Abstract
Green-synthesized metal nanoparticles show promise in nanomedicine and material engineering. In this study, the polysaccharide of Zingiber officinale (ZOP) was used as a raw material. Through single-factor experiments and a response surface methodology, the optimum synthesis protocol of Zingiber officinale polysaccharide silver nanoparticles (ZOP-NPs-AgNPs) was determined as follows: V(AgNO3):V(ZOP) = 2.98:1, 59.79 °C, 3 h, pH 9, and 20 mL NaCl, achieving a 92.51% silver chelation rate. Structure analysis revealed that ZOP-NPs-AgNPs were spherical or quasi-spherical, with a particle size < 20 nm and a face-centered cubic crystal structure, which has good thermal stability. Subsequent studies explored the antibacterial and immunomodulatory effects of ZOP-NPs-AgNPs. The minimum inhibitory concentration (MIC) of ZOP-NPs-AgNPs against Escherichia coli and Staphylococcus aureus was determined to be 0.5000 mg/mL and 0.0310 mg/mL, respectively, while the minimum bactericidal concentration (MBC) was 0.5000 mg/mL and 0.0310 mg/mL, respectively. Additionally, ZOP-NPs-AgNPs significantly enhance RAW264.7 cell proliferation and phagocytosis and boost IL−1β, IL−6, NO, and TNF-α production. This confirms that ZOP can act as a green reductant and stabilizer, offering a new method for green nano-silver synthesis. This provides a sustainable way to produce antibacterial products and functional foods, and offers useful references for eco-friendly nano-silver applications.
1. Introduction
In recent years, nanomaterials have been widely studied, among which metal nanomaterials are an important research focus. They generally refer to materials with particle sizes of 1–100 nm, which have a unique quantum-size effects and surface effects, unlike ordinary metallic substances. Silver nanoparticles (AgNPs) have unique physical and chemical properties and are widely used in medicine, furnishing materials, and other fields [1]. Because of their smaller size and larger specific surface area, they have greater advantages in the antibacterial activity. In recent years, the progression of nanotechnology has propelled the extensive application of metal nanoparticles across various domains, such as biomedicine, food packaging, and catalysis [2,3,4,5]. AgNPs, in particular, have garnered significant attention due to their potent bactericidal properties against a wide spectrum of bacteria, including both drug-resistant and drug-sensitive strains (Gram-positive and Gram-negative bacteria), positioning them as promising candidates for use in pharmaceuticals and food packaging. At present, common methods for synthesizing AgNPs encompass physical processes, chemical reduction techniques, and biological reduction approaches [6]. Physical methods typically necessitate high-performance equipment, operate under stringent conditions, and incur high costs, which poses challenges for large-scale production. Meanwhile, conventional chemical reduction techniques often employ potent reducing agents such as sodium borohydride (NaBH4) [7] or trisodium citrate [8] under rigorously controlled conditions (e.g., temperature, pH) and have been broadly applied in the synthesis of AgNPs with specific sizes and morphologies. Notwithstanding, these conventional methods frequently involve toxic chemicals, demand strict reaction control, and may leave behind harmful residues [9]. ISO international standards highlight that employing fewer reagents and more eco-friendly techniques is the optimal choice [10]. Consequently, it is of vital importance to develop a method for synthesizing AgNPs that possess low toxicity and can slowly release Ag+. Adhering to the principles of “green chemistry”, which advocates for the preferential use of natural and nontoxic components, represents an effective strategy to achieve this objective [11].
The biological reduction method has emerged as a promising approach. In this method, simple, economical, and eco-friendly natural materials serve as reducing agents for synthesizing AgNPs. These natural materials also act as stabilizing agents, preventing excessive aggregation of AgNPs, thereby enhancing their biocompatibility [12,13,14]. Currently, various biomacromolecules have been employed to stabilize dispersed AgNPs, including pectin (Akebia trifoliata var.) [15], chitosan [16], cellulose [17], fungal polysaccharides [18], and carboxymethyl cellulose [19], among others. Polysaccharides, owing to their wide availability and excellent biocompatibility, have become ideal natural reducing agents. AgNPs prepared using polysaccharides as both reducing and stabilizing agents not only possess inherent advantages but may also exhibit additional biological activities [20].
Z. officinale is a perennial herb of Zingiberaceae, which contains many active components, such as polysaccharides, polyphenols, terpenoids, gingerol, and its derivatives. It can effectively fight against many pharmacological effects, such as cardiovascular diseases, ulcers, tumors, indigestion, and inflammation [21,22]. Polysaccharides are one of the main functional active components in ginger, which has a significant developmental value and application prospect in medicine, hygiene, food, condiments, and cosmetics [23]. Numerous data show that ZOP has many biological activities, such as antitumor, antioxidant, and antiglycemic, and has the advantages of high safety and fewer toxic and side effects [24,25].
In response to the rising demand for sustainable and eco-friendly practices in nanotechnology development and considering the unique properties of ZOP and silver nanoparticles, this study employs the natural product ZOP as a reducing agent for silver nanoparticle synthesis. In preliminary studies, Z. officinale polysaccharides were extracted, and using ZOP and ZOP-NPs as reducing and stabilizing agents, ZOP-AgNPs and ZOP-NPs-AgNPs were synthesized. The physicochemical properties of ZOP, ZOP-AgNPs (0.5%, w/v; 1%, w/v), and ZOP-NPs-AgNPs (0.5%, w/v; 1%, w/v) were comprehensively analyzed using UV-visible spectrophotometer (UV-Vis), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD). Comparative studies on antioxidant and antibacterial activities revealed that ZOP-NPs-AgNPs exhibited superior performance to ZOP, ZOP-NPs, and ZOP-AgNPs [26]. To further explore the properties and potential applications of ZOP-NPs-AgNPs, through optimization using single-factor experiments and the response surface methodology (RSM), we obtained a single target sample of ZOP-NPs-AgNPs synthesized under specific optimal parameters (an AgNO3-to-polysaccharide volume ratio of 3:1, temperature of 60 °C, and time of 3 h). Based on the optimized conditions, the structure of ZOP-NPs-AgNPs was further analyzed, and its antibacterial and immunomodulatory activities were systematically evaluated. This study aims to meet the urgent need for the development of environmentally friendly and efficient alternative synthetic methods. By significantly enhancing the antibacterial and immunomodulatory properties of silver nanoparticles, it holds great potential to drive the future development of medical treatment, food preservation, and environmental protection. This study not only comprehensively and deeply analyzes the characteristics and application prospects of ZOP-NPs-AgNPs but also develops a green synthesis strategy, laying a solid theoretical foundation for the wide application of silver nanoparticles in various industries.
2. Materials and Methods
2.1. Materials and Reagents
Z. officinale (No. 2019031401) was purchased from AnguoYaoyuan Trading Co., Ltd. (Hebei, China). The sample was identified as the rhizome of Zingiber officinale Roscoe by professor Yuguang Zheng (Department of Pharmacy, Hebei University of Chinese Medicine). A voucher specimen was deposited in the College of Chemistry and Pharmaceutical Engineering, Hebei University of Science and Technology, China. AgNO3 was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China), tryptone and agar powder were purchased from Beijing Auboxing Biotechnology Co., Ltd. (Beijing, China), Escherichia coli and Staphylococcus aureus were obtained from Beijing Biological Preservation Center (Beijing, China)., and RAW264.7 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All other chemicals and reagents were analytical grade.
2.2. Preparation of ZOP
The ZOP samples used in this experiment were derived from the previously conducted experiments [26], and the initial structural characterization of ZOP was carried out.
2.3. Preparation of Z. officinale Polysaccharide Nanoparticles (ZOP-NPs)
In this experiment, ZOP-NPs were first prepared by nano-precipitation method, and then ZOP-NPs were used to synthesize AgNPs. A total of 1.5 g ZOP was dissolved in 100 mL distilled water to obtain 1.5% (w/v) polysaccharide solution. Under magnetic stirring, the polysaccharide solution was dropped dropwise into 10 times the volume of absolute ethanol, with stirring continuing for 2 h, and the solution was concentrated and dried to obtain ZOP-NPs [27].
2.4. Preparation of ZOP-NPs-AgNPs
2.4.1. Single-Factor Experiment
AgNO3 solution (0.05 mol/L), ZOP-NPs solution (1 mg/mL), and NaCl solution (1 mg/mL) were prepared. The polysaccharide solution, AgNO3 solution, and NaCl solution were measured in proportion, and pH was adjusted with ammonia water; the final solution was irradiated under 365 nm violet light and stirred for a certain period of time to obtain ZOP-NPs-AgNPs [28]. In this experiment, silver chelation rate was used as the index, and five factors were considered, namely, the volume ratio of AgNO3 solution and ZOP-NPs solution (1.5:1, 2:1, 2.5:1, 3:1, 3.5:1), the volume of NaCl (5, 10, 15, 20, 25/mL), temperature (20, 40, 60, 80, 100/°C), time (1, 2, 3, 4, 5/h), and pH value (7, 8, 9, 10, 11). While optimizing each factor, other factors remained stable. Silver ion chelation rate was determined by Volhard method. Reaction of [NH4 Fe(SO4)●12 H2O] is:
A total of 20 mL of ZOP-NPs-AgNPs solution was measured, and four times the volume of anhydrous ethanol was added to the solution. Then, the solution was allowed to stand for 20 min before being centrifuged at 8000 r/min for 10 min. After that, 20 mL of supernatant was transferred to the solution, an appropriate amount of ammonium thiocyanate standard solution (0.76 mg/mL) was added, the titration was performed, the color of the solution and the volume of the standard solution consumed were recorded, and Equation (1) was used to calculate the chelation rate of silver ions.
A represents the chelation rate (%), C0 is the concentration of silver ion in the solution before the reaction (mg/mL), and C is the concentration of silver ion in the solution after the reaction (mg/mL).
2.4.2. Box–Behnken Response Surface Methodology
Based on the results of the single-factor experiment, the Box–Behnken test factor level table was designed (Table 1). In this study, the silver chelation rate was used as the response value, 17 groups of experiments were analyzed, and a response surface model was established to obtain the best preparation process.

Table 1.
Independent variables and levels of the experimental design.
2.5. Structural Characterization of ZOP-NPs-AgNPs
2.5.1. UV-Vis Spectroscopy Analysis
During the preparation of AgNPs, 5 mL of ZOP-NPs-AgNPs solution was measured every 1 h. Scanning and recording spectra were in the range of 200–800 nm by a UV-vis spectrophotometer [29].
2.5.2. FT-IR Spectroscopy Analysis
ZOP-NPs-AgNPs sample powder and KBr were dried at 60 °C to constant weight. The sample was made into transparent and uniform tabletting by the KBr tabletting method, and FT-IR spectra were analyzed by an S−100 spectrometer (PerkinElmer, Waltham, MA, USA) in the range of 4000–400 cm−1.
2.5.3. SEM Analysis
ZOP-NPs-AgNPs were deposited on a sample table and then analyzed by a field emission scanning electron microscope (JSM-7610 F) under 3.0 kV accelerated voltage and high vacuum.
2.5.4. TEM Analysis
ZOP-NPs-AgNPs were observed at 100 keV using a transmission electron microscope analyzer (JEM-2100, JEOL, Tokyo, Japan).
2.5.5. XRD Analysis
The crystal structure of the samples was analyzed by an X-ray diffractometer model (XRD-6000, Shimadzu, Kyoto, Japan). Cu-Kα ray (λ = 0.15406 nm) was used as the target, the tube voltage was 40 kV, the tube current was 30 mA, the scanning rate was 5 °C/min, and the diffraction Angle was 2θ = 10–90° [30].
2.5.6. TGA Analysis
TGA-DSC (TA Instruments Ltd., Q600, New Castle, DE, USA) was used for thermogravimetric analysis. The temperature was increased from room temperature to 800 °C, and the heating rate was 10 °C/min.
2.6. Antibacterial Activity of ZOP-NPs-AgNPs
2.6.1. The Determination of the Diameter of the Bacteriostatic Zone
The antibacterial activity of ZOP-NPs-AgNPs was evaluated using the agar well diffusion method. In both antibacterial tests (Escherichia coli and Staphylococcus aureus), 20 mg/mL and 40 mg/mL of ZOP-NPs-AgNPs were prepared, respectively. The control groups included AgNO3 gel, ampicillin, 20 mg/mL, and 40 mg/mL of AgNO3. In the experimental procedure, after the agar in the solid culture medium had coagulated, the diluted bacterial suspension was evenly spread on the surface of the solid culture medium by streaking in a “Z” pattern with cotton swabs (three times). Subsequently, the Oxford cup was placed vertically on the solid culture medium, and 100 μL of each sample solution was dispensed into the cup. The samples were then incubated at 37 °C for 24 h in a constant temperature incubator. Eventually, the antibacterial activity of ZOP-NPs-AgNPs was evaluated and quantified by the diameter (cm) of the inhibition zones [31].
2.6.2. The Minimum Inhibitory Concentration (MIC) of ZOP-NPs-AgNPs
The MIC values, which represent the lowest extract concentrations that prevent the visible growth of microorganisms, were determined by a twofold microdilution test in combination with the colorimetric Resazurin assay. Resazurin solution, which is blue, is commonly used as an acid-base indicator (pH 3.8 (orange) − 6.5 (deep purple)) and a redox indicator. Resazurin solution can penetrate cells, and it will be irreversibly reduced to pink in the environment where bacteria grow. The concentration of the solution before it turns pink is the MIC.
Using Resazurin solution as an indicator, AgNO3 (1 mg/mL) and ampicillin (1 mg/mL) served as positive controls, while DMSO and normal saline were used as negative controls. In a 96-well plate, a twofold continuous diluted sample solution (1–1/8192 mg/mL) was prepared and dispensed into each well. Subsequently, Resazurin indicator (0.07 g/mL) and bacteria solution were added to each well. After thoroughly mixing the contents of each well, the 96-well plate was covered with a sterile plate and incubated at 37 °C in a constant temperature incubator. The color of each well in the 96-well plate was observed every 12 h. If the color in the hole turned red or pink, it indicated that bacteria grew in the well. If the color in the hole was still blue, it indicated aseptic growth, and the concentration at which the first well turned pink was considered the MIC [32].
2.6.3. The Minimum Bactericidal Concentration (MBC) of ZOP-NPs-AgNPs
Based on the aforementioned experimental results, the sample concentration corresponding to blue wells in the 96-well plate was selected for the follow-up experiment. The ZOP-NPs-AgNPs with corresponding concentrations were mixed with bacterial liquid and sterile water, and then evenly smeared on a solid culture medium. After incubation at 37 °C for 24 h in a constant temperature incubator, the bacterial growth was observed. If the number of bacterial colonies in the plate was less than 5, the corresponding sample concentration was recorded as the effective bactericidal concentration, and the effective minimum bactericidal concentration of each strain was the MBC.
2.7. Immunomodulatory Activity of ZOP-NPs-AgNPs
2.7.1. Culture of RAW264.7 Cells
RAW264.7 cells were obtained from Shanghai Cell Bank of Chinese Academy of Sciences and cultured in DMEM medium (basic medium: fetal bovine serum: penicillin-streptomycin solution = 90:10:1) at 37 °C and 5% CO2. After 24 h, the fresh culture medium was changed, and it continued to be cultured. When the cells grew to 80–90% of the bottom area of the culture bottle and the cells were in good shape, they were subcultured.
2.7.2. Cell Proliferation Experiment
The effect of ZOP-NPs-AgNPs on macrophage viability was detected by the CCK-8 method. After adjusting the concentration to 2 × 105 cells/mL, the cells were seeded into 96-well plates with 100 μL per well and cultured in a cell incubator. After 24 h, 100 μL of ZOP-NPs-AgNPs (1–500 μg/mL) with different concentrations was used to replace the culture medium, and lipopolysaccharide (LPS) (1 μg/mL) was added to the positive control group. After 20 h, 0.5 mg/mL CCK-8 solution was added to the 96-well plate. After continuing culture for 4 h, the supernatant was removed, 150 μL of DMSO was added to each well, and the plate was shaken to fully dissolve the purple formazan crystals. The absorbance value of each well was detected by a microplate reader at 490 nm [33].
2.7.3. Cell Phagocytosis Experiment
RAW264.7 cells in the logarithmic growth phase were trypsinized and diluted to a concentration of 5 × 105 cells/mL DMEM culture medium. A 150 μL suspension was placed in a 96-well plate and cultured for 24 h. Subsequently, 50 μL of ZOP-NPs-AgNPs solution (1–500 μg/mL) at varying concentrations was added to each well, followed by continued incubation for an additional 24 h. Then the culture medium was removed, and the cells were washed three times with PBS (0.01 M, pH 7.4). Then, 100 μL of cell lysis buffer was added and incubated overnight at room temperature. The absorbance was measured at 540 nm by a microplate reader. The blank control group contained only DMEM medium, while the positive control group was LPS medium [34].
2.7.4. The Effect Test of NO Secretion by Macrophages
To determine the NO production of RAW264.7 cells, RAW264.7 cells in logarithmic phase were digested with trypsin and placed in a 96-well plate. A total of 50 μL ZOP-NPs-AgNPs solution (1–500 μg/mL) was added to the culture for 24 h; then, 100 μL of the culture solution was transferred to a new plate, the Griess reagent was added to the new plate, and the absorbance was measured at 540 nm. The blank control group contained only DMEM medium, while the positive control group was LPS. The NO production was calculated according to the standard curve made with NaNO2 as the standard [35].
2.7.5. Assay of Cytokines
RAW264.7 cells were treated with varying concentrations of ZOP-NPs-AgNPs for 24 h, following the method described in the cell proliferation experiment (Section 2.7.2). The secretion of TNF-α, IL-1β, and IL-6 cytokines in the cell culture medium was detected according to the instructions of the ELISA kit (from ABclonal, Wuhan, China).
2.7.6. Data and Analysis
Excel was employed for the initial organization and sorting of the test data. Design Expert 8.0 was used to complete the response surface test design and correlation analysis. SPSS 16.0 was used to analyze the significance, and Origin 8 was used to draw the data. The level of statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Preparation of ZOP-NPs-AgNPs
3.1.1. Single-Factor Experiment Analysis
The chelation rate increased with the increase in the volume ratio of AgNO3 and ZOP-NPs solution, reaching its maximum when the volume ratio is 3:1 (Figure 1a). Before and after that, there was a downward trend. Studies have shown that as the concentration of AgNO3 increases, more silver nanoparticles are formed in the solution, resulting in a broader ultraviolet absorption peak [36]. However, with the increase in the volume ratio, the chelation rate eventually tends to become flat or even decrease, which may be due to the maximum chelation between polysaccharide and Ag+ in the solution or to the transformation of silver nanoparticles into silver oxide nanoparticles under the condition of insufficient stabilizing reagents [37,38]. Therefore, the volume ratio of AgNO3 and polysaccharide solution was selected as 3:1 for further research.
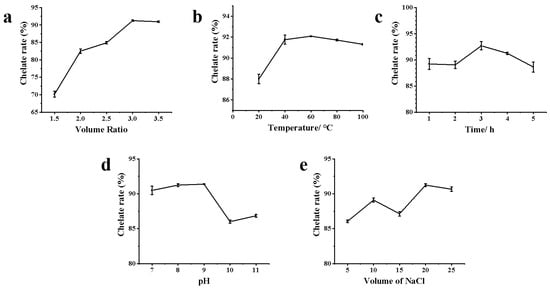
Figure 1.
The chelation rate of ZOP-NPs-AgNPs under different influencing factors: (a) the volume ratio of AgNO3 and polysaccharide; (b) temperature; (c) time; (d) pH; (e) the volume of NaCl.
The chelation rate increased with the rise in water bath temperature, peaking at 60 °C (Figure 1b). However, further temperature increases led to a decline in the chelation rate, likely due to excessive aggregation of ZOP-NPs-AgNPs, which interfered with chelation rate determination. Therefore, an extraction temperature from 50 to 70 °C was determined to be suitable for further experiments.
As shown in Figure 1c, the chelation rate increased with the increase in ultraviolet irradiation time. At the same time, the solution changed its color to dark brown or black, which reached the highest rate when the ultraviolet irradiation time was 3 h; however, the chelation rate decreased with the increase in ultraviolet irradiation time, which may be due to the agglomeration of ZOP-NPs-AgNPs with the increase in time, which affected the determination of the chelation rate.
Under alkaline conditions, Ag+ reacts with -OH to form Ag2O. The -COOH and -OH groups lose H+ to form salts, which then form complexes with Ag2O. The -CHO and -C=O groups in ZOP reduce Ag2O to AgNPs by reacting with the Ag2O complex. In this process, -CHO is the primary reducing agent, and the original -COOH, -OH, and the generated-COOH play a stable role in the formation of AgNPs [39]. Analysis of Figure 1d shows that the chelation rate increased with the solution pH. A significant pH increase facilitated AgNPs formation, likely due to a larger number of available functional groups in the polysaccharide solution for binding, thus promoting AgNPs formation [40]. However, at pH 10 and 11, the chelation rate decreased, possibly because the excessive alkalinity from added ammonia water disrupted the polysaccharide structure, affecting AgNPs formation and, consequently, the chelation rate.
Based on Figure 1e, when the volume of NaCl added was 20 mL, the chelation rate was the highest, but as the volume of NaCl increased, the chelation rate decreased. It may be that the concentration of Cl− in the solution increased, which affected the generation of AgNPs, resulting in chelation rate decrease.
3.1.2. Response Surface Results and Contour Plots
From the single-factor experiment results for the above five groups, the volume ratio of AgNO3 to polysaccharide solution, water bath temperature, and ultraviolet time have a great influence on the preparation process of ZOP-NPs-AgNPs, while the pH value and NaCl volume can be fixed at 9 and 20 mL. According to the principle of three factors and three levels, the volume ratios of AgNO3 and polysaccharide solution were determined as 2.5:1, 3:1, and 3.5:1; the experimental temperatures were 50, 60, 70 °C; The experimental times were 2, 3, 4 h. A model was established with silver chelation rate as the response value, and 17 groups of experiments were conducted (Table 2).

Table 2.
Box–Behnken experimental design and the results for silver chelation rates.
The Box–Behnken experimental design method was used to fit the relationship between each factor and response value by using the equation, and the equation was analyzed to explore the best process parameters. The fitting regression equation was obtained:
Y = 92.51 − 0.26 A − 0.76 B + 0.0075 C − 0.41 AB + 0.47 AC − 0.39 BC − 2.63 A2 − 1.3 B2 − 0.11 C2
The p-values were used as a tool to check the significance of each coefficient. From the experimental results (Table 3), the model’s p-value was less than 0.0001, indicating a significant model fit. The lack-of-fit value was 0.6649, indicating that it was insignificant relative to the pure error (p > 0.05). The p-values of A, AB, AC, BC, A2, B2, and C2 were below 0.05, indicating these factors significantly affected the chelation rate. According to the F-value, the volume ratio of AgNO3 to polysaccharide solution had the most significant impact on ZOP-NPs-AgNPs preparation, followed by water bath temperature and then violet light irradiation time. The fitting coefficient was R2 = 0.9879, which suggested that the quadratic model was significant. According to the response surface methodology, the optimum preparation technology of ZOP-NPs-AgNPs was as follows: the volume ratio of AgNO3 to polysaccharide was 2.98:1, the water bath temperature was 59.79 °C, and the irradiation time of purple light was 3 h.

Table 3.
Analysis of variance for the experimental results.
Through the three-dimensional response surface and contour map of model fitting, the relationship between different influencing factors and silver chelation rate was visualized (Figure 2). The response surface diagram illustrates the combined effects of two continuous variables on the silver chelation of ZOP-NPs-AgNPs, with the third variable held constant at zero. The results (Figure 2a) show the effects of the AgNO3 and polysaccharide solution volume ratio and temperature on chelation rate and their interactions. As the extraction temperature and volume ratio rose, the chelation rate of ZOP-NPs-AgNPs increased in a parabolic direction, indicating that the model’s maximum value was at the center.
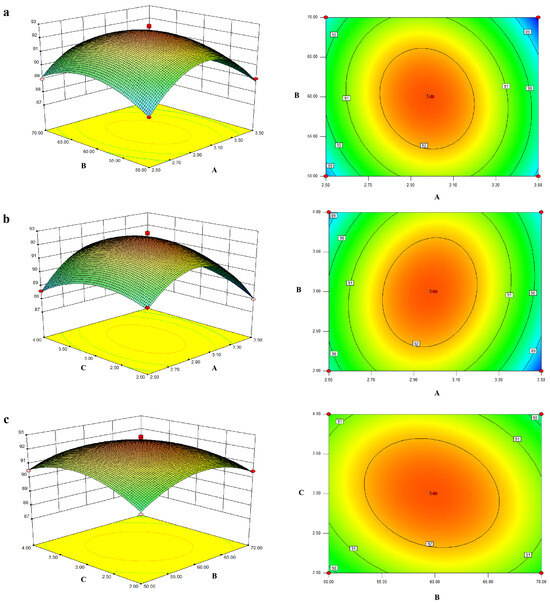
Figure 2.
(a) The effect of the interaction between AgNO3 and the polysaccharide solution volume ratio and temperature on the chelation rate. (b) The effect of AgNO3 and polysaccharide solution volume ratio and time interaction on the chelation rate. (c) The effect of the interaction between temperature and time on the chelation rate.
The contour plot also reveals a significant interaction between the volume ratio of AgNO3 and polysaccharide solution and time. In addition, the chelation rate increases with the increase in the volume ratio, but with the extension of time, the trend of the chelation rate increasing is not obvious (Figure 2b). From the response surface and contour plots of temperature and time (Figure 2c), it is evident that the chelation rate increased with higher temperatures and longer times. Overall, the order of importance for the three factors in AgNP synthesis was as follows: Volume ratio (AgNO3: polysaccharide) > Temperature > Irradiation Time.
To verify the model and assess the accuracy of the optimization results, experiments were conducted under the model-predicted conditions: an AgNO3-to-polysaccharide volume ratio of 3:1, temperature of 60 °C, and time of 3 h. The resulting chelation rate of 92.17% was very close to the predicted value of 92.51%, confirming the model’s validity.
3.2. Structural Characterization of ZOP-NPs-AgNPs
3.2.1. The Determination of the Particle Size and Silver Chelating Rate
Polysaccharides can be used not only as a reducing agent to reduce silver ions but also as a stabilizer to prevent the agglomeration of AgNPs during the formation of AgNPs [41]. The silver chelation rate of the polysaccharide was 92.17%, with a particle size below 20 nm, a PDI of 0.299, and a zeta potential of −32.83 mV. The silver chelation rate of ZOP-NPs-AgNPs prepared under the optimum conditions is higher than that of ZOP-NPs-AgNPs prepared before (68.70–82.12%) [26].
3.2.2. UV-Vis Analysis
AgNPs have characteristic absorption peaks in the wavelength range of 300–800 nm [42]. The UV-Vis spectra (Figure 3a) show that there was an obvious absorption peak at about 430 nm, which was the unique surface plasmon resonance of AgNPs [43], confirming the formation of spherical AgNPs in the solution. The intensity of the absorption peak increased gradually with the increase in time, indicating the increase in AgNPs in the solution [44]. The single broad peak in the spectrum suggests the polydispersion of ZOP-NPs-AgNPs. At the same time, we found that some researchers experienced a similar phenomenon in the study of synthesizing silver nanoparticles from Rosa roxburghii Tratt fruit polysaccharide [45].
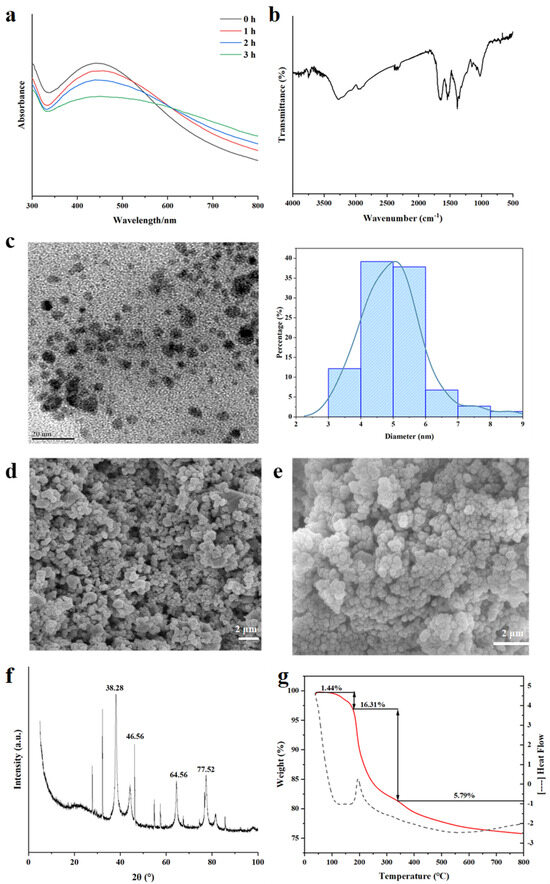
Figure 3.
Structural characterization of ZOP−NPs−AgNPs. (a) UV−vis. (b) FT−IR. (c) TEM and size statistics. (d,e) SEM. (f) XRD. (g) TGA (the red line represents weights and the dots represents heat flow).
3.2.3. FT-IR Analysis
The FT-IR spectrum of ZOP-NPs-AgNPs (Figure 3b) exhibits distinct alterations in characteristic absorption peaks compared to pure ZOP [26], confirming structural changes upon AgNPs formation. Key observations include the following: narrowing of the -OH stretching vibration at 3282 cm−1, suggesting hydroxyl group involvement in AgNPs reduction and stabilization; significant attenuation of the -CH absorption peak at 2943 cm−1, indicating strong interactions between AgNPs and aliphatic chains [41]; and intensified -COO− vibration at 1630 cm−1, coupled with weakened peaks at 1642.09 cm−1 (C=O), 1389 cm−1 (C-H), and 1017 cm−1 (C-O), collectively demonstrating the participation of surface hydroxyl and carboxyl groups in AgNPs synthesis. This trend aligns with reported mechanisms for polysaccharide-stabilized AgNPs [40,46], notably matching spectral shifts observed by Ma et al. [47] in soluble soybean polysaccharide-templated AgNPs.
3.2.4. TEM Analysis
TEM analysis of ZOP-NPs-AgNPs revealed that the solution primarily contained spherical AgNPs (Figure 3c). The dispersion of ZOP-NPs-AgNPs was good, the particle size of AgNPs was less than 20 nm, and the signs of aggregation were not obvious. Moreover, the mean diameter and standard deviation of ZOP-NPs-AgNPs were 4.99 ± 0.99 nm. The results indicated that ZOP-NPs function as a stabilizer by encapsulating the surface of nanoparticles, thereby reducing the surface activity of AgNPs and preventing their aggregation [48]. The experimental results showed that the AgNPs prepared under optimal conditions have advantages such as high silver chelation rates, small particle size, and good dispersion. Higher silver chelation rates and smaller particle sizes translate to larger specific surface areas, enabling AgNPs to have a larger contact area and, thus, exhibit better biological activity.
3.2.5. SEM Analysis
SEM of ZOP-NPs-AgNPs (Figure 3d,e) shows that the shape of AgNPs is very close to spherical, but there were also a small number of block structures. The presence of these block-like structures may be attributed to the aggregation of AgNPs during long-term storage or the freeze-drying process.
3.2.6. XRD Analysis
The crystallinity of formulated ZOP-NPs-AgNPs was tested by XRD (Figure 3f)). ZOP-NPs-AgNPs showed four prominent diffraction peaks, which were located at (2θ) 38.28° [49], 47.56° [42], 64.56° [50], and 77.52° [51], respectively, corresponding to the four planes of (111), (200), (220), and (311) of face-centered cube (FCC) [42]. The sharp and narrow peaks indicated the crystalline nature of ZOP-NPs-AgNPs, confirming the successful synthesis of AgNPs. These characteristic peaks have been widely reported by researchers and were thought to be related to the crystallization of organic phases on the surface of AgNPs [52,53]. Additionally, some minor peaks were observed in Figure 3f, which may be due to improper handling or the introduction of impurities during the synthesis process or, possibly, due to the failure to remove lipids and proteins during polysaccharide extraction [54].
3.2.7. TGA Analysis
The thermal stability of the prepared ZOP-NPs-AgNPs was investigated using thermogravimetric analysis (TGA). The process can be divided into three distinct stages (Figure 3g). The initial degradation stage of ZOP-NPs-AgNPs occurred at about 38−165 °C, in which water was mainly lost, and the weight loss rate was 1.44%. The second stage mainly occurred at about 165−330 °C, which was mainly caused by the decomposition of the polysaccharide itself, and the weight loss rate was 16.31%. The weight loss rate in the third stage was 5.79%. These observations indicate that the mass loss of AgNPs was primarily due to the volatilization of water and the degradation of the polysaccharide. This also confirms that the polysaccharide effectively encapsulated the AgNPs, which is consistent with previous results. During the synthesis of AgNPs, polysaccharide nanoparticles played a protective and stabilizing role, encapsulating AgNPs and preventing aggregation [55].
3.3. Antibacterial Activity of ZOP-NPs-AgNPs
3.3.1. Determination of Diameter of Bacteriostatic Zone
The antibacterial activity of ZOP-NPs-AgNPs was investigated. The strains Escherichia coli and Staphylococcus aureus were selected in this experiment (Figure 4). The results indicated that ZOP did not show antibacterial activity. The inhibition zones of ZOP-NPs-AgNPs against Escherichia coli (Figure 4d) and Staphylococcus aureus (Figure 4h) were 0.89 cm (20 mg/mL), 1.23 cm (40 mg/mL), 1.08 cm (20 mg/mL), and 1.61 cm (40 mg/mL), respectively. According to the results of Staphylococcus aureus, ZOP-NPs-AgNPs had better antimicrobial activity than AgNO3. This may be due to the higher content of silver in the prepared AgNPs, and because they were nanoparticles with a larger specific surface area, they can better contact the cell membrane and cell wall, destroy their structure and permeability, and at the same time, they can penetrate bacteria, attack the respiratory chain, hinder cell metabolism and cell division, and promote cell apoptosis [56]. From the determination of the inhibition zone diameter of Escherichia coli and Staphylococcus aureus, it can be seen that ZOP-NPs-AgNPs had a better inhibition effect on Escherichia coli. According to the research under different concentrations, the greater the concentration of ZOP-NPs-AgNPs, the larger the inhibition area diameter, indicating that it has a stronger inhibition effect on the growth of bacteria. Affes et al. and He et al. also observed marked dose-dependent antibacterial activities of AgNPs against Gram-positive and Gram-negative bacteria [57,58].
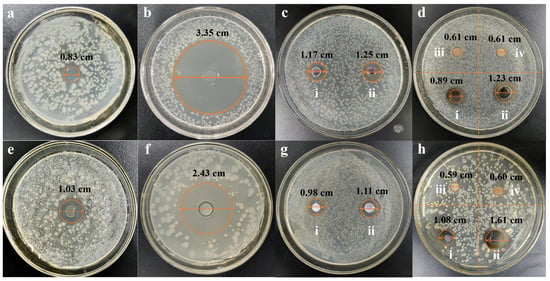
Figure 4.
Escherichia coli (a–d) and Staphylococcus aureus (e,f) inhibition zone diameter. (a,e) AgNO3 gel. (b,f) Ampicillin. (c,g) AgNO3, (i) 20 mg/mL AgNO3, and (ii) 40 mg/mL AgNO3. (d,h) ZOP and ZOP-NPs-AgNPs, (i) 20 mg/mL ZOP-NPs-AgNPs, (ii) 40 mg/mL ZOP-NPs-AgNPs, (iii) 20 mg/mL ZOP, and (iv) 40 mg/mL ZOP.
3.3.2. The MIC of ZOP-NPs-AgNPs
In this experiment, Resazurin was used as an indicator to determine the MIC of ZOP-NPs-AgNPs, and two positive controls and two negative controls were set. The positive controls were ampicillin and AgNO3, and the negative controls were DMSO and NaCl. According to the experimental results (Figure 5), DMSO and NaCl had no inhibitory effect on either of the bacteria, and the MIC of ZOP-NPs-AgNPs against Escherichia coli and Staphylococcus aureus was 0.5000 mg/mL and 0.0310 mg/mL, respectively. The results showed that ZOP-NPs-AgNPs had a better antibacterial effect on Staphylococcus aureus. The reason for this phenomenon may be related to the differences in strains, which is consistent with the findings of Liu et al. [59]. Staphylococcus aureus is Gram-positive bacteria and Escherichia coli is a Gram-positive bacterium, and Escherichia coli is a Gram-negative bacterium. There were differences in AgNPs susceptibility between the two types of bacteria, which may be related to their cellular surface properties and interactions with AgNPs. Chung et al. [60] observed that the negative charge on the cell surface of Gram-negative bacteria was higher than that of Gram-positive bacteria. Therefore, the interaction between Gram-positive bacteria and silver nanoparticles is obviously stronger than that with Gram-negative bacteria, and these conclusions were also consistent with these experimental results. In addition, Gram-negative bacteria have an outer membrane made of lipid, protein, and LPS that serves as a barrier and effectively shields antibacterial medications. However, Gram-positive bacteria do not have an outer membrane as part of their cell wall [61,62].
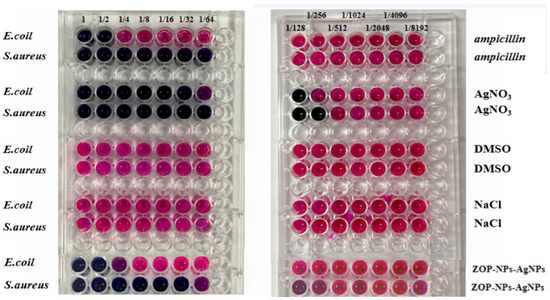
Figure 5.
The MIC of ZOP-NPs-AgNPs (E. coli: Escherichia coli, S. aureus: Staphylococcus aureus). Initial concentration is 1 mg/mL.
3.3.3. The MBC of ZOP-NPs-AgNPs
The MBC of ZOP-NPs-AgNPs against Escherichia coli and Staphylococcus aureus was determined according to MIC results. The lowest MBC of ZOP-NPs-AgNPs to the tested strain was 0.0310 mg/mL to Staphylococcus aureus, and no colonies grew in solid medium at this concentration, which indicated that ZOP-NPs-AgNPs had the strongest bactericidal effect on this strain. The MBC (0.5000 mg/mL) for Escherichia coli was higher than that for Staphylococcus aureus, and the experimental results were consistent with the MIC values.
To evaluate the antibacterial effect of silver nanoparticles synthesized from natural polysaccharides, El-Naggar et al. [63] produced silver nanoparticles by reducing AgNPs with Chlorella vulgaris soluble polysaccharides and found that the synthesized AgNPs solutions had an inhibitory effect on Bacillus, Erwinia, and Candida. It may be that AgNPs affect antibacterial potentiality by penetrating the cell wall of bacteria and causing damage through interactions with compounds containing phosphorus and sulfur, including DNA. Fang et al. [64] produced silver nanoparticles by reducing AgNPs with tea extract, and the synthesized silver nanoparticles exhibited 96.05% and 61.87% inhibition of pseudopeptalotiopsis theae (P. theae) and E. coli, respectively, indicating their good antibacterial effect. ZOP-NPs-AgNPs have a good antibacterial effect, and the main mechanism of action may be that AgNPs combine with the cell membrane and cell wall, thereby affecting the internal environment of bacteria [65] or damaging bacterial DNA and producing reactive oxygen species, thus killing bacteria. During the formation of AgNPs, some impurities in the polysaccharide were removed, which improved the polysaccharide’s reduction performance [66,67]. The ZOP-NPs-AgNPs prepared by the optimum technology have a higher silver chelating rate and better dispersion, enabling them to better combine with the surface of the bacterial cell membrane, thereby enhancing the antibacterial performance.
3.4. Immunomodulatory Activity of ZOP-NPs-AgNPs
Many studies have shown that plant polysaccharides have various biological activities. The immunological and antitumor activities of plant polysaccharides have been widely studied, and polysaccharides have also been proven to be one of the main active components of fungi. However, there are few reports on the immunological activity of plant polysaccharide silver nanoparticles. In this paper, the immunological activity of ZOP-NPs-AgNPs prepared under the optimal process was studied in vitro.
3.4.1. Cell Proliferation Activity
Macrophages can recognize pathogenic substances and remove them directly, which is a key component of the immunomodulatory system [68]. The CCK-8 method was used to study the effect of ZOP-NPs-AgNPs on the survival of RAW 264.7 cells. ZOP-NPs-AgNPs at concentrations of 10–250 μg/mL promoted the proliferation of RAW 264.7 cells without toxicity (Figure 6a). When the concentration of ZOP-NPs-AgNPs was 10–100 μg/mL, the proliferation of RAW 264.7 cells was better than that of the LPS positive control group, and there was a significant difference between ZOP-NPs-AgNPs and the blank control group (p < 0.01). When the concentration of ZOP-NPs-AgNPs was 250 and 500 μg/mL, there was no significant difference between the samples and the blank group (p > 0.05), and the cytotoxicity was lower when the concentration was 500 μg/mL. Within this concentration range, cell proliferation activity first increased and then decreased with increasing concentration, with the proliferation index reaching 1.45 at 50 μg/mL.
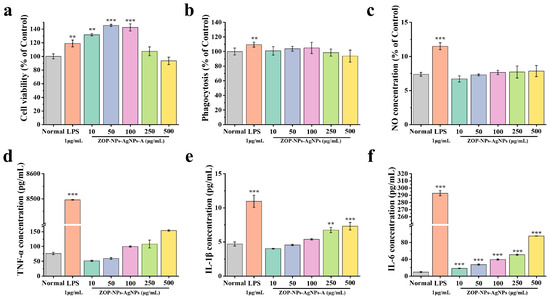
Figure 6.
(a) Cell proliferation activity. (b) Cell phagocytosis. (c) Volume of NO produced by cells. (d) TNF-α concentration. (e) IL-1β concentration. (f) IL-6 concentration. Statistical difference against the blank control group (** p < 0.01 and *** p < 0.001.).
3.4.2. Cell Phagocytic Activity
Studies have shown that macrophage phagocytosis is the first key step in the immune response. Under the induction of LPS, not only were the cell proliferation and phagocytosis abilities enhanced, but also the macrophages could release a large number of cytokines and inflammatory mediators. These mediators can resist the invasion of pathogenic microorganisms, but excessive secretion will cause an inflammatory reaction, so the LPS-induced macrophage activation model is used as an inflammatory model [69]. The neutral red uptake method was used to study the phagocytosis of RAW264.7 cells by ZOP-NPs-AgNPs. The effect of ZOP-NPs-AgNPs on phagocytic activity increased in a concentration-dependent manner in the range of 10–100 μg/mL, but the overall trend was not obvious (Figure 6b). The phagocytosis index decreased at 250 and 500 μg/mL concentrations with increasing concentration, which was lower than the normal level and showed inhibition. Compared with the blank group, there was no significant difference (p > 0.05), indicating that ZOP-NPs-AgNPs could not significantly enhance the phagocytic activity of RAW264.7 cells.
3.4.3. The Effect Test of NO Secretion by Macrophages
NO is one of the main reactive oxygen species (ROS) released by macrophages when attacking foreign bodies, which can effectively kill bacteria and play a key role in host defense [70]. In the test concentration range (Figure 6c), it can be seen that ZOP-NPs-AgNPs influenced the ability of macrophages to release NO, but there was no significant difference compared with the blank group (p > 0.05). The release of NO increased with increasing concentration and reached the highest level at 500 μg/mL, but there was still a big difference compared with the positive control group. At concentrations of 100–500 μg/mL, ZOP-NPs-AgNPs promoted the secretion of the cytotoxic molecule NO, which played an important role in apoptosis.
3.4.4. The Determination of the Cytokine Secretion Level
RAW264.7 cells are important participants in both nonspecific immunity and specific immune response and play an important role in the immunomodulatory activity [71]. They can initiate the innate immune response by recognizing infectious pathogens, thereby inhibiting the proliferation of various tumor cells and the invasion of microorganisms [72]. Once activated, macrophages can kill pathogens directly by phagocytosis and indirectly by secreting inflammatory mediators such as TNF-α, IL-1β, and IL-6 [73]. ZOP-NPs-AgNPs can promote the secretion of TNF-α, IL-1β, and IL-6 in macrophages at specific concentrations (Figure 6d–f). There was an extremely significant increase in the secretion of IL-1β and IL-6 at concentrations of 250 μg/mL and 500 μg/mL compared to the blank control (p < 0.01). The secretion of each cytokine increased in a concentration-dependent manner, and ZOP-NPs-AgNPs inhibited the secretion of IL-1β and TNF-α at low concentrations (10–100 μg/mL). When the concentration of ZOP-NPs-AgNPs was 500 μg/mL, the secretion concentrations of TNF-α, IL-1β, and IL-6 were 153.72, 7.29, and 95.41 pg/mL, respectively. However, the maximum production of cytokines was not higher in any of the three groups than in the LPS-positive control group. These results confirm that ZOP-NPs-AgNPs can promote the secretion of TNF-α, IL-1β, and IL-6 at specific concentrations and that ZOP-NPs-AgNPs have a better immune-enhancing activity at high concentrations.
This study showed that ZOP-NPs-AgNPs, as a kind of metal nanoparticles synthesized by a green pathway, not only possess the physical and chemical properties and antibacterial activity of silver nanoparticles but also acquire some immunomodulatory activities from ZOP. Their structure was characterized by UV-Vis, FT-IR, TEM, SEM, XRD, and TGA. It was found that the silver chelation rate of ZOP-NPs-AgNPs prepared by the optimum process was 92.17%, with a particle size of less than 20 nm, PDI of 0.299, and zeta potential of −32.83 mV. These characteristics are consistent with the physicochemical properties of nano-silver and indicate good thermal stability. In the antibacterial experiment, it was found that ZOP-NPs-AgNPs had certain antibacterial and bactericidal effects on Gram-negative bacteria and Gram-positive bacteria; among them, ZOP-NPs-AgNPs had the best antibacterial and bactericidal effect against Staphylococcus aureus, which may be because ginger and nano-silver have certain antibacterial and bactericidal effects. When synthesized into ZOP-NPs-AgNPs through a green method, they achieve a higher silver chelation rate, which enhances their biological activity. Furthermore, ZOP-NPs-AgNPs significantly enhanced the proliferation activity of RAW 264.7 cells and, to some extent, improved their phagocytic activity, thereby activating macrophages. They also demonstrated immunomodulatory capabilities by boosting the secretion of NO, TNF-α, IL-1β, and IL-6.
The present study explores the antibacterial and immunomodulatory activities of ZOP-NPs-AgNPs through in vitro experiments. However, the in vitro model is unable to fully replicate the complex physiological environment found in vivo, where cells are influenced by a multitude of factors, such as the circulatory and immune systems and intercellular interactions. Therefore, in vivo verification is a necessary extension as it can provide comprehensive and biologically relevant information, revealing the mechanisms of action, biodistribution, pharmacokinetic properties, and the safety profile of ZOP-NPs-AgNPs. In vivo experiments can further validate their antibacterial and immunomodulatory effects and explore their interactions with other biomolecules and cells, thereby compensating for the limitations of in vitro studies. Future work will focus on conducting in vivo experiments to provide accurate and comprehensive scientific evidence for the development and application of ZOP-NPs-AgNPs.
4. Conclusions
This study successfully optimized the synthesis process of ZOP-NPs-AgNPs using single-factor experiments and response surface methodology, and characterized the resulting nanoparticles. The optimized ZOP-NPs-AgNPs exhibited significantly stronger bacteriostatic and bactericidal efficacy against Staphylococcus aureus compared to Escherichia coli. Critically, this work demonstrates that ZOP serves as an effective, green, and operationally convenient agent for the preparation of AgNPs. Furthermore, the findings demonstrate that ZOP-NPs-AgNPs possess immunomodulatory activity, as evidenced by their ability to enhance the proliferation and phagocytic activity of RAW 264.7 macrophages and stimulate the secretion of immune mediators (NO, TNF-α, IL-1β, IL-6). While the precise mechanisms underlying this immunomodulatory activity remain to be fully elucidated, the optimized synthesis protocol established here and the demonstrated antibacterial and immunomodulatory properties of ZOP-NPs-AgNPs provide a foundation for further mechanistic studies and exploration of potential applications.
Author Contributions
Conceptualization, X.C. and H.X.; methodology, Y.J.; software, H.X. and K.Z.; validation, X.C., H.X. and M.L.; formal analysis, Y.J.; investigation, X.C.; resources, L.W.; data curation, M.L.; writing—original draft preparation, X.C. and H.X.; writing—review and editing, B.H. and Y.Z.; visualization, M.L.; supervision, K.Z.; project administration, B.H.; funding acquisition, Y.Z. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hebei Natural Science Foundation (No. H2024423098), the earmarked fund for Hebei Agriculture Research System (HARS) (HBCT2023080201, HBCT2023080205).
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Waiskopf, N.; Ben-Shahar, Y.; Galchenko, M.; Carmel, I.; Moshitzky, G.; Soreq, H.; Banin, U. Photocatalytic Reactive Oxygen Species Formation by Semiconductor–Metal Hybrid Nanoparticles. Toward Light-Induced Modulation of Biological Processes. Nano Lett. 2016, 16, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xuan, S.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Photothermal controlled antibacterial Ta4C3Tx-AgNPs/nanocellulose bioplastic food packaging. Food Chem. 2024, 448, 139126. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers 2024, 16, 941. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Shi, W.-P.; Zhou, Y.-Q.; Zhang, T.-D.; Lin, W.-J.; Guo, W.-H.; Zhou, R.-B.; Yin, D.-C. High-efficiency antibacterial calcium alginate/lysozyme/AgNPs composite sponge for wound healing. Int. J. Biol. Macromol. 2023, 256, 128370. [Google Scholar] [CrossRef]
- González-Aguiñaga, E.; Cardoso-Ávila, P.E.; Patakfalvi, R.; Pedro-Garcia, F. The morphology-dependent catalytic activity of anisotropic silver nanoparticles. Mater. Lett. 2023, 342, 134334. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef]
- Koesmawati, T.A.; Febrianti, F.; Halim, R.; Fitria, N.; Tanuwidjaja, S.; Rohman, O.; Syamsudin, A. Mercury Determination in Fish Using Cold Vapour-Atomic Absorption Spectrometry (CV-AAS) with Sodium Borohydride (NaBH4) as the Reductor. IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012026. [Google Scholar] [CrossRef]
- Sawant, P.; Chopade, S.; Shrotriya, S.; Patil, M.; Mulgund, S. Controlled Size Silver Nanoparticles Stabilised with Trisodium Citrate as Carriers for Econazole Nitrate: Synthesis, Characterization and Antifungal Activity. BioNanoScience 2025, 15, 265. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Bedlovicova, Z.; Strapac, I.; Balaz, M.; Salayova, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pelegrino, M.T.; Lima, B.d.A.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Mohammed, A.N. Resistance of bacterial pathogens to calcium hypochlorite disinfectant and evaluation of the usability of treated filter paper impregnated with nanosilver composite for drinking water purification. J. Glob. Antimicrob. Resist. 2019, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Ning, F.; Jiang, C.; Li, Y.; Peng, H.; Xiong, H. Development of antibacterial pectin from Akebia trifoliata var. australis waste for accelerated wound healing. Carbohydr. Polym. 2019, 217, 58–68. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Yaswant, G.; Kavitha, S.; Chandramohan, E.; Kowsalya, G.; Vijay, R.; Sudhagar, B.; Kumar, D.R.S. Preparation and characterization of hybrid chitosan-silver nanoparticles (Chi-Ag NPs); A potential antibacterial agent. Int. J. Biol. Macromol. 2019, 141, 290–298. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, W.; Xun, X.; Liu, M.; Han, X.; Xie, J.; Wang, M.; Zhang, Q.; Peng, Z.; Ao, H. Homogeneous silver nanoparticle loaded polydopamine/polyethyleneimine-coated bacterial cellulose nanofibers for wound dressing. Int. J. Biol. Macromol. 2023, 246, 125658. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, H.; Hu, N.; Zhang, B.; Qiu, Z.; Hu, J.; Zheng, G.; Zhang, L.; Xu, X. Construction of silver nanoparticles by the triple helical polysaccharide from black fungus and the antibacterial activities. Int. J. Biol. Macromol. 2021, 182, 1170–1178. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.d.L.Á.; Madla-Cruz, E.; Urrutia-Baca, V.H.; de la Garza-Ramos, M.A.; González-González, V.A.; Garza-Navarro, M.A. Influence of Polysaccharides’ Molecular Structure on the Antibacterial Activity and Cytotoxicity of Green Synthesized Composites Based on Silver Nanoparticles and Carboxymethyl-Cellulose. Nanomaterials 2020, 10, 1164. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Shi, M.; Yan, Y.; Liu, Y. An Insight into the Research Concerning Panax ginseng C.A. Meyer Polysaccharides: A Review. Food Rev. Int. 2020, 38, 1–17. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Cheng, H.-M.; Lo, H.-Y.; Li, C.-C.; Chou, P.-C.; Lee, Y.-C.; Ho, T.-Y. Ginger and Zingerone Ameliorate Lipopolysaccharide-Induced Acute Systemic Inflammation in Mice, Assessed by Nuclear Factor-κB Bioluminescent Imaging. J. Agric. Food Chem. 2015, 63, 6051–6058. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.R.; Mallikarjuna, K.; Kesireddy, N.; Reddy, K.S. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-T.; Yuan, B.; Wang, H.-X.; Qi, G.-H.; Cheng, S.-J. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Woźniak, Ł.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, W.; Ma, Y.; Zhang, Y.; Li, M.; Zheng, Y.; Zhang, D.; Wu, L. Structural Characterization, Antioxidant and Antibacterial Activities of a Novel Polysaccharide from Zingiber officinale and Its Application in Synthesis of Silver Nanoparticles. Front. Nutr. 2022, 9, 917094. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Heat-induced self-assembly of zein nanoparticles: Fabrication, stabilization and potential application as oral drug delivery. Food Hydrocoll. 2019, 90, 403–412. [Google Scholar] [CrossRef]
- Rangaraju, M.; Abewaa, M.; Hailemariam, E.; Abay, Y.; Prabhu, S.V.; Abdu, J.; Mengistu, A. Bacterial growth inhibition in spring water utilizing silver nanoparticles: Optimization using central composite design. Results Eng. 2024, 23, 102562. [Google Scholar] [CrossRef]
- Ghetas, H.A.; Abdel-Razek, N.; Shakweer, M.S.; Abotaleb, M.M.; Paray, B.A.; Ali, S.; Eldessouki, E.A.; Dawood, M.A.; Khalil, R.H. Antimicrobial activity of chemically and biologically synthesized silver nanoparticles against some fish pathogens. Saudi J. Biol. Sci. 2021, 29, 1298–1305. [Google Scholar] [CrossRef]
- Lian, Y.; Ma, N.; Cheng, Q.; Luo, M.; Xu, Z.; He, F.; Zhou, X.; Zhang, Y.; Jin, D.; Kong, Y.; et al. Fabrication of Nano-Silver Composite Using Amomum longiligulare Fruit Polysaccharides and Their Biological Activities. Int. J. Nanomed. 2025, 20, 1881–1898. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and an-timicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Jiang, H.; Zhang, S.; Bao, Y. Green Synthesis of Gold Nanoparticles from Polygahatous Polysaccharides and Their Anticancer Effect on Hepatic Carcinoma through Immunoregulation. ACS Omega 2024, 9, 21144–21151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Abdel-Rahman, R.M.; Hrdina, R.; Imramovský, A.; Burgert, L.; Aly, A. Antibacterial cotton fabrics treated with core–shell nanoparticles. Int. J. Biol. Macromol. 2012, 50, 1245–1253. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Abdel-Rahman, R.M.; Fouda, M.M.; Vojtova, L.; Uhrova, L.; Hassan, A.; Al-Deyab, S.S.; El-Shamy, I.E.; Jancar, J. Preparation, characterization and cytotoxicity of schizophyllan/silver nanoparticle composite. Carbohydr. Polym. 2014, 102, 238–245. [Google Scholar] [CrossRef]
- Yang, N.; Li, W.-H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind. Crop. Prod. 2013, 48, 81–88. [Google Scholar] [CrossRef]
- Wang, G.; Yang, X.; Chen, X.; Huang, J.; He, R.; Zhang, R.; Zhang, Y. Construction and antibacterial activities of walnut green husk polysaccharide based silver nanoparticles (AgNPs). Int. J. Biol. Macromol. 2024, 276, 133798. [Google Scholar] [CrossRef]
- Zhu, Y.; Pang, X.; Zhang, W.; Zhang, C.; Zhang, B.; Fu, J.; Zhao, H.; Han, W. Green synthesis of silver nanoparticles using persimmon polysaccharides for enhanced polysaccharide-based film performance. Food Res. Int. 2025, 209, 116252. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; Khojah, E.; Darwish, M.S.; Elsherbiny, E.A.; Elawady, A.A.; Dawood, D.H. Biosynthesis of Silver Nanoparticles by Polysaccharide of Leucaena leucocephala Seeds and Their Anticancer, Antifungal Properties and as Preservative of Composite Milk Sample. J. Nanomater. 2022, 2022, 7490221. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Qu, D.; Ma, Y.; Liu, C.; Zhou, J. Enhanced stability and antibacterial efficacy of a traditional Chinese medicine-mediated silver nanoparticle delivery system. Int. J. Nanomed. 2014, 9, 5491–5502. [Google Scholar] [CrossRef] [PubMed]
- Devasvaran, K.; Alallam, B.; Lee, C.; Yong, Y.K.; Lim, V. Clinacanthus nutans crude polysaccharide extract as a green platform for microwave-assisted synthesis of silver nanoparticles: Optimization, characterization, and evaluation of bioactivities. Int. J. Biol. Macromol. 2024, 278, 134893. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Zhang, P.; Li, C.; Chen, J. Polysaccharide isolated from Rosa roxburghii Tratt fruit as a stabilizing and reducing agent for the synthesis of silver nanoparticles: Antibacterial and preservative properties. J. Food Meas. Charact. 2022, 16, 1241–1251. [Google Scholar] [CrossRef]
- Cai, Z.; Dai, Q.; Guo, Y.; Wei, Y.; Wu, M.; Zhang, H. Glycyrrhiza polysaccharide-mediated synthesis of silver nanoparticles and their use for the preparation of nanocomposite curdlan antibacterial film. Int. J. Biol. Macromol. 2019, 141, 422–430. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Liu, Y.; Zheng, X.; Tang, K. Green synthesis of silver nanoparticles using soluble soybean polysaccharide and their application in antibacterial coatings. Int. J. Biol. Macromol. 2021, 166, 567–577. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef]
- Jian, W.; Ma, Y.; Wu, H.; Zhu, X.; Wang, J.; Xiong, H.; Lin, L.; Wu, L. Fabrication of highly stable silver nanoparticles using polysaccharide-protein complexes from abalone viscera and antibacterial activity evaluation. Int. J. Biol. Macromol. 2019, 128, 839–847. [Google Scholar] [CrossRef]
- Mehwish, H.M.; Liu, G.; Rajoka, M.S.R.; Cai, H.; Zhong, J.; Song, X.; Xia, L.; Wang, M.; Aadil, R.M.; Inam-Ur-Raheem, M.; et al. Therapeutic potential of Moringa oleifera seed polysaccharide embedded silver nanoparticles in wound healing. Int. J. Biol. Macromol. 2021, 184, 144–158. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V.I.; Pivovarov, A.; Makarshenko, N.P. Green Synthesis of Silver Nanoparticles in the Presence of Polysaccharide: Optimization and Characterization. J. Nanomater. 2020, 2020, 3051308. [Google Scholar] [CrossRef]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.; Khalil, M.I. Synthesis of Silver Nanoparticles Using a Novel Cyanobacteria Desertifilum sp. extract: Their Antibacterial and Cytotoxicity Effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of Gold and Silver Nanoparticles Using Emblica Officinalis Fruit Extract, Their Phase Transfer and Transmetallation in an Organic Solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Padinjarathil, H.; Joseph, M.M.; Unnikrishnan, B.; Preethi, G.; Shiji, R.; Archana, M.; Maya, S.; Syama, H.; Sreelekha, T. Galactomannan endowed biogenic silver nanoparticles exposed enhanced cancer cytotoxicity with excellent biocompatibility. Int. J. Biol. Macromol. 2018, 118, 1174–1182. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, X.; Juan, L.; Huang, L.; Li, Y.; Luo, Y.; Huang, L.; Jin, W. Comparative catalytic and bacteriostatic properties of silver nanoparticles biosynthesized using three kinds of polysaccharide. AIP Adv. 2018, 8, 065222. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Kchaou, H.; Acosta, N.; Heras, Á.; Nasri, M. Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr. Polym. 2020, 236, 116063. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhang, X.; Li, F.; Tian, Y.; Du, M.; Zhao, H.; Shao, L.; Tian, Q. Antibacterial activity of Osmunda japonica (Thunb) polysaccharides and its effect on tomato quality maintenance during storage. Int. J. Food Sci. Technol. 2020, 55, 2851–2862. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Su, Y.-P.; Chen, C.-C.; Jia, G.; Wang, H.-L.; Wu, J.C.G.; Lin, J.-G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Jiji, S.; Udhayakumar, S.; Maharajan, K.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr. Polym. 2020, 245, 116573. [Google Scholar] [CrossRef] [PubMed]
- Güneş, Ç.C.; Dündar, M.A.; Demirel, K.M.; Avcı, A. Enhancement of PCL/PLA Electrospun Nanocomposite Fibers Comprising Silver Nanoparticles Encapsulated with Thymus Vulgaris L. Molecules for Antibacterial and Anticancer Activities. ACS Biomater. Sci. Eng. 2022, 8, 3717–3732. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hong, C.; Chen, F.; Gui, F.; You, Y.; Guan, X.; Pan, X. Green synthesis of nano silver by tea extract with high anti-microbial activity. Inorg. Chem. Commun. 2021, 132, 108808. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Gopinath, K.; Gowri, S.; Arumugam, A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J. Nanostruct. Chem. 2013, 3, 68. [Google Scholar] [CrossRef]
- Islam, S.U.; Butola, B.; Kumar, A. Green chemistry based in-situ synthesis of silver nanoparticles for multifunctional finishing of chitosan polysaccharide modified cellulosic textile substrate. Int. J. Biol. Macromol. 2020, 152, 1135–1145. [Google Scholar] [CrossRef]
- Chernikov, O.V.; Chiu, H.-W.; Li, L.-H.; Kokoulin, M.S.; Molchanova, V.I.; Hsu, H.-T.; Ho, C.-L.; Hua, K.-F. Immunomodulatory Properties of Polysaccharides from the Coral Pseudopterogorgia americana in Macrophages. Cells 2021, 10, 3531. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, C.; Chen, X.; He, K.; Hu, Y. Protective effects of sinomenine against LPS-induced inflammation in piglets. Microb. Pathog. 2017, 110, 573–577. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Li, P.; Zhang, H.; Cheng, W.; Zhang, Y.; Zheng, Y.; Wu, L.; Zhang, D. Optimization of technology, structural characterization, derivatization, antioxidant and immunomodulatory activities of Salvia miltiorrhiza polysaccharides. J. Food Meas. Charact. 2022, 16, 1754–1767. [Google Scholar] [CrossRef]
- Ren, D.; Lin, D.; Alim, A.; Zheng, Q.; Yang, X. Chemical characterization of a novel polysaccharide ASKP-1 from Artemisia sphaerocephala Krasch seed and its macrophage activation via MAPK, PI3k/Akt and NF-κB signaling pathways in RAW264.7 cells. Food Funct. 2017, 8, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mohamed, H.T.; El-Husseiny, N.; Mahdy, M.M.E.; Safwat, G.; Diab, A.A.; El-Sherif, A.A.; El-Shinawi, M.; Mo-hamed, M.M. IL-8 secreted by tumor associated macrophages contribute to lapatinib resistance in HER2-positive locally ad-vanced breast cancer via activation of Src/STAT3/ERK1/2-mediated EGFR signaling. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118995. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Dayo, M.; Alahmadi, S.; Ali, S. Anti-Inflammatory and Antimicrobial Activity of Silver Nanoparticles Green-Synthesized Using Extracts of Different Plants. Nanomaterials 2024, 14, 1383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).