Cultivation Optimization and Structural Characterization of Stephanocyclus meneghinianus for Sustainable High-Quality Biosilica Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Methods
2.2. Analysis of Shape and Structure Through Microscope
2.3. Analysis of Surface Elements and Crystal Structure
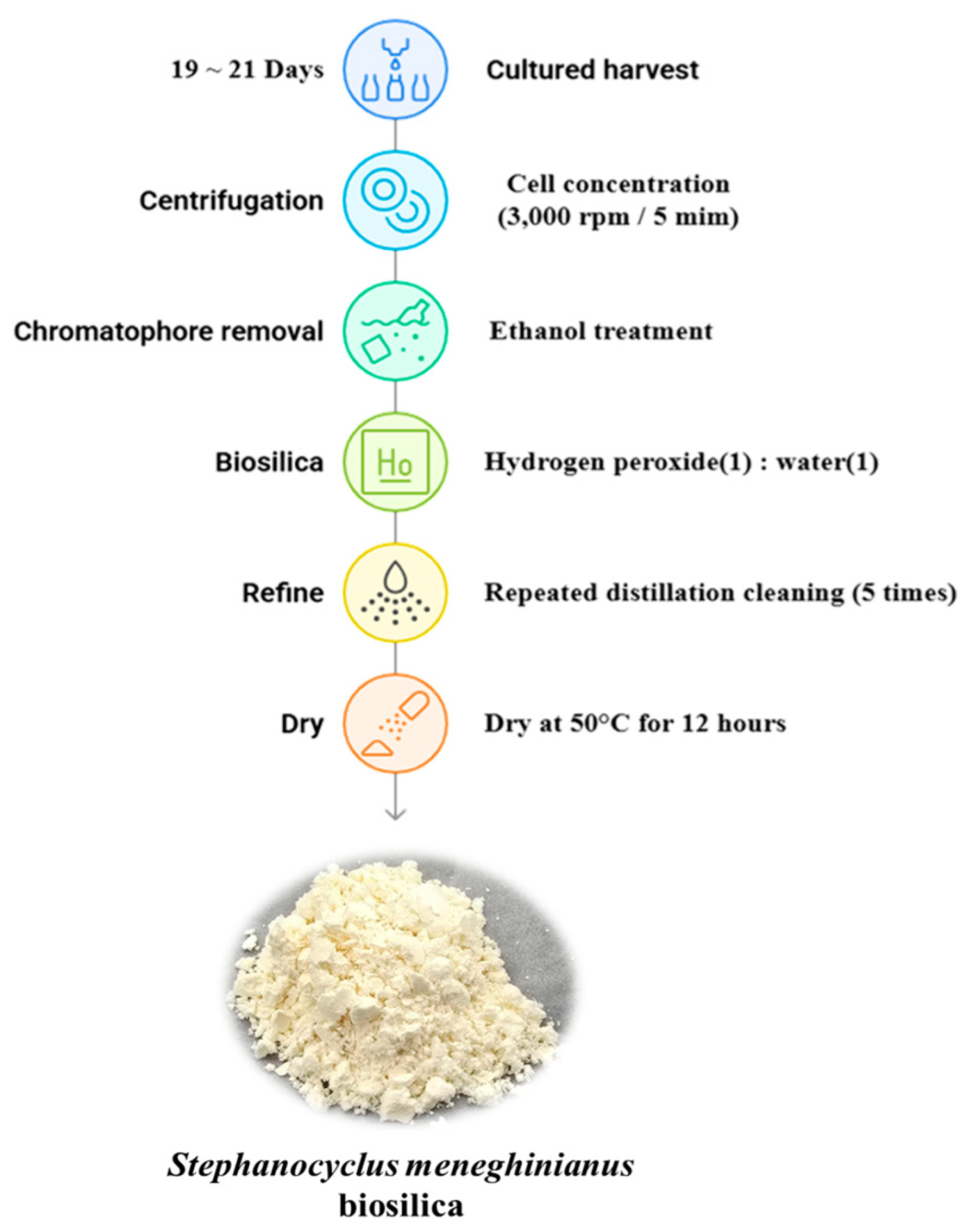
2.4. Processing Protocol for S. meneghinianus Biosilica Extraction
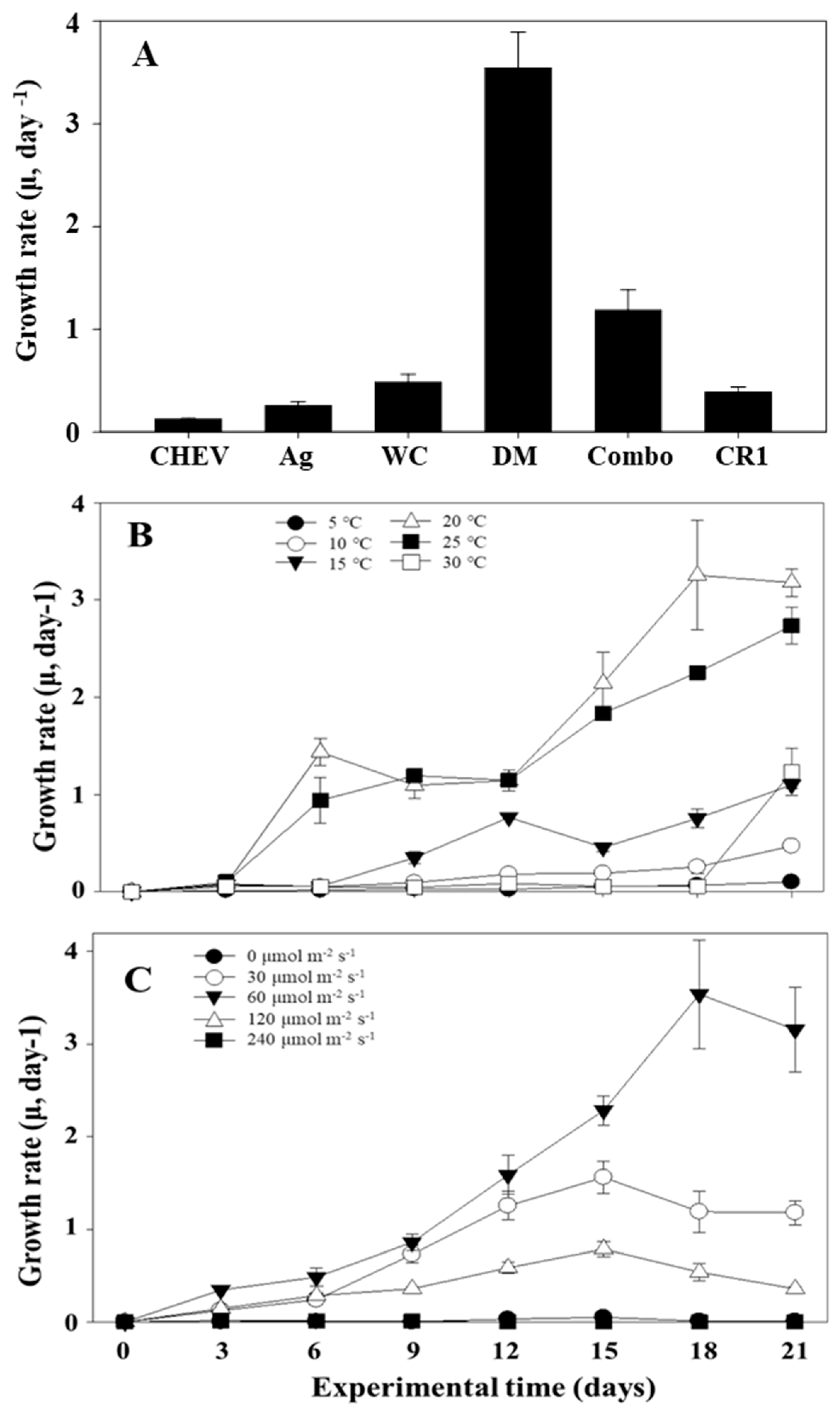
2.5. Growth Rate
3. Results
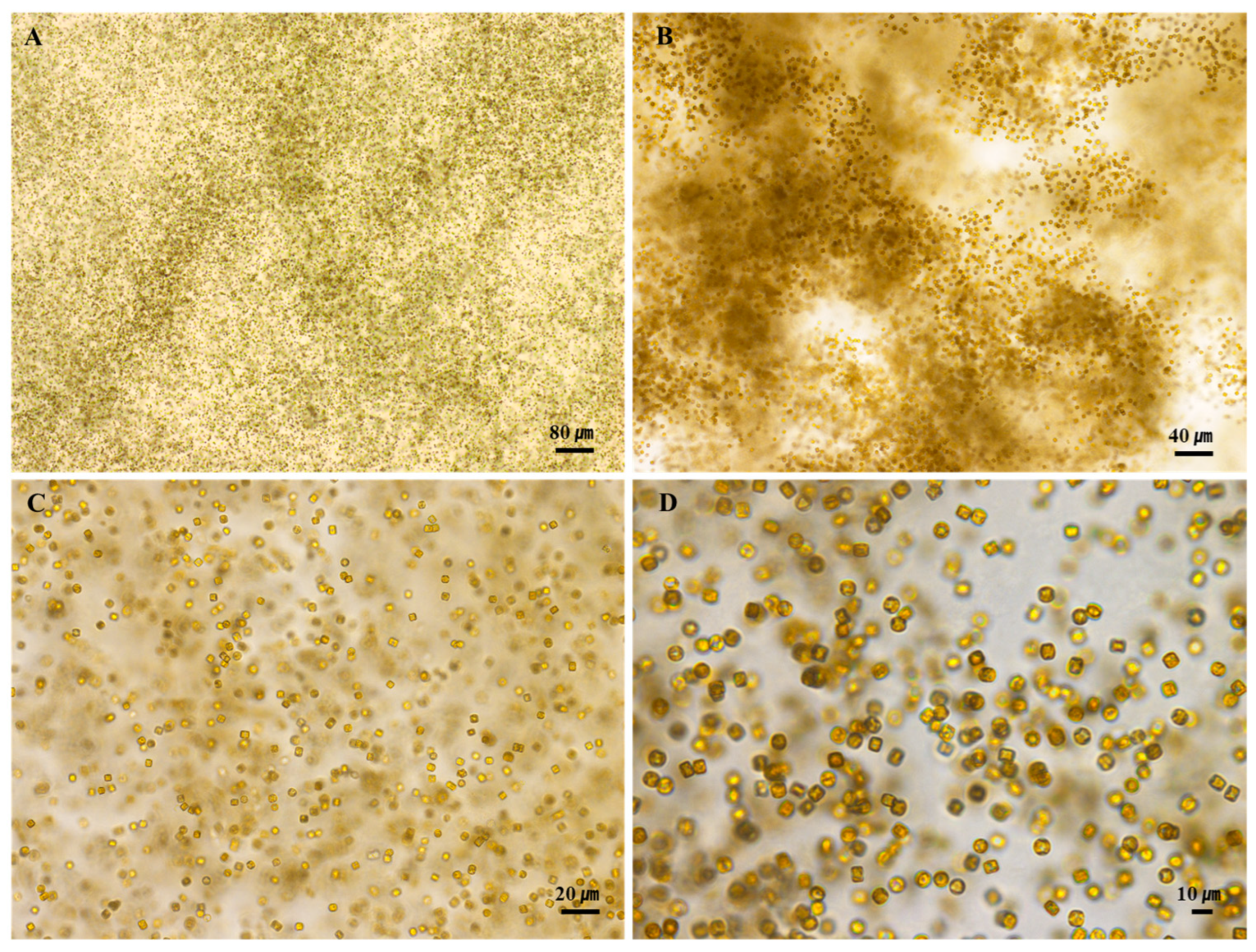
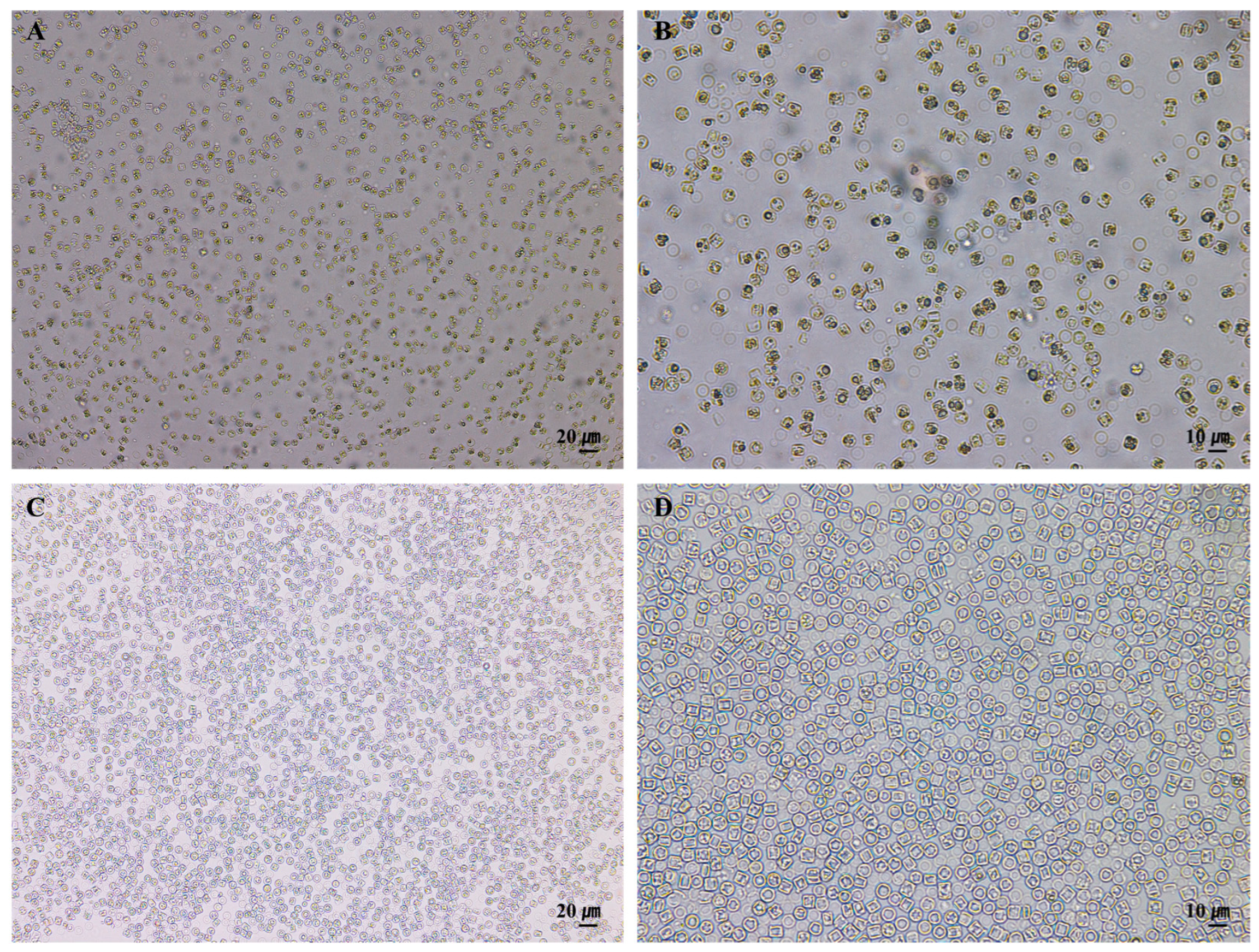
3.1. Morphology and Structure of S. meneghinianus Before Preprocessing
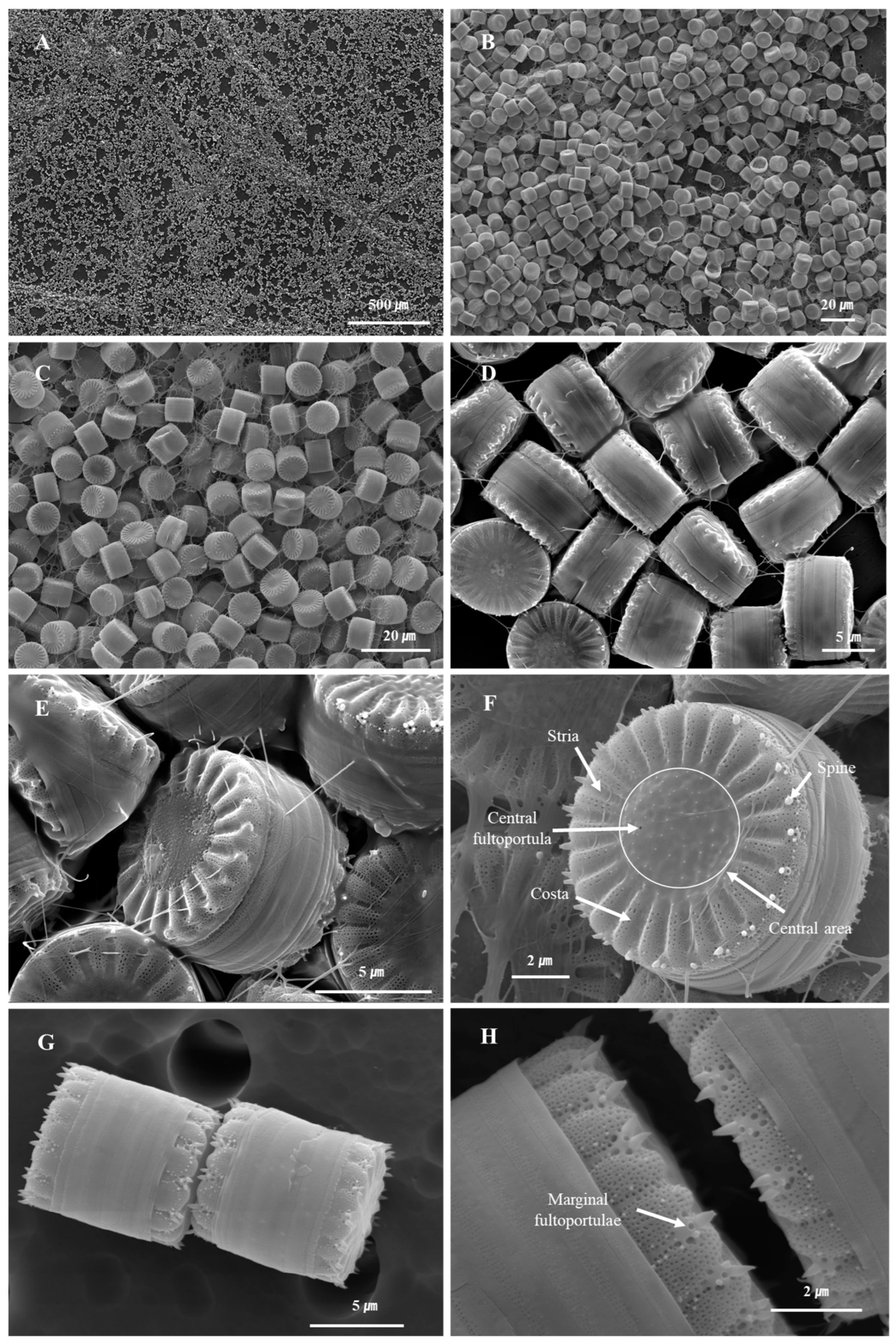
3.2. Effect of Pretreatment on the Purity and Microstructural Integrity of S. meneghinianus Biosilica
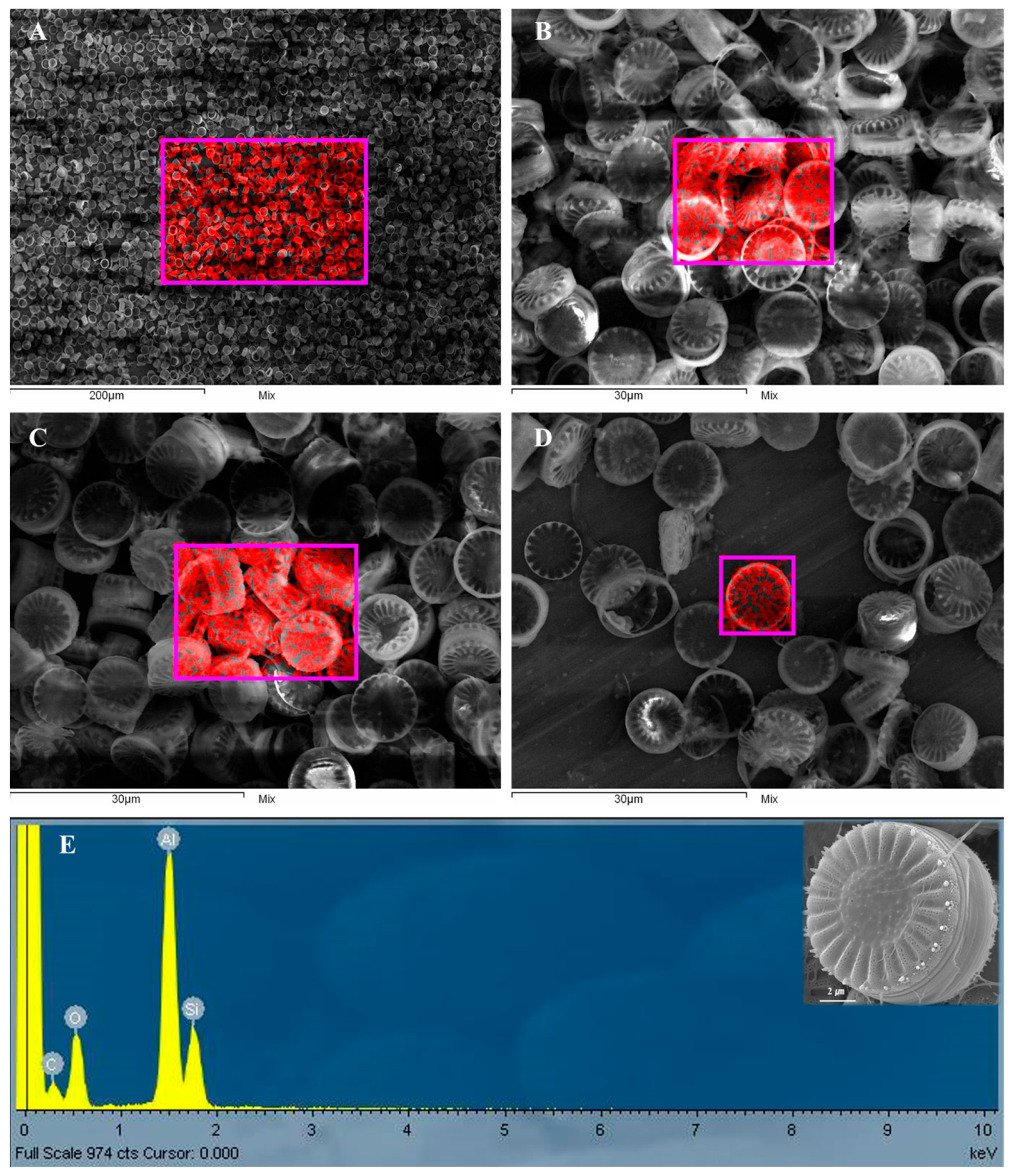
3.3. Assessment of Crystallinity and Elemental Composition in Pretreated S. meneghinianus
3.4. Optimization of Culture Conditions for S. meneghinianus Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| EDX | Energy Dispersive X-Ray Spectroscopy |
| AD | Andong Dam |
References
- Patrick, R. Ecology of freshwater diatoms and diatom communities. Biol. Diatoms 1977, 13, 284–332. [Google Scholar]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Raven, J.A. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 2009, 56, 177–192. [Google Scholar] [CrossRef]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Kröger, N.; Poulsen, N. Diatoms—From cell wall biogenesis to nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef]
- Nassif, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Townley, H.E. Diatom frustules: Physical, optical, and biotechnological applications. In The Diatom World; Springer: Berlin, Germany, 2011; pp. 273–289. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, chemical, and genetic techniques for diatom frustule modification: Applications in nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.A.D.A.A.; Abdullah, M.A. Novel drug and gene delivery system and imaging agent based on marine diatom biosilica nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef]
- Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics 2023, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic diatom biosilica and its potential for biomedical applications and prospects: A review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Villota-Enríquez, M.D.; Rodríguez-Páez, J.E. Bio-silica production from rice husk for environmental remediation: Removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2023, 301, 127671. [Google Scholar] [CrossRef]
- Kang, S.; Woo, Y.; Seo, Y.; Yoo, D.; Kwon, D.; Park, H.; Lee, S.D.; Yoo, H.Y.; Lee, T. A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System. Pharmaceutics 2024, 16, 1171. [Google Scholar] [CrossRef] [PubMed]
- Saros, J.E.; Anderson, N. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol. Rev. 2015, 90, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Kulikovskiy, M.; Genkal, S.; Maltsev, Y.; Glushchenko, A.; Kuznetsova, I.; Kapustin, D.; Gusev, E.; Martynenko, N.; Kociolek, J.P. Resurrection of the diatom genus Stephanocyclus (Coscinodiscophyceae: Stephanodiscaceae) on the basis of an integrated molecular and morphological approach. Fottea 2022, 22, 181–191. [Google Scholar] [CrossRef]
- Rabiee, N.; Khatami, M.; Jamalipour Soufi, G.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with invaluable applications in nanotechnology, biotechnology, and biomedicine: Recent advances. ACS Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef]
- Panwar, V.; Dutta, T. Diatom biogenic silica as a felicitous platform for biochemical engineering: Expanding frontiers. ACS Appl. Bio Mater. 2019, 2, 2295–2316. [Google Scholar] [CrossRef]
- Su, Y.; Lundholm, N.; Ellegaard, M. Effects of abiotic factors on the nanostructure of diatom frustules—Ranges and variability. Appl. Microbiol. Biotechnol. 2018, 102, 5889–5899. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharjya, R.; Marella, T.K.; Saxena, A.; Mishra, B.; Savio, S.; Congestri, R.; Sindhu, R.; Binod, P.; Tiwari, A. Production of lipids and proteins from marine diatoms under changing pH and silica. Bioresour. Technol. 2022, 362, 127766. [Google Scholar] [CrossRef]
- Crawford, S.A.; Higgins, M.J.; Mulvaney, P.; Wetherbee, R. Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy. J. Phycol. 2001, 37, 543–554. [Google Scholar] [CrossRef]
- Moreno, M.D.; Ma, K.; Schoenung, J.; Dávila, L.P. An integrated approach for probing the structure and mechanical properties of diatoms: Toward engineered nanotemplates. Acta Biomater. 2015, 25, 313–324. [Google Scholar] [CrossRef]
- Nocerino, V.; Miranda, B.; Tramontano, C.; Chianese, G.; Dardano, P.; Rea, I.; De Stefano, L. Plasmonic nanosensors: Design, fabrication, and applications in biomedicine. Chemosensors 2022, 10, 150. [Google Scholar] [CrossRef]
- Park, C.H.; Kwon, D.R.; Kim, K.; Sim, Y.B.; Hwang, S.J. Temperature-dependent akinete formation strategies of the harmful cyanobacterium Dolichospermum circinale. Harmful Algae 2024, 139, 102722. [Google Scholar] [CrossRef] [PubMed]
- Roubeix, V.; Lancelot, C. Effect of salinity on growth, cell size and silicification of an euryhaline freshwater diatom: Cyclotella meneghiniana Kütz. Transitional Waters Bull. 2008, 1, 1–38. [Google Scholar] [CrossRef]
- Chukhutsina, V.U.; Büchel, C.; Van Amerongen, H. Variations in the first steps of photosynthesis for the diatom Cyclotella meneghiniana grown under different light conditions. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 10–18. [Google Scholar] [CrossRef]
- Herth, W. The site of β-chitin fibril formation in centric diatoms. II. The chitin-forming cytoplasmic structures. J. Ultrastruct. Res. 1979, 68, 16–27. [Google Scholar] [CrossRef]
- Herth, W.; Barthlott, W. The site of β-chitin fibril formation in centric diatoms. I. Pores and fibril formation. J. Ultrastruct. Res. 1979, 68, 6–15. [Google Scholar] [CrossRef]
- de Brouwer, J.D.; Wolfstein, K.; Ruddy, G.K.; Jones, T.E.R.; Stal, L.J. Biogenic stabilization of intertidal sediments: The importance of extracellular polymeric substances produced by benthic diatoms. Microb. Ecol. 2005, 49, 501–512. [Google Scholar] [CrossRef]
- Bohórquez, J.; McGenity, T.J.; Papaspyrou, S.; García-Robledo, E.; Corzo, A.; Underwood, G.J. Different types of diatom-derived extracellular polymeric substances drive changes in heterotrophic bacterial communities from intertidal sediments. Front. Microbiol. 2017, 8, 245. [Google Scholar] [CrossRef]
- Cox, E.J. Morphology, cell wall, cytology, ultrastructure and morphogenetic studies: Overview and specific observations. In The Diatom World; Springer: Berlin, Germany, 2011; pp. 21–45. [Google Scholar] [CrossRef]
- Spinde, K.; Kammer, M.; Freyer, K.; Ehrlich, H.; Vournakis, J.N.; Brunner, E. Biomimetic silicification of fibrous chitin from diatoms. Chem. Mater. 2011, 23, 2973–2978. [Google Scholar] [CrossRef]
- Wolfe, A.P.; Siver, P.A. Three extant genera of freshwater thalassiosiroid diatoms from Middle Eocene sediments in northern Canada. Am. J. Bot. 2009, 96, 487–497. [Google Scholar] [CrossRef]
- LeDuff, P.; Rorrer, G.L. Formation of extracellular β-chitin nanofibers during batch cultivation of marine diatom Cyclotella sp. at silicon limitation. J. Appl. Phycol. 2019, 31, 3479–3490. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, D.; Lin, Z.; He, H.; Wen, X.; Wang, L.; Deng, F. Influences of pretreatment temperature on the surface silylation of diatomaceous amorphous silica with trimethylchlorosilane. J. Non-Cryst. Solids 2006, 352, 3762–3771. [Google Scholar] [CrossRef]
- Shabir, Q.; Pokale, A.; Loni, A.; Johnson, D.R.; Canham, L.T.; Fenollosa, R.; Tymczenko, M.; Rodríguez, I.; Meseguer, F.; Cros, A.; et al. Medically biodegradable hydrogenated amorphous silicon microspheres. Silicon 2011, 3, 173–176. [Google Scholar] [CrossRef]
- Shafik, H.M.; Herodek, S.; Presing, M.; Vörös, L.; Balogh, K.V. Growth of Cyclotella meneghiniana Kutz. II. Growth and cell composition under different growth rates with different limitingnutrient. In Annales de Limnologie-International Journal of Limnology; EDP Sciences: Les Ulis, France, 1997; Volume 33, pp. 223–233. [Google Scholar]
- Mitrovic, S.M.; Hitchcock, J.N.; Davie, A.W.; Ryan, D.A. Growth responses of Cyclotella meneghiniana (Bacillariophyceae) to various temperatures. J. Plankton Res. 2010, 32, 1217–1221. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef] [PubMed]
- NIBR. National List of Species of Korea; National Institute of Biological Resources: Incheon, Korea, 2024; Available online: https://www.kbr.go.kr/ (accessed on 29 January 2024).
- Yang, W.; Lopez, P.J.; Rosengarten, G. Diatoms: Self assembled silica nanostructures, and templates for bio/chemical sensors and biomimetic membranes. Analyst 2011, 136, 42–53. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Rosowski, J.R.; Gretz, M.R.; Roemer, S.C. Diatom extracellular polymeric substances: Function, fine structure, chemistry, and physiology. J. Phycol. 1993, 29, 537–566. [Google Scholar] [CrossRef]
- Almqvist, N.; Delamo, Y.; Smith, B.L.; Thomson, N.H.; Bartholdson, Å.; Lal, R.; Brzezinski, M.; Hansma, P.K. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 2001, 202, 518–532. [Google Scholar] [CrossRef]
- Khan, M.J.; Rai, A.; Ahirwar, A.; Sirotiya, V.; Mourya, M.; Mishra, S.; Schoefs, B.; Marchand, J.; Bhatia, S.K.; Varjani, S.; et al. Diatom microalgae as smart nanocontainers for biosensing wastewater pollutants: Recent trends and innovations. Bioengineered 2021, 12, 9531–9549. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Bose, R.; Dąbek, P.; Witkowski, A. Photonic nano-/microstructured diatom based biosilica in metal modification and removal—A review. Materials 2022, 15, 6597. [Google Scholar] [CrossRef]
- Suzuki, Y.; Takahashi, M. Growth responses of several diatom species isolated from various environments to temperature. J. Phycol. 1995, 31, 880–888. [Google Scholar] [CrossRef]
- Anderson, N.J. Diatoms, temperature and climatic change. Eur. J. Phycol. 2000, 35, 307–314. [Google Scholar] [CrossRef]
- Bruckner, C.G.; Kroth, P.G. Protocols for the removal of bacteria from freshwater benthic diatom cultures 1. J. Phycol. 2009, 45, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, W. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar. Biol. 1977, 39, 1–9. [Google Scholar] [CrossRef]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]
- Zobi, F. Diatom biosilica in targeted drug delivery and biosensing applications: Recent studies. Micro 2022, 2, 342–360. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple routes to smart nanostructured materials from diatom microalgae: A chemical perspective. Adv. Mater. 2018, 30, 1704289. [Google Scholar] [CrossRef]
- Sardo, A.; Orefice, I.; Balzano, S.; Barra, L.; Romano, G. Mini-review: Potential of diatom-derived silica for biomedical applications. Appl. Sci. 2021, 11, 4533. [Google Scholar] [CrossRef]
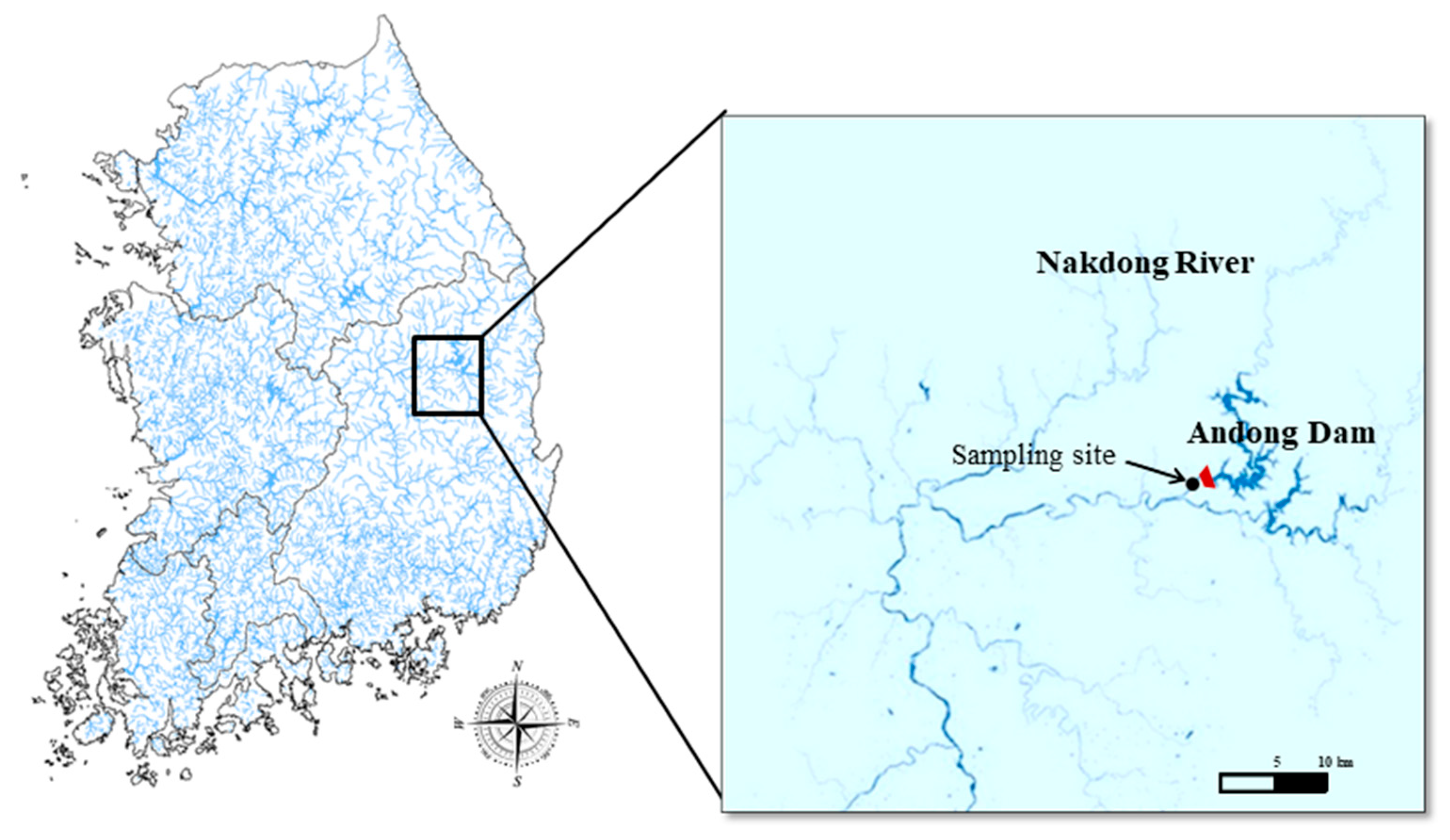


| Site | Latitude (N) | Longitude (E) | Temperature (°C) | DO (mg/L) | pH | Sample Species |
|---|---|---|---|---|---|---|
| AD | 37°33′38.69″ | 128°44′37.11″ | 12.7 | 10.13 | 7.79 | Stephanocyclus meneghinanus |
| Temperature (°C) | pH | Medium | Light Intensity (μmol/m2·s) | Analysis | |
|---|---|---|---|---|---|
| Exp. 1 | 20 | 7 | CHEV Ag WC DM Combo CR1 | 60 | Growth rate (μ, day−1) |
| Exp. 2 | 5 10 15 20 25 30 | 7 | DM medium | 60 | |
| Exp. 3 | 20 | 7 | DM medium | 0 5 15 30 50 100 |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 31.07 | 45.50 |
| Al | 47.30 | 39.26 |
| Si | 21.63 | 17.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Seo, Y.; Park, C.; Lee, S.D.; Lee, T. Cultivation Optimization and Structural Characterization of Stephanocyclus meneghinianus for Sustainable High-Quality Biosilica Production. Nanomaterials 2025, 15, 971. https://doi.org/10.3390/nano15130971
Kwon D, Seo Y, Park C, Lee SD, Lee T. Cultivation Optimization and Structural Characterization of Stephanocyclus meneghinianus for Sustainable High-Quality Biosilica Production. Nanomaterials. 2025; 15(13):971. https://doi.org/10.3390/nano15130971
Chicago/Turabian StyleKwon, Daeryul, Yoseph Seo, Chaehong Park, Sang Deuk Lee, and Taek Lee. 2025. "Cultivation Optimization and Structural Characterization of Stephanocyclus meneghinianus for Sustainable High-Quality Biosilica Production" Nanomaterials 15, no. 13: 971. https://doi.org/10.3390/nano15130971
APA StyleKwon, D., Seo, Y., Park, C., Lee, S. D., & Lee, T. (2025). Cultivation Optimization and Structural Characterization of Stephanocyclus meneghinianus for Sustainable High-Quality Biosilica Production. Nanomaterials, 15(13), 971. https://doi.org/10.3390/nano15130971









