Analysis of the Development and Thermal Properties of Chitosan Nanoparticle-Treated Palm Oil: An Experimental Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Synthesis of Chitosan Nanoparticles
2.2.2. Characterization of Chitin, Chitosan and Chitosan Nanoparticles
2.2.3. Determination of Degree of Deacetylation
2.2.4. Analysis of the Peroxide Value of Palm Oil
- Peroxide value = mEq peroxide per kg of sample
- S = volume of titrant (ml) for the sample
- B = volume of titrant (ml) for blank
- N = normality of Na2S2O3 solution (mEq/mL)
2.2.5. Determination of Free Fatty Acid Percentage (FFA%)
- % FFA = percent free fatty acid (g/100 g) expressed as Oleic acid
- V = volume of NaOH titrant (mL)
- N = normality of NaOH titrant (mol/1000 mL)
- 282 = molecular weight of oleic acid (g/mol)
- W = sample mass (g)
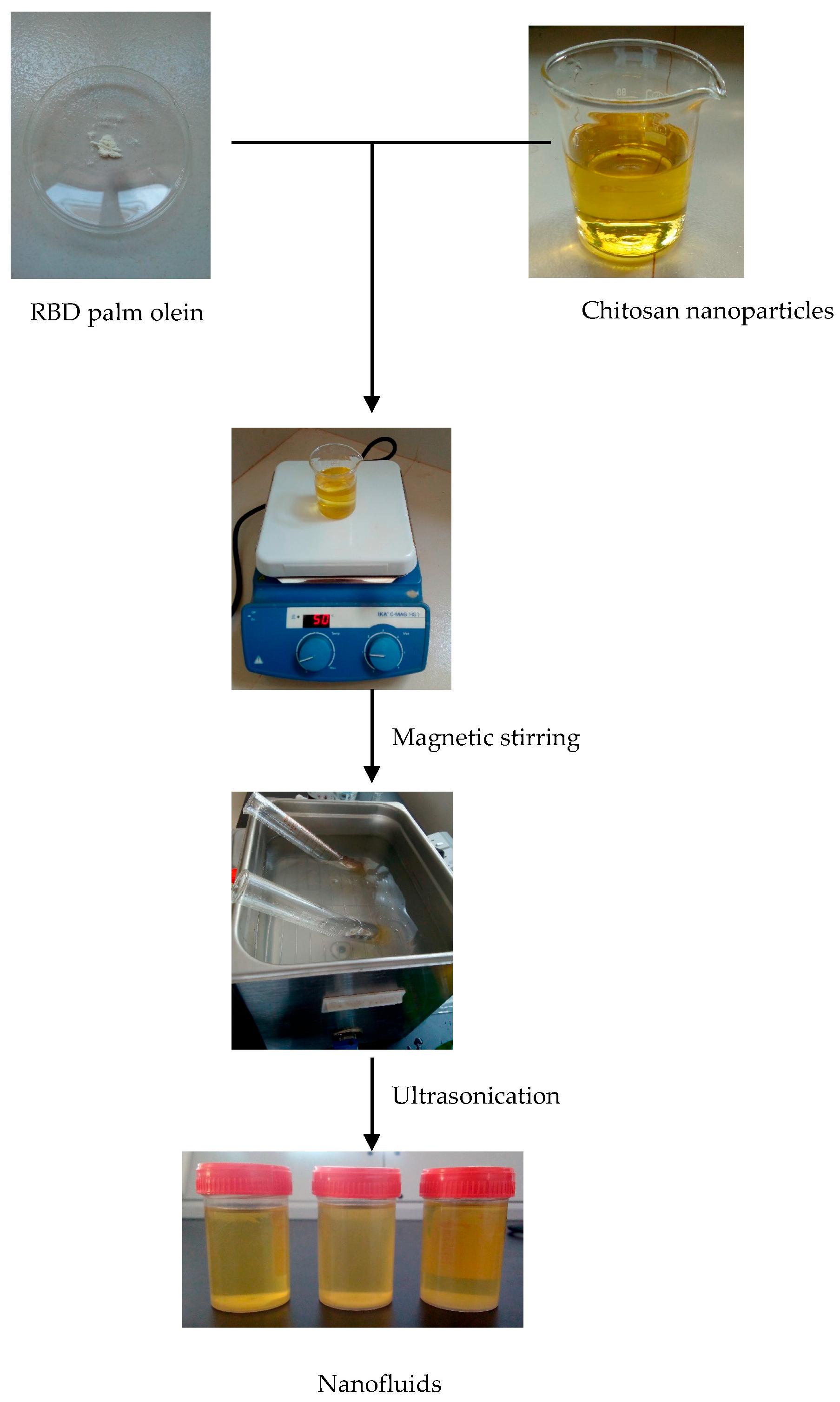
2.2.6. Preparation of Nanofluids
- mnp—mass of nanoparticles
- mbf—mass of base fluid
2.2.7. Thermal Conductivity Measurement
2.2.8. Thermal Diffusivity Measurement
2.2.9. Viscosity Measurement
3. Results and Discussion
3.1. Quality Analysis of RBD Palm Olein
3.1.1. Determination of Peroxide Value
3.1.2. Determination of FFA Percentage
3.2. Characterization of Chitin, Chitosan and Chitosan Nanoparticles
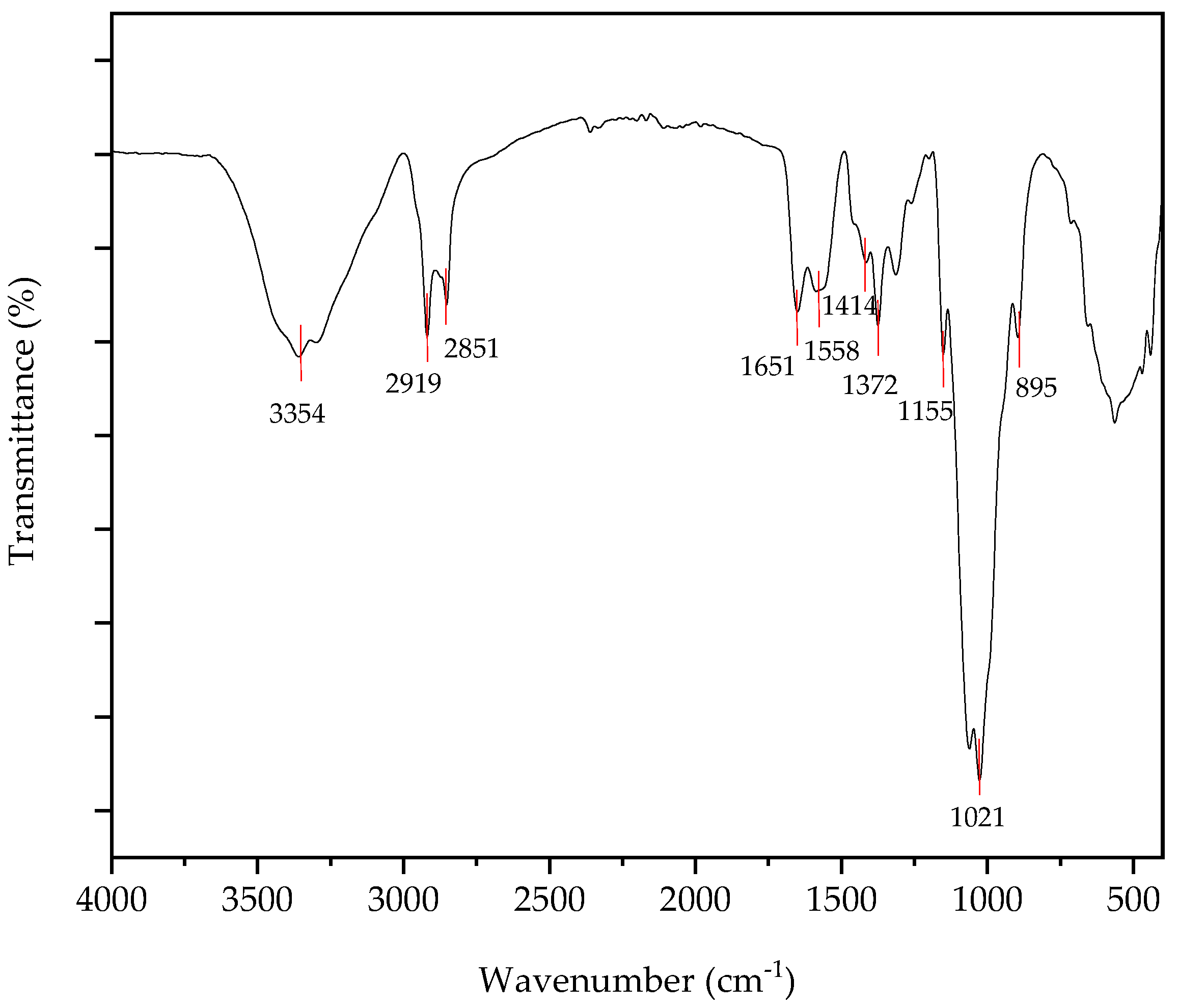
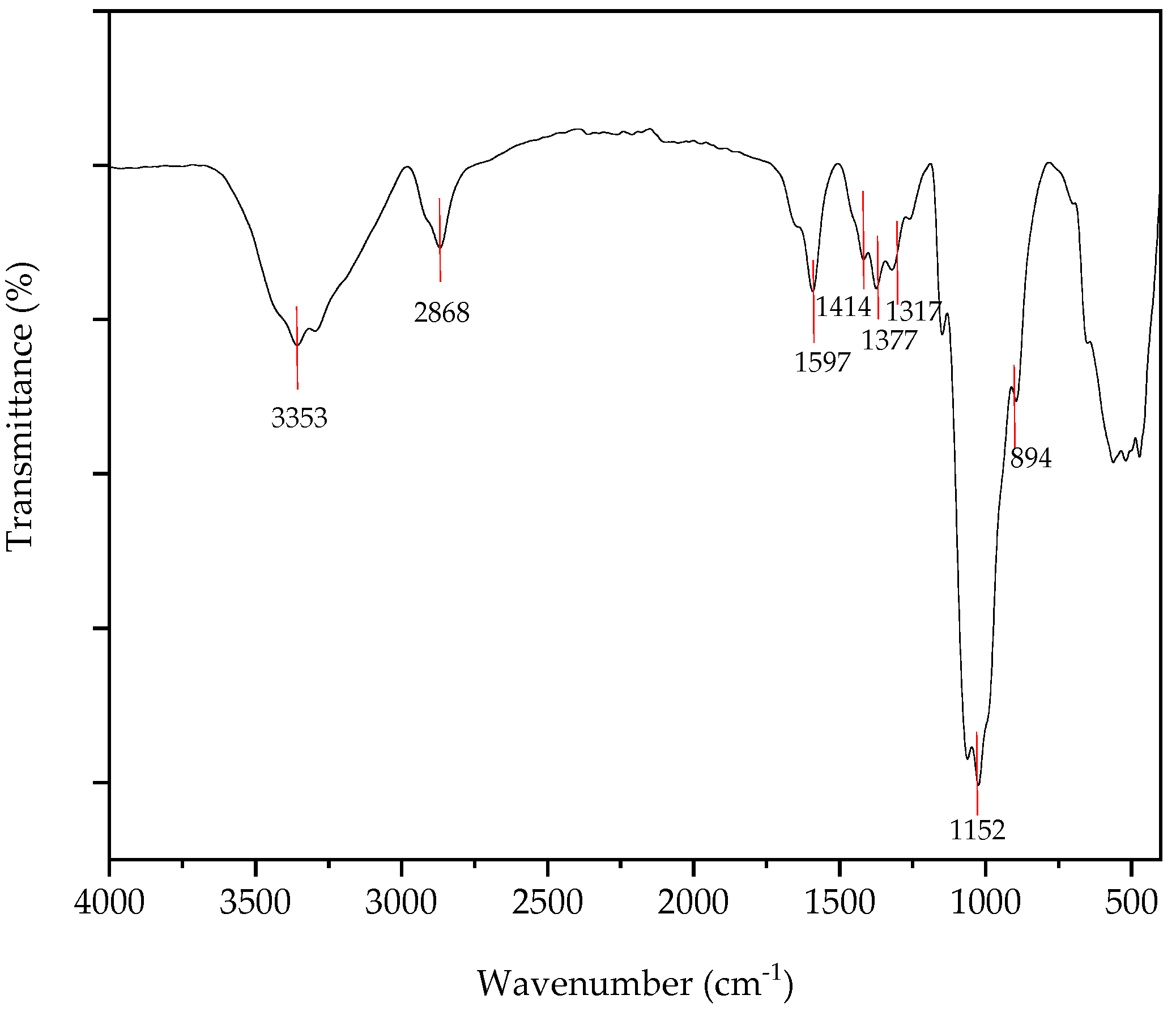
3.2.1. FTIR Analysis of Chitin, Chitosan and Chitosan Nanoparticles
3.2.2. Degree of Deacetylation
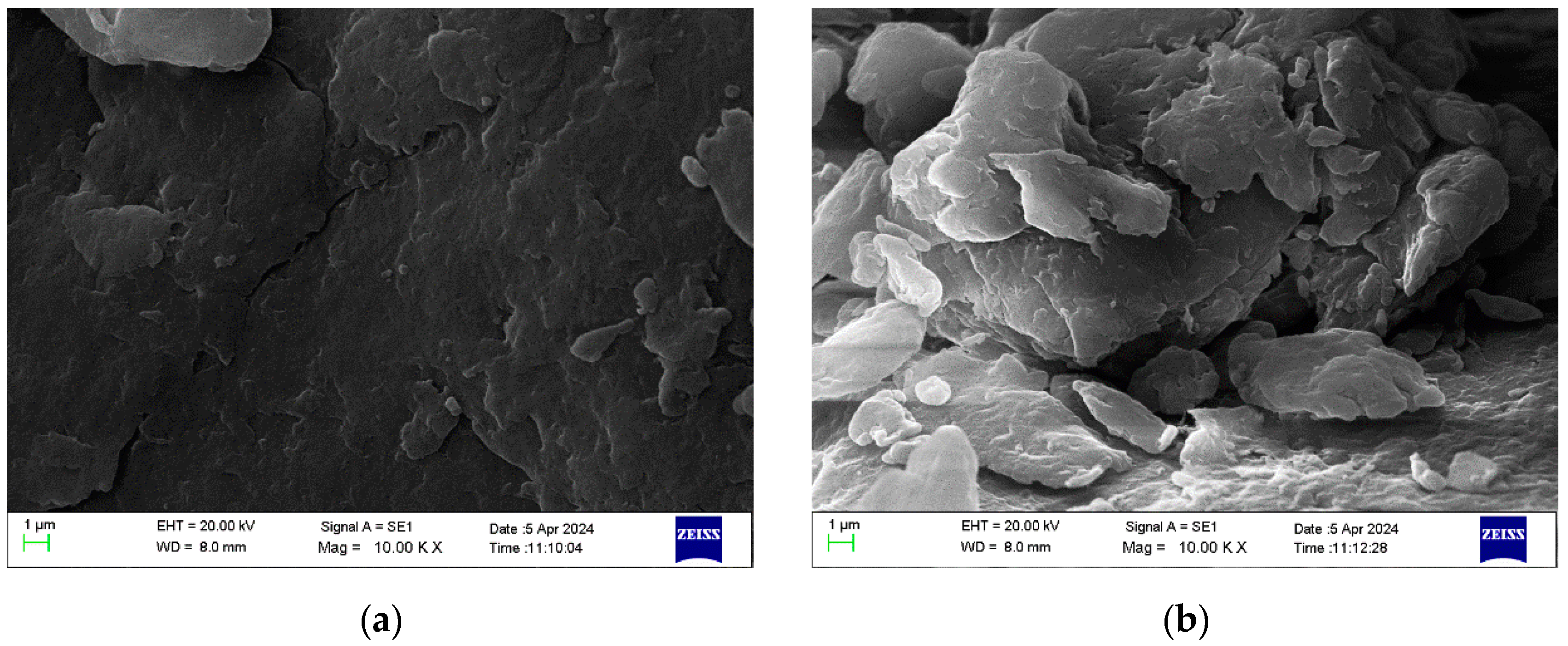
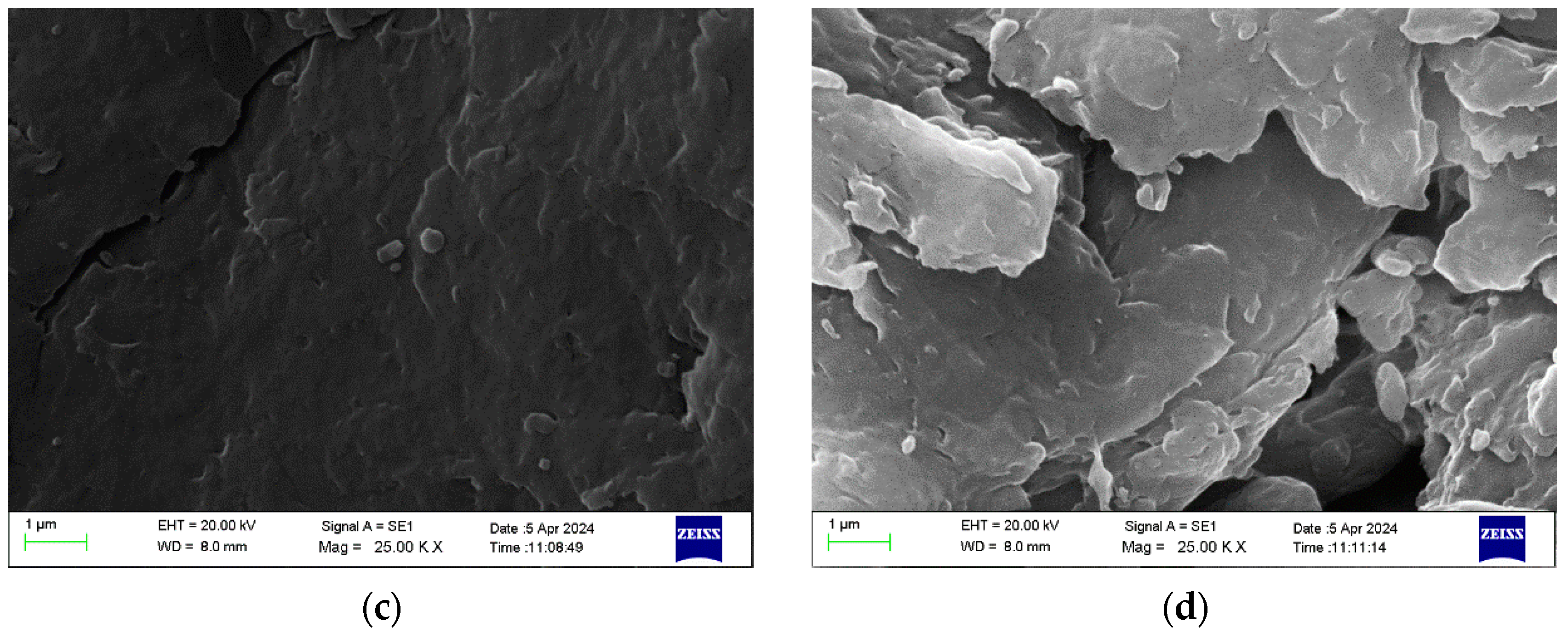
3.2.3. SEM Analysis of Chitosan Nanoparticles
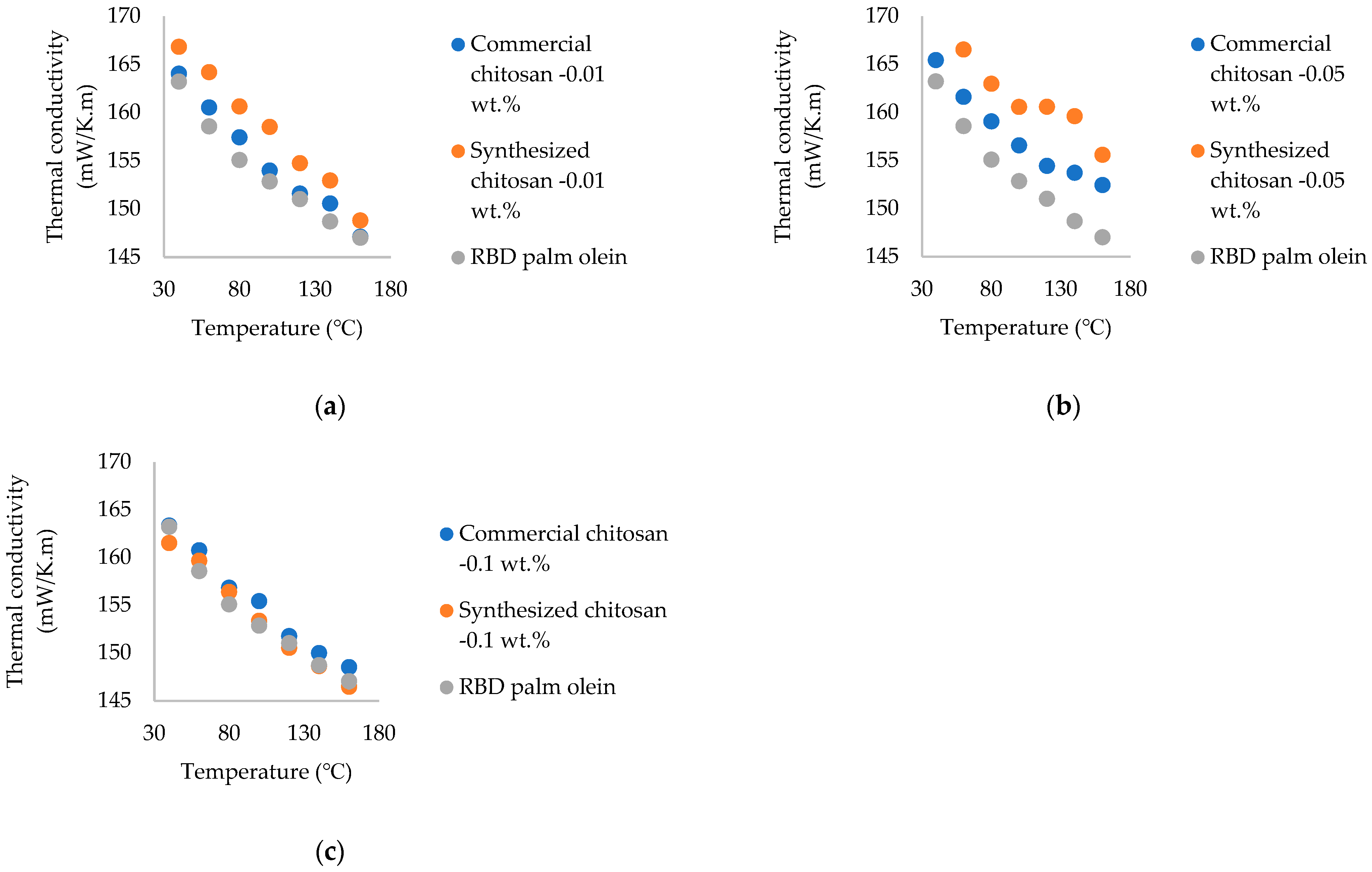
3.3. Determination of Thermal Conductivity of Chitosan Nanoparticle-Treated Nanofluids
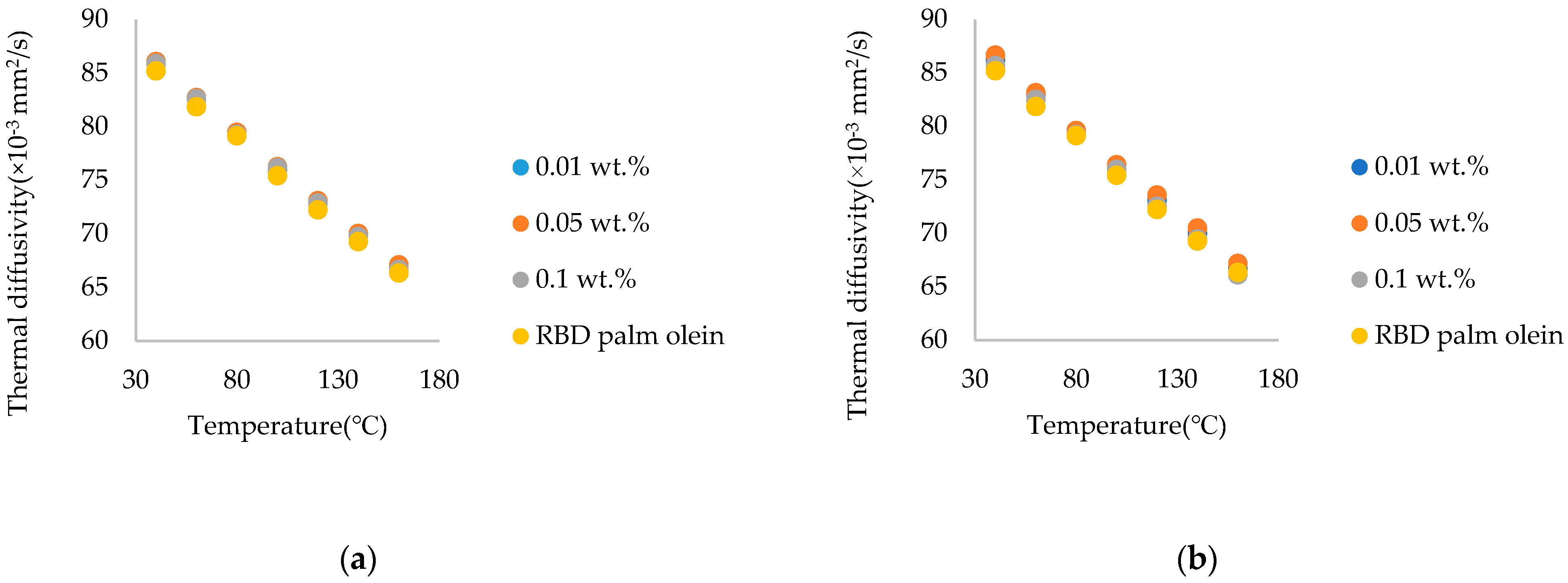
3.4. Comparison of Thermal Diffusivity of Commercial Chitosan Nanoparticle-Treated Nanofluids and Synthesized Chitosan Nanoparticle-Treated Nanofluids
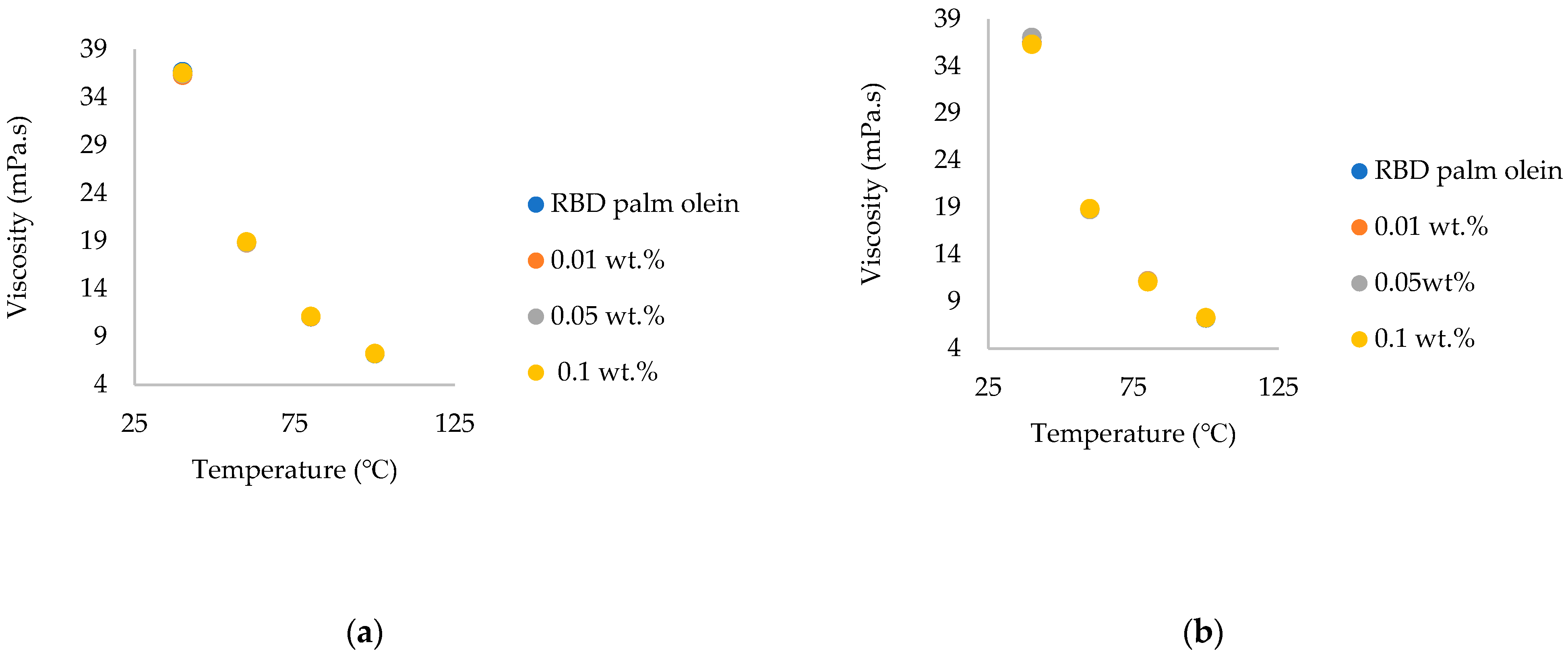
3.5. Comparison of the Viscosity of Commercial Chitosan Nanoparticles and Synthesized Chitosan Nanoparticle-Treated Nanofluids
4. Conclusions
- (a)
- The highest thermal conductivity enhancement was observed at 140 °C at 0.05 wt.% of synthesized and commercial chitosan nanoparticles, while at 160 °C, it was at 7.33% and 4.28%, respectively. Better thermal conductivity enhancement was observed throughout the temperature range of 40–160 °C. Fungal-sourced chitosan nanoparticles showed higher enhancement than marine-sourced chitosan nanoparticles. Lower enhancement was observed in 0.1 wt.% due to the higher amount of nanoparticles present in the oil. Therefore, the optimum wt.% to add chitosan nanoparticles to oil to obtain a higher enhancement of thermal conductivity is 0.05 wt.%.
- (b)
- The thermal diffusivity of both commercial chitosan and synthesized chitosan nanoparticle-treated RBD palm olein was enhanced. Comparing marine–sourced with fungal-sourced, fungal-sourced chitosan increased thermal diffusivity more than marine-sourced chitosan.
- (c)
- Observing the viscosity of commercial chitosan and synthesized chitosan nanoparticle-treated RBD palm olein, the viscosity of nanofluids increased due to the increase in the weight of nanoparticles in both cases. Viscosity enhancement by the synthesized chitosan nanoparticles was higher than by the commercial ones, and these enhancements were in an acceptable range for food applications. The findings of this novel study provide a dataset for future research on edible nanofluids, which can be applicable in the food industry. Future research work should focus on determining the biochemical changes, stability and rheological modeling of the nanofluids.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RBD | Refined, bleached, deodorized |
| GRAS | Generally recognized as safe |
| CS-NP | Chitosan nanoparticle |
| DD | Degree of deacetylation |
| MWCNT | Multi-walled carbon nanotubes |
| Eh-BN | Exfoliated hexagonal boron nitride |
| SO | Soybean oil |
| CO | Coconut oil |
| PO | Palm oil |
References
- Aguiar, L.K.; Martinez, D.C.; Caleman, S.M.Q. Consumer Awareness of Palm Oil as an Ingredient in Food and Non-Food Products. J. Food Prod. Mark. 2018, 24, 297–310. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Farrell, J.J.; Larrea, C.; Luna, E. Global Market Report: Palm Oil Prices and Sustainability; IISD: Winnipeg, MB, Canada, 2023. [Google Scholar]
- Carter, C.; Finley, W.; Fry, J.; Jackson, D.; Willis, L. Palm oil markets and future supply. Eur. J. Lipid Sci. Technol. 2007, 109, 307–314. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Choi, S.U.S. Nanofluids: A New Field of Scientific Research and Innovative Applications. Heat Transf. Eng. 2008, 29, 429–431. [Google Scholar] [CrossRef]
- Yao, W.; Huang, Z.; Li, J.; Wu, L.; Xiang, C. Enhanced Electrical Insulation and Heat Transfer Performance of Vegetable Oil Based Nanofluids. J. Nanomater. 2018, 2018, 4504208. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Alahakoon, A.M.P.D.; Polwaththa, K.P.G.D.M.; Galpaya, G.D.C.P.; Priyanjani, H.A.S.A.; Koswattage, K.R.; Senarath, W.T.P.S.K. Transforming plant tissue culture with nanoparticles: A review of current applications. Plant Nano Biol. 2024, 10, 100102. [Google Scholar] [CrossRef]
- Noorzadeh, S.; Sadegh Moghanlou, F.; Vajdi, M.; Ataei, M. Thermal conductivity, viscosity and heat transfer process in nanofluids: A critical review. J. Compos. Compd. 2020, 2, 175–192. [Google Scholar] [CrossRef]
- Vithanage, V.; Hosan, S.; Perera, H.; Galpaya, C.; Induranga, A.; Indupama, A.; Wijesekara, D.; Koswattage, K. Impact of water based nanofluids in heat exchanger type active solar PV cooling systems: A comparative CFD analysis. Int. J. Sci. Res. Arch. 2025, 14, 1139–1145. [Google Scholar] [CrossRef]
- Induranga, A.; Galpaya, C.; Vithanage, V.; Koswattage, K.R. Thermal Properties of TiO2 Nanoparticle-Treated Transformer Oil and Coconut Oil. Energies 2023, 17, 49. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Hameedha Beevi, A. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Perera, U.M.S.P.; Rajapakse, N. Chitosan Nanoparticles: Preparation, Characterization, and Applications. In Seafood Processing By-Products; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 371–387. [Google Scholar] [CrossRef]
- Savin, S.; Craciunescu, O.; Oancea, A.; Ilie, D.; Ciucan, T.; Antohi, L.S.; Toma, A.; Nicolescu, A.; Deleanu, C.; Oancea, F. Antioxidant, Cytotoxic and Antimicrobial Activity of Chitosan Preparations Extracted from Ganoderma lucidum Mushroom. Chem. Biodivers. 2020, 17, e2000175. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, quantification, characterization, and application in food packaging of chitin and chitosan from mushrooms: A review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Erdogan, S.; Kaya, M.; Akata, I. Chitin extraction and chitosan production from cell wall of two mushroom species (Lactarius vellereus and Phyllophora ribis). In Proceedings of the 6th International Advances in Applied Physics and Materials Science Congress & Exhibition: (APMAS 2016), İstanbul, Turkey, 1–3 June 2016; p. 020012. [Google Scholar]
- Ossamulu, F.; Evbouan, S.; Akanya, H.; Egwim, E.; Zobeashia, S.L.-T. Characterization of chitosan extracted from three mushroom species from Edo State, Nigeria. Ovidius Univ. Ann. Chem. 2023, 34, 22–27. [Google Scholar] [CrossRef]
- Castro Marín, A.; Colangelo, D.; Lambri, M.; Riponi, C.; Chinnici, F. Relevance and perspectives of the use of chitosan in winemaking: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3450–3464. [Google Scholar] [CrossRef]
- Mei, X.; Li, S.; Chen, Y.; Huang, X.; Cao, Y.; Guro, V.P.; Li, Y. Silica–Chitosan Composite Aerogels for Thermal Insulation and Adsorption. Crystals 2023, 13, 755. [Google Scholar] [CrossRef]
- ASTM D7896-19; Standard Test Method for Thermal Conductivity, Thermal Diffusivity, and Volumetric Heat Capacity of Engine Coolants and Related Fluids by Transient Hot Wire Liquid Thermal Conductivity Method. ASTM: West Conshohocken, PA, USA, 2019.
- Oberemko, A.; Salaberria, A.M.; Saule, R.; Saulis, G.; Kaya, M.; Labidi, J.; Baublys, V. Physicochemical and in vitro cytotoxic properties of chitosan from mushroom species (Boletus bovinus and Laccaria laccata). Carbohydr. Polym. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Sams, C.E. Chitin and ChitosanValue-Added Products from Mushroom Waste. J. Agric. Food Chem. 2004, 52, 7905–7910. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; De Silva, K.M.N. Coloration of cotton fibers using nano chitosan. Carbohydr. Polym. 2015, 134, 182–189. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q.; Shi, L. Effective thermal conductivity of carbon nanotube-based nanofluid. J. Taiwan Inst. Chem. Eng. 2015, 55, 76–81. [Google Scholar] [CrossRef]
- Younes, H.; Christensen, G.; Li, D.; Hong, H.; Ghaferi, A.A. Thermal Conductivity of Nanofluids: Review. J. Nanofluids 2015, 4, 107–132. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Role of Adsorbing Moieties on Thermal Conductivity and Associated Properties of Nanofluids. J. Phys. Chem. C 2013, 117, 9009–9019. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Vaidyanathan, A.; Sunil Kumar, S. Synthesis and characterization of kerosene–alumina nanofluids. Appl. Therm. Eng. 2013, 60, 275–284. [Google Scholar] [CrossRef]
- Ettefaghi, E.; Ahmadi, H.; Rashidi, A.; Nouralishahi, A.; Mohtasebi, S.S. Preparation and thermal properties of oil-based nanofluid from multi-walled carbon nanotubes and engine oil as nano-lubricant. Int. Commun. Heat Mass Transf. 2013, 46, 142–147. [Google Scholar] [CrossRef]
- Branson, B.T.; Beauchamp, P.S.; Beam, J.C.; Lukehart, C.M.; Davidson, J.L. Nanodiamond Nanofluids for Enhanced Thermal Conductivity. ACS Nano 2013, 7, 3183–3189. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Enhanced thermophysical properties of copper nanoparticles dispersed in gear oil. Appl. Therm. Eng. 2013, 56, 45–53. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Baruah, N.; Maharana, M.; Nayak, S.K. Performance analysis of vegetable oil-based nanofluids used in transformers. IET Sci. Meas. Technol. 2019, 13, 995–1002. [Google Scholar] [CrossRef]
- Wanatasanappan, V.V.; Rezman, M.; Abdullah, M.Z. Thermophysical Properties of Vegetable Oil-Based Hybrid Nanofluids Containing Al2O3-TiO2 Nanoparticles as Insulation Oil for Power Transformers. Nanomaterials 2022, 12, 3621. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, M.M.; Das, S.; Chattopadhyay, P.; Das, S.; Chattopadhyay, K.K. Enhancement of thermal conductivity of transformer oil by exfoliated white graphene nanosheets. In Proceedings of the 2016 IEEE 16th International Conference on Environment and Electrical Engineering (EEEIC), Florence, Italy, 7–10 June 2016; IEEE: Florence, Italy, 2016; pp. 1–5. [Google Scholar]
- Deenesh, N.K.; Mahmood, A.; Kadir, G.; Hasnan, K.; Nafarizal, N. Experimental investigation on thermal conductivity and viscosity of purified aged transformer oil based SiO2, Al2O3 and TiO2 nanofluid for Electric Multiple Unit Train. J. Phys. Conf. Ser. 2021, 1878, 012017. [Google Scholar] [CrossRef]
- Azizie, N.A.; Hussin, N.; Halim, H.A.; Irwanto, M. Experimental Investigation on Thermal Conductivity of Palm Oil and Zinc Oxide PFAE-based Nanofluids. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 108, 93–102. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Thermal conductivity and rheological properties of graphite/oil nanofluids. Colloids Surf. Physicochem. Eng. Asp. 2012, 414, 125–131. [Google Scholar] [CrossRef]
- Galpaya, C.; Induranga, A.; Vithanage, V.; Mantilaka, P.; Koswattage, K.R. Comparative Study on the Thermal Properties of Engine Oils and Their Nanofluids Incorporating Fullerene-C60, TiO2 and Fe2O3 at Different Temperatures. Energies 2024, 17, 732. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 221. [Google Scholar] [CrossRef]



| Sample | Weight Fraction of Nanoparticles (%) | Peroxide Value (meqO2/kg) |
|---|---|---|
| RBD palm olein (Initial) | - | 0.00 |
| Commercial chitosan (marine-sourced) Nanoparticle-added RBD palm olein | 0.01 | 0.00 |
| 0.05 | 0.00 | |
| 0.1 | 0.00 | |
| Synthesized chitosan (fungal-sourced) nanoparticle-added RBD palm olein | 0.01 | 0.00 |
| 0.05 | 0.00 | |
| 0.1 | 0.00 |
| Sample | Weight Fractions (%) | FFA% |
|---|---|---|
| RBD palm olein (Initial) | - | 0.10467 ± 0.0124 a |
| Commercial chitosan (marine-sourced) nanoparticle-added RBD palm olein | 0.01 | 0.10490 ± 0.0121 a |
| 0.05 | 0.10430 ± 0.0128 a | |
| 0.1 | 0.097667 ± 0.0006 a | |
| Synthesized chitosan (fungal-sourced) nanoparticle-added RBD palm olein | 0.01 | 0.097300 ± 0.0006 a |
| 0.05 | 0.10483 ± 0.0122 a | |
| 0.1 | 0.097033 ± 0.0001 a |
| Base Fluid | Nanoparticle | Concentration | Maximum Enhancement | References |
|---|---|---|---|---|
| Vegetable oil | Hexagonal boron nitride | 0.02–0.1 vol.% | 14% | [6] |
| Transformer oil | TiO2 | 0.002–0.012 vol.% | 4.2% | [10] |
| Coconut oil | TiO2 | 0.002–0.012 vol.% | 1.4% | [10] |
| Kerosene | Al2O3 | 0.05–0.5% | 1.22% | [30] |
| Engine oil | MWCNT | 0.1–0.5 wt% | 1.227% | [31] |
| Mineral oil | Diamond | 0–1.9 vol % | 11% | [32] |
| Gear oil | Cu | 0.11 and 2.0% | 24% | [33] |
| Transformer oil | AlN | 0.5 vol.% | 8% | [34] |
| Transformer oil | Al2O3 | 4 vol.% | 20% | [34] |
| Pongamia oil methyl ester (POME) | Exfoliated hexagonal boron nitride (Eh-BN) | 0.01 wt.% | 22.65% | [35] |
| Mineral oil | Eh-BN | 0.01 wt.% | 3.9% | [35] |
| Soybean oil (SO), coconut oil (CO), and palm oil (PO) | Al2O3 and TiO2 | 0.2 wt%, 0.4 wt% and 0.6 wt% | 125.3% for PO 23.3% for SO 14.1% for CO | [36] |
| Transformer oil | Amorphous graphene (a-GS) | 0.0012 wt%, 0.0025 wt%, 0.005 wt% and 0.01 wt% | 30% | [37] |
| Purified aged transformer oil | SiO2, Al2O3, and TiO2 | 0.1 vol% | 20.83% | [38] |
| Palm olein oil | ZnO | 0.0025 g/L, 0.04 g/L and 0.14 g/L | 59.5% | [39] |
| Palm fatty acid ester (PFAE) | ZnO | 0.0025 g/L, 0.04 g/L and 0.14 g/L | 27% | [39] |
| Heat transfer oil (LD320, heavy alkylbenzene | Graphite | 1.36 vol.% | 36% | [40] |
| Engine oil | TiO2 | 0.01 wt.% | 4.5% | [41] |
| Engine oil | Fe2O3 | 0.01 wt.% | 3.9% | [41] |
| RBD palm olein | Synthesized chitosan (Fungal-source) | 0.01–0.1 wt.% | 7.33% | Present study |
| RBD palm olein | Commercial chitosan (Marine-source) | 0.01–0.1 wt.% | 4.28% | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirthika, V.; Galpaya, C.; Induranga, A.; Sajiwanie, A.; Vithanage, V.; Koswattage, K.R. Analysis of the Development and Thermal Properties of Chitosan Nanoparticle-Treated Palm Oil: An Experimental Investigation. Nanomaterials 2025, 15, 972. https://doi.org/10.3390/nano15130972
Kirthika V, Galpaya C, Induranga A, Sajiwanie A, Vithanage V, Koswattage KR. Analysis of the Development and Thermal Properties of Chitosan Nanoparticle-Treated Palm Oil: An Experimental Investigation. Nanomaterials. 2025; 15(13):972. https://doi.org/10.3390/nano15130972
Chicago/Turabian StyleKirthika, Varadharaja, Chanaka Galpaya, Ashan Induranga, Amanda Sajiwanie, Vimukthi Vithanage, and Kaveenga Rasika Koswattage. 2025. "Analysis of the Development and Thermal Properties of Chitosan Nanoparticle-Treated Palm Oil: An Experimental Investigation" Nanomaterials 15, no. 13: 972. https://doi.org/10.3390/nano15130972
APA StyleKirthika, V., Galpaya, C., Induranga, A., Sajiwanie, A., Vithanage, V., & Koswattage, K. R. (2025). Analysis of the Development and Thermal Properties of Chitosan Nanoparticle-Treated Palm Oil: An Experimental Investigation. Nanomaterials, 15(13), 972. https://doi.org/10.3390/nano15130972