Synthesis of Silver Nanoparticles by Chemical Vapor Deposition Method and Its Application in Laser Desorption/Ionization Techniques
Abstract
1. Introduction
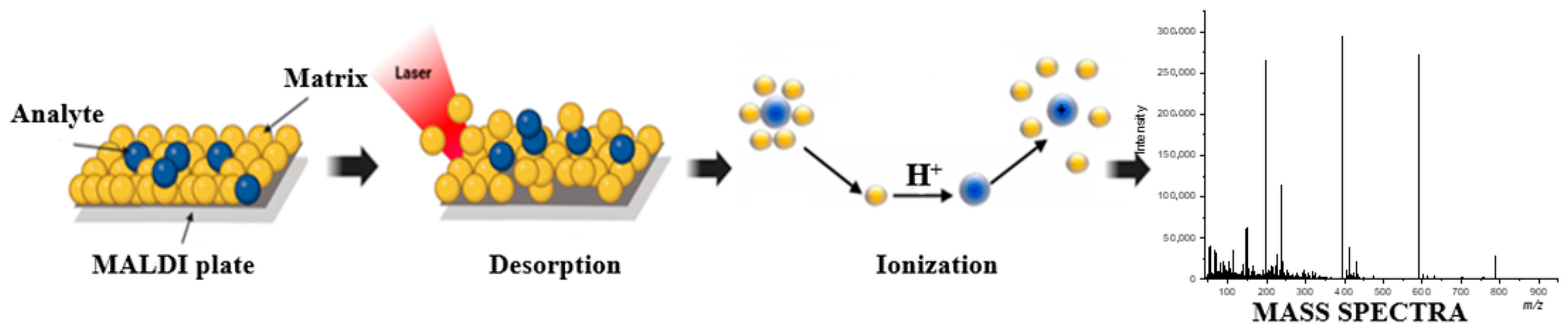
2. Organic and Nanomaterial Matrix LDI Platforms
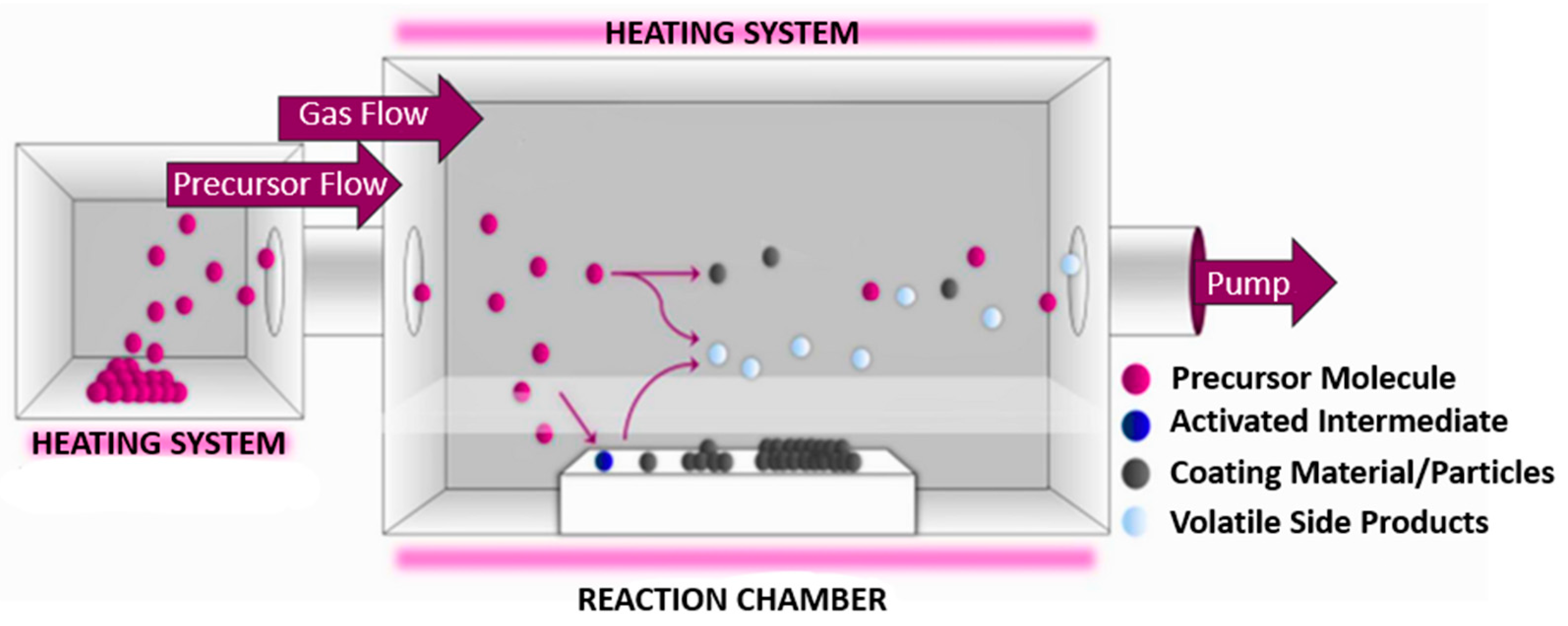
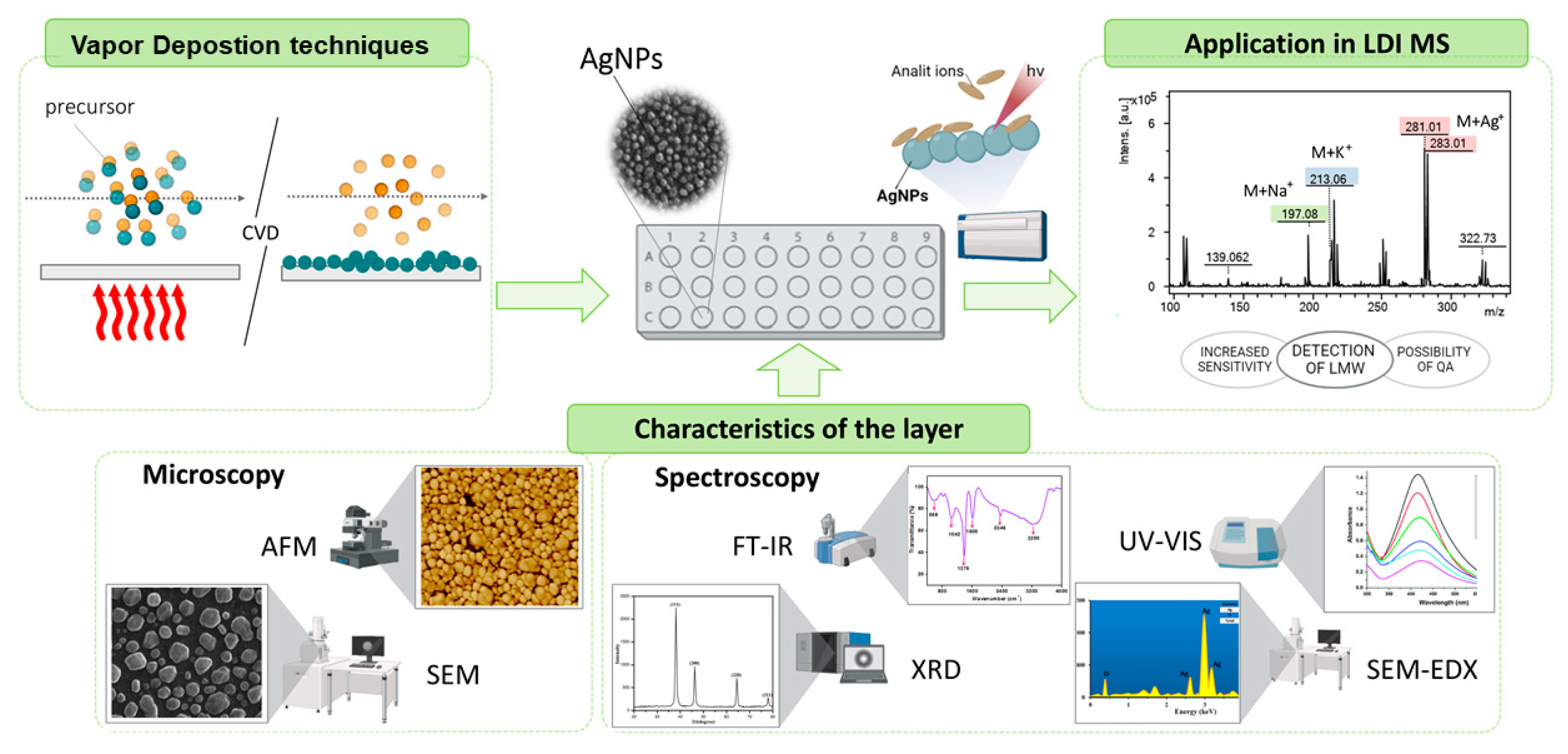
3. Chemical Vapor Deposition as a Method for Synthesizing Silver Nanoparticles
3.1. Silver Precursors
3.2. Oxidation of Silver
3.3. Functional Applications and Characterization Techniques of Silver Nanoparticles
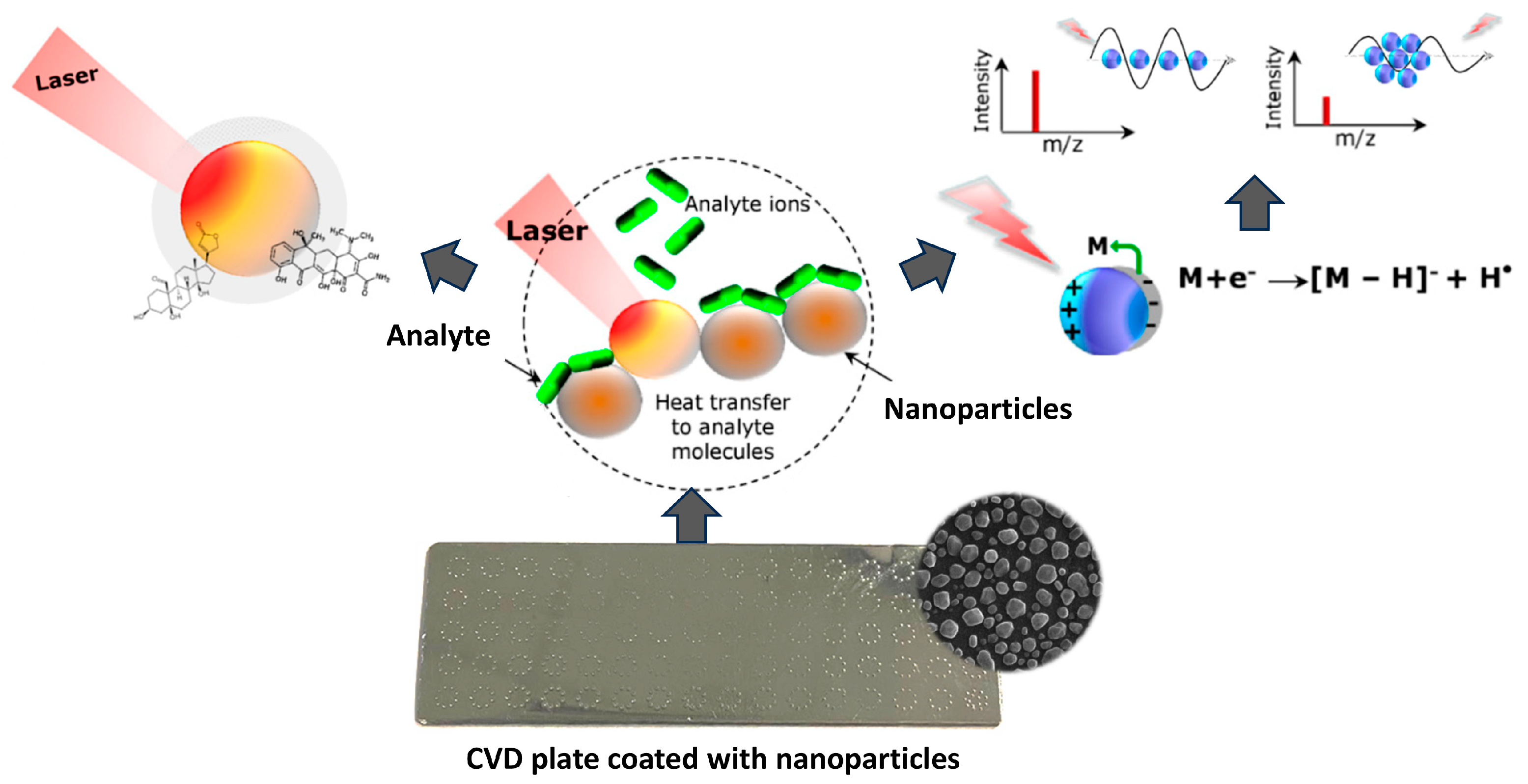
4. Application of LDI-MS Techniques
Role of Silver Nanoparticles in LDI Techniques
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korzeb, K.; Gajc, M.; Pawlak, D. Przegląd Metod Otrzymywania Materiałów Plazmonicznych Oraz Wybranych Alternatywnych Materiałów. Available online: https://rcin.org.pl/itme/Content/53503/PDF/Korzeb.pdf (accessed on 10 February 2025).
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Mutalik, C.; Sharma, S.; Yougbaré, S.; Chen, C.-Y.; Kuo, T.-R. Nanoplasmonic Biosensors: A Comprehensive Overview and Future Prospects. Int. J. Nanomed. 2025, 20, 5817–5836. [Google Scholar] [CrossRef]
- Cournut, A.; Hosu, I.S.; Braud, F.; Moustiez, P.; Coffinier, Y.; Enjalbal, C.; Bich, C. Development of nanomaterial enabling highly sensitive surface-assisted laser desorption/ionization mass spectrometry peptide analysis. Rapid Commun. Mass Spectrom. 2023, 37, e9476. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2018, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Pomastowski, P.; Buszewski, B. Complementarity of Matrix- and Nanostructure-Assisted Laser Desorption/Ionization Approaches. Nanomaterials 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- de Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications, 3rd ed.; John Wiley & Sons: West Sussex, UK, 2007. [Google Scholar]
- Kuzema, P.A. Small-molecule analysis by surface-assisted laser desorption/ionization mass spectrometry. J. Anal. Chem. 2011, 66, 1227–1242. [Google Scholar] [CrossRef]
- Fuchs, B.; Süß, R.; Schiller, J. An update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2010, 49, 450–475. [Google Scholar] [CrossRef] [PubMed]
- Hillenkamp, F.; Karas, M.; Beavis, R.C.; Chait, B.T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal. Chem. 1991, 63, 1193A–1203A. [Google Scholar] [CrossRef]
- Knochenmuss, R. Chapter 1. An Introduction to MALDI Ionization Mechanisms for Users of Mass Spectrometry Imaging. In MALDI Mass Spectrometry Imaging: From Fundamentals to Spatial Omics; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar] [CrossRef]
- Paltauf, G.; Dyer, P.E. Photomechanical Processes and Effects in Ablation. Chem. Rev. 2003, 103, 487–518. [Google Scholar] [CrossRef]
- Hosseini, S.; Martinez-Chapa, S.O. Principles and Mechanism of MALDI-ToF-MS Analysis. In Fundamentals of MALDI-ToF-MS Analysis; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wang, Y.-S. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry: Mechanistic Studies and Methods for Improving the Structural Identification of Carbohydrates. Mass Spectrom. 2017, 6, S0072. [Google Scholar] [CrossRef]
- Ehring, H.; Karas, M.; Hillenkamp, F. Role of photoionization and photochemistry in ionization processes of organic molecules and relevance for matrix-assisted laser desorption lonization mass spectrometry. J. Mass Spectrom. 1992, 27, 472–480. [Google Scholar] [CrossRef]
- Chang, W.C.; Huang, L.C.L.; Wang, Y.-S.; Peng, W.-P.; Chang, H.C.; Hsu, N.Y.; Bin Yang, W.; Chen, C.H. Matrix-assisted laser desorption/ionization (MALDI) mechanism revisited. Anal. Chim. Acta 2007, 582, 1–9. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF Mass Spectrometry in Clinical Diagnostic Microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef]
- Calvano, C.D.; Monopoli, A.; Cataldi, T.R.I.; Palmisano, F. MALDI matrices for low molecular weight compounds: An endless story? Anal. Bioanal. Chem. 2018, 410, 4015–4038. [Google Scholar] [CrossRef]
- Pavlov, J.; Attygalle, A.B. Chapter 50: Laser Ionization Mass Spectrometry of Inorganic Ions. In Mass Spectrometry Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Duan, H.; Wang, T.; Su, Z.; Pang, H.; Chen, C. Recent progress and challenges in plasmonic nanomaterials. Nanotechnol. Rev. 2022, 11, 846–873. [Google Scholar] [CrossRef]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption–ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, J.; Li, J.; Wang, Y. Graphene as a Novel Matrix for the Analysis of Small Molecules by MALDI-TOF MS. Anal. Chem. 2010, 82, 6208–6214. [Google Scholar] [CrossRef]
- Sunner, J.; Dratz, E.; Chen, Y.-C. Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions. Anal. Chem. 1995, 67, 4335–4342. [Google Scholar] [CrossRef]
- McLean, J.A.; Stumpo, K.A.; Russell, D.H. Size-Selected (2−10 nm) Gold Nanoparticles for Matrix Assisted Laser Desorption Ionization of Peptides. J. Am. Chem. Soc. 2005, 127, 5304–5305. [Google Scholar] [CrossRef]
- Gámez, F.; Hurtado, P.; Castillo, P.M.; Caro, C.; Hortal, A.R.; Zaderenko, P.; Martínez–Haya, B. UV-Vis-NIR Laser Desorption/Ionization of Synthetic Polymers Assisted by Gold Nanospheres, Nanorods and Nanostars. Plasmonics 2010, 5, 125–133. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Khurgin, J.B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotechnol. 2015, 10, 2–6. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Müller, W.H.; Verdin, A.; De Pauw, E.; Malherbe, C.; Eppe, G. Surface-assisted laser desorption/ionization mass spectrometry imaging: A review. Mass Spectrom. Rev. 2020, 41, 373–420. [Google Scholar] [CrossRef]
- Law, K.P.; Larkin, J.R. Recent advances in SALDI-MS techniques and their chemical and bioanalytical applications. Anal. Bioanal. Chem. 2010, 399, 2597–2622. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-W.; Unnikrishnan, B.; Anand, A.; Mao, J.-Y.; Huang, C.-C. Nanoparticle-based laser desorption/ionization mass spectrometric analysis of drugs and metabolites. J. Food Drug Anal. 2018, 26, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Low, B.Q.L.; Long, R.; Low, J.; Loh, H.; Tang, K.Y.; Chai, C.H.T.; Zhu, H.; Zhu, H.; Li, Z.; et al. Active Site Engineering on Plasmonic Nanostructures for Efficient Photocatalysis. ACS Nano 2023, 17, 4193–4229. [Google Scholar] [CrossRef]
- West, P.; Ishii, S.; Naik, G.; Emani, N.; Shalaev, V.; Boltasseva, A. Searching for better plasmonic materials. Laser Photon- Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Pangavhane, S.D.; Němec, P.; Wagner, T.; Janča, J.; Havel, J. Laser desorption ionization time-of-flight mass spectrometric study of binary As-Se glasses. Rapid Commun. Mass Spectrom. 2010, 24, 2000–2008. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, Y.; Qin, Y.; Shi, X.; Yang, C. The Effect of Ultraviolet Wavelength on Corrosion Behavior of 7075 Aluminum Alloy in the Marine Atmospheric Environment. Int. J. Chem. Eng. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Stiedl, J.; Green, S.; Chasse, T.; Rebner, K. Characterization of Oxide Layers on Technical Copper Material Using Ultraviolet Visible (UV-Vis) Spectroscopy as a Rapid On-Line Analysis Tool. Appl. Spectrosc. 2019, 73, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cueto, M.; Sanz, M.; Oujja, M.; Gámez, F.; Martínez−Haya, B.; Castillejo, M. Platinum Nanoparticles Prepared by Laser Ablation in Aqueous Solutions: Fabrication and Application to Laser Desorption Ionization. J. Phys. Chem. C 2011, 115, 22217–22224. [Google Scholar] [CrossRef]
- Silina, Y.E.; Koch, M.; Volmer, D.A. Influence of surface melting effects and availability of reagent ions on LDI-MS efficiency after UV laser irradiation of Pd nanostructures. J. Mass Spectrom. 2015, 50, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Schnapp, A.; Niehoff, A.-C.; Koch, A.; Dreisewerd, K. Laser desorption/ionization mass spectrometry of lipids using etched silver substrates. Methods 2016, 104, 194–203. [Google Scholar] [CrossRef]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Silver nanostructures in laser desorption/ionization mass spectrometry and mass spectrometry imaging. Anal. 2015, 140, 6195–6209. [Google Scholar] [CrossRef]
- Sibińska, E.; Walczak-Skierska, J.; Arendowski, A.; Ludwiczak, A.; Radtke, A.; Piszczek, P.; Gabryś, D.; Robotnik, K.; Pomastowski, P. Advances in LDI-MS Analysis: The Role of Chemical Vapor Deposition-Synthesized Silver Nanoparticles in Enhancing Detection of Low-Molecular-Weight Biomolecules. J. Am. Soc. Mass Spectrom. 2024, 35, 2041–2055. [Google Scholar] [CrossRef]
- Liu, N.; Guo, H.; Fu, L.; Kaiser, S.; Schweizer, H.; Giessen, H. Three-dimensional photonic metamaterials at optical frequencies. Nat. Mater. 2008, 7, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Lindquist, N.C.; Oh, S.-H.; Norris, D.J. Ultrasmooth Patterned Metals for Plasmonics and Metamaterials. Science 2009, 325, 594–597. [Google Scholar] [CrossRef]
- Gajc, M.; Surma, H.B.; Klos, A.; Sadecka, K.; Orlinski, K.; Nikolaenko, A.E.; Zdunek, K.; Pawlak, D.A. Nanoparticle Direct Doping: Novel Method for Manufacturing Three-Dimensional Bulk Plasmonic Nanocomposites. Adv. Funct. Mater. 2013, 23, 3443–3451. [Google Scholar] [CrossRef]
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties, Applications and Perspectives: Review. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Khurana, K.; Rathee, N.; Jaggi, N. Deposition of uniform plasmonic Ag nanoparticles by in-situ substrate heating using thermal evaporator. AIP Conf. Proc. 2020, 2220, 090006. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapor deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Prim. 2021, 1, 1–20. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical Vapour Deposition (CVD): Advances, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Piszczek, P. Związki Koordynacyjne Srebra i Tytanu z Ligandami Karboksylowymi Jako Prekursory Chemicznego Osadzania z Fazy Gazowej; Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 2009. [Google Scholar]
- Vikulova, E.S.; Dorovskikh, S.I.; Basova, T.V.; Zheravin, A.A.; Morozova, N.B. Silver CVD and ALD Precursors: Synthesis, Properties, and Application in Deposition Processes. Molecules 2024, 29, 5705. [Google Scholar] [CrossRef]
- Gao, L.; Härter, P.; Linsmeier, C.; Wiltner, A.; Emling, R.; Schmitt-Landsiedel, D. Silver metal organic chemical vapor deposition for advanced silver metallization. Microelectron. Eng. 2005, 82, 296–300. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Chaberski, M.; Goliński, A. Studies of thermal decomposition process of Ag(I) perfluorinated carboxylates with temperature variable IR and MS. Polyhedron 2001, 20, 2853–2861. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Jędrzejewski, T.; Wypij, M.; Golińska, P. “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. J. Clin. Med. 2019, 8, 334. [Google Scholar] [CrossRef]
- Struppert, T.; Jakob, A.; Heft, A.; Grünler, B.; Lang, H. The use of silver(I)-2-[2-(2-methoxyethoxy)ethoxy]acetate as precursor in the deposition of thin silver layers on float glass by the atmospheric pressure combustion chemical vapor deposition process. Thin Solid Films 2010, 518, 5741–5744. [Google Scholar] [CrossRef]
- Piani, R.; Beele, B.B.; Rust, J.; Lehmann, C.W.; Mohr, F. Coinage Metal Complexes Containing Perfluorinated Carboxylates. Chemistry 2023, 5, 813–833. [Google Scholar] [CrossRef]
- Jin, Q.-H.; Yuan, Y.; Yang, Y.-P.; Qiu, Q.-M.; Liu, M.; Li, Z.-F.; Zhang, Z.-W.; Zhang, C.-L. Polynuclear silver(I) complexes of diphosphine ligands and isoquinoline: Synthesis, structural characterization and spectroscopic properties. Polyhedron 2015, 101, 56–64. [Google Scholar] [CrossRef]
- Haase, T.; Kohse-Höinghaus, K.; Bahlawane, N.; Djiele, P.; Jakob, A.; Lang, H. CVD with Tri-nbutylphosphine Silver(I) Complexes: Mass Spectrometric Investigations and Depositions. Chem. Vap. Depos. 2005, 11, 195–205. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Xu, C.; Liu, D. SERS Tags for Biomedical Detection and Bioimaging. Theranostics 2022, 12, 1870–1903. [Google Scholar] [CrossRef]
- Krishna, S.B.N.; Adam, J.K.; Govender, P. Biomedical applications and toxicity of Nanosilver: A Review. J. Med. Lab. Sci. Technol. S. Afr. 2015, 29, 13–19. [Google Scholar]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; Castro, D.P.C.; Ortiz-Trujillo, I.C.; Palacio, L.E.B.; Upegui, B.J.G.; Mora, N.J.E.; Cornelio, J.A.C. Compounds of carbon nanotubes decorated with silver nanoparticles via in-situ by chemical vapor deposition (CVD). J. Mater. Res. Technol. 2019, 8, 5893–5898. [Google Scholar] [CrossRef]
- Liu, H.; Ouyang, D.; Wang, J.; Lei, C.; Shi, W.; Gilliam, T.; Liu, J.; Li, Y.; Chopra, N. Chemical Vapor Deposition Mechanism of Graphene-Encapsulated Au Nanoparticle Heterostructures and Their Plasmonics. ACS Appl. Mater. Interfaces 2021, 13, 58134–58143. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’NA, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef]
- Walczak-Skierska, J.; Monedeiro, F.; Maślak, E.; Złoch, M. Lipidomics Characterization of the Microbiome in People with Diabetic Foot Infection Using MALDI-TOF MS. Anal. Chem. 2023, 95, 16251–16262. [Google Scholar] [CrossRef] [PubMed]
- Arendowski, A. Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches. Metabolites 2024, 14, 173. [Google Scholar] [CrossRef]
- Złoch, M.; Maślak, E.; Kupczyk, W.; Pomastowski, P. Multi-Instrumental Analysis Toward Exploring the Diabetic Foot Infection Microbiota. Curr. Microbiol. 2023, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Granborg, J.R.; Handler, A.M.; Janfelt, C. Mass spectrometry imaging in drug distribution and drug metabolism studies—Principles, applications and perspectives. TrAC Trends Anal. Chem. 2022, 146. [Google Scholar] [CrossRef]
- Kafka, A.P.; Kleffmann, T.; Rades, T.; McDowell, A. The application of MALDI TOF MS in biopharmaceutical research. Int. J. Pharm. 2011, 417, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Akimowicz, M.; Bucka-Kolendo, J. MALDI-TOF MS—application in food microbiology. Acta Biochim. Pol. 2020, 67, 327–332. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1989; Volume 45. [Google Scholar]
- Zvirin, Y.; Gutman, M.; Tartakovsky, L. Fuel Effects on Emissions. In Handbook of Air Pollution From Internal Combustion Engines; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar] [CrossRef]
- Suchecki, A.; Knefel, T.; Nowakowski, J. Wpływ zanieczyszczeń w oleju napędowym na funkcjonalność pracy wtryskiwaczy paliwa w systemie Common Rail. AUTOBUSY 2016, 17, 143–148. [Google Scholar]
- Millan, M.; Behrouzi, M.; Karaca, F.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. Characterising high mass materials in heavy oil fractions by size exclusion chromatography and MALDI-mass spectrometry. Catal. Today 2005, 109, 154–161. [Google Scholar] [CrossRef]
- Pantoja, P.A.; Mendes, M.A.; Nascimento, C.A.O. Contribution of mass spectrometry in assessing quality of petroleum fractions. J. Pet. Sci. Eng. 2013, 109, 198–205. [Google Scholar] [CrossRef]
- Rudyk, S.; Ongarbayev, Y.; Spirov, P. Feature selection in GC-MS, NMR and MALDI-TOF spectra of tar sand bitumen. Unconv. Resour. 2023, 3, 61–71. [Google Scholar] [CrossRef]
- Ramopoulou, L.; Widder, L.; Brenner, J.; Ristic, A.; Allmaier, G. Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry of engine oil additive components. Rapid Commun. Mass Spectrom. 2022, 36, e9271. [Google Scholar] [CrossRef]
- Alsayegh, S.Y.; Al Disi, Z.; Al-Ghouti, M.A.; Zouari, N. Evaluation by MALDI-TOF MS and PCA of the diversity of biosurfactants and their producing bacteria, as adaption to weathered oil components. Biotechnol. Rep. 2021, 31, e00660. [Google Scholar] [CrossRef]
- Sagandykova, G.; Pryshchepa, O.; Rafińska, K.; Mametov, R.; Madajski, P.; Pomastowski, P. LDI-MS performance of gold nanostars as an inorganic matrix for low molecular weight analytes. Int. J. Mass Spectrom. 2022, 478. [Google Scholar] [CrossRef]
- Inuta, M.; Arakawa, R.; Kawasaki, H. Protein-modified silver nanoplates for the complementary analytical method of localised surface plasmon resonance and matrix assisted laser desorption/ionisation mass spectrometry. Anal. Methods 2013, 5, 5031–5036. [Google Scholar] [CrossRef]
- Arendowski, A.; Sagandykova, G.; Mametov, R.; Rafińska, K.; Pryshchepa, O.; Pomastowski, P. Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry. Materials 2022, 15, 4076. [Google Scholar] [CrossRef] [PubMed]
- Arendowski, A.; Nizioł, J.; Ruman, T. Silver-109-based laser desorption/ionization mass spectrometry method for detection and quantification of amino acids. J. Mass Spectrom. 2018, 53, 369–378. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Dycka, F.; Kratochvil, J.; Sterba, J.; Stranak, V. Gas-aggregated Ag nanoparticles for detection of small molecules using LDI MS. Anal. Bioanal. Chem. 2020, 412, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, A.; Płaza-Altamer, A.; Nizioł, J.; Ruman, T. Infrared pulsed fiber laser-produced silver-109 nanoparticles for laser desorption/ionization mass spectrometry of 3-hydroxycarboxylic acids. Rapid Commun. Mass Spectrom. 2022, 36, e9375. [Google Scholar] [CrossRef]
- Arakawa, R.; Kawasaki, H. Functionalized Nanoparticles and Nanostructured Surfaces for Surface-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Sci. 2010, 26, 1229–1240. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Dycka, F.; Kratochvil, J.; Stranak, V.; Popok, V.N. Effect of Ag Nanoparticle Size on Ion Formation in Nanoparticle Assisted LDI MS. Appl. Nano 2020, 1, 3–13. [Google Scholar] [CrossRef]
- Sagandykova, G.; Piszczek, P.; Radtke, A.; Mametov, R.; Pryshchepa, O.; Gabryś, D.; Kolankowski, M.; Pomastowski, P. Silver Nanostructured Substrates in LDI-MS of Low Molecular Weight Compounds. Materials 2022, 15, 4660. [Google Scholar] [CrossRef] [PubMed]
- Mametov, R.; Sagandykova, G.; Monedeiro, F.; Florkiewicz, A.; Piszczek, P.; Radtke, A.; Pomastowski, P. Metabolic profiling of bacteria with the application of polypyrrole-MOF SPME fibers and plasmonic nanostructured LDI-MS substrates. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maślak, E.; Arendowski, A.; Złoch, M.; Walczak-Skierska, J.; Radtke, A.; Piszczek, P.; Pomastowski, P. Silver Nanoparticle Targets Fabricated Using Chemical Vapor Deposition Method for Differentiation of Bacteria Based on Lipidomic Profiles in Laser Desorption/Ionization Mass Spectrometry. Antibiotics 2023, 12, 874. [Google Scholar] [CrossRef]
| Precursor | Type | Key Characteristics | Applications in CVD | Refs. |
|---|---|---|---|---|
| Silver nitrate (AgNO3) | Inorganic | High decomposition temperature, limited volatility | Atmospheric pressure CVD, flame-assisted CVD | [54] |
| Silver(I) fluoride (AgF) | Inorganic | High temperature required for evaporation and reaction | High-temperature CVD | [54] |
| Silver(I) β-diketonates | Organometallic | Volatile, thermally stable | MOCVD, AACVD, plasma-enhanced CVD | [55,56] |
| Silver(I) carboxylates | Metal–organic complex | Low volatility, thermally stable | Laser-activated CVD | [57] |
| Silver(I) acetate (AgOAc) | Metal–organic complex | Low volatility | Laser-activated CVD | [57] |
| Silver(I) trifluoroacetate | Metal–organic complex | Low volatility, fluorinated | Specialized techniques | [54,57] |
| Silver(I) pentafluoropropionate (Ag5(OOCC2F2)) | Metal–organic complex | High volatility, low decomposition temperature | Low-pressure CVD | [54,56,58] |
| Silver(I) pentafluoropropionate trihydrate | Metal–organic complex | Enhanced stability at ambient conditions | Low-pressure CVD | [54,55,58] |
| Silver(I)-2-[2-(2-methoxyethoxy)ethoxy]acetate | Metal–organic complex | Air-stable, polar solvent soluble, low-cost synthesis | Thin silver layers, multilayer systems | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robotnik, K.; Zieliński, T.; Walczak-Skierska, J.; Sibińska, E.; Rudzik, P.; Piszczek, P.; Radtke, A.; Pomastowski, P.P. Synthesis of Silver Nanoparticles by Chemical Vapor Deposition Method and Its Application in Laser Desorption/Ionization Techniques. Nanomaterials 2025, 15, 973. https://doi.org/10.3390/nano15130973
Robotnik K, Zieliński T, Walczak-Skierska J, Sibińska E, Rudzik P, Piszczek P, Radtke A, Pomastowski PP. Synthesis of Silver Nanoparticles by Chemical Vapor Deposition Method and Its Application in Laser Desorption/Ionization Techniques. Nanomaterials. 2025; 15(13):973. https://doi.org/10.3390/nano15130973
Chicago/Turabian StyleRobotnik, Kinga, Tomasz Zieliński, Justyna Walczak-Skierska, Ewelina Sibińska, Paulina Rudzik, Piotr Piszczek, Aleksandra Radtke, and Paweł Piotr Pomastowski. 2025. "Synthesis of Silver Nanoparticles by Chemical Vapor Deposition Method and Its Application in Laser Desorption/Ionization Techniques" Nanomaterials 15, no. 13: 973. https://doi.org/10.3390/nano15130973
APA StyleRobotnik, K., Zieliński, T., Walczak-Skierska, J., Sibińska, E., Rudzik, P., Piszczek, P., Radtke, A., & Pomastowski, P. P. (2025). Synthesis of Silver Nanoparticles by Chemical Vapor Deposition Method and Its Application in Laser Desorption/Ionization Techniques. Nanomaterials, 15(13), 973. https://doi.org/10.3390/nano15130973










