Actuator-Driven, Purge-Free Formaldehyde Gas Sensor Based on Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the HCHO Sensor
2.3. Generation of HCHO and Acetaldehyde Vapors
2.4. Generation of VOC Vapors
2.5. Evaluation of Sensors
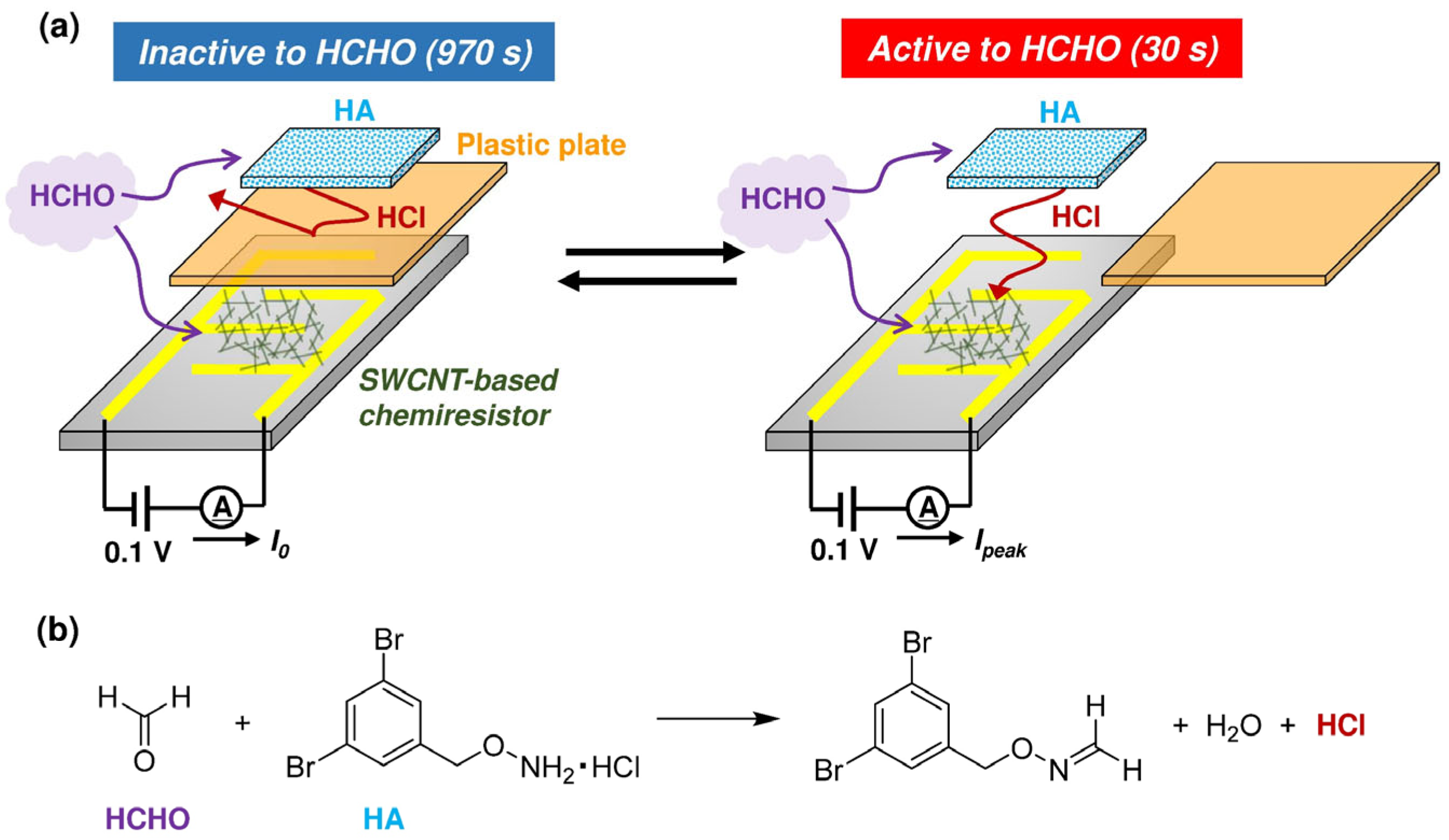
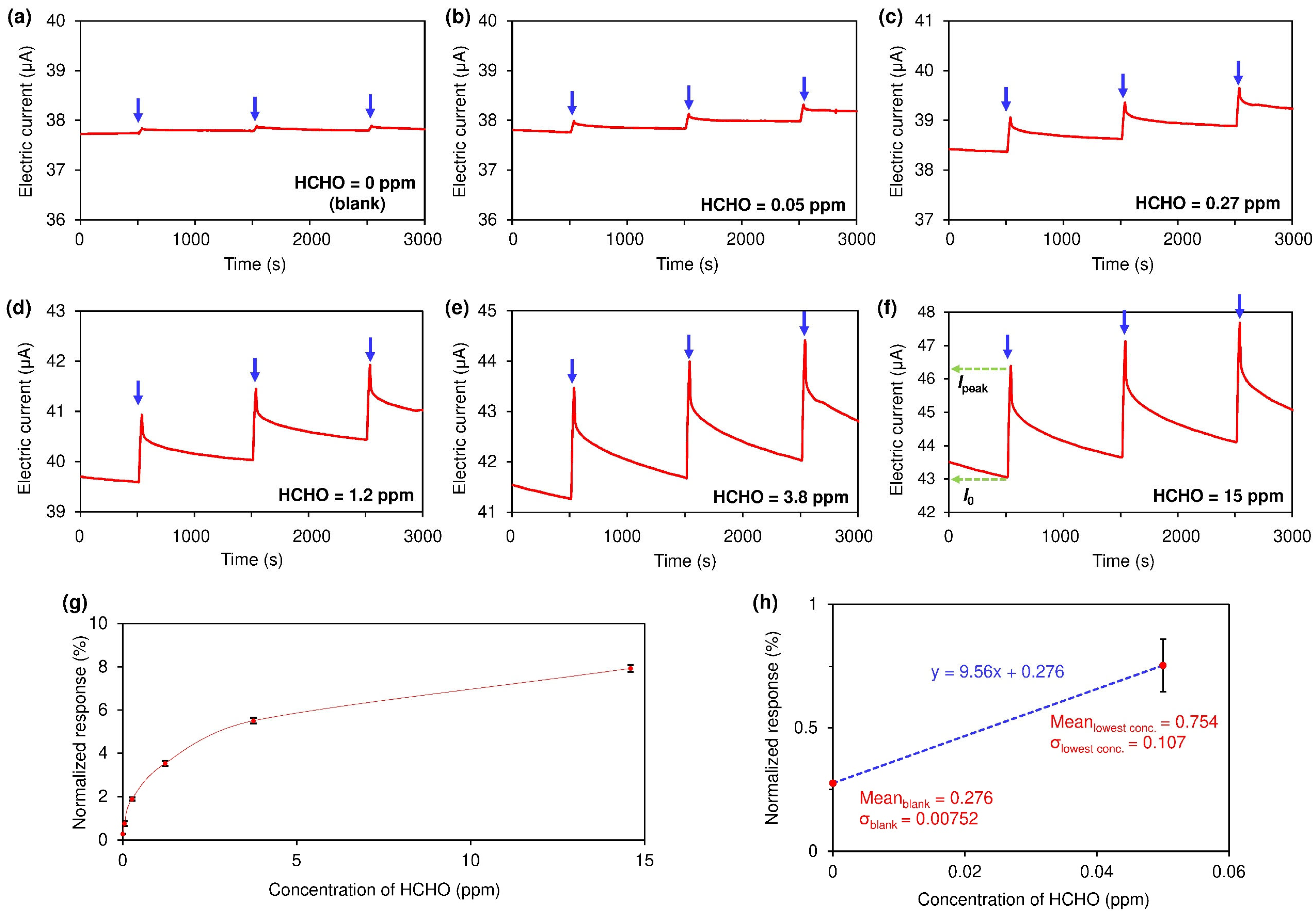
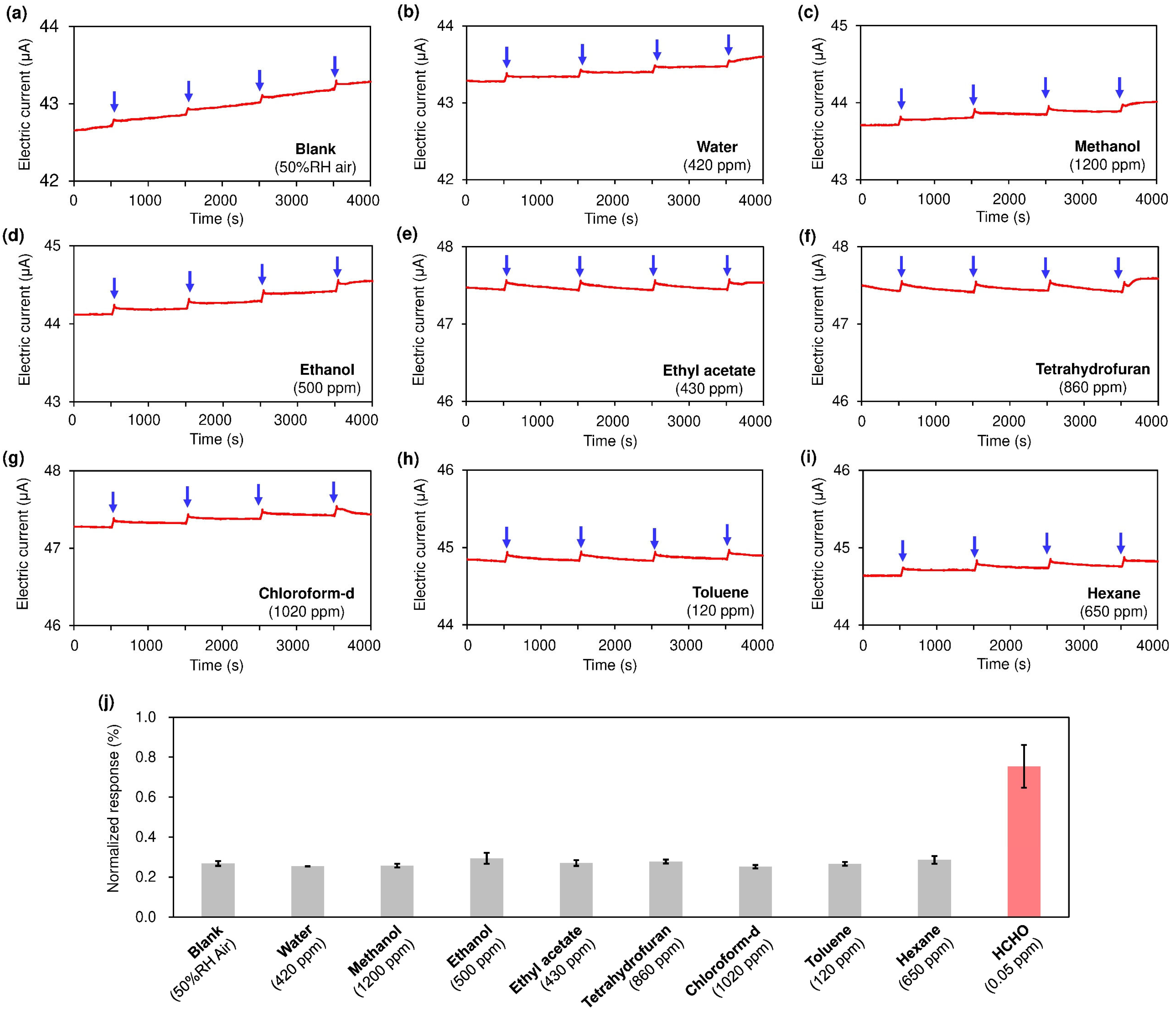
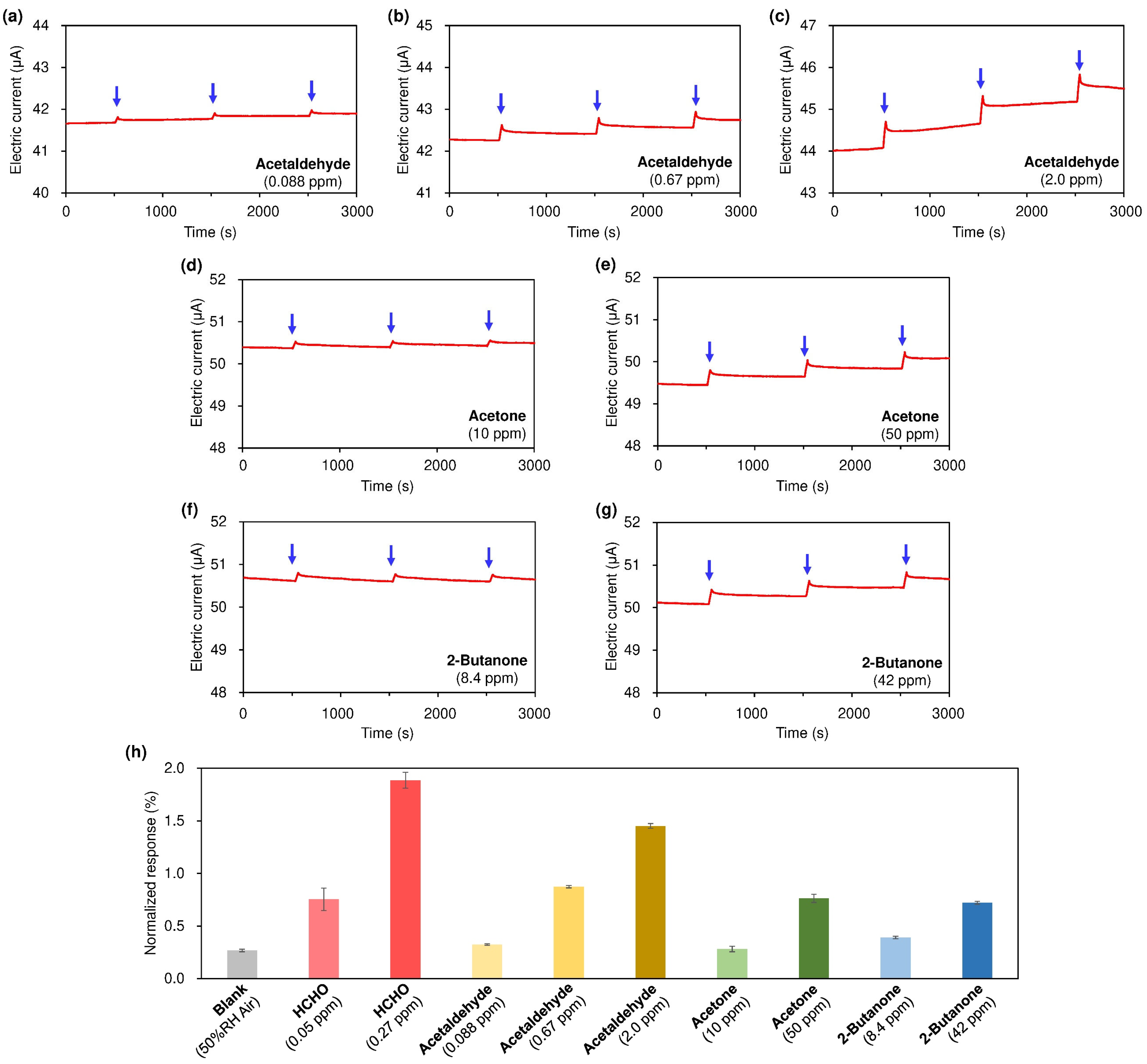
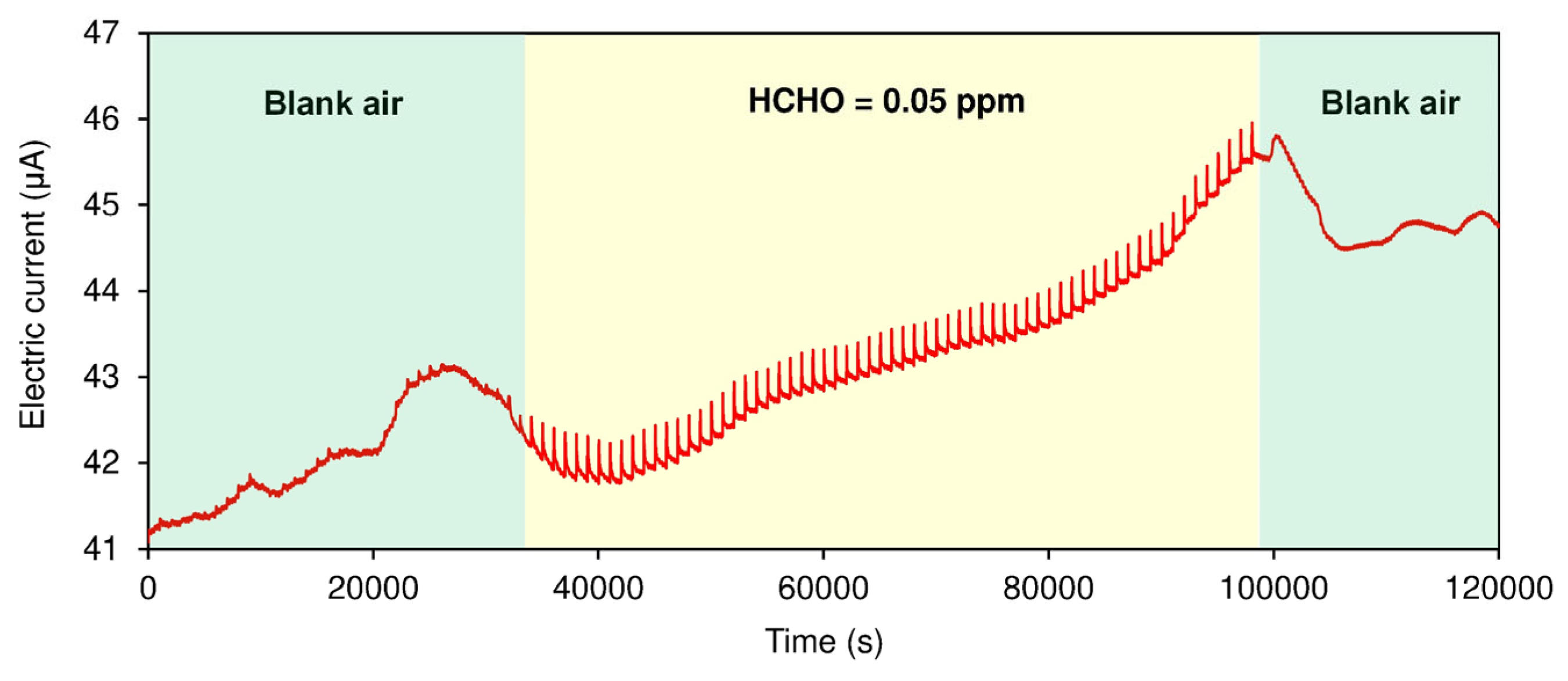
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 100, 2536–2572. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Formaldehyde (Diffusive Samplers). Available online: https://www.osha.gov/sites/default/files/methods/osha-1007.pdf (accessed on 11 April 2025).
- Formaldehyde HCHO. Available online: https://www.gastec.co.jp/files/user/asset/pdf/en/detector_tube/91LL.pdf (accessed on 11 April 2025).
- Chung, P.-R.; Tzeng, C.-T.; Ke, M.-T.; Lee, C.-Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yuan, Y.J. Thin-Film Sensors for Detection of Formaldehyde: A Review. IEEE Sens. J. 2015, 15, 6749–6760. [Google Scholar] [CrossRef]
- Kukkar, D.; Vellingiri, K.; Kaur, R.; Bhardwaj, S.K.; Deep, A.; Kim, K.-H. Nanomaterials for Sensing of Formaldehyde in Air: Principles, Applications, and Performance Evaluation. Nano Res. 2019, 12, 225–246. [Google Scholar] [CrossRef]
- Roy, S.; Pan, S.; De, P. Recent Progress on Polymeric Probes for Formaldehyde Sensing: A Comprehensive Review. Sci. Technol. Adv. Mater. 2024, 25, 2423597. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D. Organic-Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Lim, H.-R.; Kim, H.S.; Qazi, R.; Kwon, Y.-T.; Jeong, J.-W.; Yeo, W.-H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, 190192. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Recent Advances in Applications of Sorted Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Tang, D.-M.; Cretu, O.; Ishihara, S.; Zheng, Y.; Otsuka, K.; Xiang, R.; Maruyama, S.; Cheng, H.-M.; Liu, C.; Golberg, D. Chirality Engineering for Carbon Nanotube Electronics. Nat. Rev. Electr. Eng. 2024, 1, 149–162. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Preparation of Layered Organic-Inorganic Nanohybrid Thin Films of Molybdenum Trioxide with Polyaniline Derivatives for Aldehyde Gases Sensors of Several Tens ppb Level. Sens. Actuators B 2008, 128, 512–520. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Gong, L.; Li, L. High Sensitive and Selective Formaldehyde Sensors Based on Nanoparticle-assembled ZnO Micro-Octahedrons Synthesized by Homogeneous Precipitation Method. Sens. Actuators B 2011, 160, 364–370. [Google Scholar] [CrossRef]
- Lu, Y.; Meyyappan, M.; Li, J. A Carbon-Nanotube-Based Sensor Array for Formaldehyde Detection. Nanotechnology 2011, 22, 055502. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.; Hong, S.; Chung, T.D. Formaldehyde Gas Sensing Chip Based on Single-Walled Carbon Nanotubes and Thin Water Layer. Chem. Commun. 2011, 47, 2892–2894. [Google Scholar] [CrossRef]
- Xie, H.; Sheng, C.; Chen, X.; Wang, X.; Li, Z.; Zhou, J. Multi-Wall Carbon Nanotube Gas Sensors Modified with Amino-Group to Detect Low Concentration of Formaldehyde. Sens. Actuator B-Chem. 2012, 168, 34–38. [Google Scholar] [CrossRef]
- Shi, D.; Wei, L.; Wang, J.; Zhao, J.; Chen, C.; Xu, D.; Geng, H.; Zhang, Y. Solid Organic Acid Tetrafluorohydroquinone Functionalized Signle-Walled Carbon Nanotube Chemiresistive Sensors for Highly Sensitive and Selective Formaldehyde Detection. Sens. Actuator B Chem. 2013, 177, 370–375. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Nakanishi, T.; Tanaka, T.; Kataura, H. Amperometric Detection of Sub-ppm Formaldehyde Using SWCNTs and Hydroxylamines: A Referenced Chemiresistive System. ACS Sens. 2017, 2, 1405–1409. [Google Scholar] [CrossRef]
- Gu, F.; Di, M.; Han, D.; Hong, S.; Wang, Z. Atomically Dispersed Au on In2O3 Nanosheets for Highly Sensitive and Selective Detection of Formaldehyde. ACS Sens. 2020, 5, 2611–2619. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Y.; Xu, D.; Zi, B.; Zeng, J.; Lu, Q.; Xiong, K.; Zhang, J.; Zhao, J.; Liu, Q. Ultrasensitive Formaldehyde Sensor Based on SnO2 with Rich Adsorbed Oxygen Derived from a Metal Organic Framework. ACS Sens. 2022, 7, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Snow, E.S.; Perkins, F.K.; Robinson, J.A. Chemical Vapor Detection Using Single-Walled Carbon Nanotubes. Chem. Soc. Rev. 2006, 35, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Ishihara, S.; Bahuguna, A.; Kumar, S.; Krishnan, V.; Labuta, J.; Nakanishi, T.; Tanaka, T.; Kataura, H.; Kon, Y.; Hong, D. Cascade Reaction-Based Chemiresistive Array for Ethylene Sensing. ACS Sens. 2020, 5, 1405–1410. [Google Scholar] [CrossRef]
- Luo, S.-X.L.; Swager, T.M. Chemiresistive Sensing with Functionalized Carbon Nanotubes. Nat. Rev. Methods Primers 2023, 3, 73. [Google Scholar] [CrossRef]
- Luo, K.; Peng, H.; Zhang, B.; Chen, L.; Zhang, P.; Peng, Z.; Fu, X. Advances in Carbon Nanotube-Based Gas Sensors: Exploring the Path to the Future. Coord. Chem. Rev. 2024, 518, 216049. [Google Scholar] [CrossRef]
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef]
- Chahal, M.K.; Sumita, M.; Labuta, J.; Payne, D.T.; Hill, J.P.; Yamauchi, Y.; Nakanishi, T.; Tanaka, T.; Kataura, H.; Koga, K.; et al. Selective Detection of Toxic C1 Chemicals Using a Hydroxylamine-Based Chemiresistive Sensor Array. ACS Sens. 2023, 8, 1585–1592. [Google Scholar] [CrossRef]
- Yomogida, Y.; Tanaka, T.; Zhang, M.; Yudasaka, M.; Wei, X.; Kataura, H. Industrial-Scale Separation of High-Purity Single-Chirality Single-Wall Carbon Nanotubes for Biological Imaging. Nat. Commun. 2016, 7, 12056. [Google Scholar] [CrossRef]
- Ishihara, S.; Azzarelli, J.M.; Krikorian, M.; Swager, T.M. Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc. 2016, 138, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; O’Kelly, C.J.; Tanaka, T.; Kataura, H.; Labuta, J.; Shingaya, Y.; Nakayama, T.; Ohsawa, T.; Nakanishi, T.; Swager, T.M. Metallic Versus Semiconducting SWCNT Chemiresistors: A Case for Separated SWCNTs Wrapped by a Metallosupramolecular Polymer. ACS Appl. Mater. Interface 2017, 9, 38062–38067. [Google Scholar] [CrossRef] [PubMed]
- Graupner, R.; Abraham, J.; Vencelová, A.; Seyller, T.; Hennrich, F.; Kappes, M.M.; Hirsch, A.; Ley, L. Doping of Single-Walled Carbon Nanotube Bundles by Brønsted Acids. Phys. Chem. Chem. Phys. 2003, 5, 5472–5476. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Li, J.; Cabezas, A.L.; Zhang, S.-L. Characterization of Acid-Treated Carbon Nanotube Thin Films by Means of Raman Spectroscopy and Field-Effect Response. Chem. Phys. Lett. 2009, 476, 258–261. [Google Scholar] [CrossRef]
- Tey, J.N.; Ho, X.; Wei, J. Effect of Doping on Single-Walled Carbon Nanotubes Network of Different Metallicity. Nanoscale Res. Lett. 2012, 7, 548. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- McMurry, J. Organic Chemistry, 4th ed.; Brooks/Cole Publishing Company: Pacific Grove, CA, USA, 1996; pp. 726–727. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, S.; Chahal, M.K.; Labuta, J.; Tanaka, T.; Kataura, H.; Hill, J.P.; Nakanishi, T. Actuator-Driven, Purge-Free Formaldehyde Gas Sensor Based on Single-Walled Carbon Nanotubes. Nanomaterials 2025, 15, 962. https://doi.org/10.3390/nano15130962
Ishihara S, Chahal MK, Labuta J, Tanaka T, Kataura H, Hill JP, Nakanishi T. Actuator-Driven, Purge-Free Formaldehyde Gas Sensor Based on Single-Walled Carbon Nanotubes. Nanomaterials. 2025; 15(13):962. https://doi.org/10.3390/nano15130962
Chicago/Turabian StyleIshihara, Shinsuke, Mandeep K. Chahal, Jan Labuta, Takeshi Tanaka, Hiromichi Kataura, Jonathan P. Hill, and Takashi Nakanishi. 2025. "Actuator-Driven, Purge-Free Formaldehyde Gas Sensor Based on Single-Walled Carbon Nanotubes" Nanomaterials 15, no. 13: 962. https://doi.org/10.3390/nano15130962
APA StyleIshihara, S., Chahal, M. K., Labuta, J., Tanaka, T., Kataura, H., Hill, J. P., & Nakanishi, T. (2025). Actuator-Driven, Purge-Free Formaldehyde Gas Sensor Based on Single-Walled Carbon Nanotubes. Nanomaterials, 15(13), 962. https://doi.org/10.3390/nano15130962






