Fabrication of Metal–Organic Framework-Mediated Heterogeneous Photocatalyst Using Sludge Generated in the Classical Fenton Process
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of HO-MIL-88/C Composites
2.3. Characterization
2.4. Photo-Fenton Experiments
2.5. Solution Free Radicals’ Detection
3. Results and Discussion
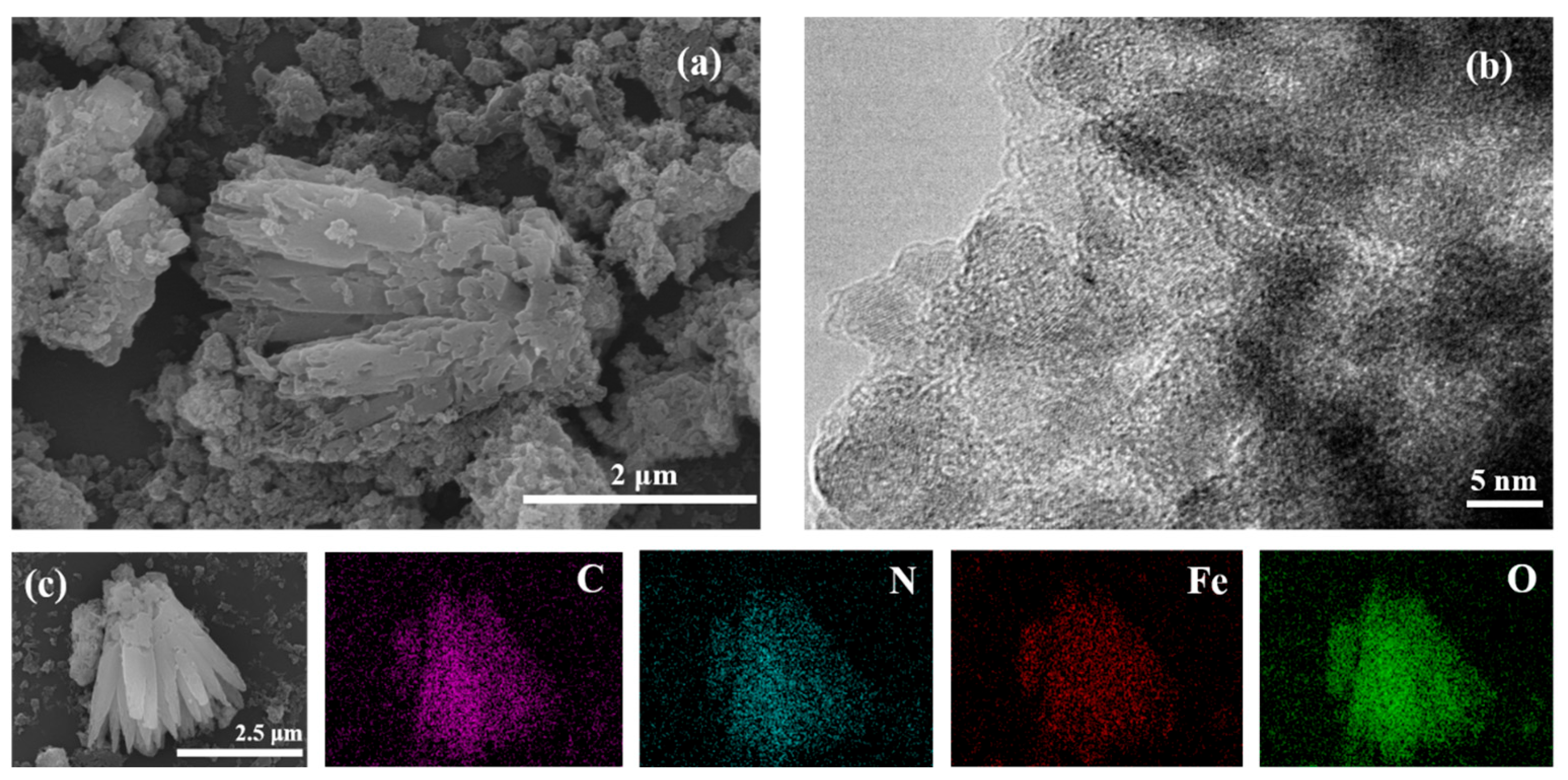
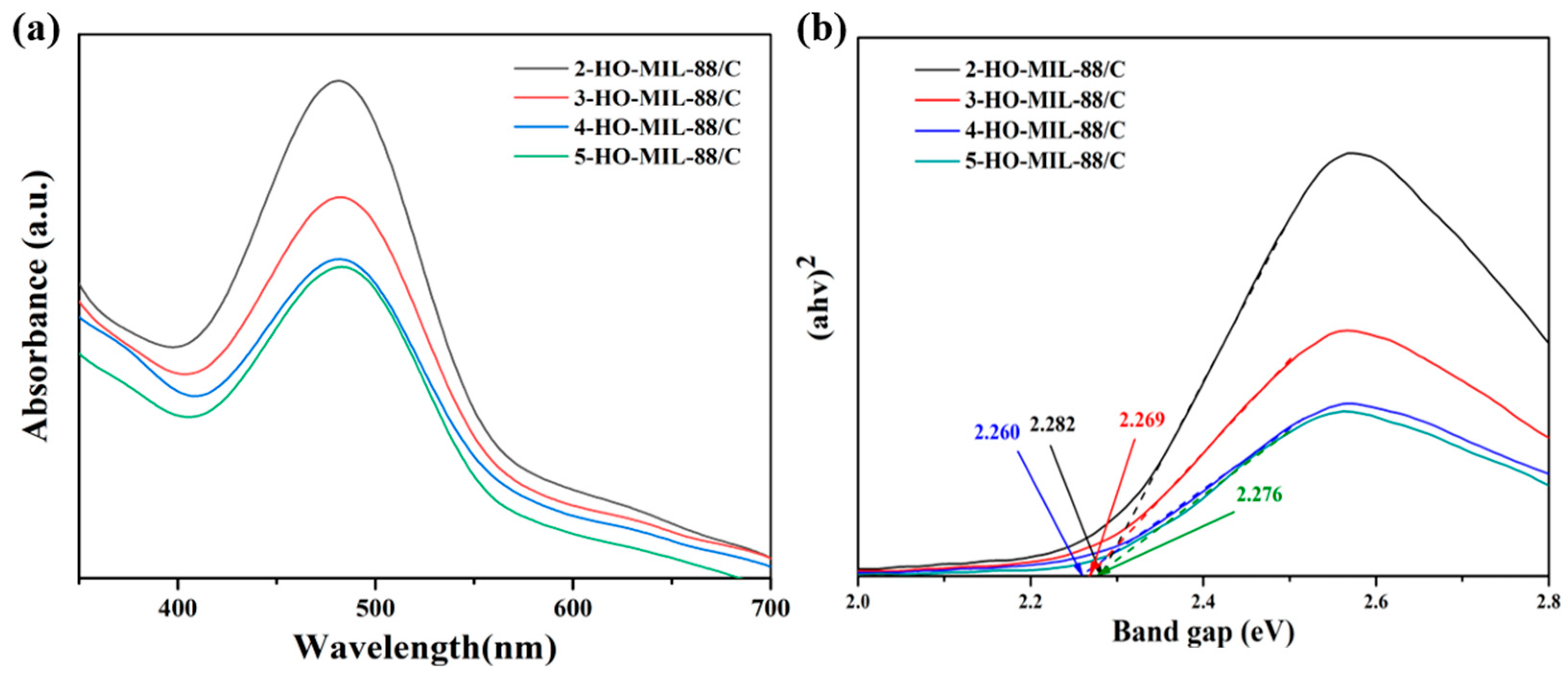
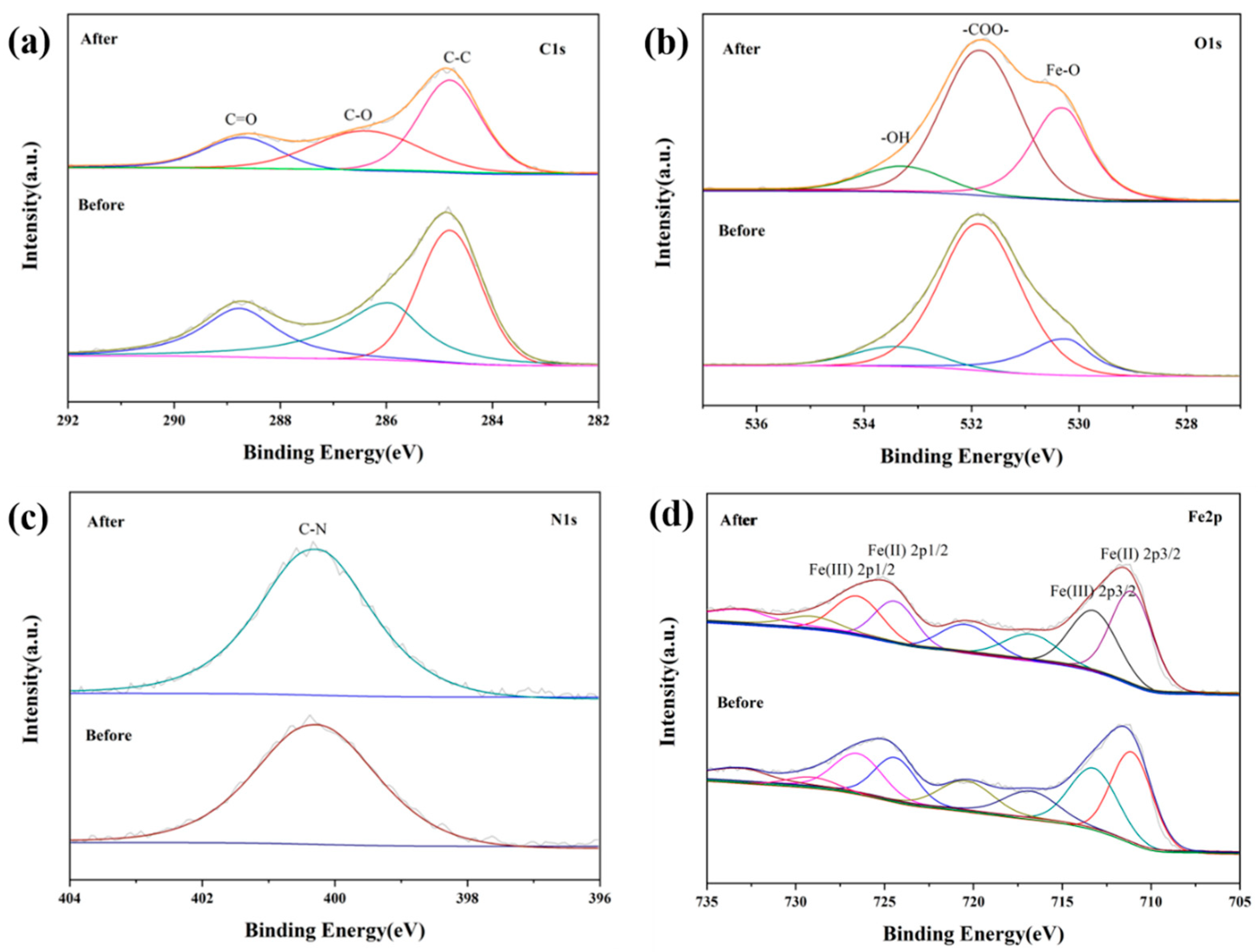
3.1. Structural and Morphological Characterizations
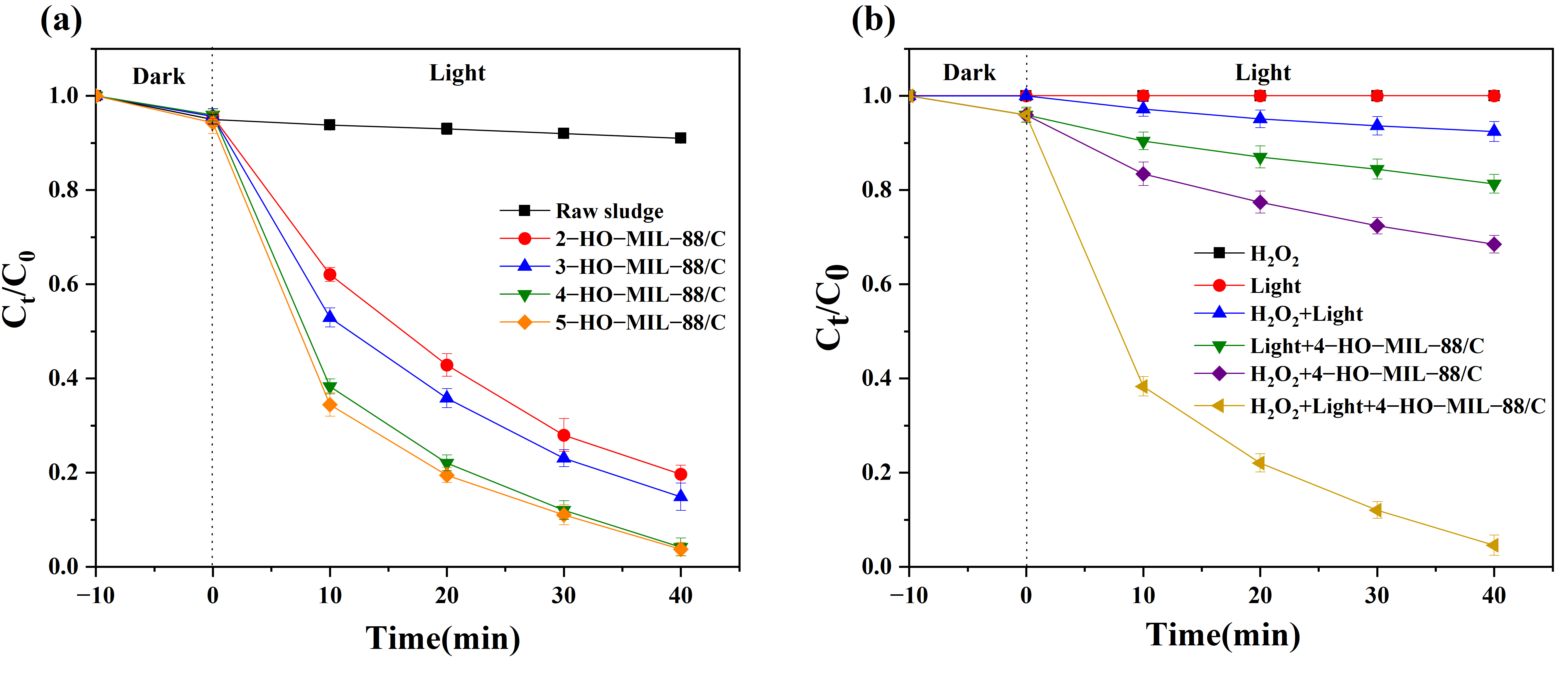
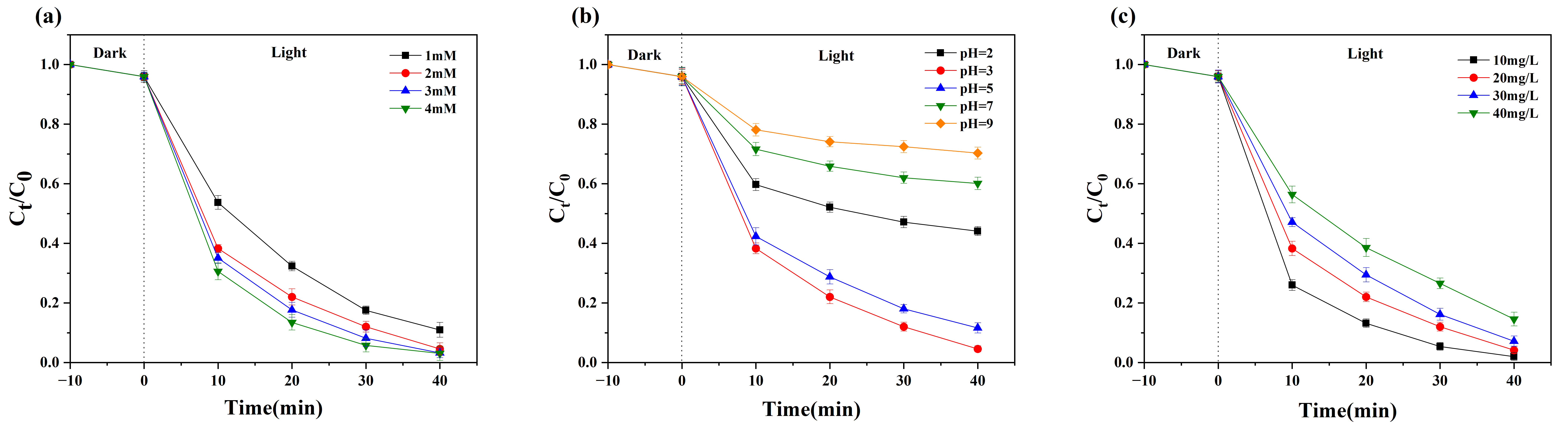
3.2. Photo-Fenton Catalytic Activity Analysis
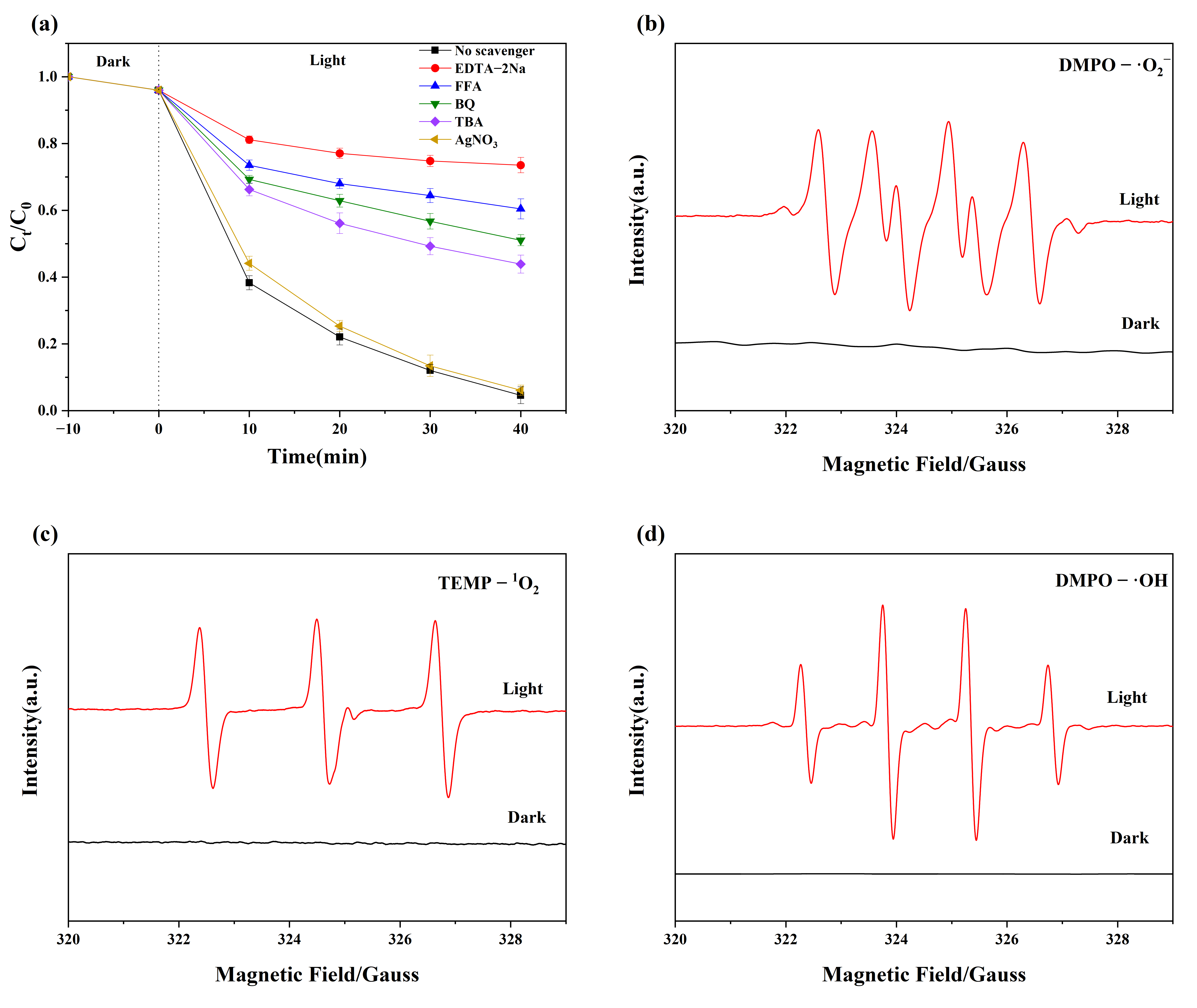
3.3. Active Species Detection
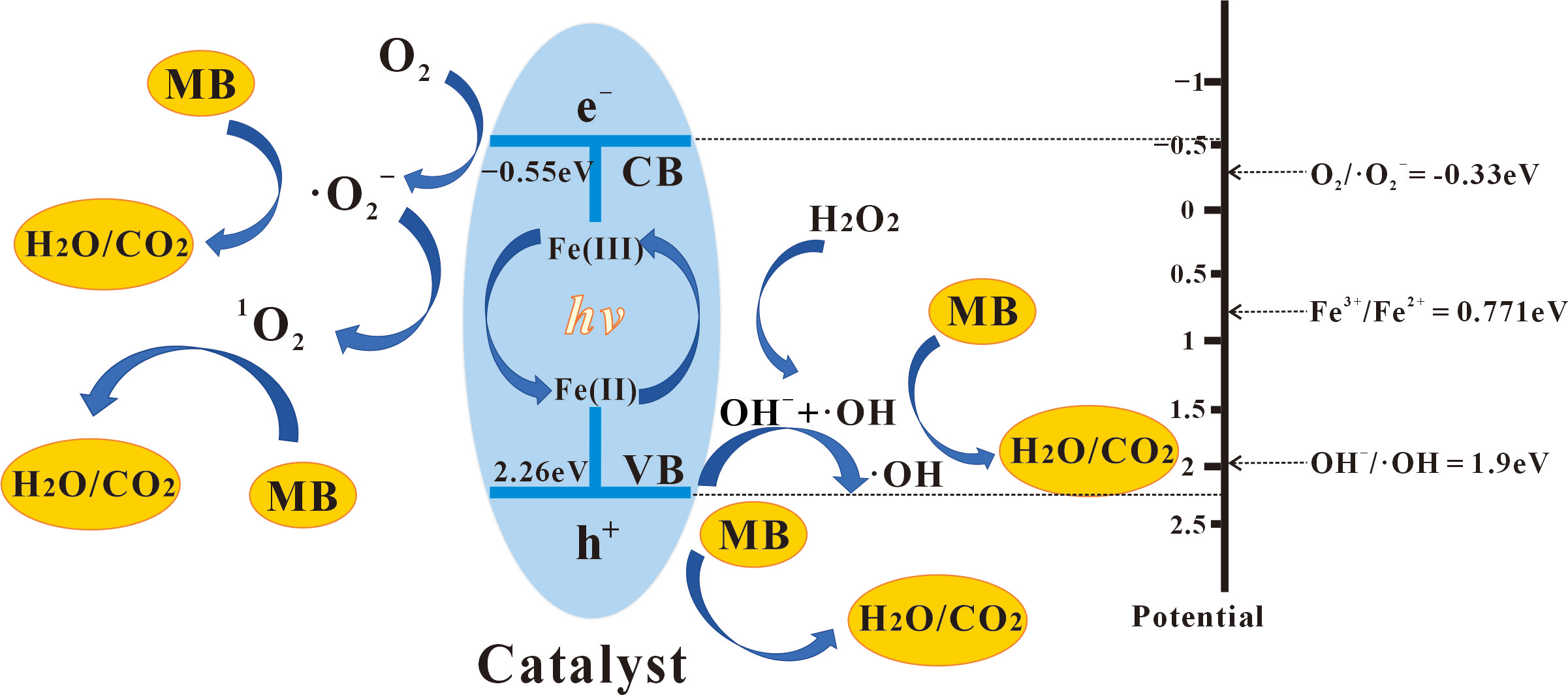
3.4. Mechanism of Photo-Fenton Catalytic Degradation
3.5. Stability and Reusability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Yin, W.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. High-efficient removal of organic dyes from model wastewater using Mg(OH)2-MnO2 nanocomposite: Synergistic effects of adsorption, precipitation, and photodegradation. Sep. Purif. Technol. 2021, 272, 118901. [Google Scholar] [CrossRef]
- Yadav, D.; Karki, S.; Gohain, M.B.; Ingole, P.G. Development of micropollutants removal process using thin-film nanocomposite membranes prepared by green new vapour-phase interfacial polymerization method. Chem. Eng. J. 2023, 472, 144940. [Google Scholar] [CrossRef]
- Yuksekdag, A.; Korkut, S.; Kaya, R.; Emin Pasaoglu, M.; Turken, T.; Agtas, M.; Evren Ersahin, M.; Ozgun, H.; Koyuncu, I. Upgrading of conventional water treatment plant by nanofiltration for enhanced organic matter removal. Sep. Purif. Technol. 2023, 325, 124766. [Google Scholar] [CrossRef]
- Cui, M.; Gu, W.; Yang, X.; Li, D.; Zhang, L.; Yang, N.; Wang, X.; Zhan, G. Microbial electrochemical driven anaerobic ammonium oxidation coupling to denitrification in a single-chamber stainless steel reactor for simultaneous nitrogen and carbon removal. Bioelectrochemistry 2022, 145, 108097. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Cao, Y. A novel strategy for enhancing heterogeneous Fenton degradation of dye wastewater using natural pyrite: Kinetics and mechanism. Chemosphere 2021, 272, 129883. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Gao, B.; Sillanpää, M. Landfill leachate treatment in-depth by bio-chemical strategy: Microbial activation and catalytic ozonation mechanism. Chem. Eng. J. 2022, 444, 136464. [Google Scholar] [CrossRef]
- Gao, Q.; Li, L.; Zhang, Y.; Zhou, H.; Jiang, J.; Wei, L.; Wang, G.; Ding, J.; Zhao, Q. Advanced oxidation processes (AOPs)-based sludge pretreatment techniques for enhanced short-chain fatty acids production: A critical review. Chem. Eng. J. 2024, 489, 151496. [Google Scholar] [CrossRef]
- Shitu, A.; Liu, G.; Muhammad, A.I.; Zhang, Y.; Tadda, M.A.; Qi, W.; Liu, D.; Ye, Z.; Zhu, S. Recent advances in application of moving bed bioreactors for wastewater treatment from recirculating aquaculture systems: A review. Aquac. Fish. 2022, 7, 244–258. [Google Scholar] [CrossRef]
- Li, X.; Jia, X.; Zhang, C.; Jiang, X.; Jiang, F.; Hu, Y.; Liu, Z.; Liu, Z. Advances in heterogeneous electro-Fenton processes for water treatment: A comprehensive review. Sep. Purif. Technol. 2025, 361, 131470. [Google Scholar] [CrossRef]
- Lu, H.; Hou, L.; Zhang, Y.; Cao, X.; Xu, X.; Shang, Y. Pilot-scale and large-scale Fenton-like applications with nano-metal catalysts: From catalytic modules to scale-up applications. Water Res. 2024, 266, 122425. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Wu, B.-X.; Huang, X.-W.; Hu, Y.; Hou, D.; Ren, W.; Liu, L.-L.; Chen, Y.; Tian, L.; Zhang, L.-S.; et al. Highly selective in-situ generation of 1O2 via electronic structure regulation of single atom sites in electro-Fenton system. Appl. Catal. B Environ. Energy 2025, 366, 125040. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Xiao, X.; Liu, B.; Huang, W.; Xie, Q.; Lan, C.; Luo, S.; Tang, L. Recent advancements in antibiotics removal by bio-electrochemical systems (BESs): From mechanisms to application of emerging combined systems. Water Res. 2025, 268, 122683. [Google Scholar] [CrossRef]
- Cai, C.; Fan, G.; Du, B.; Chen, Z.; Lin, J.; Yang, S.; Lin, X.; Li, X. Metal–organic-framework-based photocatalysts for microorganism inactivation: A review. Catal. Sci. Technol. 2022, 12, 3767–3777. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Zhang, H.; Chen, Z.; Ling, Y.; Yang, Y.; Liu, X.; Jia, Y.; Zhou, Y. TEA-assistant synthesis of MOF-74 nanorods for drug delivery and in-vitro magnetic resonance imaging. Microporous Mesoporous Mater. 2021, 315, 110900. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Liu, H.; Yuan, R.; Wang, X.; Feng, Z.; Chen, Z.; Chen, H. Strategies to improve adsorption and photocatalytic performance of metal-organic frameworks (MOFs) for perfluoroalkyl and polyfluoroalkyl substances (PFASs) removal from water: A review. Environ. Res. 2024, 240, 117483. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, W.; Li, Q.; Qu, S.; Zhang, L.; Tan, L.-L.; Shang, L. Plasmon-enhanced visible-light photocatalytic antibacterial activity of metal–organic framework/gold nanocomposites. J. Mater. Chem. A 2023, 11, 2391–2401. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Xing, L.; Li, J.; Xu, M.; Pan, G.; Li, J. Superoxide radical mediated persulfate activation by nitrogen doped bimetallic MOF (FeCo/N-MOF) for efficient tetracycline degradation. Sep. Purif. Technol. 2022, 282, 120124. [Google Scholar] [CrossRef]
- Gholizadeh Khasevani, S.; Gholami, M.R. Novel MIL-88A/g-C3N4 nanocomposites: Fabrication, characterization and application as a photocatalyst. Inorg. Chem. Commun. 2019, 102, 221–228. [Google Scholar] [CrossRef]
- Hu, P.; Yao, C.; Yang, L.; Xin, Y.; Miao, Y. Boosted photodegradation of tetracycline hydrochloride over Z-scheme MIL-88B(Fe)/Bi2WO6 composites under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127248. [Google Scholar] [CrossRef]
- Lei, Z.-D.; Xue, Y.-C.; Chen, W.-Q.; Li, L.; Qiu, W.-H.; Zhang, Y.; Tang, L. The Influence of Carbon Nitride Nanosheets Doping on the Crystalline Formation of MIL-88B(Fe) and the Photocatalytic Activities. Small 2018, 14, 1802045. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Zhang, Y.; Yang, L.; Liu, J.; Wang, Y.; He, Q. Constructing uniform Fe3O4@C@MnO2 microspheres with yolk-shell interior toward enhancement in microwave absorption. J Alloy Compd 2020, 817, 152795. [Google Scholar] [CrossRef]
- Jiang, H.; Zhong, Y.; Tian, K.; Pang, H.; Hao, Y. Enhanced photocatalytic degradation of bisphenol A over N,S-doped carbon quantum dot-modified MIL-101(Fe) heterostructure composites under visible light irradiation by persulfate. Appl. Surf. Sci. 2021, 577, 151902. [Google Scholar] [CrossRef]
- Li, Y.H.; Yi, X.H.; Li, Y.X.; Wang, C.C.; Wang, P.; Zhao, C.; Zheng, W.W. Robust Cr(VI) reduction over hydroxyl modified UiO-66 photocatalyst constructed from mixed ligands: Performances and mechanism insight with or without tartaric acid. Environ. Res. 2021, 201, 111596. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Zhang, X.D.; Bi, F.K.; Zheng, Z.H.; Sheng, L.J.; Xu, J.C.; Wang, Z.; Yang, Y.Q. Effective toluene adsorption over defective UiO-66-NH2: An experimental and computational exploration. J. Mol. Liq. 2020, 316, 113812. [Google Scholar] [CrossRef]
- Hua, J.; Chen, F.-y.; Tang, Y.-b.; Zhao, S.-g.; Yang, J.-t.; Wu, R. Synthesis of type II heterojunction 2D/3D Bi2S3/MIL-88(A) with enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. Solids 2023, 177, 111299. [Google Scholar] [CrossRef]
- Gotic, M.; Music, S. Mossbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J. Mol. Struct. 2007, 834, 445–453. [Google Scholar] [CrossRef]
- Liu, H.; Cui, M.; Liu, Y.; Kong, D.; Li, Z.; Weerasooriya, R.; Chen, X. Fabrication of novel Fe2O3/MXene cathode for heterogeneous electro-Fenton degradation of sulfamethoxazole. J. Ind. Eng. Chem. 2023, 125, 360–369. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Kong, D.; Chen, X. FeCN/MXene as novel heterogeneous electro-Fenton catalysts for the degradation of sulfathiazole: Performance and mechanism investigation. J. Environ. Sci. 2025, 154, 300–313. [Google Scholar] [CrossRef]
- Xu, R.; Ji, Q.; Zhao, P.; Jian, M.; Xiang, C.; Hu, C.; Zhang, G.; Tang, C.; Liu, R.; Zhang, X. Hierarchically porous UiO-66 with tunable mesopores and oxygen vacancies for enhanced arsenic removal. J. Mater. Chem. A 2020, 8, 7870–7879. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Liu, X.; Kuang, W.; Xiong, H.-B. Fabrication of Metal–Organic Framework-Mediated Heterogeneous Photocatalyst Using Sludge Generated in the Classical Fenton Process. Nanomaterials 2025, 15, 1069. https://doi.org/10.3390/nano15141069
Wang X-Y, Liu X, Kuang W, Xiong H-B. Fabrication of Metal–Organic Framework-Mediated Heterogeneous Photocatalyst Using Sludge Generated in the Classical Fenton Process. Nanomaterials. 2025; 15(14):1069. https://doi.org/10.3390/nano15141069
Chicago/Turabian StyleWang, Xiang-Yu, Xu Liu, Wu Kuang, and Hong-Bin Xiong. 2025. "Fabrication of Metal–Organic Framework-Mediated Heterogeneous Photocatalyst Using Sludge Generated in the Classical Fenton Process" Nanomaterials 15, no. 14: 1069. https://doi.org/10.3390/nano15141069
APA StyleWang, X.-Y., Liu, X., Kuang, W., & Xiong, H.-B. (2025). Fabrication of Metal–Organic Framework-Mediated Heterogeneous Photocatalyst Using Sludge Generated in the Classical Fenton Process. Nanomaterials, 15(14), 1069. https://doi.org/10.3390/nano15141069




