Impact of Cetyl-Containing Ionic Liquids on Metal Halide Perovskite Structure and Photoluminescence
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herz, L.M. Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Lim, J.; Kober-Czerny, M.; Lin, Y.-H.; Ball, J.M.; Sakai, N.; Duijnstee, E.A.; Hong, M.J.; Labram, J.G.; Wenger, B.; Snaith, H.J. Long-range charge carrier mobility in metal halide perovskite thin-films and single crystals via transient photo-conductivity. Nat. Commun. 2022, 13, 4201. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, M.; Plochocka, P. Excitons in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 1903659. [Google Scholar] [CrossRef]
- Adjokatse, S.; Fang, H.-H.; Loi, M.A. Broadly tunable metal halide perovskites for solid-state light-emission applications. Mater. Today 2017, 20, 413–424. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Cho, H.; Lee, T.-W. Metal halide perovskite light emitters. Proc. Natl. Acad. Sci. USA 2016, 113, 11694–11702. [Google Scholar] [CrossRef]
- Richter, J.M.; Abdi-Jalebi, M.; Sadhanala, A.; Tabachnyk, M.; Rivett, J.P.H.; Pazos-Outón, L.M.; Gödel, K.C.; Price, M.; Deschler, F.; Friend, R.H. Enhancing photoluminescence yields in lead halide perovskites by photon recycling and light out-coupling. Nat. Commun. 2016, 7, 13941. [Google Scholar] [CrossRef]
- Goetz, K.P.; Taylor, A.D.; Paulus, F.; Vaynzof, Y. Shining Light on the Photoluminescence Properties of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 1910004. [Google Scholar] [CrossRef]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites For Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Niu, T.; Chao, L.; Gao, W.; Ran, C.; Song, L.; Chen, Y.; Fu, L.; Huang, W. Ionic Liquids-Enabled Efficient and Stable Perovskite Photovoltaics: Progress and Challenges. ACS Energy Lett. 2021, 6, 1453–1479. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Mudring, A.-V. Ionic liquids and deep eutectics as a transformative platform for the synthesis of nanomaterials. Chem. Commun. 2022, 58, 3865–3892. [Google Scholar] [CrossRef]
- Li, G.; Su, Z.; Li, M.; Yang, F.; Aldamasy, M.H.; Pascual, J.; Yang, F.; Liu, H.; Zuo, W.; Girolamo, D.D.; et al. Ionic Liquid Stabilizing High-Efficiency Tin HalidePerovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2101539. [Google Scholar] [CrossRef]
- Kim, G.-W.; Petrozza, A. Defect Tolerance and Intolerance in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 2001959. [Google Scholar] [CrossRef]
- Paek, S.; Schouwink, P.; Athanasopoulou, E.N.; Cho, K.T.; Grancini, G.; Lee, Y.; Zhang, Y.; Stellacci, F.; Nazeeruddin, M.K.; Gao, P. From Nano- to Micrometer Scale: The Role of Antisolvent Treatment on High Performance Perovskite Solar Cells. Chem. Mater. 2017, 29, 3490–3498. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, Y.; Hao, M.; Xin, F.; Zhou, Z.; Zhou, Y. Harnessing chemical functions of ionic liquids for perovskite solar cells. J. Energy Chem. 2022, 68, 797–810. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.; He, K.; Sun, Y.; Li, B.; Zhao, Q.; Du, B. Progress and challenges: A review of ionic liquid treatment for efficient and stable perovskite solar cells. J. Mater. Chem. C 2024, 12, 10837–10856. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Zhang, F.; Kang, C.; Li, Y. Ionic Liquids for Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2201292. [Google Scholar] [CrossRef]
- Luo, J.; Lin, F.; Yuan, J.; Wan, Z.; Jia, C. Application of Ionic Liquids and Derived Materials to High-Efficiency and Stable Perovskite Solar Cells. ACS Mater. Lett. 2022, 4, 1684–1715. [Google Scholar] [CrossRef]
- Deng, X.; Xie, L.; Wang, S.; Li, C.; Wang, A.; Yuan, Y.; Cao, Z.; Li, T.; Ding, L.; Hao, F. Ionic liquids engineering for high-efficiency and stable perovskite solar cells. Chem. Eng. J. 2020, 398, 125594. [Google Scholar] [CrossRef]
- Wang, F.; Duan, D.; Singh, M.; Sutter-Fella, C.M.; Lin, H.; Li, L.; Naumov, P.; Hu, H. Ionic Liquid Engineering in Perovskite Photovoltaics. Energy Environ. Mater. 2023, 6, e12435. [Google Scholar] [CrossRef]
- Yang, X.; Quan, K.; Wang, J.; Liu, J.; Liu, B.; Chen, J.; Guan, M.; Qiu, H. Particle size and pore adjustment of dendritic mesoporous silica using different long alkyl-chain imidazolium ionic liquids as templates. Microporous Mesoporous Mater. 2022, 345, 112249. [Google Scholar] [CrossRef]
- Wang, T.; Kaper, H.; Antonietti, M.; Smarsly, B. Templating Behavior of a Long-Chain Ionic Liquid in the Hydrothermal Synthesis of Mesoporous Silica. Langmuir 2007, 23, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, F.; Shahrokhian, S.; Mirzaei, Y. Effect of Long-Chain Ionic Liquids on the Capacitive Performance of Carbon Nanotube-Sulfonated Polyaniline Hydrogels for Energy Storage Applications. J. Phys. Chem. C 2020, 124, 9810–9821. [Google Scholar] [CrossRef]
- Shen, J.; Wang, D.; Yang, L.; Wang, T.; Yang, Q.; Wang, J. Synthesis of a bilayered SDS/ionic liquid stabilized ferrofluid and magnetic cubic mesoporous silica using a long chain ionic liquid. New J. Chem. 2023, 47, 362–372. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.; Liang, X.; Wang, F.; Xu, Y.; Lin, H.; Hu, R.; Hu, H. Long-chain organic molecules enable mixed dimensional perovskite photovoltaics: A brief view. Front. Chem. 2024, 11, 1341935. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Di, J.; Zhang, P.; Xia, J.; Dai, S.; Li, H. Ionic liquid-induced strategy for porous perovskite-like PbBiO2Br photocatalysts with enhanced photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2017, 206, 127–135. [Google Scholar] [CrossRef]
- Nandwani, S.K.; Malek, N.I.; Lad, V.N.; Chakraborty, M.; Gupta, S. Study on interfacial properties of Imidazolium ionic liquids as surfactant and their application in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 383–393. [Google Scholar] [CrossRef]
- Chadha, C.; Singh, G.; Singh, G.; Kumar, H.; Kang, T.S. Modulating the mixed micellization of CTAB and an ionic liquid 1-hexadecyl-3-methylimidazollium bromide via varying physical states of ionic liquid. RSC Adv. 2016, 6, 38238–38251. [Google Scholar] [CrossRef]
- Zech, O.; Thomaier, S.; Bauduin, P.; Rück, T.; Touraud, D.; Kunz, W. Microemulsions with an Ionic Liquid Surfactant and Room Temperature Ionic Liquids As Polar Pseudo-Phase. J. Phys. Chem. B 2009, 113, 465–473. [Google Scholar] [CrossRef]
- Xia, X.; Wan, R.; Wang, P.; Huo, W.; Dong, H.; Du, Q. Toxicity of imidazoles ionic liquid [C16mim]Cl to Hela cells. Ecotoxicol. Environ. Saf. 2018, 162, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Nancharaiah, Y.V.; Venugopalan, V.P. Long alkyl-chain imidazolium ionic liquids: Antibiofilm activity against phototrophic biofilms. Colloids Surf. B Biointerfaces 2017, 155, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-H.; Ho, W.-Y.; Yeh, L.-H.; Cheng, Y.-S.; Chou, T.-H. Effects of 1-hexadecyl-3-methylimidazolium ionic liquids on the physicochemical characteristics and cytotoxicity of phosphatidylcholine vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1083–1091. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Kullmer, C.N.P.; Ta, D.; Chen, C.Y.; Cieker, C.J.; Annunziata, O.; Dzyuba, S.V. Hexadecyl-Containing Organic Salts as Novel Organogelators forIonic, Eutectic, and Molecular Liquids. ACS Omega 2019, 4, 9400–9406. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, F.; Lu, L.; Wang, T.; Yang, G.; Liang, X.; Zhou, X.; Sun, X.; Li, Q.; Li, Y.; et al. Enhancing the humidity resistance of perovskite photovoltaics: Comprehensive PbI2 management utilizing long-chain ionic liquid. Nano Energy 2025, 136, 110716. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Wang, Y.; Wang, Z.; Wong, W.; Li, C. Halide perovskite based on hydrophobic ionic liquid for stability improving and its application in high-efficient photovoltaic cell. Electrochim. Acta 2019, 303, 133–139. [Google Scholar] [CrossRef]
- Brittman, S.; Adhyaksa, G.W.P.; Garnett, E.C. The Expanding World of Hybrid Perovskites: Materials Properties and Emerging Applications. MRS Commun. 2015, 5, 7–26. [Google Scholar] [CrossRef]
- Chang, C.; Zou, X.; Cheng, J.; Ling, T.; Yao, Y.; Chen, D. Influence of Solution Deposition Process on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films for Solar Cells. Materials 2019, 12, 2494. [Google Scholar] [CrossRef]
- Leyre, S.; Coutino-Gonzalez, E.; Ryckaert, J.J.J.J.; Poelman, Y.M.D.; Smet, P.F.; Durinck, G.; Hofkens, J.; Deconinck, G.; Hanselaer, P. Absolute Determination of Photoluminescence Quantum Efficiency Using an Integrating Sphere Setup. Rev. Sci. Instrum. 2014, 85, 123115. [Google Scholar] [CrossRef]
- Labsphere. Integrating Sphere Theory and Applications. Available online: https://www.labsphere.com/wp-content/uploads/2021/09/Integrating-Sphere-Theory-and-Applications.pdf (accessed on 14 March 2025).
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 16, Revision C.01; Gaussian, Inc.: Pittsburgh, PA, USA, 2019.
- Mannino, G.; Deretzis, I.; Smecca, E.; La Magna, A.; Alberti, A.; Ceratti, D.; Cahen, D. Temperature-Dependent Optical Band Gap in CsPbBr3, MAPbBr3, and FAPbBr3 Single Crystals. J. Phys. Chem. Lett. 2020, 11, 2490–2496. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ottesen, M.; Wiedmann, S.; Wharmby, M.; Küchler, R.; Yuan, H.; Debroye, E.; Steele, J.A.; Martens, J.; Hussey, N.E.; et al. Tracking Structural Phase Transitions in Lead-Halide Perovskites by Means of Thermal Expansion. Adv. Mater. 2019, 31, 1900521. [Google Scholar] [CrossRef]
- Wang, K.-H.; Li, L.-C.; Shellaiah, M.; Sun, K.W. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef]
- Mahapatra, A.; Chakraborty, M.; Barik, S.; Sarkar, M. Comparison between pyrrolidinium-based and imidazolium-based dicationic ionic liquids: Intermolecular interaction, structural organization, and solute dynamics. Phys. Chem. Chem. Phys. 2021, 23, 21029–21041. [Google Scholar] [CrossRef]
- Yu, J.C.; Kim, D.B.; Jung, E.D.; Lee, B.R.; Song, M.H. High-performance perovskite light-emitting diodes via morphological control of perovskite films. Nanoscale 2016, 8, 7036–7042. [Google Scholar] [CrossRef] [PubMed]

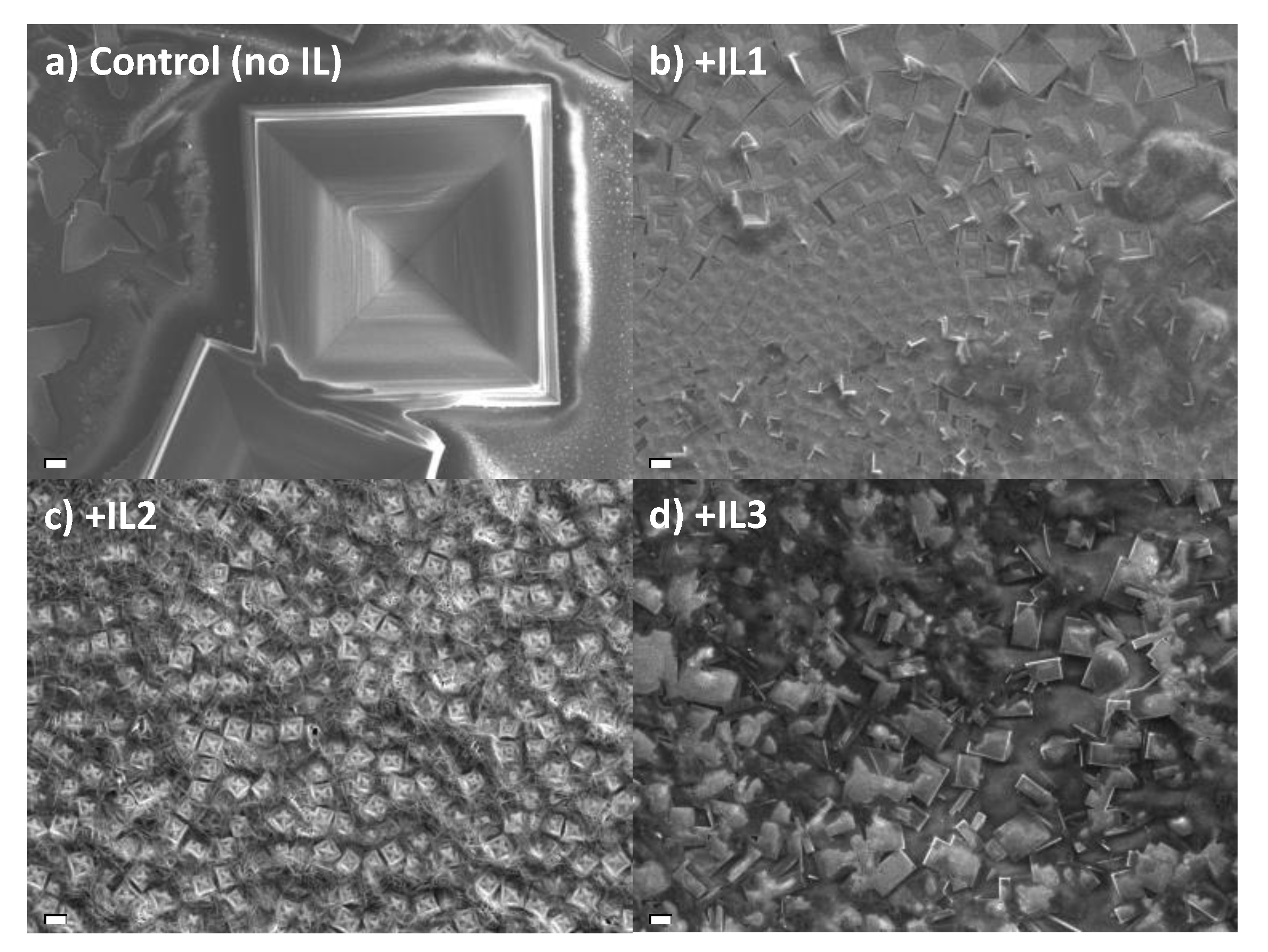
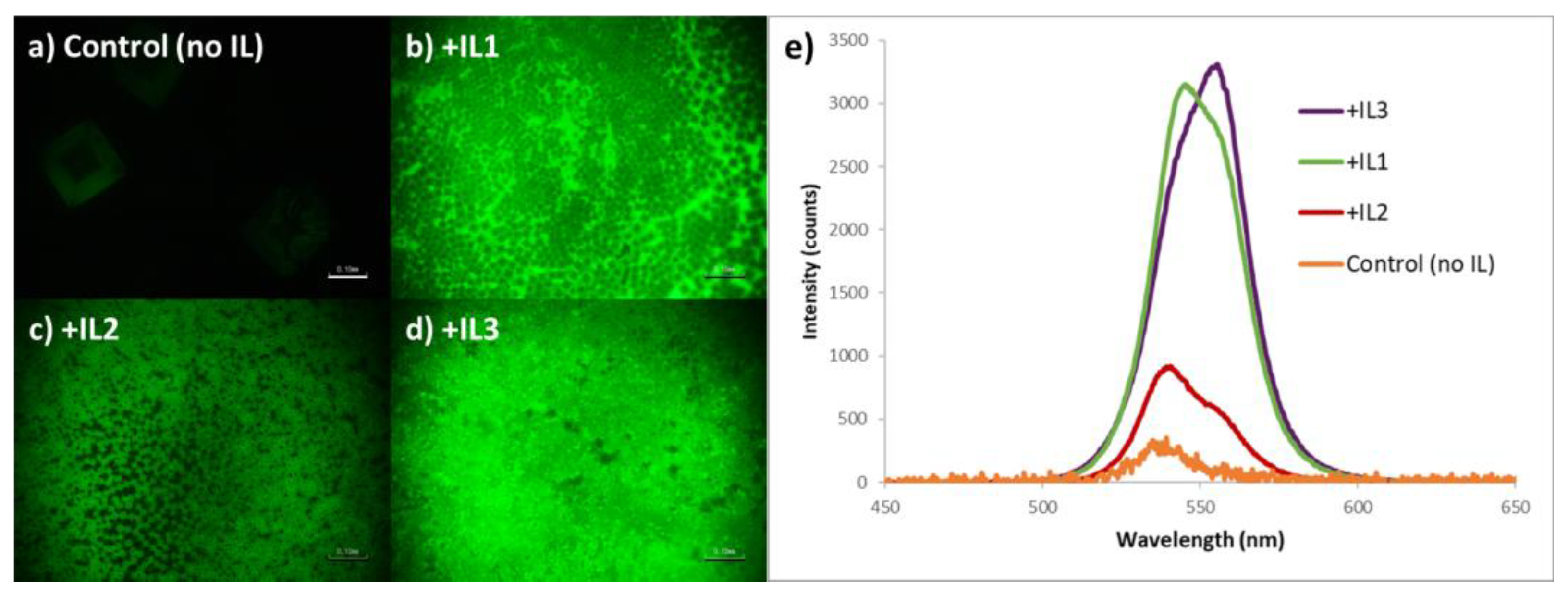
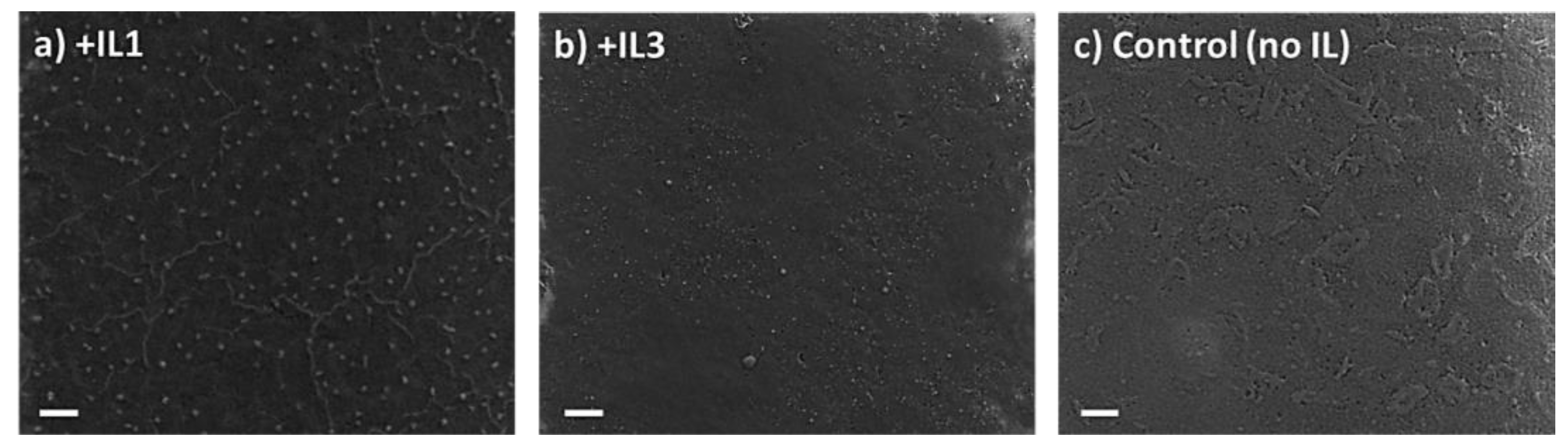
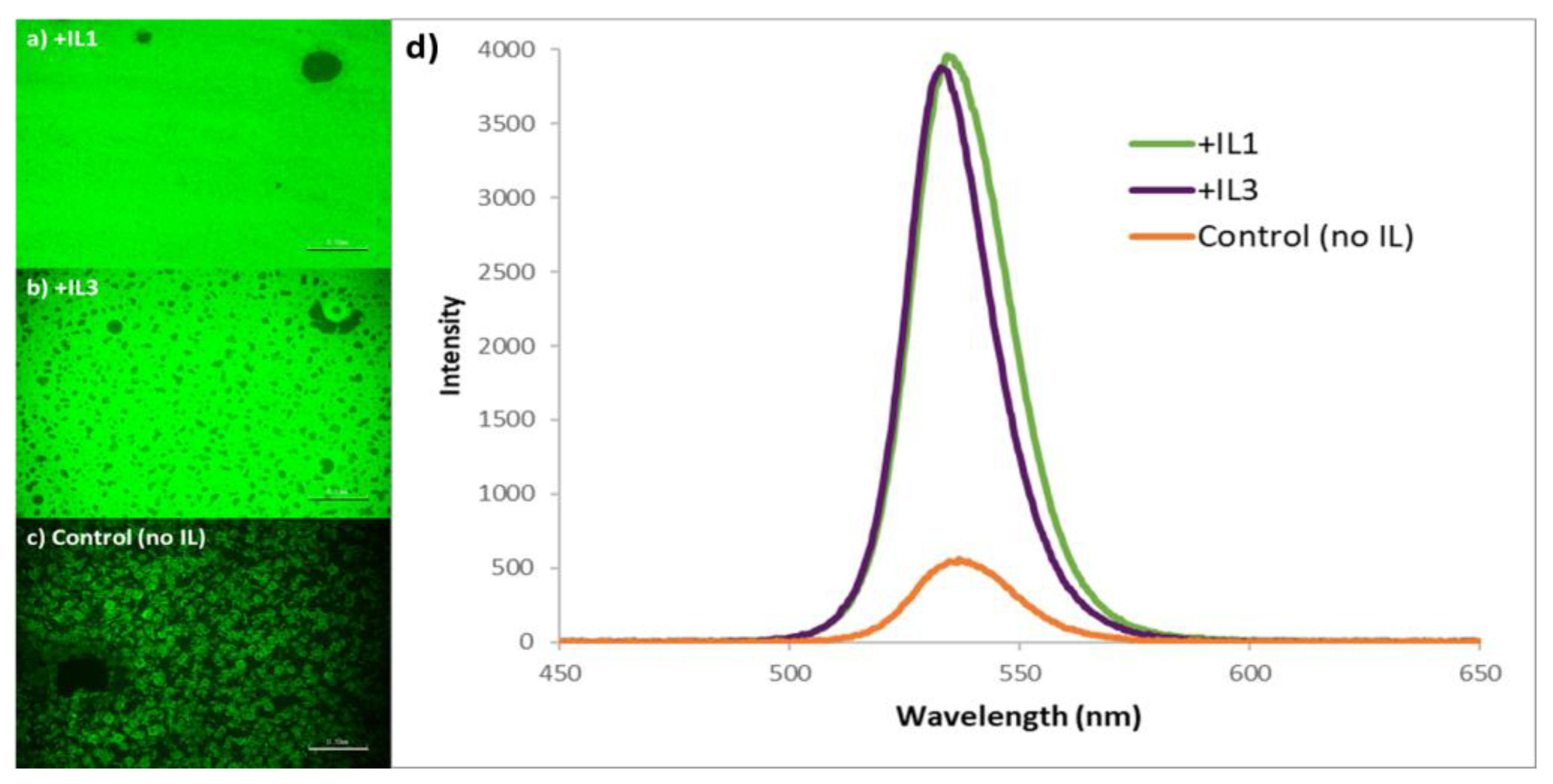


| Sample | Crystallite Size (nm) |
|---|---|
| One-Step Static Method | |
| Control (no IL) | 114.9 (4.1) |
| +IL1 | 124.5 (2.8) |
| +IL2 | 84.9 (1.4) |
| +IL3 | 77.0 (1.3) |
| Sample | Crystallite Size (nm) |
|---|---|
| Two-Step Spin Coat Method | |
| Control (no IL) | 85.5 (7.5) |
| +IL1 | 46.1 (7.6) |
| +IL3 | 52.3 (12.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubbs, M.A.; Gonzalez-Rodriguez, R.; Dzyuba, S.V.; Janesko, B.G.; Coffer, J.L. Impact of Cetyl-Containing Ionic Liquids on Metal Halide Perovskite Structure and Photoluminescence. Nanomaterials 2025, 15, 964. https://doi.org/10.3390/nano15130964
Grubbs MA, Gonzalez-Rodriguez R, Dzyuba SV, Janesko BG, Coffer JL. Impact of Cetyl-Containing Ionic Liquids on Metal Halide Perovskite Structure and Photoluminescence. Nanomaterials. 2025; 15(13):964. https://doi.org/10.3390/nano15130964
Chicago/Turabian StyleGrubbs, Maegyn A., Roberto Gonzalez-Rodriguez, Sergei V. Dzyuba, Benjamin G. Janesko, and Jeffery L. Coffer. 2025. "Impact of Cetyl-Containing Ionic Liquids on Metal Halide Perovskite Structure and Photoluminescence" Nanomaterials 15, no. 13: 964. https://doi.org/10.3390/nano15130964
APA StyleGrubbs, M. A., Gonzalez-Rodriguez, R., Dzyuba, S. V., Janesko, B. G., & Coffer, J. L. (2025). Impact of Cetyl-Containing Ionic Liquids on Metal Halide Perovskite Structure and Photoluminescence. Nanomaterials, 15(13), 964. https://doi.org/10.3390/nano15130964









