Abstract
Titanium dioxide (TiO2) is a benchmark photocatalyst for environmental applications, but its limited visible-light activity due to a wide band gap and fast charge recombination restricts its practical efficiency. This study presents the development of heterostructured Ag (Au)/MoS2-TiO2 inverse opal (IO) films that synergistically integrate photonic, plasmonic, and semiconducting functionalities to overcome these limitations. The materials were synthesized via a one-step evaporation-induced co-assembly approach, embedding MoS2 nanosheets and plasmonic nanoparticles (Ag or Au) within a nanocrystalline TiO2 photonic framework. The inverse opal architecture enhances light harvesting through slow-photon effects, while MoS2 and plasmonic nanoparticles improve visible-light absorption and charge separation. By tuning the template sphere size, the photonic band gap was aligned with the TiO2-MoS2 absorption edge and the localized surface plasmon resonance of Ag, enabling optimal spectral overlap. The corresponding Ag/MoS2-TiO2 photonic films exhibited superior photocatalytic and photoelectrocatalytic degradation of tetracycline under visible light. Ultraviolet photoelectron spectroscopy and Mott–Schottky analysis confirmed favorable band alignment and Fermi level shifts that facilitate interfacial charge transfer. These results highlight the potential of integrated photonic–plasmonic-semiconductor architectures for efficient solar-driven water treatment.
1. Introduction
Titanium dioxide (TiO2) nanomaterials have been long investigated as benchmark semiconductor photocatalysts due to their high redox potential, chemical stability, and low cost [1,2]. In addition, TiO2 is largely considered non-toxic, though recent studies have raised concerns on the toxicity of TiO2 nanoparticles (NPs) to human health and the environment [3,4], indicating that their immobilization on suitable supports, which prevents material leaching, is the safest approach towards environmentally benign TiO2 photocatalytic nanomaterials [5]. However, their practical use in environmental remediation is limited by their wide band gap (3.0–3.2 eV) and low quantum efficiency, which depend critically on the structural, electronic, and morphological properties of TiO2, i.e., the nanomaterial phase, crystallinity, defect content, texture, size, and shape that largely impact charge carrier recombination and the process efficiency [6,7]. To overcome these limitations, heterojunction engineering, particularly with narrow-band-gap semiconductors and suitable co-catalysts, has emerged as an effective strategy to enhance visible-light absorption, promote charge separation, and facilitate surface redox reactions [8,9] for the abatement of contaminants of emerging concern by means of photocatalysis [10,11] and photoelectrocatalysis [12,13].
Molybdenum disulfide (MoS2), the prototype transition metal dichalcogenide, has emerged as a promising co-catalyst for TiO2 due to its distinctive electronic properties [14,15]. A key advantage of MoS2 lies in the tunability of its band gap, from 1.3 (indirect) to 1.9 eV (direct), which can be modulated by reducing the number of nanosheets (NSs) from bulk to monolayer levels, as a result of weak van der Waals interlayer coupling [16]. This band gap tunability, together with MoS2’s exceptional optoelectronic and mechanical characteristics, has expanded its application potential to hydrogen evolution and water remediation [17,18,19]. Despite these advantages, the performance of MoS2 photocatalysts is hindered by its low oxidation potential for hydroxyl radical formation and the limited number of active sites on its inert basal planes [20]. To circumvent these drawbacks, heterojunctions with nanostructured TiO2 supports have been extensively explored [21,22,23,24,25] in order to enhance visible-light absorption and facilitate charge separation for photocatalytic organics degradation.
Among various architectures, inverse opal (IO) structures stand out due to their three-dimensional photonic crystal (PC) framework [26,27], which enables slow-photon effects at the photonic band gap (PBG) edges. The overlap of slow photons with spectral regions of weak materials’ electronic absorption offers a unique structural approach for improving light harvesting of photocatalytic nanomaterials, especially TiO2 photocatalysts [28,29,30]. Additionally, the IO morphology offers a hierarchical porous structure with interconnected macropores and mesoporous skeletal walls [31], facilitating mass transport and pollutant adsorption that can be further combined with compositional and morphological modifications for visible-light-activated TiO2-based PC catalysts [32,33,34,35,36]. The co-assembly of templating colloids with metal oxide precursors has emerged as an advanced strategy that integrates template self-assembly and liquid-phase infiltration into a single-step process, leading to the fabrication of high-quality IO photocatalytic films [37,38]. This approach was further enhanced by incorporating noble metal NPs as a third component in the co-assembly mixture, enabling the creation of composite IO structures with plasmonic functionality [39,40,41]. More recently, a three-phase co-assembly method involving polymer colloids and water-soluble metal oxide precursors enabled the fabrication of compositionally tunable WO3/TiO2 IOs, featuring uniformly distributed nanoscale type II heterojunctions within the skeletal walls [42]. This single-step method was also successfully applied to incorporate MoS2 NSs into the nanocrystalline framework of TiO2 IOs, resulting in highly active MoS2–TiO2 photocatalysts for the visible-light degradation of pharmaceutical contaminants [43].
Plasmonic-assisted photocatalysis via metal–semiconductor heterojunctions has emerged as a powerful strategy to evade the limitations of poor light harvesting and inefficient charge separation in wide-band-gap semiconductors like TiO2 [44]. Beyond their long-established role as electron scavengers that suppress electron–hole recombination [9], metal co-catalysts such as Au and Ag NPs can enhance photocatalytic activity through both radiative and non-radiative decay of their localized surface plasmon resonance (LSPR) [45,46]. Radiative decay can induce strong near-field enhancement, significantly increasing electron–hole pair generation in adjacent semiconductors [47]. For larger NPs (typically >50 nm), light scattering becomes dominant [44,45], extending the photon path length and promoting further charge carrier generation. However, such enhancements are spectrally confined to the overlap between the semiconductor band gap and LSPR absorption. Non-radiative LSPR decay can lead to hot electron injection over the Schottky barrier at the metal–semiconductor interface [48,49] or to plasmon-induced resonant energy transfer [50], both of which enable visible-light activation of TiO2 without direct band gap excitation. Efforts to align the LSPR of Au and Ag NPs with slow-photon regions in photonic TiO2 structures have aimed to synergistically combine plasmonic and photonic effects for improved light harvesting [51]. Enhanced photocatalytic performance of PBG engineered IOs has been largely attributed to LSPR excitation facilitated by slow photons and efficient hot electron injection into the TiO2 conduction band [52,53,54,55,56]. More recently, electron scavenging due to interfacial band alignment and radiative near-field enhancement by plasmonic NPs have been identified as key factors in the improved (photo)electrocatalytic performance of plasmonic modified IOs [57,58,59].
In this work, heterostructured Ag(Au)/MoS2–TiO2 IO films were developed and investigated as photoelectrocatalysts for the degradation of tetracycline (TC), a broad-spectrum antibiotic of significant environmental concern [10]. The plasmonic–photonic structures were fabricated via the evaporation-induced self-assembly of sacrificial colloidal spheres with a water-soluble Ti precursor and aqueous dispersions of Ag(Au) NPs and MoS2 NSs. This approach enabled their effective incorporation into the nanocrystalline TiO2 IO framework and the formation of abundant, stable heterojunctions. Two distinct colloidal template sizes were employed to tune the slow-photon spectral regions to the TiO2 absorption edge and the visible absorption of MoS2, respectively, in order to explore the optimal amplification spectral range. The incorporation of MoS2 NSs and Ag NPs into the TiO2 IO structure led to a marked enhancement in both photocatalytic and photoelectrocatalytic activity under visible light. The best performance in TC degradation was achieved with Ag/MoS2–TiO2 IO films, where the red-shifted slow photons matched both the Ag LSPR absorption and the MoS2–TiO2 absorption edge, assisted by electron transfer from MoS2 NSs and Ag NPs acting as visible light sensitizers of TiO2 along with the local electromagnetic field enhancement at the metal–semiconductor interface due to LSPR.
2. Materials and Methods
2.1. Chemicals and Reagents
Monodisperse polystyrene (PS) microspheres with mean diameters of 211 and 418 nm (SD = 5–10 nm) were obtained from Microparticles GmbH as 5% (w/v) colloidal dispersions in deionized water (2.7–3.0% CV). Titanium(IV) bis(ammonium lactato) dihydroxide (TiBALDH, 50 wt.% in water), molybdenum disulfide dispersion (1 mg/mL in H2O, lateral size 50–1000 nm), Au NPs (5 nm, OD 1, citrate-stabilized, λmax ≈ 510–521 nm), and HellmanexTM III were purchased from Sigma-Aldrich, St. Louis, MO, USA. Ag NPs (10 nm, 0.02 mg/mL in 2 mM sodium citrate, λmax ≈ 390–400 nm) were sourced from Thermo Scientific Chemicals, Waltham, MA, USA. All other reagents, including ethanol (absolute, 99.8%), acetone (ACS reagent, ≥99.5%), and hydrochloric acid (ACS reagent, fuming, ≥37%), were of analytical grade.
2.2. Materials Fabrication and Characterization
Plasmonic–photonic Ag(Au)/MoS2–TiO2 IO films were synthesized via evaporation-induced co-assembly of monodisperse PS spheres (211 and 418 nm in diameter) with a titania precursor mixture (0.25 mL TiBALDH, 0.5 mL 0.1 M HCl, and 1 mL ethanol), MoS2 NS dispersion, and citrate-stabilized suspensions of 10 nm Ag or 5 nm Au NPs (Figure 1). The smaller PS spheres (211 nm) were chosen based on prior results demonstrating optimal photocatalytic activity in unmodified TiBALDH-derived TiO2 IO films, owing to the spectral overlap of slow-photon modes near the red PBG edge with the anatase TiO2 absorption edge [60].
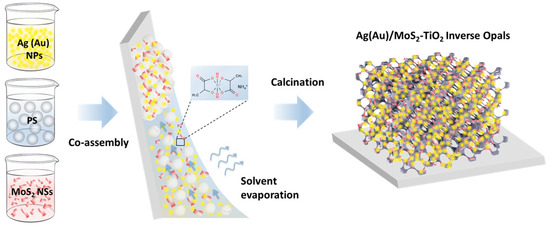
Figure 1.
Schematic fabrication of Ag(Au)/MoS2–TiO2 IO films.
In contrast, the larger PS spheres (418 nm) were selected to maximize light harvesting in MoS2–TiO2 composites, where the photonic band gap aligns between the two prominent excitonic absorption peaks of MoS2 NSs [43]. To improve uniformity and co-assembly compatibility, cascade centrifugation was applied to the commercial MoS2 dispersion to narrow the lateral size distribution of the nanosheets and minimize aggregation, which otherwise hinders the formation of well-ordered IO structures [43]. Following probe sonication, the initial MoS2 dispersion was subjected to a stepwise centrifugation process. An initial low-speed spin at 1000 rpm was used to remove large aggregates, and the resulting supernatant was subsequently centrifuged at increasing speeds up to 8000 rpm. This gradual process reduced both the concentration and average size of MoS2 NS aggregates. Aliquots of 0.05 mL and 0.15 mL from the refined MoS2 dispersions were then used to partially replace Milli-Q water in 211 nm and 418 nm PS sphere suspensions, respectively. These suspensions were diluted to 0.125 wt.% and treated with ultrasound to ensure homogeneity before being mixed with 0.07 mL of a freshly prepared titania precursor. Plasmonic NPs (Ag or Au) were introduced into the mixture using an optimized addition volume of 0.2 mL from their respective colloidal dispersions. Clean fluorine-doped tin oxide (FTO) substrates (2.2 mm thick, 7 Ω/sq surface resistivity, Sigma Aldrich) or plain glass slides were then immersed vertically into 10 mL of the prepared colloidal mixtures. Solvent evaporation was carried out at 60 °C, allowing self-assembly of the PS opal template filled with the mixed precursor. The resulting dry composite films were calcined in air at 400 °C for 2 h (ramp rate 1 °C/min) to remove the PS spheres and crystallize the inverse opal TiO2 framework containing embedded MoS2 NSs and Ag/Au NPs. The plasmonic modified MoS2-TiO2 PC films were designated as Ag/Au 0.2-PCYYY-MoS2 0.ZZ, with 0.2 and 0.ZZ representing the volumes (in mL) of the Ag/Au and MoS2 suspensions, respectively, while YYY denotes the diameter of the templating PS microspheres. Reference TiO2 IO films, namely PC211 and PC418, prepared from 211 and 418 nm PS microspheres as well as Ag/Au 0.2-PC211 and Ag/Au 0.2-PC418 IO films, were also prepared by the same method for comparison purposes.
The morphology and phase composition of the films were characterized using scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray (EDX) analyzer, as well as an FEI Talos F200i field-emission scanning/transmission electron microscope (S/TEM) (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a windowless energy-dispersive spectroscopy (EDS) microanalyzer. The structural properties were investigated by X-ray powder diffraction (XRD) by a SmartLab Rigaku θ/θ Bragg–Brentano diffractometer (Rigaku, Tokyo, Japan), using a pyrolytic graphite monochromator and Cu Kα radiation. The continuous step-scanning mode was used at 0.03° steps with 11 s/step. The phase composition was further studied by micro-Raman spectroscopy using a confocal Raman microscope (LabRAM Soleil™, Horiba Scientific, Longjumeau, France) with 532 nm laser excitation, focused on the films’ surface using a 100× (NA = 0.9) objective. Optical characterization was conducted via diffuse and specular reflectance measurements using a Cary60 UV-Vis spectrophotometer (Agilent, Santa Clara, CA, USA), equipped with a fiber-optic diffuse reflectance probe and a 15° specular reflectance accessory. A Halon reference and a UV-enhanced aluminum mirror were employed for background calibration in the respective configurations. Ultraviolet photoelectron spectroscopy (UPS) was performed using He I excitation (21.22 eV photon energy). The work function (WF), referenced to the vacuum level, was calculated by subtracting the secondary electron cut-off energy from the incident photon energy. The cut-off was determined by extrapolating the linear portion of the low-binding energy edge of the spectrum to its intersection with the baseline. Similarly, the valence band maximum (VBM) position relative to the Fermi level (EF) was estimated from the difference EF-EVBM, obtained by extending the linear region of the valence band edge near EF to intersect with the baseline of the spectrum.
2.3. Photocatalytic and Photoelectrochemical Evaluation
The photocatalytic activity of the Ag(Au)/MoS2–TiO2 IO films was initially evaluated via the visible-light-induced degradation of salicylic acid (SA) as a model pollutant [60]. SA was selected as it is a recalcitrant, colorless water contaminant, which, in contrast to dye pollutants, absorbs in the UV range, well below the PBG of the IO films and thus avoids slow photon contributions by spectral overlap with the target molecule absorption. The photodegradation tests were carried out at acidic pH = 3 that assists the chemisorption of SA molecules on titania and favors direct oxidation by valence band holes [60]. PC films (1 cm2) were placed horizontally in vials containing 4 mL of a 30 μM aqueous SA solution (pH = 3) and stirred in the dark for 60 min to establish adsorption–desorption equilibrium. A 150 W Xenon lamp equipped with a 305 nm long-pass filter and a heat-reflective mirror was used as the illumination source. For visible-light-only irradiation, an additional 400 nm long-pass filter was applied. The incident beam was directed onto the film using a UV-enhanced Al mirror at an intensity of 70 mW/cm2. Aliquots (0.5 mL) were periodically collected and analyzed using a UV-Vis spectrophotometer with a 10 mm quartz microcell. All photocatalytic tests were conducted in triplicate, and the mean kinetic constants were reported with standard errors.
Photoelectrochemical measurements were carried out in a standard three-electrode configuration using a CS350 potentiostat/galvanostat (Corrtest Instruments, Wuhan, China). The IO films on FTO substrates (4 cm2) served as working electrodes, with a Pt foil as the counter electrode and Ag/AgCl as the reference electrode. The electrolyte was an aqueous 0.5 M NaHCO3 solution. Visible light illumination (90 mW/cm2) was provided by a 300 W Xe lamp combined with a 400 nm long-pass filter. Electrochemical impedance spectroscopy (EIS) was carried out at open-circuit voltage over the 104–10−2 Hz frequency range with a 10 mV AC amplitude. Mott–Schottky measurements were conducted at 500 Hz with a scan rate of 10 mV/s. Flat band potentials were determined from Mott–Schottky plots (1/C2 vs. applied potential) using the following equation:
where C is the space-charge capacitance, e is the elementary charge, A is the electrode area, ND is the donor density, ε is the permittivity of the semiconductor, ε0 is the vacuum permittivity, Vfb is the flat band potential, T is the temperature, and k is Boltzmann’s constant.
The photoelectrocatalytic degradation of TC was also examined using the same three-electrode configuration under visible light (300 W Xe lamp (Corrtest Instruments, Wuhan, China), 400 nm long-pass filter, 90 mW/cm2), in a 40 mL solution of 0.1 M NaHCO3 containing 20 mg/L TC. The solution was gently stirred during irradiation. Aliquots were withdrawn periodically for UV-Vis analysis to monitor TC degradation. All experiments were repeated in triplicate, and the reported rate constants include standard error estimates. Furthermore, TC degradation was investigated by high-performance liquid chromatography (HPLC) using a Shimadzu Prominence-i LC-2030C 3D Plus (Kyoto, Japan) HPLC-DAD equipped with a quaternary pump. Chromatographic separation was achieved with a ZORBAX eclipse plus C18 column (150 mm × 4.6 mm, 5 μm), provided by Agilent. The separation mobile phase consisted of Solution (A), an acetonitrile–methanol mixture (60:40), and Solution (B), formic acid 1% in isocratic conditions. The total run was 12 min and the retention time was 5.9 min for TC and 5.7 min for epi-TC, the unavoidable epimer of TC. The column temperature was maintained at 25 °C, the injection volume was 20 μL, and the flow rate was 1 mL/min. For the determination of samples, solutions were directly injected to the chromatographic system. The results were expressed as the sum of TC and epi-TC and they were quantified using a standard solution of 11.5 ppm.
3. Results
3.1. Structural and Optical Properties
Co-assembly of Ag and Au NP aqueous suspensions with polymer colloidal spheres and a hydrolyzed titania precursor was carried out for two selected MoS2–TiO2 IOs with distinct macropore sizes. This approach aimed to align the slow-photon spectral regions with the TiO2 absorption edge and the visible-light absorption of MoS2, respectively. Figure 2a,b display SEM images of the PC211-MoS2 0.05 and PC418-MoS2 0.15 films, fabricated using PS spheres of 211 and 418 nm in diameter. Both samples exhibit well-ordered, periodic IO architectures with interconnected macropores through smaller ones that appear at the contact points of adjacent PS spheres after calcination. The average macropore diameters that were determined for the two IOs were approximately 140 and 250 nm, respectively. Specular reflectance (R%) measurements at a 15° angle of incidence revealed distinct Bragg reflections, indicative of PBG formation, centered at 332 nm for PC211-MoS2 0.05 and 512 nm for PC418-MoS2 0.15 (Figure 2c).
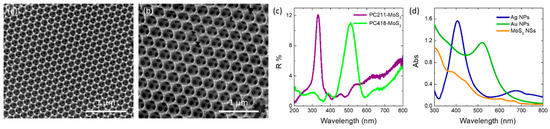
Figure 2.
SEM images of the (a) PC211-MoS2 0.05 and (b) PC418-MoS2 0.15 co-assembled IO films, as well as (c) the corresponding Bragg reflection R% peaks in comparison to (d) the LSPR extinction of Ag, Au NP, and MoS2 NS suspensions.
The PBG positions can be estimated using the modified Bragg’s law for first-order diffraction from the (111) planes of a face-centered cubic (fcc) IO structure [60]:
where
is the PBG wavelength,
is the interplanar spacing, and
is the macropore diameter. The effective refractive index
is calculated as a volume-weighted average of the refractive indices of the colloidal spheres
and solid matrix
consisting primarily of TiO2, following
Here,
is the volume filling fraction of the spheres (
for close-packed fcc lattices), and
is the angle of incidence relative to the [111] direction.
By applying Equation (2) to the experimental R% peak positions at
and using the measured diameters, the effective refractive indices and the corresponding void fractions
were determined in air (with
and
, Table 1). The calculated
values were consistently lower than the theoretical 0.26, indicating incomplete filling of the IO lattice. Notably, this effect was less pronounced in samples with smaller macropores, in agreement with previous studies on co-assembled TiO2 IOs [31], which reported increased surface area and mesopore volume related to enhanced skeletal wall porosity. Furthermore, using the determined void fractions and a refractive index of
, the PBGs were recalculated for aqueous environments, where photocatalytic reactions occur, shifting to 381 nm and 622 nm for the PC211-MoS2 0.05 and PC418-MoS2 0.15 films, respectively (Table 1).

Table 1.
Structural and optical properties of the MoS2-TiO2 IO films.
The smaller-diameter IOs were selected for plasmonic modification because the unmodified TiO2 PC211 films exhibited the highest photocatalytic activity in SA degradation, attributed to the spectral overlap of red-edge slow photons with the anatase TiO2 absorption edge [60]. This spectral match could be further enhanced by the close proximity of the Ag NPs’ LSPR and the enhanced MoS2 absorption around 400 nm (Figure 2d). Larger-diameter PC418 IOs were also chosen, as they showed superior light-trapping properties, with the PBG positioned between the MoS2 excitonic absorption peaks at approximately 605 and 664 nm (Figure 2d), leading to the highest photocatalytic degradation rates for SA pollutants by co-assembled MoS2-TiO2 IO films under visible light [43]. TEM imaging confirmed that simultaneous incorporation of plasmonic NPs and MoS2 NSs into the PC418-MoS2 0.15 films did not disrupt the IO structure after calcination (Figure 3), while EDX elemental mapping revealed uniform distributions of Mo, S, and Ag/Au species within the TiO2 skeleton.
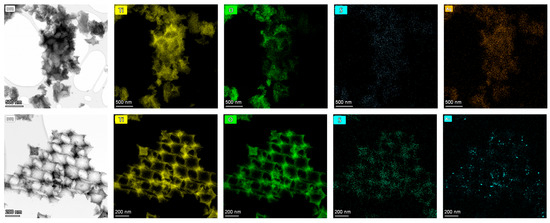
Figure 3.
TEM images of (upper row) Ag 0.2-PC418-MoS2 0.15 and (bottom row) Au 0.2-PC418-MoS2 0.15 samples and the corresponding EDX elemental maps for Ti, O, S, and Ag/Au species.
Further TEM analysis was performed on samples prepared by 211 nm PS spheres (Figure 4) to verify whether the presence of Ag/Au NPs and MoS2 NSs impacted the IO structural integrity at smaller pore sizes. TEM images confirmed successful IO formation, although a lower MoS2 NS concentration was required during synthesis to preserve periodicity, due to the denser skeleton (higher filling fraction) of PC211 films. EDX mapping showed uniform Ag NP distribution in both films. However, quantitative EDX analysis indicated that the Ag 0.2-PC211-MoS2 0.05 films accommodated significantly higher Ag NP loading compared to Ag 0.2-PC418-MoS2 0.15 (Table 2), likely due to the increased surface area associated with smaller macropores and a higher density of skeletal interfaces.
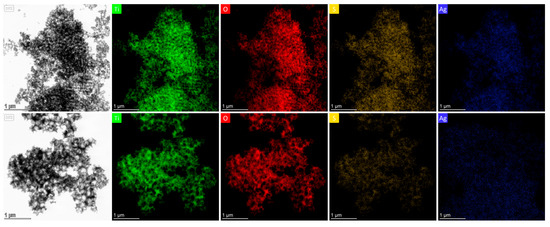
Figure 4.
TEM images and the corresponding EDX elemental maps for Ti, O, S, and Ag species for (upper row) Ag 0.2-PC211-MoS2 0.05 and (bottom row) Ag 0.2-PC418-MoS2 0.15 IO samples.

Table 2.
EDX analysis for the Ag-modified PC films.
Table 2.
EDX analysis for the Ag-modified PC films.
| Z | Element | Family | Atomic Fraction (%) | Atomic Error (%) | Mass Fraction (%) | Mass Error (%) |
|---|---|---|---|---|---|---|
| Ag 0.2-PC211 | ||||||
| 8 | O | K | 66.25 | 3.78 | 39.60 | 4.04 |
| 22 | Ti | K | 33.71 | 3.78 | 60.30 | 4.05 |
| 47 | Ag | L | 0.04 | 0.01 | 0.10 | 0.02 |
| Ag 0.2-PC211-MoS2 0.05 | ||||||
| 8 | O | K | 66.05 | 3.60 | 39.24 | 4.02 |
| 16 | S | K | 0.45 | 0.09 | 0.56 | 0.12 |
| 22 | Ti | K | 33.20 | 3.63 | 59.2 | 4.14 |
| 42 | Mo | K | 0.20 | 0.02 | 0.60 | 0.06 |
| 47 | Ag | L | 0.10 | 0.01 | 0.40 | 0.06 |
| Ag 0.2-PC418 | ||||||
| 8 | O | K | 66.04 | 4.16 | 39.29 | 3.54 |
| 22 | Ti | K | 33.94 | 4.17 | 60.65 | 3.55 |
| 47 | Ag | L | 0.02 | 0.00 | 0.06 | 0.01 |
| Ag 0.2-PC418-MoS2 0.15 | ||||||
| 8 | O | K | 64.87 | 3.79 | 38.18 | 3.93 |
| 16 | S | K | 0.47 | 0.09 | 0.55 | 0.12 |
| 22 | Ti | K | 34.43 | 3.82 | 60.54 | 4.03 |
| 42 | Mo | K | 0.20 | 0.01 | 0.62 | 0.06 |
| 47 | Ag | L | 0.03 | 0.00 | 0.11 | 0.02 |
Comparative TEM and EDX analyses of reference Ag 0.2-PC211 and Ag 0.2-PC418 films (without MoS2) further revealed a notably higher Ag content in the PC211 films (Figure 5), reflecting the higher volume filling fraction of PC211 IOs with the smaller macropores, which present increased surface area and mesoporosity and thus favor NP loading [31]. This effect was significantly amplified with the incorporation of MoS2 NSs, in line with recent BET measurements showing that the addition MoS2 NSs increases the mesoporosity of the nanocrystalline TiO2 IO skeleton [43].
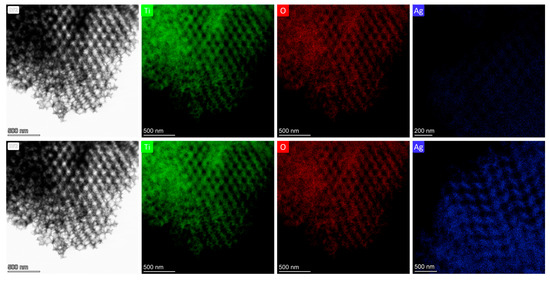
Figure 5.
TEM images and the corresponding EDX elemental maps and spectra for Ti, O, and Ag species for (upper row) Ag 0.2-PC211 and (bottom row) Ag 0.2-PC418 IO films.
XRD measurements on the Ag(Au) 0.2-PC211-MoS2 0.05 IO films in comparison to their constituents (Figure 6a) did not show any distinct diffraction peaks, different from those of the FTO substrate. A featureless hump could be only traced at ~25°, which can be related to the excessively broadened (101) diffraction peak of the anatase TiO2 (space group I41/amd) due to the small size of the anatase NPs (below ca. 10 nm) that form in co-assembled TiO2 IOs using the TiBALDH precursor [33,43]. In addition, no observable contribution could be detected from either the Ag(Au) NPs or the MoS2 NSs, because of their low content. This was verified by the corresponding Raman spectra (Figure 6b), where the characteristic Raman-active modes of anatase TiO2 were observed at 147 (Eg), 199 (Eg), 398 (B1g), 518 (A1g + B1g), and 640 cm−1 (Eg) with no signal from the characteristic Raman vibrations of MoS2 NSs, similar to the XRD results. All anatase Raman bands showed appreciable shifts and broadening with respect to bulk anatase [61], especially the most intense low frequency Eg mode that shifted to 147 cm−1 with a full width at half maximum (FWHM) of 16 cm−1. This effect can be related to the formation of small anatase NPs, which leads to the breakdown of the q = 0 selection rule for Raman scattering [61]. Specifically, using the Eg frequency and FWHM vs. NP size correlation curves [61], the formation of 8–9 nm anatase NPs can be predicted for the PC211 films, in agreement with previous reports on TiO2 IO films using TiBALDH [62].
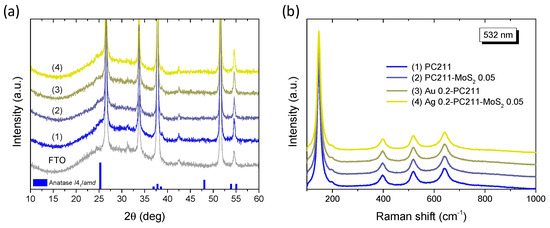
Figure 6.
(a) XRD patterns and (b) Raman spectra at 532 nm of (1) Ag(Au) 0.2-PC211-MoS2 0.05 compared to the pristine PC211 and PC211-MoS2 0.05 IO films. The XRD patterns of the FTO substrate and the anatase TiO2 (JCPDS No. 21-1272) are also shown in (a).
The optical properties of the IO films were analyzed by diffuse reflectance (DR%) UV-Vis spectroscopy (Figure 7). Comparing the DR% spectra of the reference PC418 and PC418-MoS2 0.15 samples with those of the plasmon-modified counterparts, a distinct decrease in reflectance was observed, appearing as local minima around 490 nm and 586 nm for the Ag- and Au-containing samples, respectively. These minima correspond closely to the LSPR absorption regions of Ag and Au NPS, though slightly red-shifted, likely due to aggregation effects of the plasmonic NPs [63].
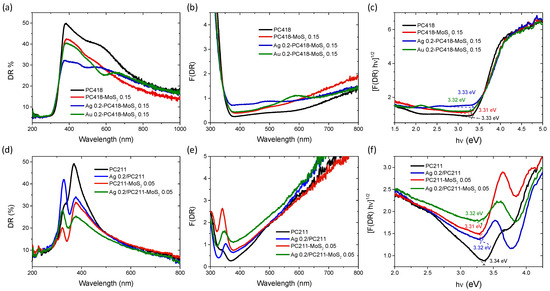
Figure 7.
(a,d) Diffuse reflectance (DR%), (b,e) Kubelka–Munk absorbance spectra (F(DR)) and (c,f) the corresponding (indirect band gap) Tauc plots for the (upper row) Ag (Au) 0.2-PC418-MoS2 0.15 IO films compared to the unmodified ones, as well as (bottom row) the Ag 0.2-PC211-MoS2 0.05 and reference IO films.
Furthermore, the DR% of the Ag 0.2-PC418-MoS2 0.15 films exhibited a significant reduction at wavelengths beyond 400 nm, above the anatase absorption edge, compared to the unmodified PC418-MoS2 0.15 film. In contrast, the DR% decrease for the Au 0.2-PC418-MoS2 0.15 film was confined to a narrower spectral range, reflecting the different spectral overlap of the LSPR with the DR% of the unmodified PC418 IO support. These variations were also observed in the Kubelka–Munk absorbance spectra (F(DR)) (Figure 7b), whereas only a slight decrease in the indirect band gap of anatase TiO2, reaching 2 meV, was derived for the Ag (Au) 0.2-PC418-MoS2 0.15 IO films from the corresponding Tauc plots (Figure 7c). For the PC211 IO films, the diffuse reflectance of TiO2 dropped markedly due to the absorbance of the Ag NPs’ LSPR and/or MoS2 NSs around its band gap edge (Figure 2d), resulting in the clearer appearance of the IO skeletal Bragg reflection, which was barely discernible in the unmodified PC211 IO film. The synergistic absorbance of Ag NPs and MoS2 NSs accordingly produced the most pronounced DR% decrease at around 400 nm for the Ag 0.2-PC211-MoS2 0.05 photonic films. These effects were further reflected in the corresponding F(DR) absorbance spectra (Figure 7e), while the derived Tauc plots indicated slight variations of the anatase band gap for the Ag 0.2-PC211-MoS2 0.05 and the constituent IO films (Figure 7e), similar to the PC418 ones.
3.2. Photocatalytic Evaluation
The plasmonic modified MoS2-TiO2 PC films were initially evaluated on the degradation of SA as model pharmaceutical pollutant under visible-light irradiation (λ > 400 nm) (Figure 8). Control experiments in the absence of films as well as in the presence of the pristine PC418 IO films showed negligible SA degradation under visible light (Figure 8b). In contrast, visible light illumination after dark adsorption in the presence of the IO films led to a continuous decrease in SA concentration (C), monitored spectrophotometrically via the SA absorption band at 300 nm (Figure 8a). The ln(C/C0) vs. time (t) plots, where C0 is the initial SA concentration after dark adsorption, exhibited linear behavior, indicating pseudo-first-order kinetics (Figure 8c). The kinetic constants (k, min−1) were derived from the slopes of these linear fits. Plasmonic modification enhanced the degradation rates, with k increasing by 50% for Ag 0.2-PC418-MoS2 0.15, while only a modest ~10% improvement was observed for Au 0.2-PC418-MoS2 0.15 (Figure 8d). The weak improvement of SA degradation by the Au-modified MoS2-TiO2 IO films, which are preferable due their higher stability, could be mainly related to the spectral mismatch of LSPR with the composite semiconductor absorption that precludes near-field amplification effects from the strongly absorbing 5 nm Au NPs [58] as well as the non-optimal spectral overlap of the observed PBGs (Table 1) with Au plasmonic absorption leading to weak enhancement of the hot electron injection mechanism that has been reported to be the key factor in Au-TiO2 IO photocatalysts [52,55,64,65].
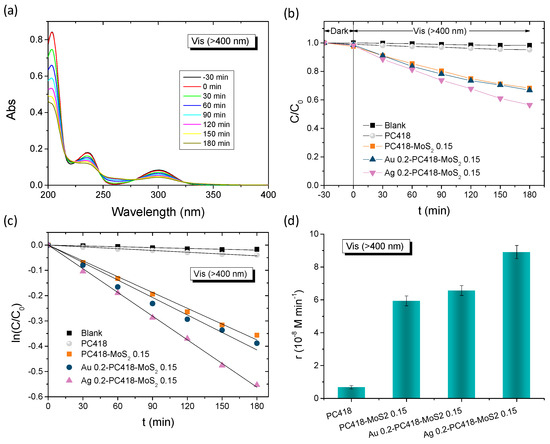
Figure 8.
(a) Time dependence of SA absorbance spectra in the presence of Ag 0.2-PC418-MoS2 0.15. (b,c) SA photodegradation kinetics and (d) reaction rates for the Au and Ag modified PC418-MoS2 0.15 films in comparison to the reference ones under visible-light irradiation.
The best-performing Ag-modified MoS2-TiO2 PC films were subsequently selected for further photocatalytic evaluation of the degradation of TC under visible light for both IO diameters. Figure 9 summarizes the TC degradation results for the Ag-modified PC211-MoS2 0.05 and PC418-MoS2 0.15 films, compared to the corresponding Ag-modified PC211 and PC418 reference ones. After dark adsorption, the TC concentration, monitored via its 370 nm absorption band (Figure 9a), decreased steadily under illumination, again following first-order kinetics with k values extracted from the ln(C/C0) vs. t plots (Figure 9c). It should be noted that the highest TC dark adsorption was observed for Ag-modified MoS2-TiO2 PCs, reflecting the increase of surface area after the integration of MoS2 NSs in the TiO2 IO skeleton [43], whereas the incorporation of Ag NPs did not result in any appreciable variation in dark adsorption, because of the low content of plasmonic NPs. The visible-light reaction rates rvis were then calculated as r = k C0, making them independent of dark adsorption variations. Notably, both Ag-modified MoS2-TiO2 PC211 and PC418 films exhibited significantly higher reaction rates compared to the corresponding Ag-modified PC211 and PC418 controls, confirming the essential role of MoS2 NSs in enhancing visible-light-driven photocatalysis. Interestingly, although the larger-diameter Ag-modified PC418-MoS2 0.15 films, whose unmodified TiO2-MoS2 IO supports demonstrated optimal visible-light trapping and superior SA degradation [43], were outperformed by approximately 30% by the smaller-diameter Ag 0.2-PC211-MoS2 0.05 films in TC degradation (Figure 9d).
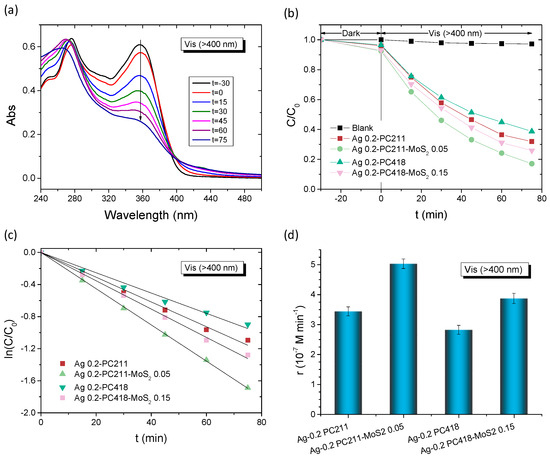
Figure 9.
(a) Time dependence of TC absorbance in the presence of Ag 0.2-PC211. (b,c) TC photodegradation kinetics and (d) reaction rates for the Ag modified TiO2-MoS2 IO films in comparison to the Ag-modified TiO2 IOs under visible-light irradiation.
This size-selective performance suggests that tuning the PBG closer to the TiO2 absorption edge and the LSPR absorption of Ag NPs, rather than matching the MoS2 visible-light absorption, is more effective for enhancing VLA photocatalytic activity. Assuming a PBG width corresponding to the full width at half maximum of the Bragg reflection R% peak of ≈40 nm (Figure 2c), the PBG centered at ~380 nm in water (Table 1) would extend between 360 and 400 nm. The associated red-edge slow photons, spanning roughly 20–30 nm above 400 nm, would closely match both Ag LSPR absorption and MoS2-TiO2 absorption. Thus, plasmonic effects, including local field enhancement for the strongly absorbing small-diameter Ag NPs [44] and/or hot electron injection from Ag NPs into the MoS2-TiO2 composite, together with electron transfer from MoS2 NSs [43], could be proposed as the primary contributors to the enhanced visible-light photocatalytic activity of the Ag-modified MoS2-TiO2 photonic crystals.
An advanced application of the Ag-modified MoS2-TiO2 PC films was further explored through photoelectrocatalytic degradation of TC using a three-electrode electrochemical setup under visible light (λ > 400 nm). The experiments employed an Ag/AgCl reference electrode and a 40 mL working solution—ten times larger than the volume used in the previous photocatalytic tests—consisting of 0.1 M NaHCO3 as supporting electrolyte containing 20 mg/L of TC under mild stirring. Initial tests were carried out on the Ag 0.2-PC418-MoS2 0.15 photoelectrodes under three different conditions: without external bias and under applied potentials of +0.5 V and +1.0 V vs. Ag/AgCl (Figure 10a–c). A clear enhancement in TC degradation kinetics was observed with increasing applied potential, reaching optimal performance at +1.0 V vs. Ag/AgCl, indicative of more efficient electron–hole separation facilitated by the external bias.
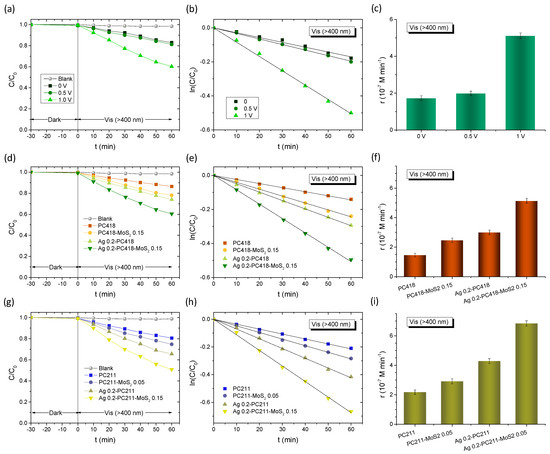
Figure 10.
Visible-light-activated photoelectrocatalytic TC degradation kinetics and the corresponding reaction rates for (a–c) Ag 0.2-PC418-MoS2 0.15 under no bias and under +0.5 and +1.0 V vs. Ag/AgCl, (d–f) Ag 0.2-PC418-MoS2 0.15, and (g–i) Ag 0.2-PC211-MoS2 0.05 photoelectrodes compared to the corresponding constituent IO films at +1.0 V vs. Ag/AgCl.
It should be noted that in this case, no appreciable dark adsorption could be traced on the TC absorbance due to the relatively small size (4 cm2) of the photolectrode with respect to the reaction solution volume (40 mL).
Subsequent comparative evaluations were performed for both Ag 0.2-PC418-MoS2 0.15 (Figure 10d–f) and Ag 0.2-PC211-MoS2 0.05 (Figure 10g–i) IO films, alongside their corresponding reference samples, at an applied bias of +1.0 V vs. Ag/AgCl under visible light. In all cases, TC degradation followed first-order kinetics, with rate constants determined from linear fits of the ln(C/C0) vs. time plots. The incorporation of MoS2 NSs and Ag NPs into the TiO2 IO frameworks consistently resulted in enhanced reaction rates compared to the unmodified reference films, with the Ag-modified TiO2 photoelectrodes exhibiting higher performance. Moreover, the synergistic effect of combining MoS2 NSs and Ag NPs was evident, particularly for the Ag 0.2-PC211-MoS2 0.05 films, which achieved reaction rates that surpassed those of the unmodified PC211-MoS2 and Ag 0.2-PC211 IO films by factors of 2.3 and 1.6, respectively (Figure 10i). These results strongly support the synergistic amplification mechanism involving slow-photon-enhanced light harvesting through spectral matching of the low-energy (red) edge of the inverse opal PBG with the Ag LSPR and the close-lying TiO2 absorption edge. This effect is further amplified by the contribution of the MoS2 NSs that enable visible-light activation and effective charge separation during the photoelectrocatalytic process. The obtained kinetic constant k for the Ag 0.2-PC211-MoS2 0.05 IO films are comparable to the best values reported by visible-light-responsive photoelectrodes, including benchmark BiVO4 photoelectrodes (Table 3).

Table 3.
Performance comparison on the photoelectrocatalytic degradation of tetracycline antibiotics under visible-light irradiation.
Moreover, in order to validate the performance of the optimal Ag 0.2-PC211-MoS2 0.05 IO photoanodes, the photoelectrocatalytic degradation of TC was also monitored by HPLC under visible and UV-Vis light for up to 3 h at +1.0 V vs. Ag/AgCl (Figure 11). The corresponding HPLC chromatograms show that TC degradation reaches 71.1% and 93.0% after 1.5 and 3 h under visible light, whereas much higher TC degradation levels of 98.0% and 100% could be achieved after 1.5 and 3 h under UV–visible light, supporting the high photocatalytic activity of the optimized Ag/MoS2-TiO2 IO photoanodes.
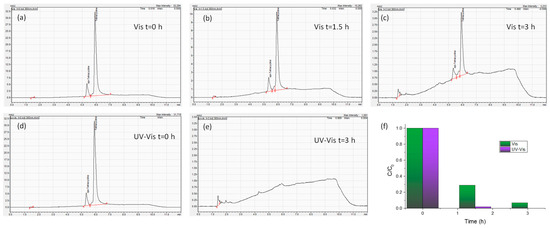
Figure 11.
HPLC chromatograms of TC obtained during photoelectrocatalytic degradation experiments in the presence of Ag 0.2-PC211-MoS2 0.05 IO films under visible light for (a) 0 h, (b) 1.5 h, and (c) 3 h as well as UV-Vis light for (d) 0 h and (e) 3 h at +1.0 V vs. Ag/AgCl. (f) The corresponding TC degradation kinetics up to 3 h under both illumination conditions.
3.3. Band Alignment and Charge Separation
To investigate band alignment and charge separation at the photoelectrode/electrolyte interface, UPS measurements were performed on the Ag 0.2-PC211-MoS2 0.05 IO film and its individual components. Figure 12a,b display the secondary electron cut-off and valence band regions, respectively, from which the WF and valence band maximum (VBM) positions relative to the Fermi level were extracted vs. the vacuum level. For the unmodified PC211 film, the WF and EF-EVBM were determined as 4.80 eV and 2.96 eV, respectively, consistent with previously reported values for co-assembled TiO2 IOs [42]. Upon modification, the WF decreased by 0.40 eV (PC211-MoS2 0.05), 0.45 eV (Ag 0.2-PC211), and 0.33 eV (Ag 0.2-PC211-MoS2 0.05), while the VBM positions shifted only marginally (≈0.02 eV), indicating an upward shift of the TiO2 Fermi level due to heterojunction formation with MoS2 NSs or Ag NPs.
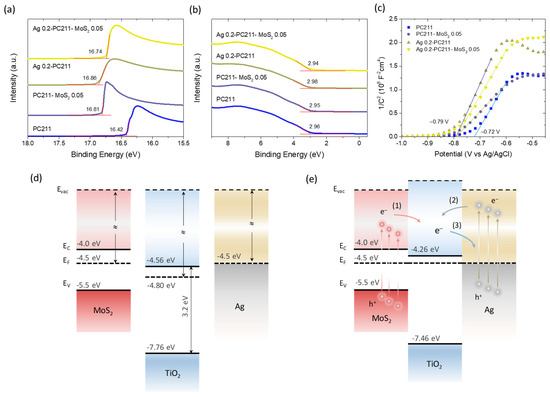
Figure 12.
(a) Secondary electron cut-off spectra and (b) valence band UP spectra of Ag 0.2-PC211-MoS2 0.05 and its constituent IO films, used to determine the WF and VBM positions relative to the Fermi level. (c) Mott–Schottky plots for the same films, showing flat band potential shifts after modification. (d) Schematic energy band alignment and (e) charge transfer pathways in the Ag-TiO2-MoS2 heterojunction system, before and after interfacial contact and EF equilibration: (1) electron transfer from MoS2 to TiO2, (2) hot electron injection to TiO2 via the non-radiative LSPR decay of Ag NPs, and (3) electron transfer from TiO2 to Ag NPs acting as electron scavengers.
To further probe this behavior in the electrolyte environment, Mott–Schottky analysis was carried out to determine the flat band potentials (Vfb) of the IO photoelectrodes (Figure 12c). For the unmodified PC211 film, Vfb was determined at −0.72 V vs. Ag/AgCl using the x-intercept of the best linear regression curve on the corresponding Mott–Schottky plot. All modified films (PC211-MoS2 0.05, Ag 0.2-PC211, and Ag 0.2-PC211-MoS2 0.05) showed similar negative shifts to −0.79 V vs. Ag/AgCl, supporting the EF shift toward more negative potentials observed by UPS.
Based on these findings and values from the literature—WF~4.5 eV for multilayer MoS2 NSs on TiOx [73], a band gap of 1.5 eV, and EF~0.5 eV below the conduction band minimum due to n-doping [74], as well as WF~4.5 eV for citrate-capped Ag NPs [75]—a tentative energy band diagram of the Ag-TiO2-MoS2 system can be proposed (Figure 12c,d). Since both MoS2 NSs and Ag NPs have higher WFs than TiO2, Fermi level equilibration after heterojunction formation induces an upward shift in the EF of TiO2, consistent with both UPS and Mott–Schottky results. In the TiO2-MoS2 heterojunction, visible-light excitation of MoS2 can promote electron transfer to the TiO2 conduction band, enabling the formation of superoxide radicals, which are major reactive species in the degradation of TC under visible light [43].
For Ag–TiO2 interactions, two electron transfer pathways are plausible: (i) the injection of hot electrons into TiO2 from Ag NPs via the non-radiative decay of LSPR or (ii) electron transfer from TiO2 to Ag NPs, which can act as electron sinks to suppress recombination [9]. Given the improved photocatalytic performance of both TiO2-MoS2 and Ag-TiO2 IO films (Figure 10), visible-light sensitization of the TiO2 skeleton by both components appears highly likely. Moreover, the close spatial proximity of Ag NPs to the TiO2-MoS2 interface may lead to significant local field enhancement at LSPR wavelengths. Considering the spectral alignment among red-edge slow photons, Ag LSPR absorption, and the TiO2-MoS2 absorption edge, a synergistic radiative enhancement of charge carrier generation at the interface is proposed. This mechanism aligns with the higher performance observed for the Ag 0.2-PC211-MoS2 0.05 IO films. On the other hand, in the case of Au/MoS2-TiO2 IO films, the higher work function of ~5.2 eV for bulk Au, which could be further increased by ~0.2 eV with citrate capping [75], would lead to a Schottky barrier at the Au-TiO2 interfaces with height of WF(Au)-χ(TiO2) = 0.84 eV, where χ is the electron affinity of 4.56 eV measured for the TiO2 PCs (Figure 12d). In the absence of appreciable spectral overlap of Au LSPR with TiO2-MoS2 absorption and the PBGs of the IO films to allow for local-field and slow-photon amplifications, the formation of a sizeable Schottky barrier could obstruct hot electron injection from the Au NPs to TiO2 under visible light, limiting the efficiency of visible light sensitization of the semiconductor system by plasmonic Au. It should be though noted that decreasing the size of Au NPs may cause a decrease in their WF [76,77], which may approach that of Ag NPs, leading to a similar band alignment. However, the absence of slow-photon effects and local-field enhancement may still impede photocatalytic performance.
4. Conclusions
In summary, heterostructured Ag (Au)/MoS2-TiO2 IO films were successfully fabricated via a one-step co-assembly approach, enabling the simultaneous incorporation of plasmonic NPs and MoS2 NSs into a highly ordered, porous TiO2 photonic framework. The resulting architectures act as efficient visible-light-responsive photoelectrocatalysts for TC degradation. Optical and structural characterization revealed that the synergistic interplay among slow-photon effects, plasmonic resonance, and semiconductor heterojunction formation plays a central role in enhancing visible light harvesting, charge separation, and catalytic efficiency. Among all tested systems, Ag/MoS2-TiO2 PC211 films showed superior photocatalytic and photoelectrocatalytic performance. This enhancement was attributed to optimal spectral alignment between the red-edge slow photons of the TiO2 IO PBG, the LSPR of Ag NPs, and the TiO2-MoS2 absorption edge. UPS and Mott–Schottky analysis confirmed that the incorporation of MoS2 NSs and Ag NPs leads to an upward shift in the Fermi level of TiO2, facilitating efficient electron transfer. The proposed energy band alignment suggests multiple contributing mechanisms: (i) visible-light excitation of MoS2 followed by electron injection into the TiO2 conduction band, (ii) plasmon-induced hot electron transfer from Ag NPs to TiO2, and (iii) local electromagnetic field enhancement at the TiO2-MoS2-Ag interface due to LSPR coupling. These effects are further amplified by slow-photon effects in the IO structure, which concentrate the optical field at these spectral regions and promote radiative enhancement near the interface. This work underscores the potential of integrating photonic, plasmonic, and semiconductor functionalities into a unified nanostructure for advanced water treatment applications and provides a route for designing multifunctional photocatalysts responsive to visible light.
Author Contributions
Conceptualization, V.L.; methodology, S.L. and V.L.; investigation, S.L., E.S., P.T., S.G., V.P., M.G.K. and N.S.T.; formal analysis, S.L., E.S., P.T., S.G., V.P. and M.G.K.; validation, S.L., E.S., P.T., S.G., M.G.K. and N.S.T.; resources, V.L., V.P. and N.S.T.; writing—original draft preparation, S.L. and V.L.; writing—review and editing, V.L. and S.L.; supervision, V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “First Call for H.F.R.I. Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment grant” (Project Number: 543).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Rashid, M.M.; Forte Tavčer, P.; Tomšič, B. Influence of titanium dioxide nanoparticles on human health and the environment. Nanomaterials 2021, 11, 2354. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-based nanostructures as a promising material for diverse environmental applications: A review. Reactions 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Valério, A.; Sárria, M.P.; Rodriguez-Lorenzo, L.; Hotza, D.; Espiña, B.; Gómez González, S.Y. Are TiO2 nanoparticles safe for photocatalysis in aqueous media? Nanoscale Adv. 2020, 2, 4951–4960. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase polymorph of TiO2 (anatase, rutile, brookite, TiO2(B)) for efficient photocatalyst: Fabrication and activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Rega, R.; Fioravanti, A.; Hejazi, S.M.H.; Shahrezaei, M.; Kment, Š.; Maddalena, P.; Naldoni, A.; Lettieri, S. Charge carrier recombination processes, intragap defect states, and photoluminescence mechanisms in stoichiometric and reduced TiO2 brookite nanorods: An interpretation scheme through in situ photoluminescence excitation spectroscopy in controlled environment. Nanoscale 2024, 16, 11296–11309. [Google Scholar] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Recent progress of applied TiO2 photoelectrocatalysis for the degradation of organic pollutants in wastewaters. J. Environ. Chem. Eng. 2023, 11, 109635. [Google Scholar] [CrossRef]
- Weng, B.; Zhang, M.; Lin, Y.; Yang, J.; Lv, J.; Han, N.; Xie, J.; Jia, H.; Su, B.; Roeffaers, M.; et al. Photo-assisted technologies for environmental remediation. Nat. Rev. Clean Technol. 2025, 1, 201–215. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef]
- Wang, K.; Fu, X.; Zhang, Q.; Yin, G.; Wei, Z.; Su, W. When MoS2 meets TiO2: Facile synthesis strategies, hybrid nanostructures, synergistic properties, and photocatalytic applications. J. Mater. Chem. C 2021, 9, 8466–8482. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.; Kumar, A.; Patel, S.; Singh, J. 2D layered transition metal dichalcogenides (MoS2): Synthesis, applications and theoretical aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Sivaranjani, P.; Janani, B.; Thomas, A.; Raju, L.; Khan, S. Recent development in MoS2-based nano-photocatalyst for the degradation of pharmaceutically active compounds. J. Clean. Prod. 2022, 352, 131506. [Google Scholar] [CrossRef]
- Rahman, A.; Jennings, J.R.; Tan, A.L.; Khan, M.M. Molybdenum disulfide-based nanomaterials for visible-light-induced photocatalysis. ACS Omega 2022, 7, 22089–22110. [Google Scholar] [CrossRef]
- Lei, L.; Huang, D.; Zeng, G.; Cheng, M.; Jiang, D.; Zhou, C.; Chen, S.; Wang, W. A fantastic two-dimensional MoS2 material based on the inert basal planes activation: Electronic structure, synthesis strategies, catalytic active sites, catalytic and electronic properties. Coord. Chem. Rev. 2019, 399, 213020. [Google Scholar] [CrossRef]
- Gopal, R.; Chinnapan, M.M.; Bojarajan, A.K.; Rotte, N.K.; Ponraj, J.S.; Ganesan, R.; Atanas, I.; Nadarajah, M.; Manavalan, R.K.; Gaspar, J. Facile synthesis and defect optimization of 2D-layered MoS2 on TiO2 heterostructure for industrial effluent, wastewater treatments. Sci. Rep. 2020, 10, 21625. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Liu, Z.; Xu, Y. Visible-light-driven photocatalysis-enhanced nanozyme of TiO2 nanotubes@MoS2 nanoflowers for efficient wound healing infected with multidrug-resistant bacteria. Small 2021, 17, 2103348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.M.; Lu, X.L.; Yu, X.; Kong, M.H.; Duan, X.D.; Qin, G.; Zhao, Y.H.; Wang, Z.L.; Dionysiou, D.D. Molybdenum disulfide nanosheets vertically grown on self-supported titanium dioxide/nitrogen-doped carbon nanofiber film for effective hydrogen peroxide decomposition and “memory catalysis. J. Colloid Interface Sci. 2021, 596, 384–395. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Mady, A.H.; Mahadadalkar, M.A.; Baynosa, M.L.; Kumar, D.R.; Rabie, A.M.; Lee, J.; Kim, W.K.; Shim, J.-J. Highly active Z-scheme heterojunction photocatalyst of anatase TiO2 octahedra covered with C-MoS2 nanosheets for efficient degradation of organic pollutants under solar light. J. Colloid Interface Sci. 2022, 606, 337–352. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kin, H. Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J. Colloid Interface Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, A.; O’Dwyer, C. Many facets of photonic crystals: From optics and sensors to energy storage and photocatalysis. Adv. Mater. Technol. 2023, 8, 2201410. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, X.; Fu, X.; Teng, D.; Murtaza, G.; Meng, Z.; Jia, Z.; Qiu, L. Slow light effect enhances the photocatalytic effect of inverse opal TiO2-based photonic nanocrystals. ACS Appl. Nano Mater. 2024, 7, 15376–15386. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Y.; Li, A.; Cheng, C. Recent advances on three-dimensional ordered macroporous metal oxide-based photoelectrodes for photoelectrochemical water splitting. Mater. Chem. Front. 2024, 8, 1230–1249. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Ye, M.; Cheng, H. Fabrication of a TiO2 inverse opal film-modified photocatalytic microreactor for highly efficient degradation of dyes. Langmuir 2025, 41, 8138–8143. [Google Scholar] [CrossRef]
- Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Tsoutsou, D.; Gardelis, S.; Romanos, G.E.; Ioannidis, N.; Boukos, N.; Dimoulas, A.; Falaras, P.; et al. Boosting visible light harvesting and charge separation in surface modified TiO2 photonic crystal catalysts with CoOx nanoclusters. Mater. Adv. 2020, 1, 2310–2322. [Google Scholar] [CrossRef]
- Madanu, T.L.; Mouchet, S.R.; Deparis, O.; Liu, J.; Li, Y.; Su, B.-L. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photocatalysis. J. Colloid Interface Sci. 2022, 634, 290–299. [Google Scholar] [CrossRef]
- Pylarinou, M.; Toumazatou, A.; Sakellis, E.; Xenogiannopoulou, E.; Gardelis, S.; Boukos, N.; Dimoulas, A.; Likodimos, V. Visible light trapping against charge recombination in FeOx–TiO2 photonic crystal photocatalysts. Materials 2021, 14, 7117. [Google Scholar] [CrossRef] [PubMed]
- Piwoński, J.; Kisielewska, A.; Piwoński, I. Preparation and photocatalytic activity of TiO2 photonic crystals modified by bimetallic Ag–Pt nanostructures. Catal. Sci. Technol. 2024, 14, 4274–4292. [Google Scholar]
- Toumazatou, A.; Sakellis, E.; Likodimos, V. Improving visible light photocatalysis using optical defects in CoOx-TiO2 photonic crystals. Materials 2024, 17, 5996. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Teng, D.; Meng, Z.; Qiu, L. Inverse opal TiO2-CdS photonic crystal beads with slow light effect for photocatalytic degradation. Appl. Surf. Sci. 2025, 682, 161719. [Google Scholar] [CrossRef]
- Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354–10359. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, Y.J.; Teng, J.; Lu, X. Fabrication of large domain crack-free colloidal crystal heterostructures with superposition bandgaps using hydrophobic polystyrene spheres. ACS Appl. Mater. Interfaces 2012, 4, 5562–5569. [Google Scholar] [CrossRef]
- Vasquez, Y.; Kolle, M.; Mishchenko, L.; Hatton, B.D.; Aizenberg, J. Three-phase co-assembly: In situ incorporation of nanoparticles into tunable, highly ordered, porous silica films. ACS Photon. 2014, 1, 53–60. [Google Scholar] [CrossRef]
- Cai, Z.; Xiong, Z.; Lu, X.; Teng, J. In situ gold-loaded titania photonic crystals with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 545–553. [Google Scholar] [CrossRef]
- Bijl, M.; Lim, K.R.G.; Garg, S.; Nicolas, N.; Visser, N.L.; Aizenberg, M.; Van Der Hoeven, J.E.S.; Aizenberg, J. Controlling nanoparticle placement in Au/TiO2 inverse opal photocatalysts. Nanoscale 2024, 16, 13867–13873. [Google Scholar] [CrossRef] [PubMed]
- Apostolaki, M.-A.; Sakellis, E.; Tsipas, P.; Giannouri, M.; Gardelis, S.; Boukos, N.; Dimoulas, A.; Likodimos, V. Three-phase co-assembly of compositionally tunable WO3/TiO2 inverse opal photoelectrodes. Appl. Surf. Sci. 2022, 613, 155919. [Google Scholar] [CrossRef]
- Loukopoulos, S.; Sakellis, E.; Kostakis, M.G.; Gerokonstantis, D.-T.; Tsipas, P.; Gardelis, S.; Kontos, A.G.; Katsaros, F.K.; Sideratou, Z.; Romanos, G.E.; et al. Co-assembled MoS2–TiO2 inverse opal photocatalysts for visible light-activated pharmaceutical photodegradation. ACS Omega 2023, 8, 33639–33650. [Google Scholar] [CrossRef] [PubMed]
- Mascaretti, L.; Dutta, A.; Kment, Š.; Shalaev, V.M.; Boltasseva, A.; Zbořil, R.; Naldoni, A. Plasmon-enhanced photoelectrochemical water splitting for efficient renewable energy storage. Adv. Mater. 2019, 31, 1805513. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, T.G.U.; Ghobadi, A.; Ozbay, E.; Karadas, F. Strategies for plasmonic hot-electron-driven photoelectrochemical water splitting. ChemPhotoChem 2018, 2, 161–182. [Google Scholar] [CrossRef]
- Nianqiang, W. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nature Photon. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photon. 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic crystals for plasmonic photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.; Chen, S.; Quan, X.; Zhao, H. Integrating plasmonic nanoparticles with TiO2 photonic crystal for enhancement of visible-light-driven photocatalysis. Environ. Sci. Technol. 2012, 46, 1724–1730. [Google Scholar] [CrossRef]
- Zhang, L.W.; Lin, C.Y.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic enhancement in visible-light-driven photocatalysis. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [PubMed]
- Temerov, F.; Pham, K.; Juuti, P.; Mäkelä, J.M.; Grachova, E.V.; Kumar, S.; Eslava, S.; Saarinen, J.J. Tailoring Plasmonic photocatalysts with aerosol techniques. ACS Appl. Mater. Interfaces 2020, 12, 41200–41210. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. One-pot synthesis of noble-metal-modified TiO2 photocatalysts. Chem. Lett. 2021, 50, 711–713. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.-F.; Hu, Z.-Y.; Liu, J.; Li, Y.; Hu, J.; Van Tendeloo, G.; Chen, L.-H.; Su, B.-L. Size effect of bifunctional gold in hierarchical titanium oxide-gold-cadmium sulfide with slow photon effect for unprecedented visible-light hydrogen production. J. Colloid Interface Sci. 2021, 604, 131–140. [Google Scholar] [CrossRef]
- Collins, G.; Lonergan, A.; McNulty, D.; Glynn, C.; Buckley, D.; Hu, C.; O’Dwyer, C. Semiconducting metal oxide photonic crystal plasmonic photocatalysts. Adv. Mater. Interfaces 2020, 7, 1901805. [Google Scholar] [CrossRef]
- Pylarinou, M.; Sakellis, E.; Tsipas, P.; Gardelis, S.; Psycharis, V.; Dimoulas, A.; Stergiopoulos, T.; Likodimos, V. Light concentration and electron transfer in plasmonic–photonic Ag,Au modified Mo-BiVO4 inverse opal photoelectrocatalysts. Nanoscale 2024, 16, 10366–10376. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Grasset, F.; Ishii, S.; Fudouzi, H.; Uchikoshi, T. Tunable Slow photon effect and local surface plasmon in Ag-immobilized TiO2 inverse opal films for enhancing pollutant photodegradation. Mater. Adv. 2024, 5, 8615–8628. [Google Scholar] [CrossRef]
- Apostolaki, M.-A.; Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Xenogiannopoulou, E.; Gardelis, S.; Boukos, N.; Falaras, P.; Dimoulas, A.; Likodimos, V. Graphene quantum dot-TiO2 photonic crystal films for photocatalytic applications. Nanomaterials 2020, 10, 2566. [Google Scholar] [CrossRef]
- Balaji, S.Y.D.J.R.; Djaoued, Y.; Robichaud, J. Phonon confinement studies in nanocrystalline anatase-TiO2 thin films by micro Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1416–1422. [Google Scholar] [CrossRef]
- Loukopoulos, S.; Toumazatou, A.; Sakellis, E.; Xenogiannopoulou, E.; Boukos, N.; Dimoulas, A.; Likodimos, V. Heterostructured CoOx–TiO2 mesoporous/photonic crystal bilayer films for enhanced visible-light harvesting and photocatalysis. Materials 2020, 13, 4305. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.; Kontoleta, E.; Digdaya, I.A.; Jonsson, M.P.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. the role of size and dimerization of decorating plasmonic silver nanoparticles on the photoelectrochemical solar water splitting performance of BiVO4 photoanodes. ChemNanoMat 2016, 2, 739–747. [Google Scholar] [CrossRef]
- Rahul, T.K.; Sandhyarani, N. Plasmonic and photonic effects on hydrogen evolution over chemically modified titania inverse opals. ChemNanoMat 2018, 4, 642–648. [Google Scholar] [CrossRef]
- Temerov, F.; Ankudze, B.; Saarinen, J.J. TiO2 inverse opal structures with facile decoration of precious metal nanoparticles for enhanced photocatalytic activity. Mater. Chem. Phys. 2020, 242, 122471. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Li, L.; Bai, J.; Xia, L.; Zhou, B. Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation. Appl. Catal. B Environ. 2017, 217, 21–29. [Google Scholar] [CrossRef]
- Xia, L.; Li, J.; Bai, J.; Li, L.; Zeng, Q.; Xu, Q.; Zhou, B. Preparation of a BiVO4 nanoporous photoanode based on peroxovanadate reduction and conversion for efficient photoelectrochemical performance. Nanoscale 2018, 10, 2848–2855. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.; Zhang, J.; Okoth, O.K.; Chen, F. Preparation of BiVO4/ZnO composite film with enhanced visible-light photoelectrocatalytic activity. Ceram. Int. 2018, 44, 3672–3677. [Google Scholar] [CrossRef]
- Changanaqui, K.; Brillas, E.; Alarcón, H.; Sirés, I. ZnO/TiO2/Ag2Se Nanostructures as Photoelectrocatalysts for the degradation of oxytetracycline in water. Electrochim. Acta 2020, 331, 135194. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Chen, P.; Lv, W.; Liu, G. In-situ stabilizing surface oxygen vacancies of TiO2 nanowire array photoelectrode by N-doped carbon dots for enhanced photoelectrocatalytic activities under visible light. J. Catal. 2020, 382, 212–227. [Google Scholar] [CrossRef]
- Guo, Y.; Han, W.; Zhao, K.; Hao, S.; Shia, S.; Ding, Y. Promoting effects of Y doping and FeOOH loading for efficient photoelectrochemical activity on BiVO4 electrodes. New J. Chem. 2022, 46, 13178–13183. [Google Scholar]
- Cong, S.; Yu, J.; Liu, B.; Teng, W.; Tang, Y. Preparing a dual-function BiVO4/NiFe-LDH composite photoanode for enhanced photoelectrocatalytic wastewater treatment and simultaneous hydrogen evolution. New J. Chem. 2022, 46, 15227–15243. [Google Scholar] [CrossRef]
- Lattyak, C.; Gehrke, K.; Vehse, M. Layer-thickness-dependent work function of MoS2 on metal and metal oxide substrates. J. Phys. Chem. C 2022, 126, 13929–13935. [Google Scholar] [CrossRef]
- Park, Y.; Li, N.; Jung, D.; Singh, L.T.; Baik, J.; Lee, E.; Oh, D.; Kim, Y.D.; Lee, J.Y.; Woo, J.; et al. Unveiling the origin of n-type doping of natural MoS2: Carbon. Npj 2D Mater. Appl. 2023, 7, 60. [Google Scholar] [CrossRef]
- Schnippering, M.; Carrara, M.; Foelske, A.; Kötz, R.; Fermín, D.J. Electronic properties of Ag nanoparticle arrays. A Kelvin probe and high resolution XPS study. Phys. Chem. Chem. Phys. 2007, 9, 725–730. [Google Scholar] [CrossRef]
- Zhang, Y.; Pluchery, O.; Caillard, L.; Lamic-Humblot, A.-F.; Casale, S.; Chabal, Y.J.; Salmeron, M. Sensing the charge state of single gold nanoparticles via work function measurements. Nano Lett. 2015, 15, 51–55. [Google Scholar] [CrossRef]
- Kao, K.-C.; Huang, S.-J.; Hsia, Y.-F.; Huang, J.-H.; Mou, C.-Y. Supported Au nanoparticles on TiO2 for visible light photocatalytic H2O2 production: Effects of au particle size and density. ACS Appl. Nano Mater. 2024, 7, 218–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).