process. Eng. 2024, 59, 105030. [Google Scholar] [CrossRef]
- Gong, H.; Pang, T.; Yang, X.; Chen, F.; Jiang, N.; Li, Y.; Chen, C.; Cai, C. Rapid visual detection of tobacco mosaic virus using a portable paper-based molecularly imprinted sensor. Sens. Actuators B Chem. 2025, 424, 136909. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, D.; Zhang, K.; Camarada, M.B.; Xiong, J.; Liao, X. A novel portable electrochemical sensor based on post-synthetic modified Cu-MOF for highly sensitive electrochemical detection of pefloxacin in food samples. Microchem. J. 2025, 209, 112895. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhang, D.; Yang, Y.; Mi, Q.; Zhang, J.H.; Yu, L.D. Multifunctional latex/polytetrafluoroethylene-based triboelectric nanogenerator for self-powered organ-like MXene/metal-organic framework-derived CuO nanohybrid ammonia sensor. ACS Nano 2025, 15, 2911–2919. [Google Scholar] [CrossRef]
- Tie, Y.; Ma, S.Y.; Pei, S.T.; Zhang, Q.X.; Zhu, K.M.; Zhang, R.; Xu, X.H.; Han, T.; Liu, W.W. Pr doped BiFeO3 hollow nanofibers via electrospinning method as a formaldehyde sensor. Sens. Actuator B Chem. 2020, 308, 127689. [Google Scholar] [CrossRef]
- Wang, B.B.; Xing, Y.L.; Zhang, K.W.; Wang, Z.; Xia, Y.Z.; Long, X.J. Electron-deficient organic molecules based on B←N unit: A N-type room-temperature chemiresistive sensors with moisture resistance. Adv. Sci. 2024, 11, 2409890. [Google Scholar] [CrossRef]
- Pflüger, T.; Gschell, M.; Zhang, L.; Shnitsar, V.; Zabadné, A.J.; Zierep, P.; Günther, S.; Einsle, O.; Andrade, S.L.A. How sensor Amt-like proteins integrate ammonium signals. Sci. Adv. 2024, 10, eadm9441. [Google Scholar] [CrossRef]
- Fu, F.M.; Zhang, X.Y.; Wang, W.; Xie, X.J. Colorimetric optode sensor with tripodal ionophore for rapid urinary ammonium determination. ACS Sens. 2025, 10, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Q.; Fuh, H.R.; Bau, J.Y.; Cho, J.; Abid, M.; Bae, T.S.; Chung, H.S.; Coileáin, C.Ó.; Chang, C.R.; Wu, H.C. Impact of molecule-molecule interactions when discerning low-concentration hazardous gas mixtures. ACS Nano 2025, 19, 7202–7212. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, B.; Gupta, S.K.; Mandal, D.; Gor, A.A.; Bandyopadhyay, R.; Mukherjee, K. Probing the p-type chemiresistive response of NiFe2O4 nanoparticles for potential utilization as ethanol sensor. Chem. Asian J. 2024, 19, e202300841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.X.; Qiu, C.K.; Jia, P.L.; An, F.; Zhou, L.N.; Zhu, L.; Zhang, D.Z. Polyaniline/ZnO heterostructure-based ammonia sensor self-powered by electrospinning of PTFE-PVDF/MXene piezo-tribo hybrid nanogenerator. Chem. Eng. J. 2024, 496, 154226. [Google Scholar] [CrossRef]
- Rezaeipour, A.; Dehghani, S.; Hoghoghifard, S. Acetone sensor based on porous nickel ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 26276–26285. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jia, C.W.; Wang, Q.Y.; Kong, Q.; Chen, G.; Guan, H.T.; Dong, C.J. MOFs-derived porous NiFe2O4 nano-octahedrons with hollow interiors for an excellent toluene gas sensor. Nanomaterials 2019, 9, 1059. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, D.; Tang, M.; Zhang, H.; Wang, Z.; Jia, P.; Zhai, J. Amorphous Ag catalytic layer-SnO2 sensitive layer-graphite carbon nitride electron supply layer synergy-enhanced hydrogen gas sensor. Chem. Eng. J. 2024, 495, 153676. [Google Scholar] [CrossRef]
- Tang, M.X.; Qin, C.; Sun, X.Y.; Li, M.W.; Wang, Y.H.; Cao, J.L.; Wang, Y. Enhanced H2 gas sensing performances by Pd-loaded In2O3 microspheres. Appl. Phys. A 2024, 130, 741. [Google Scholar] [CrossRef]
- Praveen, L.L.; Singh, N.P.; Vardhan, R.V.; Mandal, S. All-printed WO3 films on an Ag-interdigitated electrode derived from aqueous screen-printable inks for room-temperature ammonia gas detection. Flex. Print. Electron. 2025, 10, 015008. [Google Scholar] [CrossRef]
- Chao, J.F.; Liu, Z.D.; Xing, S.M.; Gao, Q.Q.; Zhao, J.Z. Enhanced ammonia detection of gas sensors based on square-like tungsten oxide loaded by Pt nanoparticles. Sens. Actuators B Chem. 2021, 347, 130621. [Google Scholar] [CrossRef]
- Zhai, H.C.; Wu, Z.Y.; Xiao, K.; Ge, M.Y.; Liu, C.X.; Tian, P.F.; Wan, J.; Wang, J.L.; Kang, J.Y.; Chu, J.H.; et al. p-Type β-Ga2O3 film room-temperature NH3 gas sensors with fast gas sensing and a low limit of detection. J. Mater. Chem. C 2024, 12, 19526–19535. [Google Scholar] [CrossRef]
- Karmakar, S.; Sett, A.; Maity, P.C.; Karmakar, G.; Sha, R.; Bhattacharyya, T.K.; Lahiri, I. A room-temperature gas sensor based on 2D Ni-Co-Zn ternary oxide nanoflakes for selective and sensitive ammonia detection. Dalton Trans. 2023, 52, 16500–16512. [Google Scholar] [CrossRef]
- Haridas, V.; Sukhananazerin, A.; Sneha, J.M.; Pullithadathil, B.; Narayanan, B. α-Fe2O3 loaded less-defective graphene sheets as chemiresistive gas sensor for selective sensing of NH3. Appl. Surf. Sci. 2020, 517, 146158. [Google Scholar] [CrossRef]
- Punetha, D.; Pandey, S.K. Enhancement and optimization in sensing characteristics of ammonia gas sensor based on light assisted nanostructured WO3 thin film. IEEE Sens. J. 2020, 20, 14617–14623. [Google Scholar] [CrossRef]
- Qian, X.J.; Chen, Y.P.; Tao, Y.Y.; Zhang, J.; Zhang, G.F.; Xu, H.Y. Facile synthesis of NiFe2O4-based nanoblocks for low-temperature detection of trace n-butanol. RSC Adv. 2024, 14, 2214–2225. [Google Scholar] [CrossRef]
- Xia, Y.D.; Dai, J.H.; Cao, Y.R.; Ni, Y.X.; Ou, K.; Wang, H.Y. High-performance room temperature NH3 sensor based on zigzag morphology TiO2/Fe2O3 heterojunction. Eur. Phys. J. Plus 2025, 140, 138. [Google Scholar] [CrossRef]
- Alagarasan, D.; Hegde, S.S.; Naik, R.; Shetty, H.D.; Prasad, H.B.S.; Alshahrani, T.; AlFaify, S.; Shkir, M. Remarkable NH3 gas sensing performance of spray deposited Tb doped WO3 thin films at room temperature. J. Photoch. Photobio. A Chem. 2024, 459, 116087. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.J.; Wang, X.W.; Huang, J.K.; Pan, W.J.; Zhang, J.H.; Meteku, B.E.; Zeng, J.B. UV illumination-enhanced ultrasensitive ammonia gas sensor based on (001)TiO2/MXene heterostructure for food spoilage detection. J. Hazard. Mater. 2021, 423, 127160. [Google Scholar] [CrossRef]



| Sensing Materials | Temp. (°C) | Conc. (ppm) | Response | Res./Rec. Time (s) | Ref. |
|---|---|---|---|---|---|
| WO3 porous nanoplates | RT | 100 | ~340 | 90 s/30 s | [21] |
| 1%Pt/WO3 | 270 °C | 100 | ∼10 | 7 s/7 s | [22] |
| β-Ga2O3 | RT | 50 | 219.1% | 42.3 s/60 s | [23] |
| NiCo2ZnO4 | RT | 25 | 22.69 | 74.84 s/240 s | [24] |
| α-Fe2O4/graphene | 250 °C | 10 | 13.5% | 648 s/152 s | [25] |
| WO3 thin film | 250 °C | 50 | 12.58 | 13 s/34 s | [26] |
| 10 wt% Ag-NiFe2O4 | 280 °C | 30 | 4.59 | 3 s/9 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Sun, Y.; Liu, Z.; Jiao, G.; Zhang, D. High-Sensitivity, Low Detection Limit, and Fast Ammonia Detection of Ag-NiFe2O4 Nanocomposite and DFT Study. Nanomaterials 2025, 15, 1088. https://doi.org/10.3390/nano15141088
Hao X, Sun Y, Liu Z, Jiao G, Zhang D. High-Sensitivity, Low Detection Limit, and Fast Ammonia Detection of Ag-NiFe2O4 Nanocomposite and DFT Study. Nanomaterials. 2025; 15(14):1088. https://doi.org/10.3390/nano15141088
Chicago/Turabian StyleHao, Xianfeng, Yuehang Sun, Zongwei Liu, Gongao Jiao, and Dongzhi Zhang. 2025. "High-Sensitivity, Low Detection Limit, and Fast Ammonia Detection of Ag-NiFe2O4 Nanocomposite and DFT Study" Nanomaterials 15, no. 14: 1088. https://doi.org/10.3390/nano15141088
APA StyleHao, X., Sun, Y., Liu, Z., Jiao, G., & Zhang, D. (2025). High-Sensitivity, Low Detection Limit, and Fast Ammonia Detection of Ag-NiFe2O4 Nanocomposite and DFT Study. Nanomaterials, 15(14), 1088. https://doi.org/10.3390/nano15141088






