Strategic Doping for Precise Structural Control and Intense Photocurrents Under Visible Light in Ba2M0.4Bi1.6O6 (M = La, Ce, Pr, Pb, Y) Double Perovskites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Powder Samples
2.2. Preparation of Film Photoelectrodes
2.3. Material Characterization
3. Results and Discussion
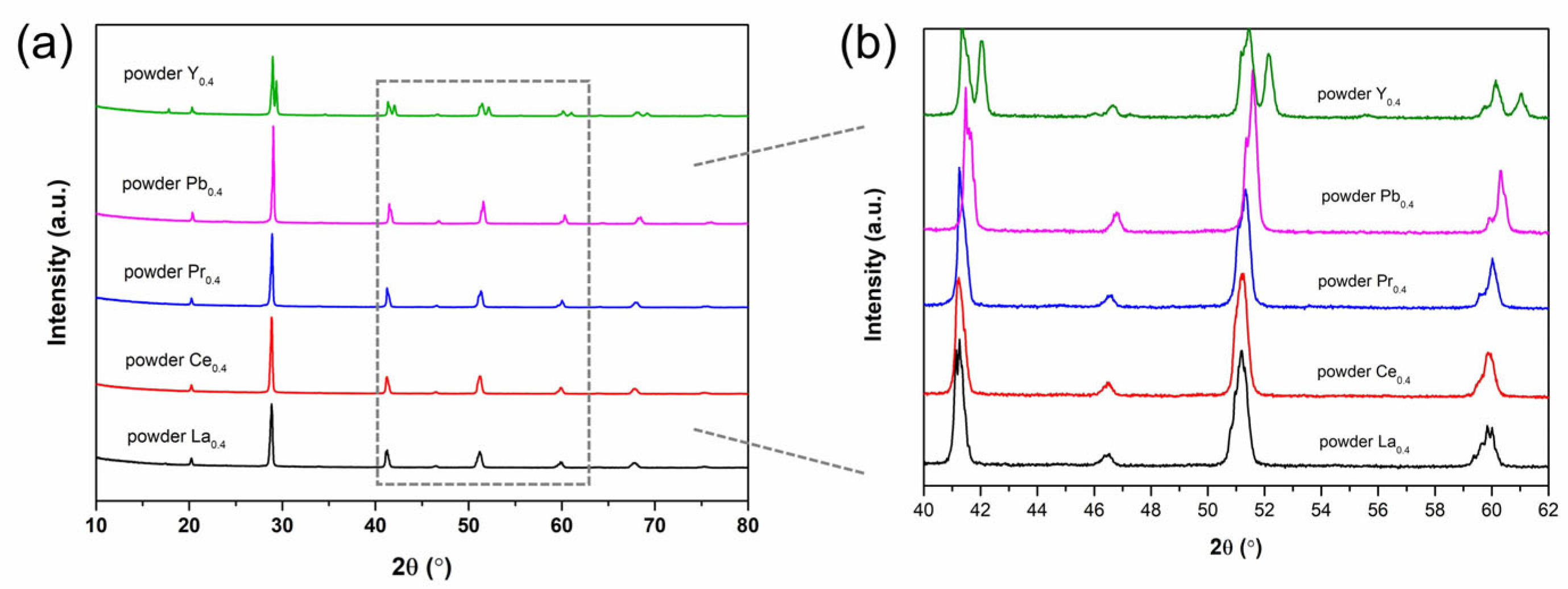
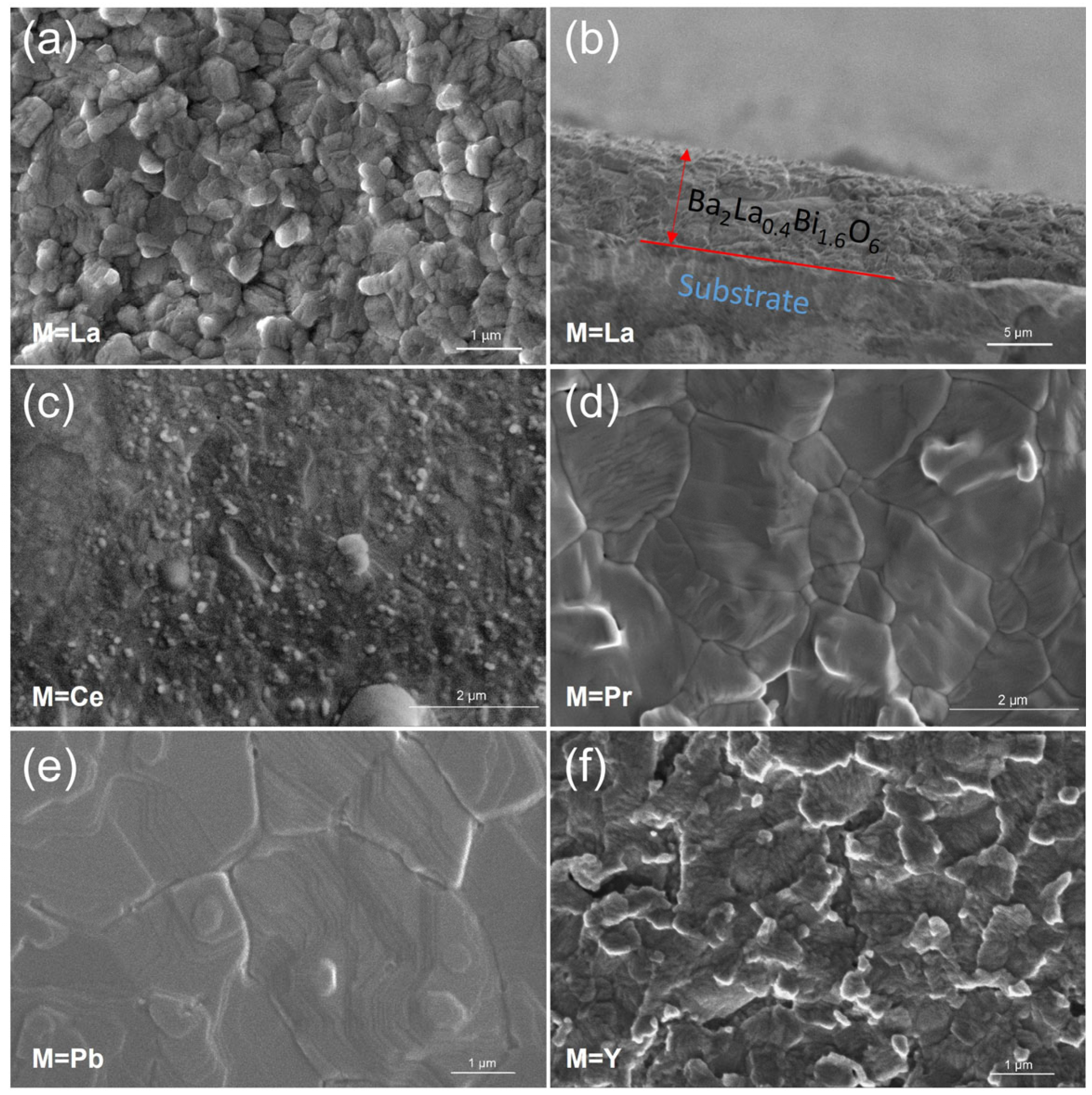
3.1. Crystal Structure
3.2. Optical Properties and Band Structure
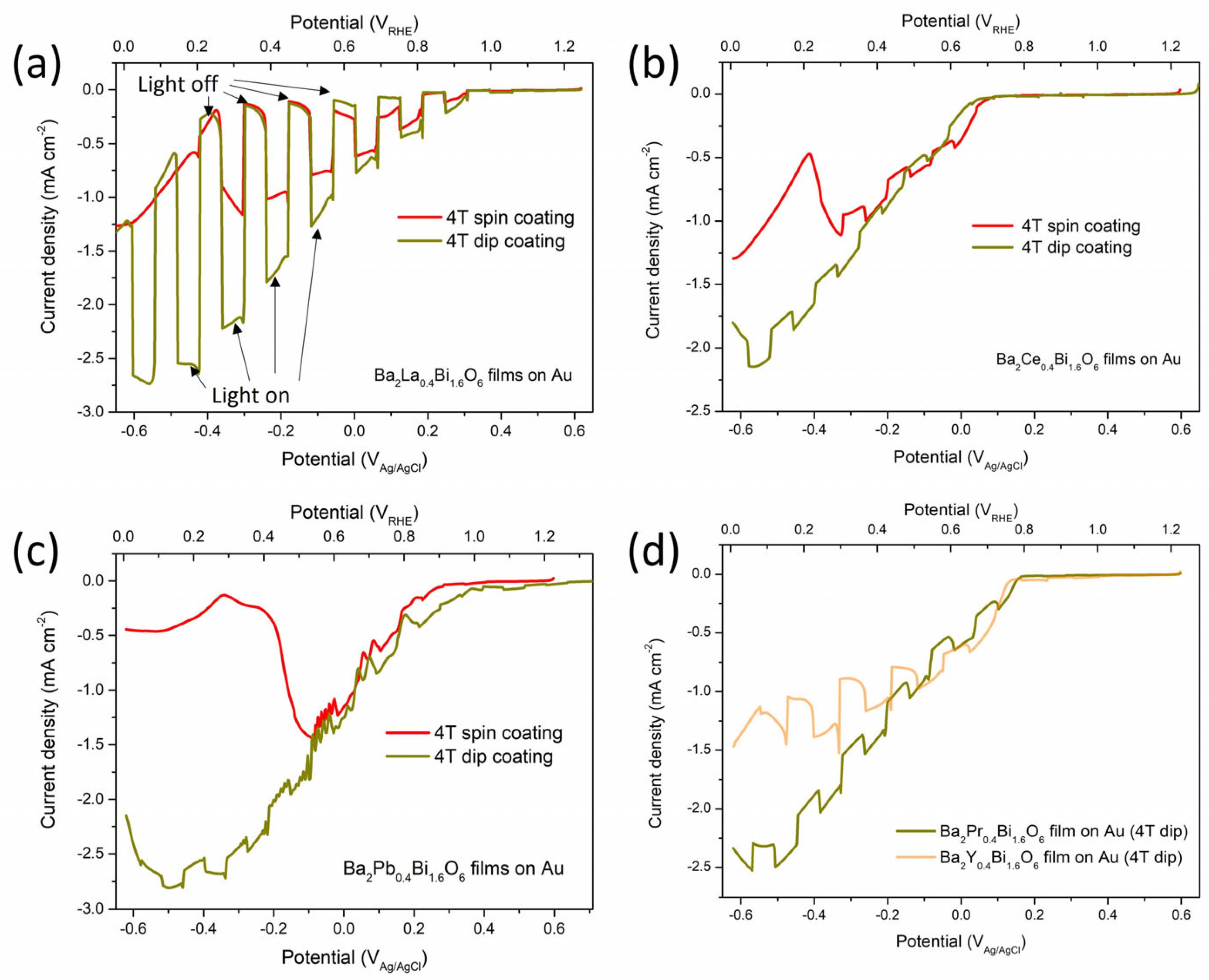
3.3. Photoelectrodes Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Sanga, L.; Lalengmawia, C.; Renthlei, Z.; Chanu, S.T.; Hima, L.; Singh, N.S.; Yvaz, A.; Bhattarai, S.; Rai, D.P. A review on perovskite materials for photovoltaic applications. Next Mater. 2025, 7, 100494. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, K.; Peng, Z.; Li, S.; Zou, F.; Wang, F.; Li, J.; Zhang, Z.; Chen, C.; Zhu, Q.; et al. Durable all-inorganic perovskite tandem photovoltaics. Nature 2025, 637, 1111–1117. [Google Scholar] [CrossRef]
- Ghani, M.U.; Junaid, M.; Batoo, K.M.; Ijaz, M.F.; Zazoum, B. An extensive study of structural, electronic, optical, mechanical, and thermodynamic properties of inorganic oxide perovskite materials ScXO3 (X=Ga, In) for optoelectronic applications: A DFT study. Inorg. Chem. Commun. 2025, 172, 113459. [Google Scholar] [CrossRef]
- Wang, C.; Ke, X.; Wang, J.; Liang, R.; Luo, Z.; Tian, Y.; Yi, D.; Zhang, Q.; Wang, J.; Han, X.-F.; et al. Ferroelastic switching in a layered-perovskite thin film. Nat. Commun. 2016, 7, 10636. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, H.; Wang, J.; Zhang, Q.; Jin, L.; Wang, C.; Zhang, Y.; Su, N.; Meng, F.; Yang, Y.; et al. Large Switchable Photoconduction within 2D Potential Well of a Layered Ferroelectric Heterostructure. Adv. Mater. 2020, 32, 2003033. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, W.-J. High-throughput computational screening of oxide double perovskites for optoelectronic and photocatalysis applications. J. Energy Chem. 2021, 57, 351–358. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Chen, H.; Li, X.; Liu, D.; Li, X.; Li, W.; Wei, G.; Shen, S.; Chi, B.; et al. Applications of perovskite oxides for oxygen evolution and oxygen reduction reactions in alkaline media. Energy Rev. 2025, 4, 100139. [Google Scholar] [CrossRef]
- Scholder, R.; Ganter, K.W.; Gläser, H.; Merz, G. Über Alkali-und Erdalkalioxobismutate(V). Z. Anorg. Allg. Chem. 1963, 319, 375–386. [Google Scholar] [CrossRef]
- Cox, D.E.; Sleight, A.W. Crystal structure of Ba2Bi3+Bi5+O6. Solid State Commun. 1976, 19, 969–973. [Google Scholar] [CrossRef]
- Mattheiss, L.F.; Hamann, D.R. Electronic Structure of the High-Tc Superconductor Ba1-xKxBiO3. Phys. Rev. Lett. 1988, 60, 2681–2684. [Google Scholar] [CrossRef]
- Cox, D.E.; Sleight, A.W. Mixed-valent Ba2Bi3+Bi5+O6: Structure and properties vs temperature. Acta Crystallogr. B 1979, 35, 1–10. [Google Scholar] [CrossRef]
- Papaconstantopoulos, D.A.; Pasturel, A.; Julien, J.P.; Cyrot-Lackmann, F. Calculations of the electronic structure and superconducting properties of the Ba(K)Pb(Bi)O3 system. Phys. Rev. B Condens. Matter 1989, 40, 8844–8851. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.T.; Polderman, M.J.; Mulder, F.M. Structural and transport properties of the BaBi1−xInxO3 system. Mater. Res. Bull. 2000, 35, 1205–1211. [Google Scholar] [CrossRef]
- Fu, W.T. A neutron powder diffraction study on BaBi0.5Sb0.5O3. Solid State Commun. 2000, 116, 461–464. [Google Scholar] [CrossRef]
- Yasukawa, M.; Shiga, Y.; Kono, T. Electrical conduction and thermoelectric properties of perovskite-type BaBi1−xSbxO3. Solid State Commun. 2012, 152, 964–967. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, W.; Hu, X.; Weng, B. Sulfur Tuning Oxygen Vacancy of Ba2Bi1.4Ta0.6O6 for Boosted Photocatalytic Tetracycline Hydrochloride Degradation and Hydrogen Evolution. J. Colloid Interface Sci. 2023, 636, 470–479. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalysis on BaBiO3 Driven by Visible Light. J. Phys. Chem. C 2007, 111, 12779–12785. [Google Scholar] [CrossRef]
- Matsushita, A.; Nakane, T.; Naka, T.; Isago, H.; Yamada, Y.; Yamada, Y. Valence States of Rare-Earth Ions and Band Gaps in RBiBa2O6 (R = La, Ce, Pr, Nd, Sm, Gd, Eu, and Dy) Photocatalysts. Jpn. J. Appl. Phys. 2012, 51, 121802. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Takeda, S.; Ishikawa, F.; Ohmura, A.; Nakayama, A.; Yamada, Y.; Matsushita, A.; Yea, J. Photocatalytic Activities of Ba2RBiO6 (R = La, Ce, Nd, Sm, Eu, Gd, Dy) under Visible Light Irradiation. J. Ceram. Soc. Jpn. 2010, 118, 91–95. [Google Scholar] [CrossRef]
- Ge, J.; Yin, W.-J.; Yan, Y. Solution-Processed Nb-Substituted BaBiO3 Double Perovskite Thin Films for Photoelectrochemical Water Reduction. Chem. Mater. 2018, 30, 1017–1031. [Google Scholar] [CrossRef]
- Weng, B.; Xiao, Z.; Meng, W.; Grice, C.R.; Poudel, T.; Deng, X.; Yan, Y. Bandgap Engineering of Barium Bismuth Niobate Double Perovskite for Photoelectrochemical Water Oxidation. Adv. Energy Mater. 2017, 7, 1602260. [Google Scholar] [CrossRef]
- Weng, B.; Grice, C.R.; Ge, J.; Poudel, T.; Deng, X.; Yan, Y. Barium Bismuth Niobate Double Perovskite/Tungsten Oxide Nanosheet Photoanode for High-Performance Photoelectrochemical Water Splitting. Adv. Energy Mater. 2018, 8, 1701655. [Google Scholar] [CrossRef]
- Shi, J.; Rubinstein, E.A.; Li, W.; Zhang, J.; Yang, Y.; Lee, T.L.; Qin, C.; Yan, P.; MacManus-Driscoll, J.L.; Scanlon, D.O.; et al. Modulation of the Bi3+ 6s2 Lone Pair State in Perovskites for High-Mobility p-Type Oxide Semiconductors. Adv. Sci. 2022, 9, 2104141. [Google Scholar] [CrossRef]
- Yin, W.-J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide Perovskites, Double Perovskites and Derivatives for Electrocatalysis, Photocatalysis, and Photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. [Google Scholar] [CrossRef]
- Guo, T.; Fu, W.T.; de Groot, H.J.M. Highly Reversible Ambipolar Photocurrents by Modulating Ta in Ba2Bi2–xTaxO6 Double Perovskites. J. Phys. Chem. C 2024, 128, 13177–13189. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Guo, T.; Fu, W.T.; de Groot, H.J.M. Engineering Ba2Bi2O6 Double Perovskite with La3+ for High Current Density Visible Light Photoelectrochemical Hydrogen Evolution. Small 2024, 20, 2308781. [Google Scholar] [CrossRef] [PubMed]
- Horvat-Radošević, V.; Kvastek, K. Role of Pt-Probe Pseudo-Reference Electrode in Impedance Measurements of Pt and Polyaniline (PANI) Modified Pt Electrodes. J. Electroanal. Chem. 2006, 591, 217–222. [Google Scholar] [CrossRef]
- Sleight, A.W.; Gillson, J.L.; Bierstedt, P.E. High-temperature superconductivity in the BaPb1-xBixO3 systems. Solid State Commun. 1975, 17, 27–28. [Google Scholar] [CrossRef]
- Mattheiss, L.F.; Hamann, D.R. Electronic Structure of BaPb1−xBixO3. Phys. Rev. B 1983, 28, 4227–4241. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Reis, K.P.; Jacobson, A.J.; Schneemeyer, L.F.; Waszczak, J.V. Syntheses, Structures, and Magnetism of Barium/Rare-Earth/Bismuth Double Perovskites. Crystal Structures of Ba2MBiO6 (M = Ce, Pr, Nd, Tb, Yb) by Powder Neutron Diffraction. Chem. Mater. 1995, 7, 2161–2167. [Google Scholar] [CrossRef]
- Poltavets, V.; Kazin, P.; Jansen, M. Syntheses and structures of BaPrxBi1−xO3 perovskites. Solid State Sci. 2006, 8, 1152–1159. [Google Scholar] [CrossRef]
- Sato, A.; Matsukawa, M.; Taniguchi, H.; Tsuji, S.; Nishidate, K.; Aisawa, S.; Matsushita, A.; Zhang, K. Structural, physical and photocatalytic properties of mixed-valence double-perovskite Ba2Pr(Bi,Sb)O6 semiconductor synthesized by citrate pyrolysis technique. Solid State Sci. 2020, 107, 106352. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Ding, W.; Feng, L.; Gao, Y.; Guo, Z. Formability of ABX3 (X = F, Cl, Br, I) Halide Perovskites. Acta Crystallogr. B 2008, 64, 702–707. [Google Scholar] [CrossRef]
- Khalfin, S.; Bekenstein, Y. Advances in Lead-Free Double Perovskite Nanocrystals, Engineering Band-Gaps and Enhancing Stability through Composition Tunability. Nanoscale 2019, 11, 8665–8679. [Google Scholar] [CrossRef]
- Otsuka, S.; Hinatsu, Y. Structures and magnetic properties of rare earth double perovskites containing antimony or bismuth Ba2LnMO6 (Ln=rare earths; M=Sb, Bi). J. Solid State Chem. 2015, 227, 132–141. [Google Scholar] [CrossRef]
- Drost, R.J.; Fu, W.T. Preparation and Characterization of the Perovskites BaCe1−xBixO3. Mater. Res. Bull. 1995, 30, 471–478. [Google Scholar] [CrossRef]
- Marx, D.T.; Radaelli, P.G.; Jorgensen, J.D.; Hitterman, R.L.; Hinks, D.G.; Pei, S.; Dabrowski, B. Metastable behavior of the superconducting phase in the BaBi1−xPbxO3 system. Phys. Rev. B Condens. Matter 1992, 46, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Blower, S.K.; Greaves, C. Bismuth additions to YBa2Cu3O7: Structural and electrical characterisation. Solid State Commun. 1988, 68, 765–767. [Google Scholar] [CrossRef]
- Ritter, H.; Ihringer, J.; Maichle, J.K.; Prandl, W.; Hoser, A.; Hewat, A.W. The crystal structure of the prototypic ceramic superconductor BaPbO3: An X-ray and neutron diffraction study. Z. Phys. B Condensed Matter 1989, 75, 297–302. [Google Scholar] [CrossRef]
- Bharath, M.; Sharma, P.; Brar, J.; Sagdeo, P.R.; Bindu, R. Electronic and optical properties of Y-doped BaBiO3. Eur. Phys. J. B 2021, 94, 59. [Google Scholar] [CrossRef]
- Dongol, M.; El-Nahass, M.M.; El-Denglawey, A.; Elhady, A.F.; Abuelwafa, A.A. Optical Properties of Nano 5,10,15,20-Tetraphenyl-21H,23H-Prophyrin Nickel (II) Thin Films. Curr. Appl. Phys. 2012, 12, 1178–1184. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; El-Gohary, Z.; Soliman, H.S. Structural and optical studies of thermally evaporated CoPc thin films. Opt. Laser Technol. 2003, 35, 523–531. [Google Scholar] [CrossRef]
- Zarrabi, N.; Sandberg, O.J.; Meredith, P.; Armin, A. Subgap Absorption in Organic Semiconductors. J. Phys. Chem. Lett. 2023, 14, 3174–3185. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Wang, C.; Chen, F.; Ma, T.; Huang, H. Defects in photoreduction reactions: Fundamentals, classification, and catalytic energy conversion. eScience 2024, 4, 100228. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Weng, Y. Defect States and Polarons in Photocatalytic Semiconductors Revealed via Time-Resolved Spectroscopy. J. Phys. Chem. C 2024, 128, 16275–16290. [Google Scholar] [CrossRef]
- Kangsabanik, J.; Borkar, H.; Bhawna; Siddiqui, M.S.; Aslam, M.; Alam, A. Origin of High Nonradiative Recombination and Relevant Optoelectronic Properties of Ba2Bi1+xNb1−xO6: Candidate for Photo(electro)catalysis and Photovoltaic Applications? Adv. Opt. Mater. 2020, 8, 2000901. [Google Scholar] [CrossRef]
- Prokofiev, A.V.; Shelykh, A.I.; Melekh, B.T. Periodicity in the band gap variation of Ln2X3 (X = O, S, Se) in the lanthanide series. J. Alloys Compd. 1996, 242, 41–44. [Google Scholar] [CrossRef]
- Aycibin, M.; Erdinc, B.; Akkus, H. Electronic Structure and Lattice Dynamics of BaCeO3 Compound in Cubic Phase. J. Electron. Mater. 2014, 43, 4301–4307. [Google Scholar] [CrossRef]
- Machida, M.; Yabunaka, J.-i.; Kijima, T.; Matsushima, S.; Arai, M. Electronic structure of layered tantalates photocatalysts, RbLnTa2O7 (Ln=La, Pr, Nd, and Sm). Int. J. Inorg. Mater. 2001, 3, 545–550. [Google Scholar] [CrossRef]
- Ryzhkov, M.V.; Gubanov, V.A.; Teterin, Y.A.; Baev, A.S. Electronic structure, chemical bonding and X-ray photoelectron spectra of light rare-earth oxides. Z. Phys. B Condensed Matter 1985, 59, 1–6. [Google Scholar] [CrossRef]
- Nishidate, K.; Adiko, A.; Matsukawa, M.; Taniguchi, H.; Sato, A.; Matsushita, A.; Tanibayashi, S.; Hasegawa, M. Electronic properties and crystal structures of double-perovskites, Ba2BiIIIBiVO6, Ba2PrBiO6, and Ba2PrSbO6: First-principles study. Mater. Res. Express 2020, 7, 065505. [Google Scholar] [CrossRef]
- Wang, L.; Marin, C.M.; Mei, W.-N.; Li Cheung, C. Electronic structures of lanthanum, samarium, and gadolinium sulfides. AIMS Mater. Sci. 2015, 2, 97–105. [Google Scholar] [CrossRef]
- Lakshminarasimhan, N.; Park, Y.; Choi, W. Role of valency ordering on the visible light photocatalytic activity of BaBi0.53+Bi0.55+O3. Chem. Phys. Lett. 2008, 452, 264–268. [Google Scholar] [CrossRef]
- Hiramatsu, H.; Yanagi, H.; Kamiya, T.; Ueda, K.; Hirano, M.; Hosono, H. Crystal Structures, Optoelectronic Properties, and Electronic Structures of Layered Oxychalcogenides MCuOCh (M = Bi, La; Ch = S, Se, Te): Effects of Electronic Configurations of M3+ Ions. Chem. Mater. 2007, 20, 326–334. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Antuch, M.; Millet, P.; Iwase, A.; Kudo, A. Water reduction into hydrogen using Rh-doped SrTiO3 photoelectrodes surface-modified by minute amounts of Pt: Insights from heterogeneous kinetic analysis. Electrochim. Acta 2019, 297, 696–704. [Google Scholar] [CrossRef]
- Freeman, E.; Kumar, S.; Celorrio, V.; Park, M.S.; Kim, J.H.; Fermin, D.J.; Eslava, S. Strategies for the deposition of LaFeO3 photocathodes: Improving the photocurrent with a polymer template. Sustain. Energy Fuels 2020, 4, 884–894. [Google Scholar] [CrossRef]
- Harris-Lee, T.R.; Marken, F.; Bentley, C.L.; Zhang, J.; Johnson, A.L. A chemist’s guide to photoelectrode development for water splitting—The importance of molecular precursor design. EES Catal. 2023, 1, 832–873. [Google Scholar] [CrossRef]
- Govoni, M.; Marri, I.; Ossicini, S. Auger recombination in Si and GaAs semiconductors: Ab initio results. Phys. Rev. B 2011, 84, 075215. [Google Scholar] [CrossRef]
- Martínez-García, A.; Vendra, V.K.; Sunkara, S.; Haldankar, P.; Jasinski, J.; Sunkara, M.K. Tungsten oxide-coated copper oxide nanowire arrays for enhanced activity and durability with photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 15235–15241. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard Electrode-Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Vivier, V.; Regis, A.; Sagon, G.; Nedelec, J.Y.; Yu, L.T.; Cachet-Vivier, C. Cyclic Voltammetry Study of Bismuth Oxide Bi2O3 Powder by Means of a Cavity Microelectrode Coupled with Raman Microspectrometry. Electrochim. Acta 2001, 46, 907–914. [Google Scholar] [CrossRef]
- Lopes, T.; Andrade, L.; Le Formal, F.; Gratzel, M.; Sivula, K.; Mendes, A. Hematite Photoelectrodes for Water Splitting: Evaluation of the Role of Film Thickness by Impedance Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515–16523. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Liu, B.; Wang, S.; Huang, W. Imperfect makes perfect: Defect engineering of photoelectrodes towards efficient photoelectrochemical water splitting. Chem. Commun. 2023, 59, 10044–10066. [Google Scholar] [CrossRef]
- Johnson, N.A.; Gould, E.S. Metal ion catalysis of oxygen transfer reactions. III. Transition metal chelates as catalysts in the oxidation of nitrosobenzene. Oxygen transfer from alkyl peroxy radicals. J. Am. Chem. Soc. 1973, 95, 5198–5204. [Google Scholar] [CrossRef]
- De Gryse, R.; Gomes, W.P.; Cardon, F.; Vennik, J. On the Interpretation of Mott-Schottky Plots Determined at Semiconductor/Electrolyte Systems. J. Electrochem. Soc. 1975, 122, 711–712. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat Band Potential Determination: Avoiding the Pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Cardon, F.; Gomes, W.P. On the Determination of the Flat-Band Potential of a Semiconductor in Contact with a Metal or an Electrolyte from the Mott-Schottky Plot. J. Phys. D Appl. Phys. 1978, 11, L63–L67. [Google Scholar] [CrossRef]
- Tan, C.C.; Feteira, A.; Sinclair, D.C. Ba2Bi1.4Nb0.6O6: A Nonferroelectric, High Permittivity Oxide. Chem. Mater. 2012, 24, 2247–2249. [Google Scholar] [CrossRef]
- Takahashi, A.; Kumagai, Y.; Miyamoto, J.; Mochizuki, Y.; Oba, F. Machine learning models for predicting the dielectric constants of oxides based on high-throughput first-principles calculations. Phys. Rev. Mater. 2020, 4, 103801. [Google Scholar] [CrossRef]
- Sivula, K. Mott–Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-R.F.; Bard, A.J. Semiconductor Electrodes: XXXVI. Characteristics of n-MoSe2, n- and p-WSe2, Electrodes in Aqueous Solution. J. Electrochem. Soc. 1981, 128, 945–952. [Google Scholar] [CrossRef]





| Ion | La3+ | Ce4+ | Pr3+ | Pr4+ | Pb4+ | Y3+ |
|---|---|---|---|---|---|---|
| Coordination number | 6 | 6 | 6 | 6 | 6 | 6 |
| Ionic radius (Å) | 1.032 | 0.87 | 0.99 | 0.85 | 0.775 | 0.9 |
| Formula | Ba2La0.4Bi1.6O6 | Ba2Ce0.4Bi1.6O6 | Ba2Pr0.4Bi1.6O6 | Ba2Pb0.4Bi1.6O6 | Ba2Y0.4Bi1.6O6 | |
| t | 0.927 | 0.929 | 0.931 | 0.937 | 0.938 | |
| µ | 0.640 | 0.636 | 0.633 | 0.622 | 0.621 | |
| Sample | La0.4 | Ce0.4 | Pr0.4 | Pb0.4 | Y0.4 | |
|---|---|---|---|---|---|---|
| Phase | 1 | 1 | 1 | 1 | 1 | 2 |
| Space group | I2/m | Fmm | I2/m | |||
| a (Å) | 6.2156 (2) | 6.1559 (5) | 6.1942 (3) | 6.1176 (3) | 8.5827 (2) | 6.1838 (3) |
| b (Å) | 6.1598 (3) | 6.1953 (4) | 6.1469 (3) | 6.1566 (3) | 6.1409 (4) | |
| c (Å) | 8.7137 (4) | 8.7045 (6) | 8.6898 (4) | 8.6401 (4) | 8.6775 (6) | |
| β (°) | 90.305 (4) | 90.02 (2) | 89.884 (8) | 90.0229 (0) | 90 | 90.1599 (0) |
| Rwp (%) | 5.64 | 6.93 | 6.68 | 7.35 | 8.69 | |
| 1.74 | 2.15 | 2.01 | 2.56 | 2.56 | ||
| Sample | Rs/Ω | Rct/Ω | Cdl/F | Rsc/Ω | Csc/F | |
|---|---|---|---|---|---|---|
| La0.4-dip | 278.3 (±1.1) | 47.1 (±3.3) × 103 | 1.5 (±0.0) × 10−5 | 1203.5 (±18.3) | 1.4 (±0.1) × 10−5 | 0.025 |
| La0.4-spin | 277.0 (±1.7) | 28.5 (±1.0) × 103 | 1.4 (±0.0) × 10−5 | 1100.0 (±10.0) | 2.1 (±0.2) × 10−5 | 0.014 |
| Pb0.4-spin | 274.8 (±0.4) | 17.6 (±0.4) × 103 | 3.1 (±0.0) × 10−5 | 102.0 (±1.4) | 1.4 (±0.2) × 10−5 | 0.003 |
| BBO-spin | 283.9 (±0.8) | 56.5 (±1.4) × 103 | 2.8 (±0.0) × 10−5 | 27.0 (±2.3) | 2.4 (±0.4) × 10−5 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Fu, W.T.; de Groot, H.J.M. Strategic Doping for Precise Structural Control and Intense Photocurrents Under Visible Light in Ba2M0.4Bi1.6O6 (M = La, Ce, Pr, Pb, Y) Double Perovskites. Nanomaterials 2025, 15, 1039. https://doi.org/10.3390/nano15131039
Guo T, Fu WT, de Groot HJM. Strategic Doping for Precise Structural Control and Intense Photocurrents Under Visible Light in Ba2M0.4Bi1.6O6 (M = La, Ce, Pr, Pb, Y) Double Perovskites. Nanomaterials. 2025; 15(13):1039. https://doi.org/10.3390/nano15131039
Chicago/Turabian StyleGuo, Tirong, Wen Tian Fu, and Huub J. M. de Groot. 2025. "Strategic Doping for Precise Structural Control and Intense Photocurrents Under Visible Light in Ba2M0.4Bi1.6O6 (M = La, Ce, Pr, Pb, Y) Double Perovskites" Nanomaterials 15, no. 13: 1039. https://doi.org/10.3390/nano15131039
APA StyleGuo, T., Fu, W. T., & de Groot, H. J. M. (2025). Strategic Doping for Precise Structural Control and Intense Photocurrents Under Visible Light in Ba2M0.4Bi1.6O6 (M = La, Ce, Pr, Pb, Y) Double Perovskites. Nanomaterials, 15(13), 1039. https://doi.org/10.3390/nano15131039







