Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen
Abstract
1. Introduction
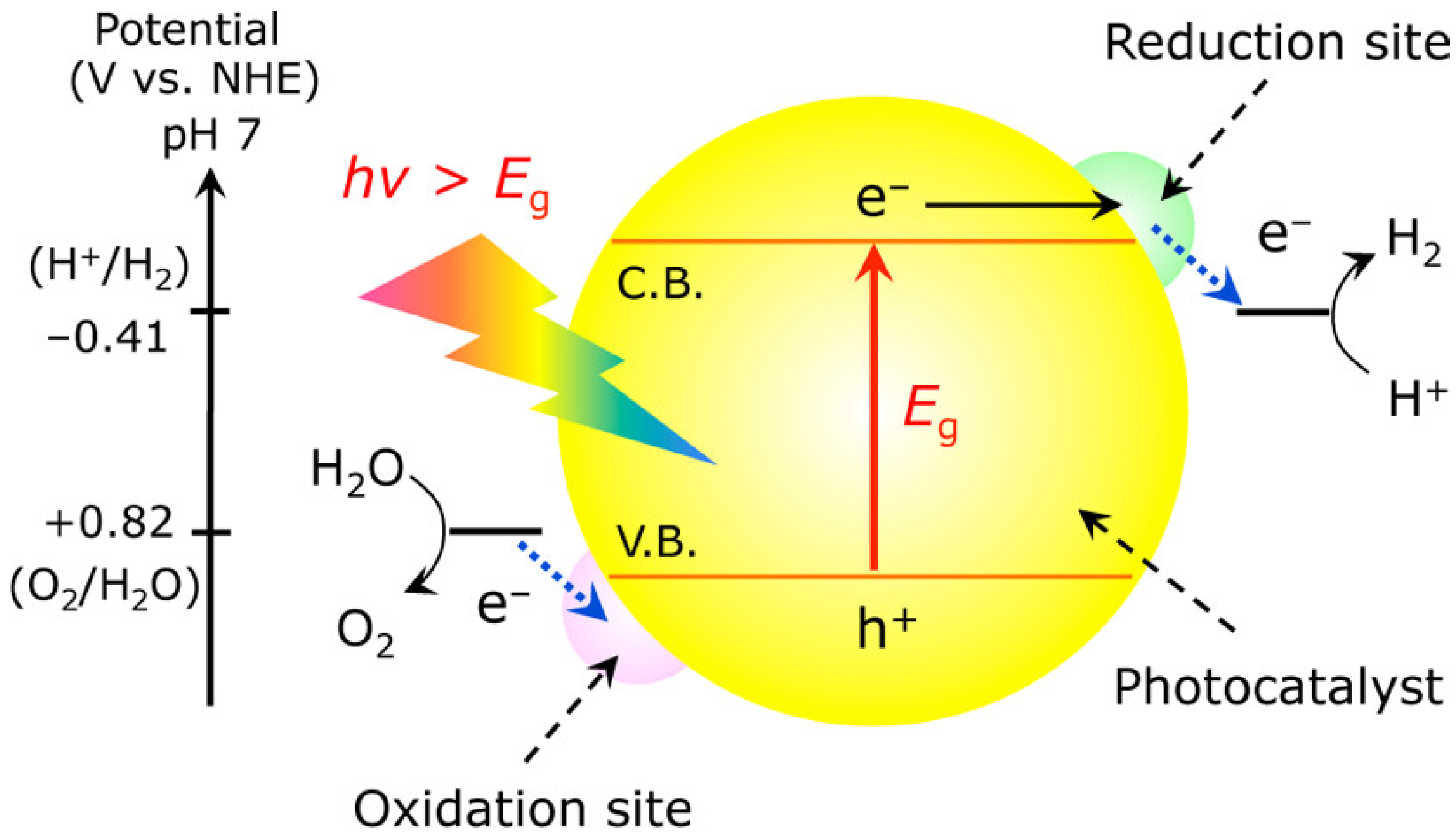
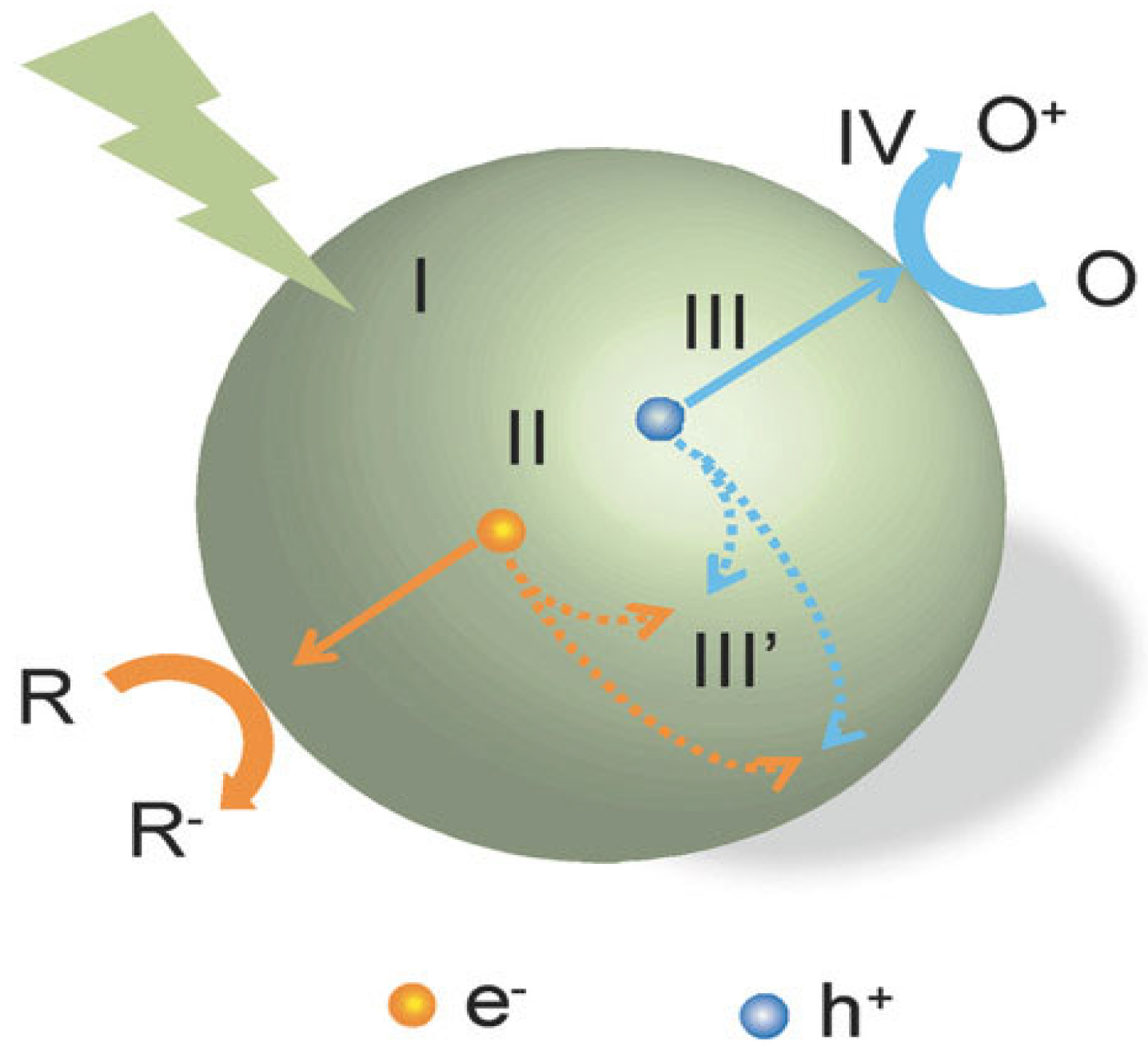
2. Photochemical Reaction
3. Basics of Photochemical Water Splitting
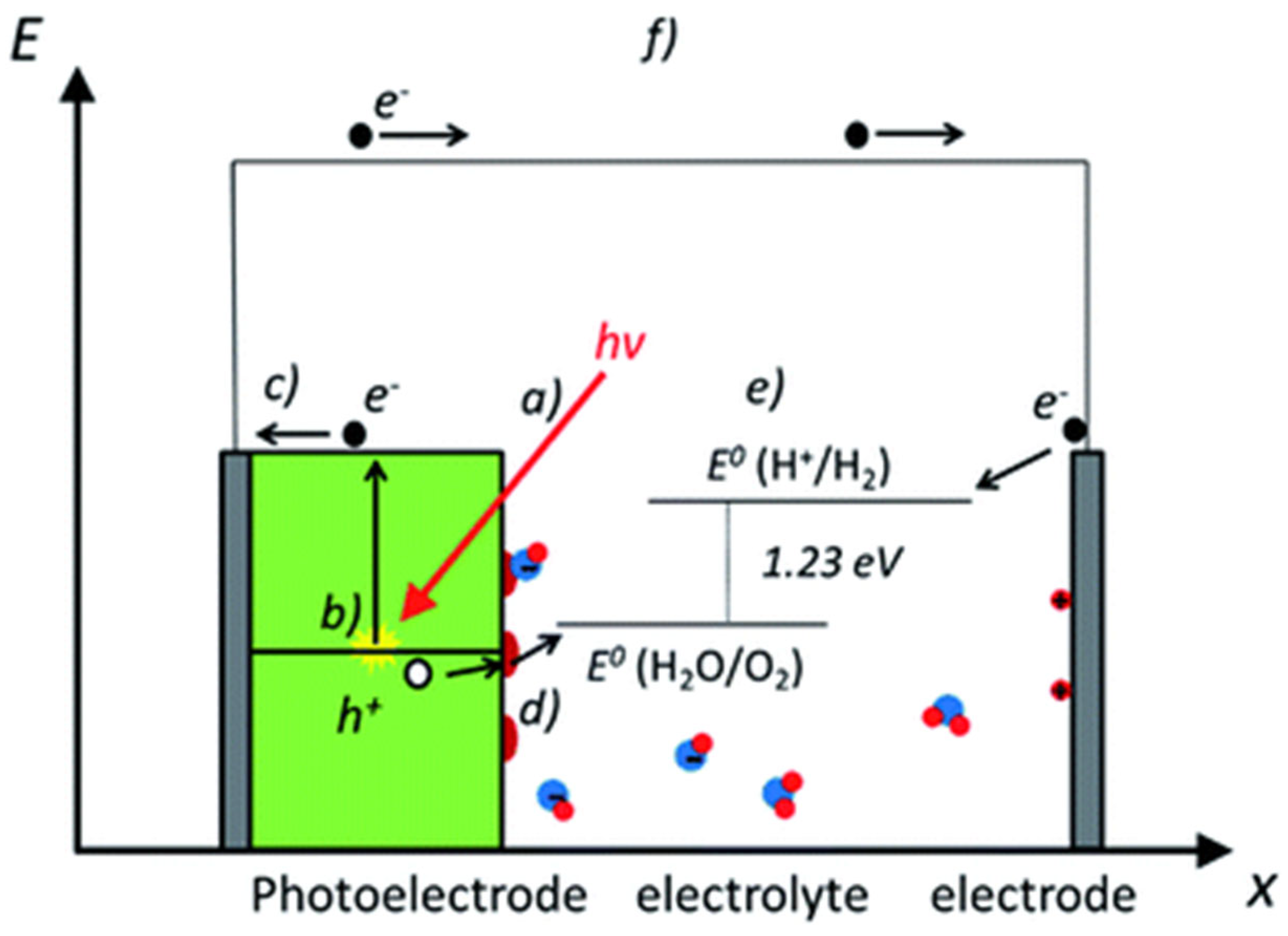
4. Photoelectrochemical Reaction
5. The Basic Concept Involves in PEC Water Splitting
6. Factors Affecting the Photochemical (PC) and Photoelectrochemical (PEC) Performance
6.1. Crystalline Structure and Morphology
6.2. Bandgap
6.3. The Dependence on Temperature and Pressure
6.4. Effect of pH
6.5. Limitations of Photochemical vs. Photoelectrochemical Approaches
7. Titanium Dioxide (TiO2)
Role of TiO2 in HER
8. TiO2 Modification Strategies
8.1. Doping of Metal and Non-Metal
8.2. Creation of Heterojunction
8.2.1. Type I Heterojunction
8.2.2. Type II Heterojunction
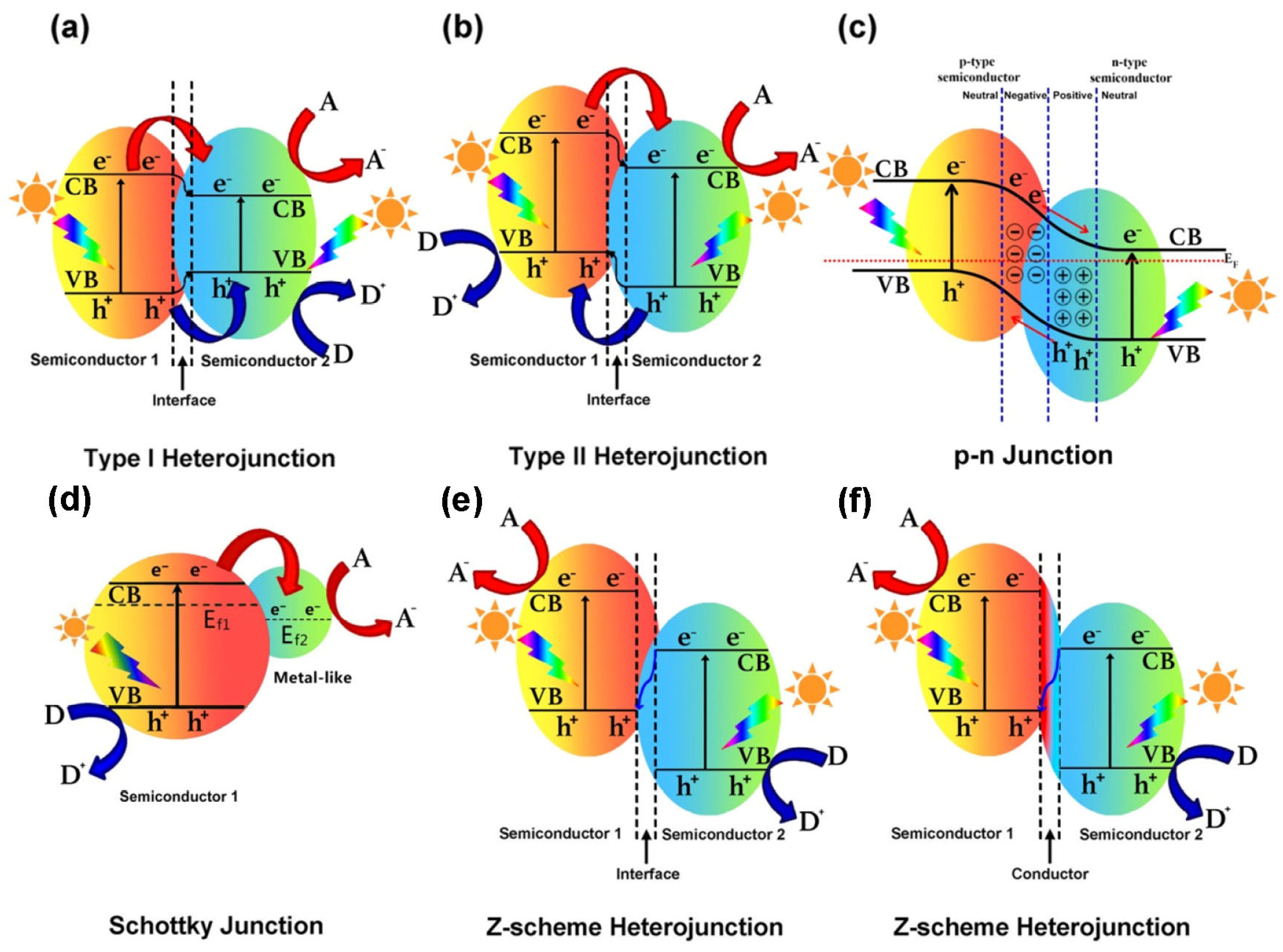
8.2.3. p-n Heterojunction
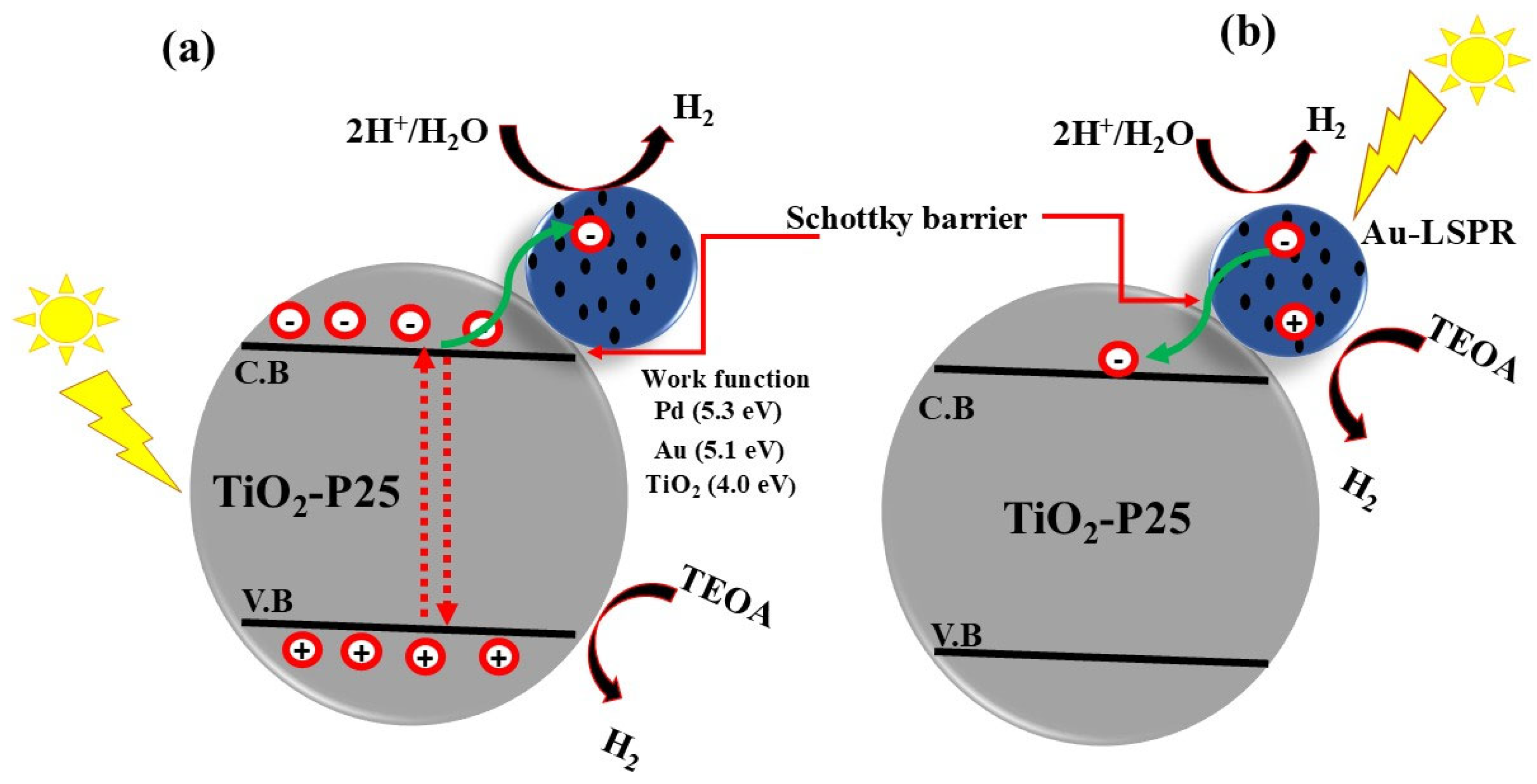
8.2.4. Schottky Junction
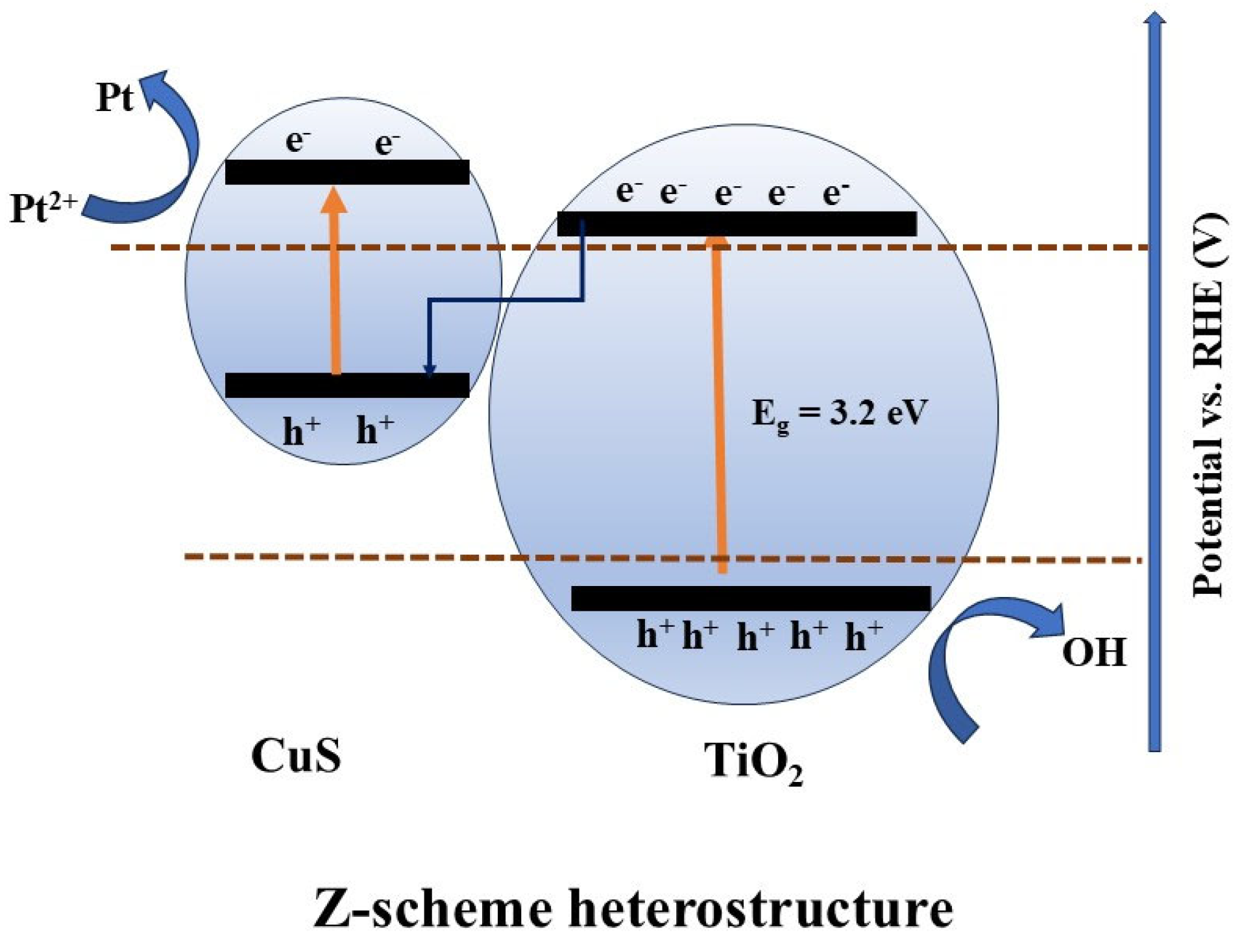
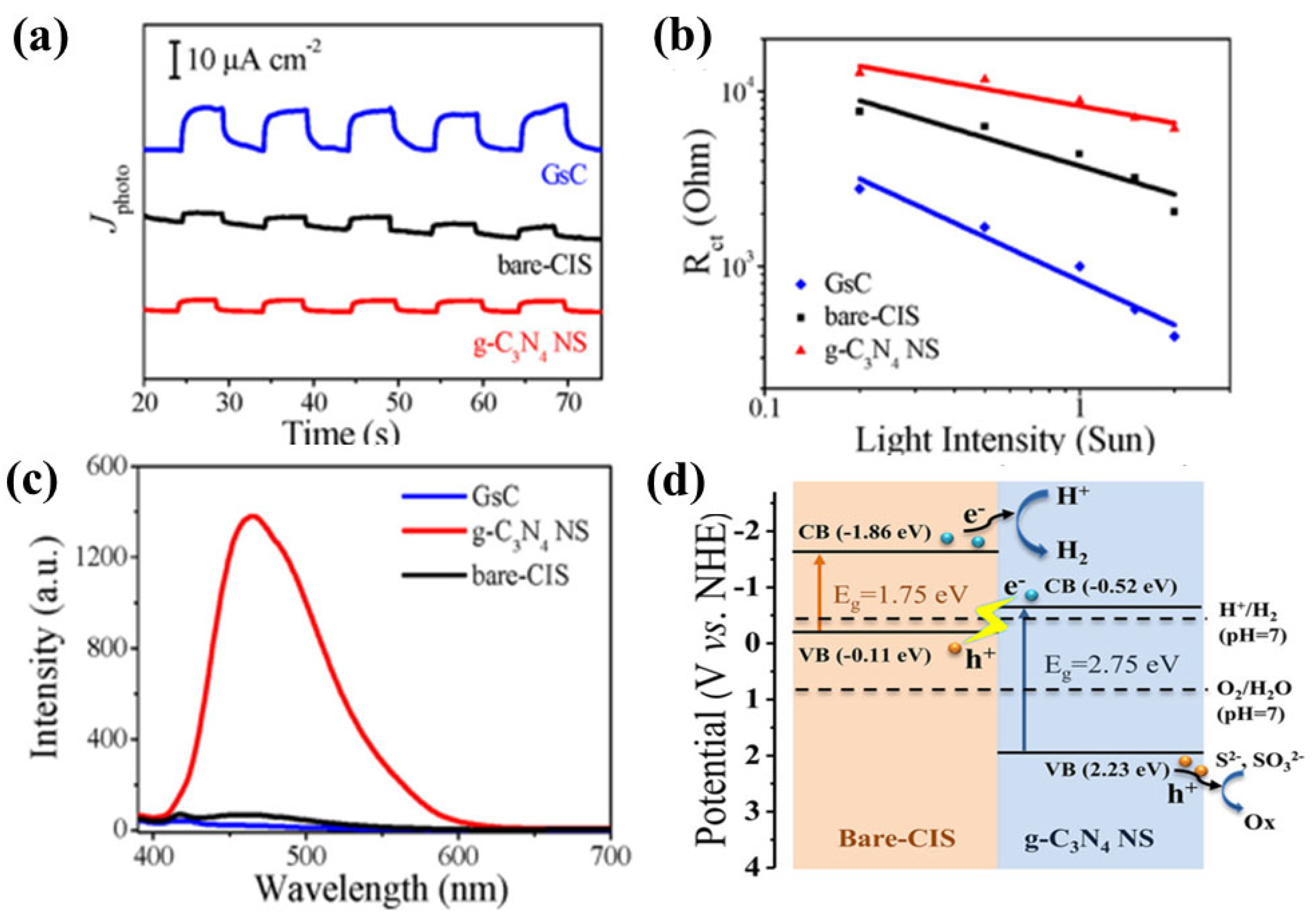
8.2.5. Z-Scheme Heterojunction
8.3. Inorganic Acid Modification of TiO2
8.4. Modification of TiO2 by Creating Oxygen Vacancies
9. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef]
- 2020 World Population Data Sheet; Population Reference Bureau: Washington, DC, USA, 2020; p. 4.
- Reddy, C.V.; Reddy, K.R.; Shetti, N.P.; Shim, J.; Aminabhavi, T.M.; Dionysiou, D.D. Hetero-nanostructured metal oxide-based hybrid photocatalysts for enhanced photoelectrochemical water splitting—A review. Int. J. Hydrogen Energy 2020, 45, 18331–18347. [Google Scholar] [CrossRef]
- Nisar, M.; Bernard, F.L.; Duarte, E.; Chaban, V.; Einloft, S. New polysulfone microcapsules containing metal oxides and ([BMIM][NTf2]) ionic liquid for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 104781. [Google Scholar] [CrossRef]
- Nisar, M.; Thue, P.S.; Maghous, M.B.; Geshev, J.; Lima, E.C.; Einloft, S. Polysulfone metal-activated carbon magnetic nanocomposites with enhanced CO2capture. RSC Adv. 2020, 10, 34595–34604. [Google Scholar] [CrossRef]
- Lewis, E.; Chamel, O.; Mohsenin, M.; Ots, E.; White, E.T. Intergovernmental Panel on Climate Change. In Sustainaspeak, 1st ed.; Routledge: New York, NY, USA, 2018; pp. 153–154. [Google Scholar] [CrossRef]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-Driven Sustainable Hydrogen Production Utilizing TiO2 Nanostructures: A Review. Small Methods 2018, 3, 1800184. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, S.-Y.; Li, Y.-R. Advances in solar hydrogen production via two-step water-splitting thermochemical cycles based on metal redox reactions. Renew. Energy 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Nocera, D.G. The Artificial Leaf. Acc. Chem. Res. 2012, 45, 767–776. [Google Scholar] [CrossRef]
- Wang, M.; Han, K.; Zhang, S.; Sun, L. Integration of organometallic complexes with semiconductors and other nanomaterials for photocatalytic H2 production. Co-ord. Chem. Rev. 2015, 287, 1–14. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar thermochemical production of hydrogen—A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Graf, D.; Monnerie, N.; Roeb, M.; Schmitz, M.; Sattler, C. Economic comparison of solar hydrogen generation by means of thermochemical cycles and electrolysis. Int. J. Hydrogen Energy 2008, 33, 4511–4519. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. Process. Intensif. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Balayeva, N.O. Mamiyev, Chapter 5—Integrated processes involving adsorption, photolysis, and photocatalysis. In Hybrid and Combined Processes for Air Pollution Control: Methodologies, Mechanisms and Effect of Key Parameters; Assadi, A., Amrane, A., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–153. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Solid Solution of GaN and ZnO as a Stable Photocatalyst for Overall Water Splitting under Visible Light. Chem. Mater. 2010, 22, 612–623. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Yang, H.; Chen, F.; Wang, X.; Qian, J.; Wang, J.; Li, J.; Lv, C.; Li, L.; Bandaru, S.; Gao, J. Self-Reconstructed Spinel with Enhanced SO42− Adsorption and Highly Exposed Co3+ From Heterostructure Boosts Activity and Stability at High Current Density for Overall Water Splitting. Adv. Funct. Mater. 2025, 35, 2419978. [Google Scholar] [CrossRef]
- Wang, J.-L.; Wang, C.; Lin, W. Metal–Organic Frameworks for Light Harvesting and Photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic hydrogen production from water using porous material [Ru2(p-BDC)2]n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; Dekrafft, K.E.; Lin, W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Maeda, K. Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, H.; Jia, Z.; Cao, J.; John, R. Photoelectrochemical behaviour of methanol oxidation at nanoporous TiO2 film electrodes. J. Photochem. Photobiol. A Chem. 2001, 144, 197–204. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Minggu, L.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Josny, J.; Jinu, M.; Soney, C.G. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Wilke, T.; Schricker, D.; Rolf, J.; Kleinermanns, K. Solar Water Splitting by Semiconductor Nanocomposites and Hydrogen Storage with Quinoid Systems. Open J. Phys. Chem. 2012, 2, 195–203. [Google Scholar] [CrossRef]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical Water Splitting Using Dense and Aligned TiO2 Nanorod Arrays. Small 2009, 5, 104–111. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Meshram, S.; Adhyapak, P.; Mulik, U.; Amalnerkar, D. Facile synthesis of CuO nanomorphs and their morphology dependent sunlight driven photocatalytic properties. Chem. Eng. J. 2012, 204–206, 158–168. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Optimization of photoelectrochemical water splitting performance on hierarchical TiO2 nanotube arrays. Energy Environ. Sci. 2012, 5, 6506–6512. [Google Scholar] [CrossRef]
- Liu, N.; Albu, S.P.; Lee, K.; So, S.; Schmuki, P. Water annealing and other low temperature treatments of anodic TiO2 nanotubes: A comparison of properties and efficiencies in dye sensitized solar cells and for water splitting. Electrochim. Acta 2012, 82, 98–102. [Google Scholar] [CrossRef]
- Yin, W.-J.; Tang, H.; Wei, S.-H.; Al-Jassim, M.M.; Turner, J.; Yan, Y. Band structure engineering of semiconductors for enhanced photoelectrochemical water splitting: The case ofTiO. Phys. Rev. B Condens. Matter Mater Phys 2010, 82. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band Structure Tuning of TiO2 for Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. C 2014, 118, 7451–7457. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.; Srivastava, O. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrogen Energy 2017, 42, 22677–22686. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Lopes, T.; Andrade, L.; Mendes, A. Temperature effect on water splitting using a Si-doped hematite photoanode. J. Power Sources 2014, 272, 567–580. [Google Scholar] [CrossRef]
- Nie, Q.; Yang, L.; Cao, C.; Zeng, Y.; Wang, G.; Wang, C.; Lin, S. Interface optimization of ZnO nanorod/CdS quantum dots heterostructure by a facile two-step low-temperature thermal treatment for improved photoelectrochemical water splitting. Chem. Eng. J. 2017, 325, 151–159. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Al-Agel, F.A.; Suleiman, J.; Khan, S.A. Studies on silicon quantum dots prepared at different working pressure. Results Phys. 2017, 7, 1128–1134. [Google Scholar] [CrossRef]
- Nada, A.; Hamed, H.; Barakat, M.; Mohamed, N.; Veziroglu, T. Enhancement of photocatalytic hydrogen production rate using photosensitized TiO2/RuO2-MV2+. Int. J. Hydrogen Energy 2008, 33, 3264–3269. [Google Scholar] [CrossRef]
- Fekete, M.; Riedel, W.; Patti, A.F.; Spiccia, L. Photoelectrochemical water oxidation by screen printed ZnO nanoparticle films: Effect of pH on catalytic activity and stability. Nanoscale 2014, 6, 7585–7593. [Google Scholar] [CrossRef]
- Yanagi, R.; Zhao, T.; Solanki, D.; Pan, Z.; Hu, S. Charge Separation in Photocatalysts: Mechanisms, Physical Parameters, and Design Principles. ACS Energy Lett. 2022, 7, 432–452. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Y.; Yue, T.; Lu, Y. Improving built-in electric fields for effective photocatalytic activity in the rationally designed electron transfer pathway of TiO2@MoS2/Bi2S. J. Mater. Chem. A 2024, 12, 10838–10851. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Khan, N.; Stelo, F.; Santos, G.H.; Rossi, L.M.; Gonçalves, R.V.; Wender, H. Recent advances on Z-scheme engineered BiVO4-based semiconductor photocatalysts for CO2 reduction: A review. Appl. Surf. Sci. Adv. 2022, 11, 100289. [Google Scholar] [CrossRef]
- Khan, N.; Koche, A.; Khan, W.; Nogueira, P.A.; Jamil, A.; Pieper, Y.; Toma, F.M.; Nascimento, O.R.; Gonçalves, R.V.; Khan, S. Optimizing Photocatalytic Water Oxidation by FeMnOx Bimetallic Cocatalyst on Zn-Doped BiVO4 via Magnetron Sputter Deposition. Appl. Catal. A Gen. 2025, 704, 120389. [Google Scholar] [CrossRef]
- Khan, N.; Koche, A.; Centurion, H.A.; Rabelo, L.; Bettini, J.; dos Santos, G.T.; Souza, F.L.; Gonçalves, R.V.; Khan, S. Triggering Synergy between p-Type Sputter-Deposited FeMnOx or FeNiOx and W-Doped BiVO4 for Enhanced Oxygen Evolution. ACS Appl. Energy Mater. 2024, 7, 2129–2141. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, selectivity, and stability of earth-abundant CuO/Cu2O/Cu0-based photocatalysts toward CO2 reduction. Chem. Eng. J. 2022, 429. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Isimjan, T.T.; Rohani, S.; Ray, A.K. Photoelectrochemical water splitting for hydrogen generation on highly ordered TiO2 nanotubes fabricated by using Ti as cathode. Int. J. Hydrogen Energy 2012, 37, 103–108. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef]
- Ismail, A.A. Mesoporous PdO–TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B. Environ. 2012, 117–118, 67–72. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Uyar, T.; Ramakrishna, S. Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 2960–2986. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Lu, J.; Wu, X.; Chu, W. Silver sulfide nanoparticles sensitized titanium dioxide nanotube arrays synthesized by in situ sulfurization for photocatalytic hydrogen production. J. Colloid Interface Sci. 2014, 413, 17–23. [Google Scholar] [CrossRef]
- Lalitha, K.; Reddy, J.K.; Sharma, M.V.P.; Kumari, V.D.; Subrahmanyam, M. Continuous hydrogen production activity over finely dispersed Ag2O/TiO2 catalysts from methanol:water mixtures under solar irradiation: A structure–activity correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Wu, N.-L.; Lee, M.-S. Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int. J. Hydrogen Energy 2004, 29, 1601–1605. [Google Scholar] [CrossRef]
- Zhu, Z.; Kao, C.-T.; Tang, B.-H.; Chang, W.-C.; Wu, R.-J. Efficient hydrogen production by photocatalytic water-splitting using Pt-doped TiO2 hollow spheres under visible light. Ceram. Int. 2016, 42, 6749–6754. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Zhou, Z.; Jiang, Y.; Hu, Q.; Xue, C.; Guo, W. Efficient photocatalytic hydrogen production over Rh and Nb codoped TiO2 nanorods. Chem. Eng. J. 2018, 337, 282–289. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Hou, X.; Wang, C.-W.; Zhu, W.-D.; Wang, X.-Q.; Li, Y.; Wang, J.; Chen, J.-B.; Gan, T.; Hu, H.-Y.; Zhou, F. Preparation of nitrogen-doped anatase TiO2 nanoworm/nanotube hierarchical structures and its photocatalytic effect. Solid State Sci. 2014, 29, 27–33. [Google Scholar] [CrossRef]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of S-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A Chem. 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Luo, H.; Takata, T.; Lee, Y.; Zhao, J.; Domen, K.; Yan, Y. Photocatalytic Activity Enhancing for Titanium Dioxide by Co-doping with Bromine and Chlorine. Chem. Mater. 2004, 16, 846–849. [Google Scholar] [CrossRef]
- Khan, S.; Ruwer, T.L.; Khan, N.; Köche, A.; Lodge, R.W.; Coelho-Júnior, H.; Sommer, R.L.; Santos, M.J.L.; Malfatti, C.F.; Bergmann, C.P.; et al. Revealing the true impact of interstitial and substitutional nitrogen doping in TiO2 on photoelectrochemical applications. J. Mater. Chem. A 2021, 9, 12214–12224. [Google Scholar] [CrossRef]
- Fazil, M.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Enhanced Photo/Electrocatalytic Hydrogen Evolution by Hydrothermally Derived Cu-Doped TiO2 Solid Solution Nanostructures. Langmuir 2024, 40, 4063–4076. [Google Scholar] [CrossRef]
- Thabet, S.M.; Abdelhamid, H.N.; Ibrahim, S.A.; El-Bery, H.M. Boosting photocatalytic water splitting of TiO2 using metal (Ru, Co, or Ni) co-catalysts for hydrogen generation. Sci. Rep. 2024, 14, 1–10. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Mani, S.S.; Rajendran, S.; Mathew, T.; Gopinath, C.S. A review on the recent advances in the design and structure–activity relationship of TiO2-based photocatalysts for solar hydrogen production. Energy Adv. 2024, 3, 1472–1504. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef]
- Raziq, F.; Qu, Y.; Zhang, X.; Humayun, M.; Wu, J.; Zada, A.; Yu, H.; Sun, X.; Jing, L. Enhanced Cocatalyst-Free Visible-Light Activities for Photocatalytic Fuel Production of g-C3N4 by Trapping Holes and Transferring Electrons. J. Phys. Chem. C 2015, 120, 98–107. [Google Scholar] [CrossRef]
- Ghasemi, S.; Esfandiar, A.; Setayesh, S.R.; Habibi-Yangjeh, A.; Gholami, M.R. Synthesis and characterization of TiO2–graphene nanocomposites modified with noble metals as a photocatalyst for degradation of pollutants. Appl. Catal. A Gen. 2013, 462–463, 82–90. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Kim, W.J.; Jang, E.; Park, T.J. Enhanced visible-light photocatalytic activity of ZnS/g-C3N4 type-II heterojunction nanocomposites synthesized with atomic layer deposition. Appl. Surf. Sci. 2017, 419, 159–164. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wang, H.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, W.; Zeng, G. Facile synthesis of Sb2S3/ultrathin g-C3N4 sheets heterostructures embedded with g-C3N4 quantum dots with enhanced NIR-light photocatalytic performance. Appl. Catal. B Environ. 2016, 193, 36–46. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.; Xing, C.; Zhang, Z.; Meng, S.; Chen, M. Two-Dimensional CaIn2S4/g-C3N4 Heterojunction Nanocomposite with Enhanced Visible-Light Photocatalytic Activities: Interfacial Engineering and Mechanism Insight. ACS Appl. Mater. Interfaces 2015, 7, 19234–19242. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.-Y.; Huang, T.; Liu, X.-H.; Yang, X.-J. Novel mesoporous P-doped graphitic carbon nitride nanosheets coupled with ZnIn2S4nanosheets as efficient visible light driven heterostructures with remarkably enhanced photo-reduction activity. Nanoscale 2016, 8, 3711–3719. [Google Scholar] [CrossRef]
- Chen, W.; Hua, Y.-X.; Wang, Y.; Huang, T.; Liu, T.-Y.; Liu, X.-H. Two-dimensional mesoporous g-C3N4 nanosheet-supported MgIn2S4 nanoplates as visible-light-active heterostructures for enhanced photocatalytic activity. J. Catal. 2017, 349, 8–18. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, D.; Ong, W.-J. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: A review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. Facile fabrication of a CoO/g-C3N4p–n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017, 7, 3325–3331. [Google Scholar] [CrossRef]
- Chu, J.; Han, X.; Yu, Z.; Du, Y.; Song, B.; Xu, P. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production on CdS/Cu7S4/g-C3N4 Ternary Heterostructures. ACS Appl. Mater. Interfaces 2018, 10, 20404–20411. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, G.; Zhou, W.; Liu, Y.; Pang, H.; Zhang, H.; Hao, D.; Meng, X.; Li, P.; Kako, T.; et al. In Situ Bond Modulation of Graphitic Carbon Nitride to Construct p–n Homojunctions for Enhanced Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2016, 26, 6822–6829. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, L.; Zhu, J.; Chen, M.; Shi, W.; Xie, J. Novel p–n heterojunction photocatalyst constructed by porous graphite-like C3N4 and nanostructured BiOI: Facile synthesis and enhanced photocatalytic activity. Dalton Trans. 2013, 42, 15726–15734. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, M.; Li, R.; Chen, Y. Novel Cu3P/g-C3N4 p-n heterojunction photocatalysts for solar hydrogen generation. Sci. China Mater. 2018, 61, 861–868. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.-J.; Ong, W.-J.; Lee, H.W.; Chang, W.S.; Chai, S.-P. Engineering nanoscale p–n junction: Via the synergetic dual-doping of p-type boron-doped graphene hybridized with n-type oxygen-doped carbon nitride for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 3181–3194. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Guo, E.; Yin, L. Tailored SrTiO3/TiO2 heterostructures for dye-sensitized solar cells with enhanced photoelectric conversion performance. J. Mater. Chem. A 2015, 3, 13390–13401. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Fan, Y.; Yu, Y.; Li, Q.; Zhou, J.; Gu, H.; Shi, K.; Jiang, B. P-N Heterojunction Embedded CuS/TiO2 Bifunctional Photocatalyst for Synchronous Hydrogen Production and Benzylamine Conversion. Small 2024, 20, e2306344. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Zhou, D.; Qin, X.; Yan, W. The formation of S-scheme anatase/rutile TiO2 heterojunction and the enhancement of solar-to-hydrogen efficiency driven by dual electric field synergy. Fuel 2025, 388. [Google Scholar] [CrossRef]
- Hong, J.W.; Wi, D.H.; Lee, S.-U.; Han, S.W. Metal–Semiconductor Heteronanocrystals with Desired Configurations for Plasmonic Photocatalysis. J. Am. Chem. Soc. 2016, 138, 15766–15773. [Google Scholar] [CrossRef]
- Qiu, P.; Zhang, Y.; Cheng, G. Precursor self-derived Cu@TiO2 hybrid Schottky junction for enhanced solar-to-hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 10628–10637. [Google Scholar] [CrossRef]
- Méndez-Medrano, A.A.; Bahena-Uribe, D.; Dragoe, D.; Clavaguéra, C.; Colbeau-Justin, C.; Báez, J.P.P.; Rodríguez-López, J.L.; Remita, H. Enhanced Photocatalytic Activity of Surface-Modified TiO2 with Bimetallic AuPd Nanoalloys for Hydrogen Generation. Sol. RRL 2024, 8. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Wang, M.; Han, Q.; Li, L.; Tang, L.; Li, H.; Zhou, Y.; Zou, Z. Construction of an all-solid-state artificial Z-scheme system consisting of Bi2WO6/Au/CdS nanostructure for photocatalytic CO2 reduction into renewable hydrocarbon fuel. Nanotechnology 2017, 28, 274002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xie, S.; Weng, B.; Xu, Y.-J. Vertically aligned ZnO–Au@CdS core–shell nanorod arrays as an all-solid-state vectorial Z-scheme system for photocatalytic application. J. Mater. Chem. A 2016, 4, 18804–18814. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, L.; Zhang, M.; Ai, Z.; Xi, H.; Li, Y.; Shi, Q.; Chen, J. Enhanced photocatalytic activity of all-solid-state g-C3N4 /Au/P25 Z-scheme system for visible-light-driven H2 evolution. Int. J. Hydrogen Energy 2016, 41, 6277–6287. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, K.; Song, L.; Zhao, M.; Zhang, Z. Enhanced Photocarrier Separation in Hierarchical Graphitic-C3N4-Supported CuInS2 for Noble-Metal-Free Z-Scheme Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 24577–24583. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Song, C.; Wang, D. Oxygen vacancy stimulated direct Z-scheme of mesoporous Cu2O/TiO2 for enhanced photocatalytic hydrogen production from water and seawater. J. Alloys Compd. 2021, 868. [Google Scholar] [CrossRef]
- Ohno, T.; Murakami, N.; Koyanagi, T.; Yang, Y. Photocatalytic reduction of CO2 over a hybrid photocatalyst composed of WO3 and graphitic carbon nitride (g-C3N4) under visible light. J. CO2 Util. 2014, 6, 17–25. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Yang, Y.; Li, J.; Liu, W.; Shen, Y.; Tian, X. Photocatalytic Hydrogen Evolution Using Ternary-Metal-Sulfide/TiO2 Heterojunction Photocatalysts. ChemCatChem 2022, 14, e202101439. [Google Scholar] [CrossRef]
- Li, Z.; Luan, Y.; Qu, Y.; Jing, L. Modification Strategies with Inorganic Acids for Efficient Photocatalysts by Promoting the Adsorption of O2. ACS Appl. Mater. Interfaces 2015, 7, 22727–22740. [Google Scholar] [CrossRef]
- Cui, H.; Cao, Y.; Jing, L.; Luan, Y.; Li, N. Effects of Inorganic Acid Modification on Photocatalytic Performance of TiO2 and Its Activity-Enhanced Mechanism Related to Adsorbed O2. ChemPlusChem 2013, 79, 318–324. [Google Scholar] [CrossRef]
- Cao, Y.; Jing, L.; Shi, X.; Luan, Y.; Durrant, J.R.; Tang, J.; Fu, H. Enhanced photocatalytic activity of nc-TiO2 by promoting photogenerated electrons captured by the adsorbed oxygen. Phys. Chem. Chem. Phys. 2012, 14, 8530–8536. [Google Scholar] [CrossRef]
- Montoya, A.T.; Gillan, E.G. Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2. ACS Omega 2018, 34, 2947–2955. [Google Scholar] [CrossRef]
- Li, L.; Rohrer, G.S.; Salvador, P.A.; Dickey, E. Heterostructured Ceramic Powders for Photocatalytic Hydrogen Production: Nanostructured TiO2 Shells Surrounding Microcrystalline (Ba,Sr) TiO3 Cores. J. Am. Ceram. Soc. 2012, 95, 1414–1420. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Li, J.; Yang, X. Photocatalytic hydrogen production from methanol aqueous solution under visible-light using Cu/S–TiO2 prepared by electroless plating method. Catal. Commun. 2015, 59, 189–194. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, Y.; Guo, L. Study on the synthesis of Ni doped mesoporous TiO2 and its photocatalytic activity for hydrogen evolution in aqueous methanol solution. Chem. Phys. Lett. 2005, 415, 74–78. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Sang, H.; Wang, K. Synthesis of Bi-doped TiO2 Nanotubes and Enhanced Photocatalytic Activity for Hydrogen Evolution from Glycerol Solution. Chin. J. Chem. 2013, 31, 415–420. [Google Scholar] [CrossRef]
- Kong, X.; Peng, Z.; Jiang, R.; Jia, P.; Feng, J.; Yang, P.; Chi, Q.; Ye, W.; Xu, F.; Gao, P. Nanolayered Heterostructures of N-Doped TiO2, and N-Doped Carbon for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1373–1381. [Google Scholar] [CrossRef]
- Sayed, F.N.; Jayakumar, O.D.; Sasikala, R.; Kadam, R.M.; Bharadwaj, S.R.; Kienle, L.; Schürmann, U.; Kaps, S.; Adelung, R.; Mittal, J.P.; et al. Photochemical Hydrogen Generation Using Nitrogen-Doped TiO2–Pd Nanoparticles: Facile Synthesis and Effect of Ti3+ Incorporation. J. Phys. Chem. C 2012, 116, 12462–12467. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Z.; Wu, X.; Wang, G.; Zhou, W. In-situ S-doped porous anatase TiO2 nanopillars for high-efficient visible-light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2016, 41, 1535–1541. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Cortés, J.A.; Fernández-García, M.; Kubacka, A. UV and visible light driven H2 photo-production using Nb-doped TiO2: Comparing Pt and Pd co-catalysts. Mol. Catal. 2017, 437, 1–10. [Google Scholar] [CrossRef]
- Sreethawong, T.; Yoshikawa, S. Impact of photochemically deposited monometallic Pt and bimetallic Pt–Au nanoparticles on photocatalytic dye-sensitized H2 production activity of mesoporous-assembled TiO2–SiO2 mixed oxide nanocrystal. Chem. Eng. J. 2012, 197, 272–282. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, L.; Liu, X.; Sun, X.; Wang, J.; Du, H. Special Z-scheme Cu3P/TiO2 hetero-junction for efficient photocatalytic hydrogen evolution from water. J. Alloys Compd. 2022, 894, 162331. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, F.; Sun, B.; Gao, T.; Zhou, G. Hierarchical S-scheme heterojunctions of ZnIn2S4-decorated TiO2 for enhancing photocatalytic H2 evolution. Chin. J. Catal. 2024, 59, 334–345. [Google Scholar] [CrossRef]
- Yu, C.; Li, M.; Yang, D.; Pan, K.; Yang, F.; Xu, Y.; Yuan, L.; Qu, Y.; Zhou, W. NiO nanoparticles dotted TiO2 nanosheets assembled nanotubes P-N heterojunctions for efficient interface charge separation and photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 568. [Google Scholar] [CrossRef]
- Yang, S.; Wang, K.; Chen, Q.; Wu, Y. Enhanced photocatalytic hydrogen production of S-scheme TiO2/g-C3N4 heterojunction loaded with single-atom Ni. J. Mater. Sci. Technol. 2024, 175, 104–114. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Su, X.; Chen, S.; Zhang, L.J.; Yang, H.; Pei, Y. Activating a TiO2/BiVO4 Film for Photoelectrochemical Water Splitting by Constructing a Heterojunction Interface with a Uniform Crystal Plane Orientation. ACS Appl. Mater. Interfaces 2022, 14, 2316–2325. [Google Scholar] [CrossRef]
- Lee, M.G.; Yang, J.W.; Park, H.; Moon, C.W.; Andoshe, D.M.; Park, J.; Moon, C.-K.; Lee, T.H.; Choi, K.S.; Cheon, W.S.; et al. Crystal Facet Engineering of TiO2 Nanostructures for Enhancing Photoelectrochemical Water Splitting with BiVO4 Nanodots. Nano-Micro Lett. 2022, 14, 48. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yang, W.; Balaghi, S.E.; Triana, C.A.; Mavrokefalos, C.K.; Patzke, G.R. The Role of Surface States on Reduced TiO2@BiVO4 Photoanodes: Enhanced Water Oxidation Performance through Improved Charge Transfer. ACS Catal. 2021, 11, 7637–7646. [Google Scholar] [CrossRef]
- Chen, W.; Wang, T.; Xue, J.; Li, S.; Wang, Z.; Sun, S. Cobalt–Nickel Layered Double Hydroxides Modified on TiO2 Nanotube Arrays for Highly Efficient and Stable PEC Water Splitting. Small 2017, 13. [Google Scholar] [CrossRef]
- Wang, J.; Xue, C.; Yao, W.; Liu, J.; Gao, X.; Zong, R.; Yang, Z.; Jin, W.; Tao, D. MOF-derived hollow TiO2@C/FeTiO3 nanoparticles as photoanodes with enhanced full spectrum light PEC activities. Appl. Catal. B Environ. 2019, 250, 369–381. [Google Scholar] [CrossRef]
- Jung, E.S.; Basit, M.A.; Abbas, M.A.; Ali, I.; Kim, D.W.; Park, Y.M.; Bang, J.H.; Park, T.J. Improved CdS quantum dot distribution on a TiO2 photoanode by an atomic-layer-deposited ZnS passivation layer for quantum dot-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2020, 218. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Kiong, T.S.; Ismail, A.F.; Teridi, M.A.M. Novel gamma-ray enhanced TiO2 nanoparticles photoanode for efficient photoelectrochemical (PEC) water splitting. Appl. Surf. Sci. 2024, 642, 158602. [Google Scholar] [CrossRef]
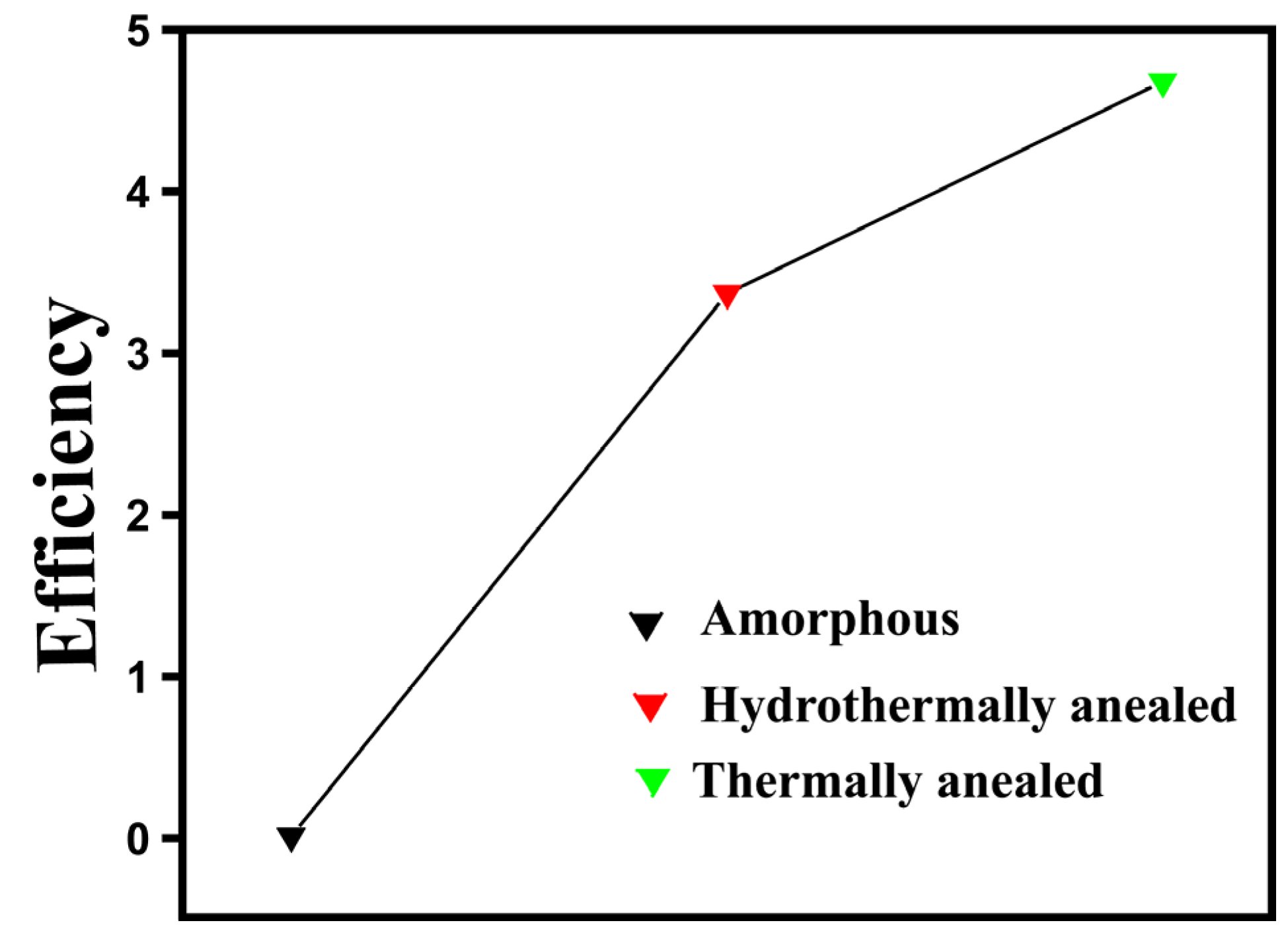

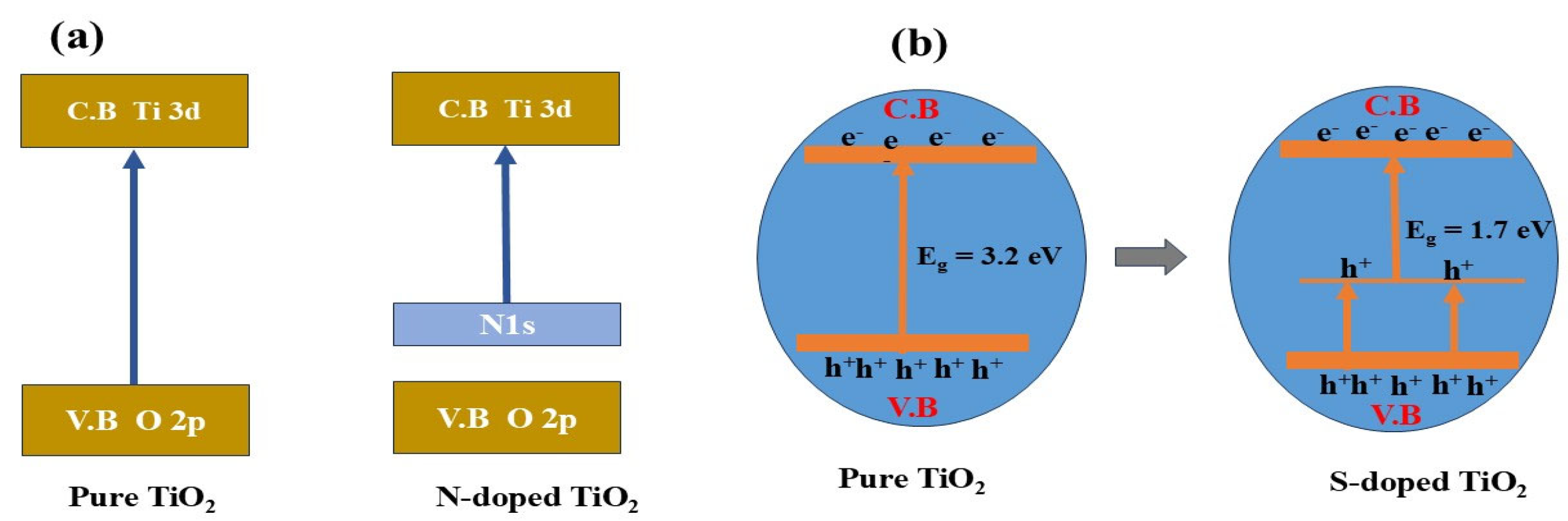
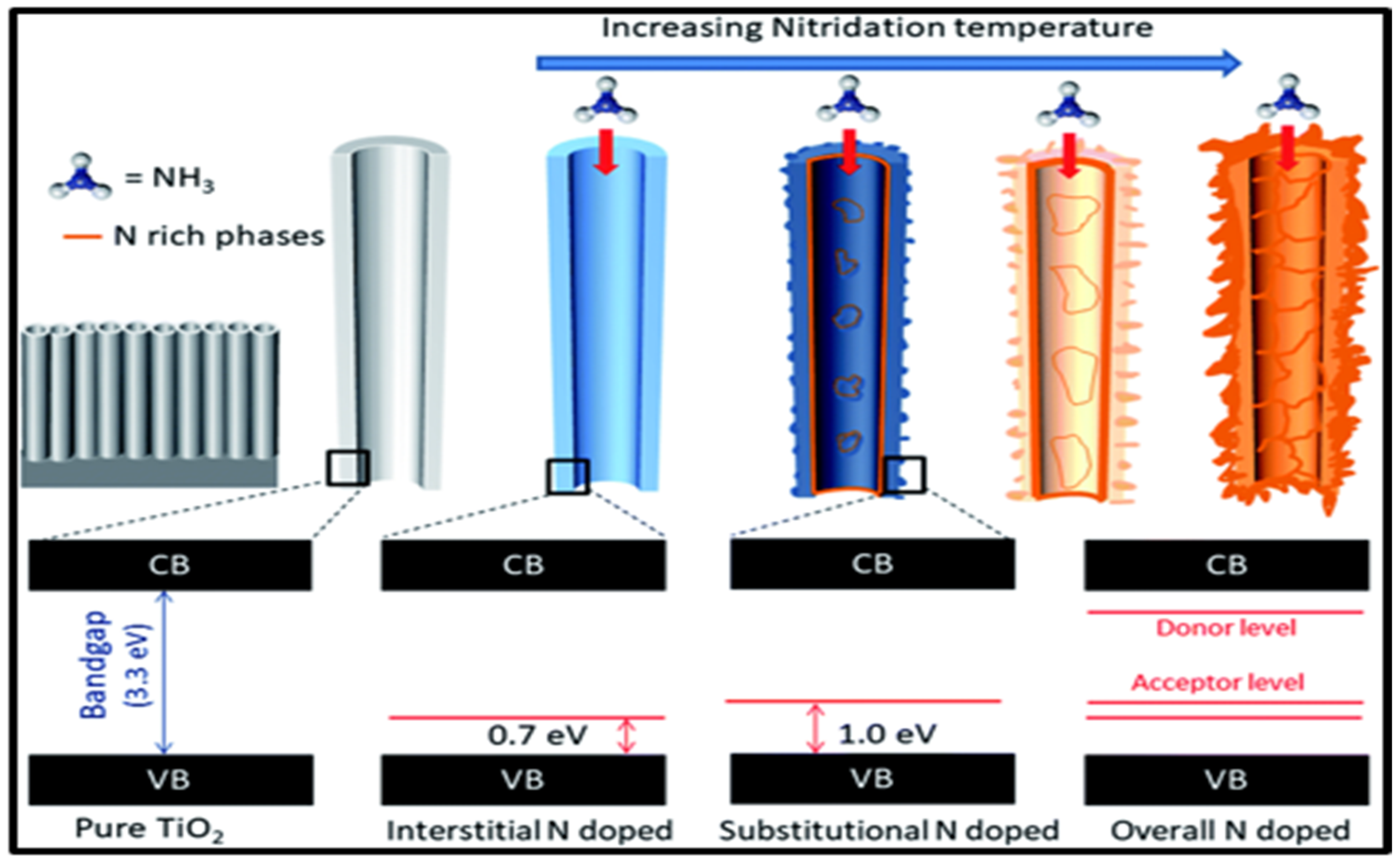


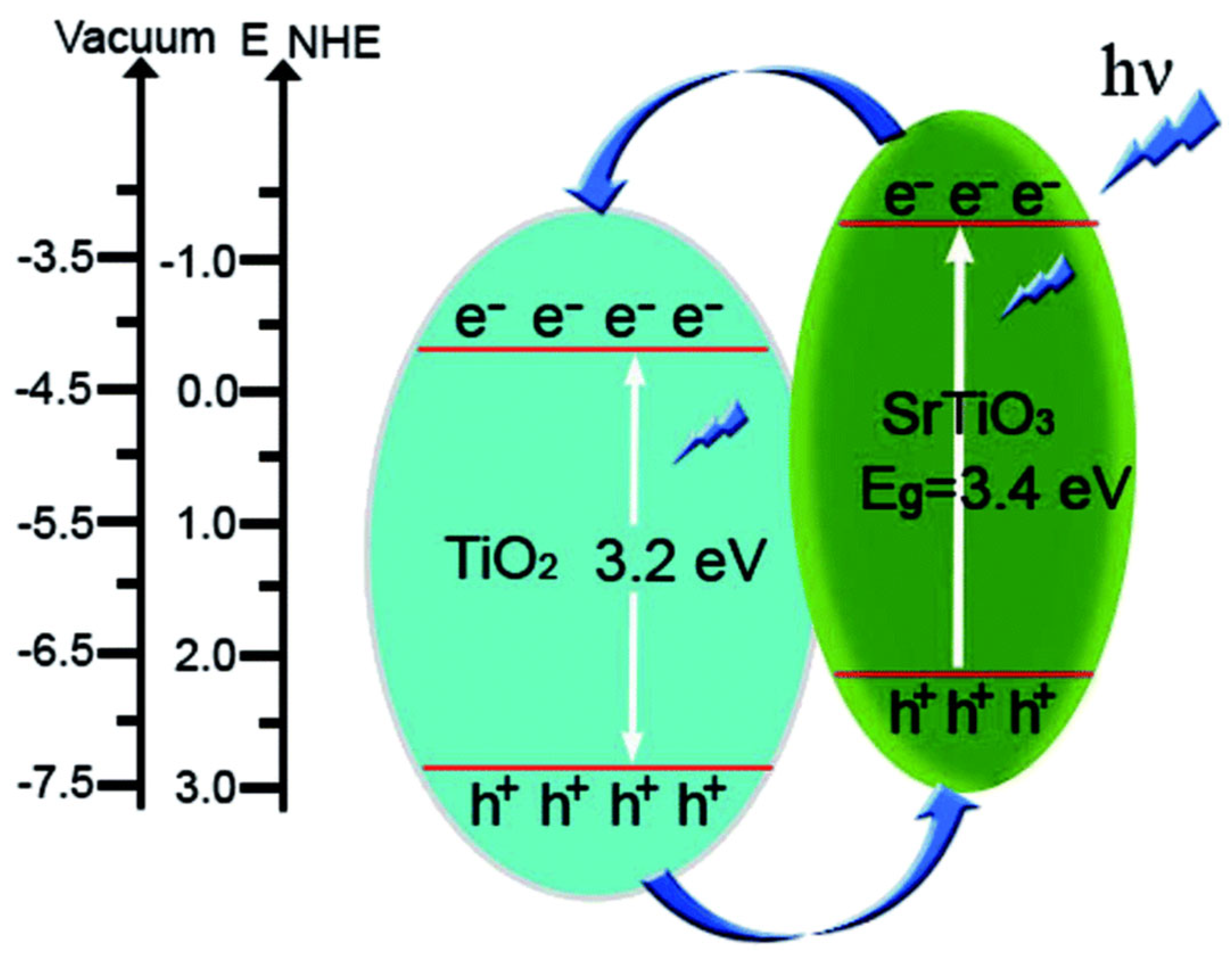
| Entry | Catalysts | H2 Production | Parameters | Ref |
|---|---|---|---|---|
| 1 | Co, Ni, and Cu-doped TiO2 | 85 µmol h−1 | UV irradiation Methanol | [123] |
| 2 | Ga or Ni co-doped TiO2 | 35 µmol h−1 | 125 W Hg lamp ≥ 400 nm Methanol | [124] |
| 3 | Cu and S-TiO2 | 7.5 mmol g−1 h−1 | 500 W Xe lamp, using quartz reactor | [125] |
| 4 | Ni-doped TiO2 | 180 µmol h−1 | 300 W Hg lamp methanol | [126] |
| 5 | Bi-doped TiO2 | 4500 µmol g−1 h−1 | 500 W, using Xe lamp | [127] |
| 6 | N-doped TiO2 | 102 µmol g−1 h−1 | Visible light irradiations | [128] |
| 7 | B-N-TiO2 | 85 µmol/50 mg methanol | 36 W light lamp | [129] |
| 8 | S-doped TiO2 | 164 mmol g−1 h−1 | Visible light (1.5 G) irradiation ≥ 400 nm | [130] |
| 9 | Nb-doped TiO2 | 0.7 mmol g−1 h−1 | Hg(UV)-Xe (Vis) lamp (500 W) | [131] |
| 10 | TiO2-SiO2 | 1.7 cm3/h.gcat | 300 W Xe lamp, visible light irradiation (λ > 420 nm) | [132] |
| 11 | Cu3P/TiO2 Z-scheme | 607 µmol g−1 h−1 | 300 W Xe lamp Water, methanol | [133] |
| 12 | Znln2S4/ TiO2 Z-scheme | 6.5 mmol g−1 h−1 | 300 W Xe lamp irradiation TEOA | [134] |
| 13 | NiO-TiO2 p-n heterojunction | 228 µmol g−1 h−1 | 300 W Xe lamp AM 1.5 G filter Water, methanol | [135] |
| 14 | Ru-doped TiO2 | 23.9 mmol g−1 h−1 | UV-LED lamp, methanol | [77] |
| 15 | g-C3N4/ TiO2 S-scheme | 134 µmol g−1 h−1 | 300 W Xe lamp TEOA | [136] |
| Entry | Photoelectrode | Photocurrent Density | Parameters | References |
|---|---|---|---|---|
| 1 | Pure TiO2 and N-TiO2 | 180 µA/cm2 | <0.3 V vs. Ag/AgCl, AM 1.5 G, 1 Sun illumination, and 1 M KOH | [75] |
| 2 | TiO2-BiFeO3/rGO | 1.7 mA/cm2 | 0.6 V vs. Ag/AgCl, AM 1.5 G, 1 Sun illumination, 0.1 M NaOH | [100] |
| 3 | TiO2/BiVO4 | 2.2 mA/cm2 | at 1.23 Vs RHE, 1 Sun illumination, AM 1.5 G | [137] |
| 4 | BiVO4/TiO2 NFs | 1.7 mA/cm2 | at 1.23 V vs. RHE, AM 1.5 G illumination, 1 M Na2SO3 | [138] |
| 5 | R-TiO2@BiVO4 | 2.1 mA/cm2 | 0.45 V vs. RHE, AM 1.5 G illumination. | [139] |
| 6 | TiO2@CoNi-LDHs | 4.4 mA/cm2 | 1.23 V vs. RHE, AM 1.5 G illumination, 1 M Na2SO3, pH 6.8 | [140] |
| 7 | TiO2%40CoNi-LDHsTiO2@c/FeTiO3 | 6.03 mA/cm2 | 1.5 V vs. SCE | [141] |
| 8 | ZnS/CdS/TiO2 | 7.45 mA/cm2 | 0.25 M S/1 M Na2S | [142] |
| 9 | TiO2 NPs | 100.12 μA cm−2 | 1.23 V vs. RHE, 0.5 M Na2SO4, 1.5 G illumination | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, M.; Khan, N.; Qadir, M.I.; Shah, Z. Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen. Nanomaterials 2025, 15, 984. https://doi.org/10.3390/nano15130984
Nisar M, Khan N, Qadir MI, Shah Z. Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen. Nanomaterials. 2025; 15(13):984. https://doi.org/10.3390/nano15130984
Chicago/Turabian StyleNisar, Muhammad, Niqab Khan, Muhammad I. Qadir, and Zeban Shah. 2025. "Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen" Nanomaterials 15, no. 13: 984. https://doi.org/10.3390/nano15130984
APA StyleNisar, M., Khan, N., Qadir, M. I., & Shah, Z. (2025). Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen. Nanomaterials, 15(13), 984. https://doi.org/10.3390/nano15130984







