Novel Core–Shell Metal Oxide Nanofibers with Advanced Optical and Magnetic Properties Deposited by Co-Axial Electrospinning
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Solution Fabrication
2.3. Electrospinning
2.4. Characterization
3. Results
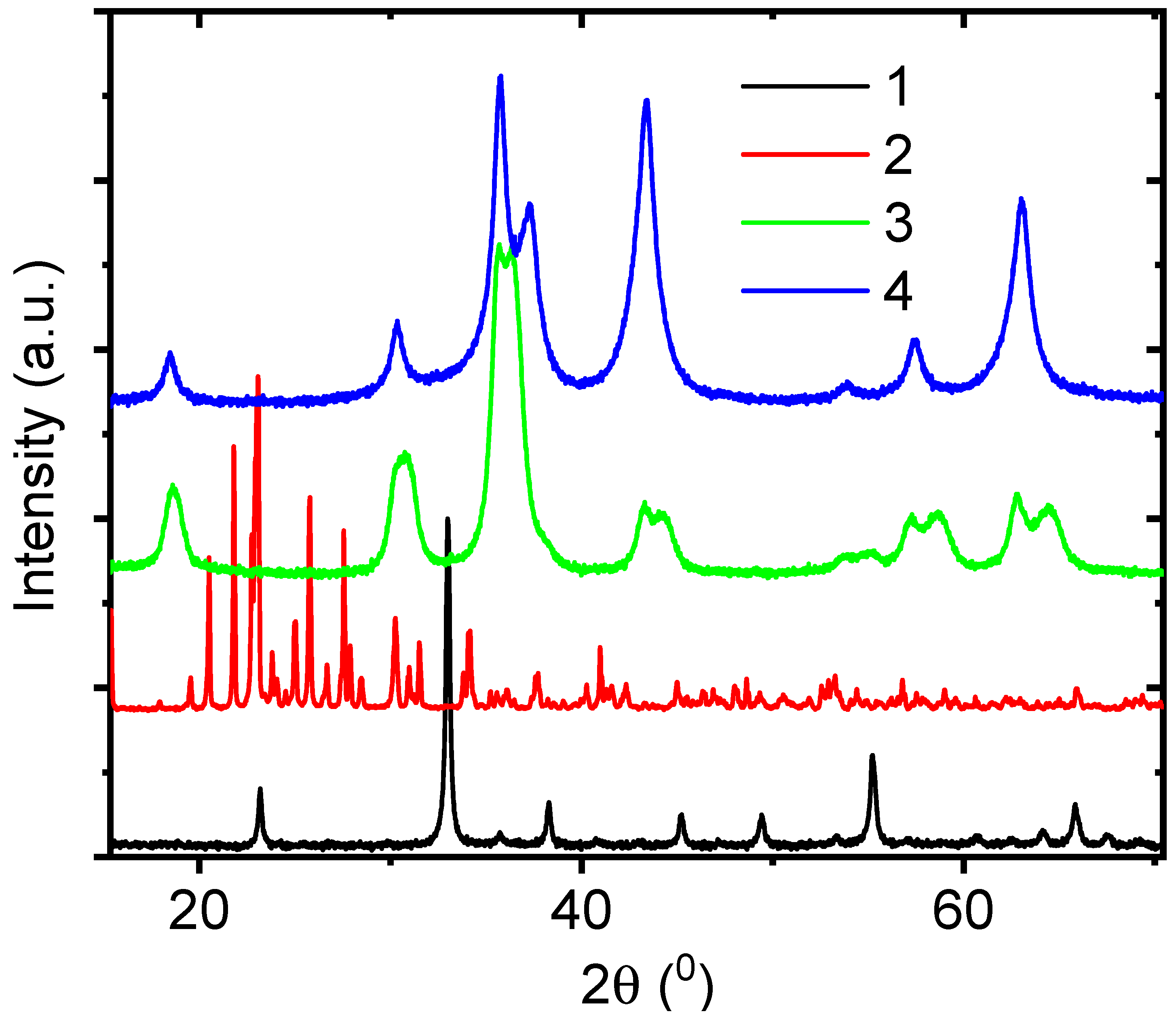
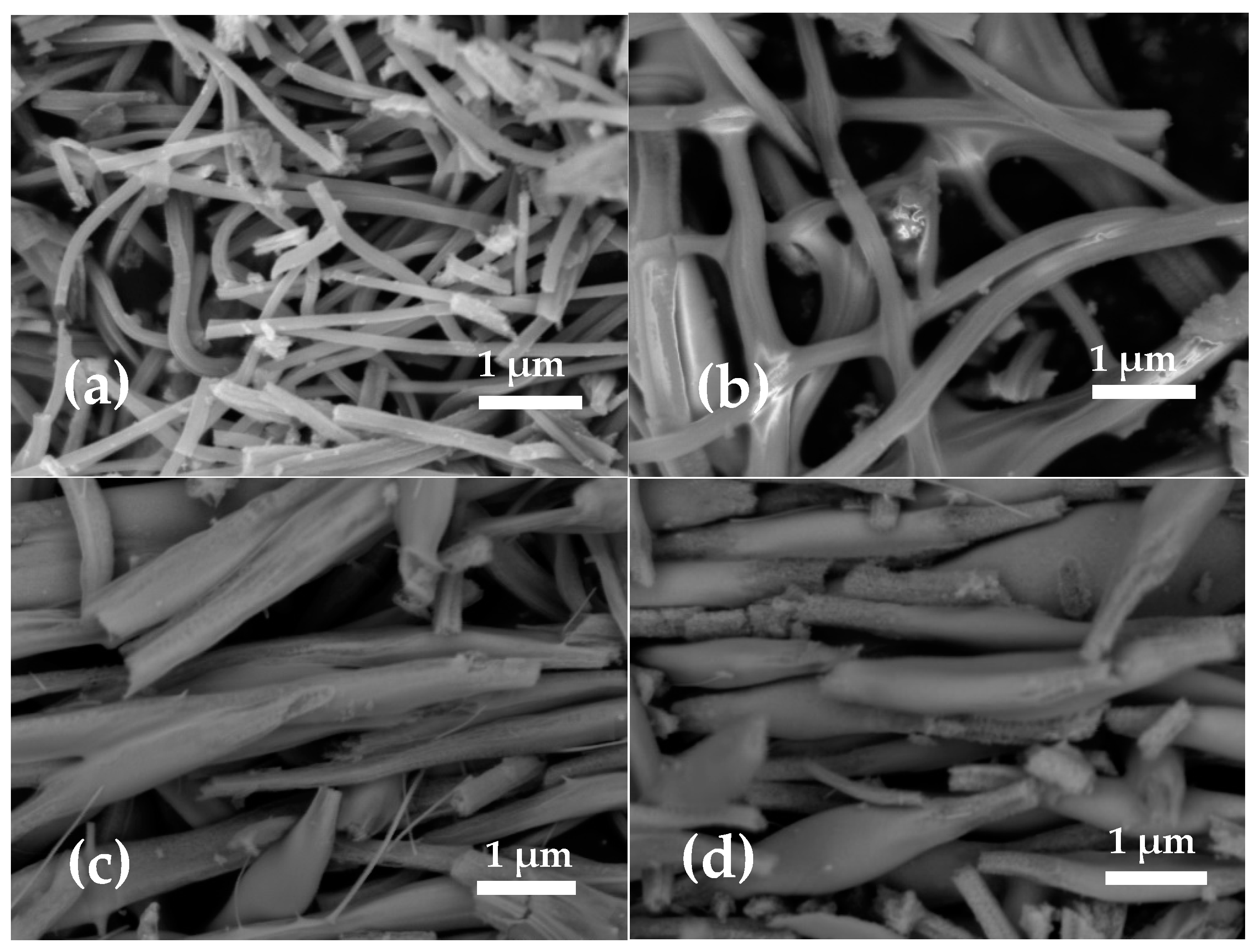
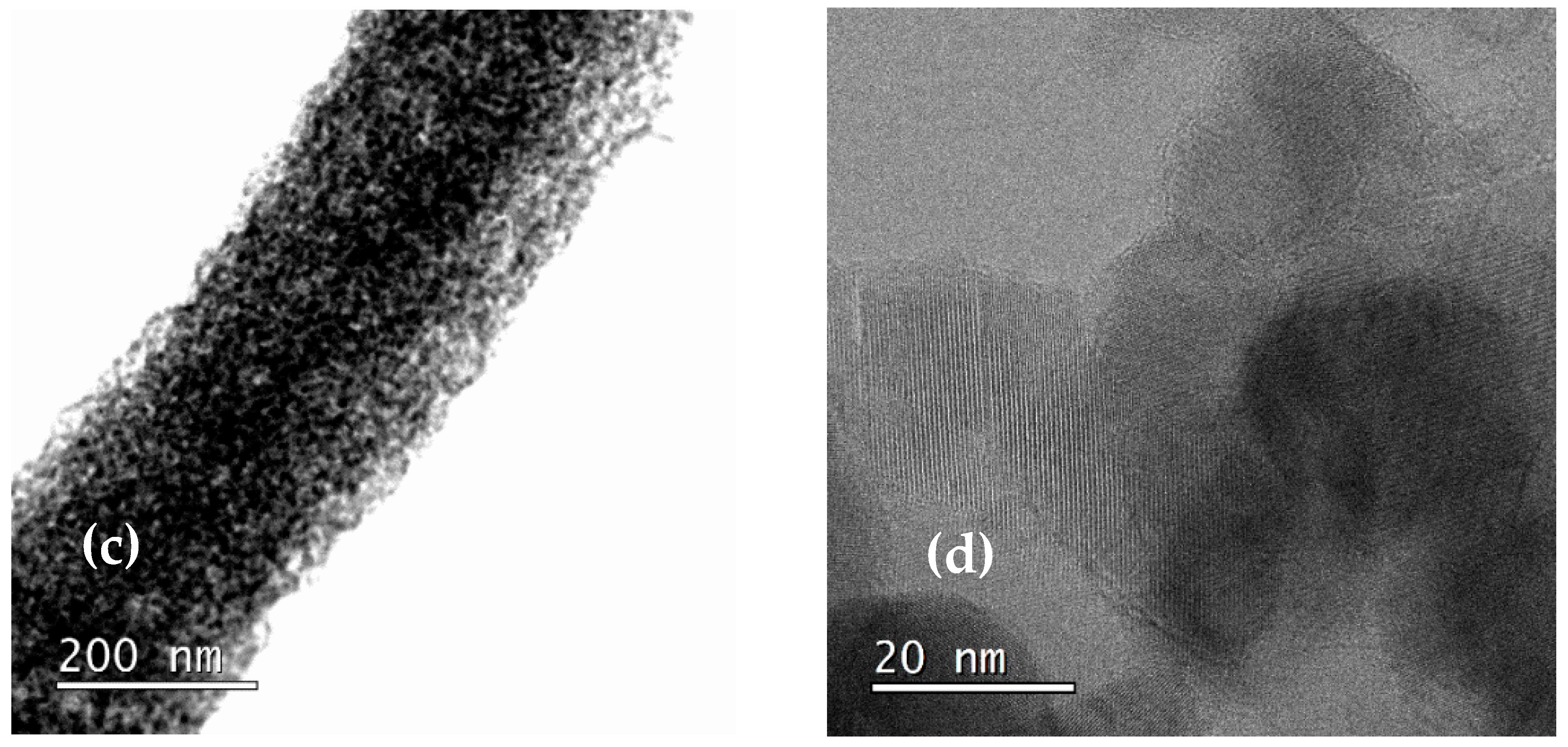
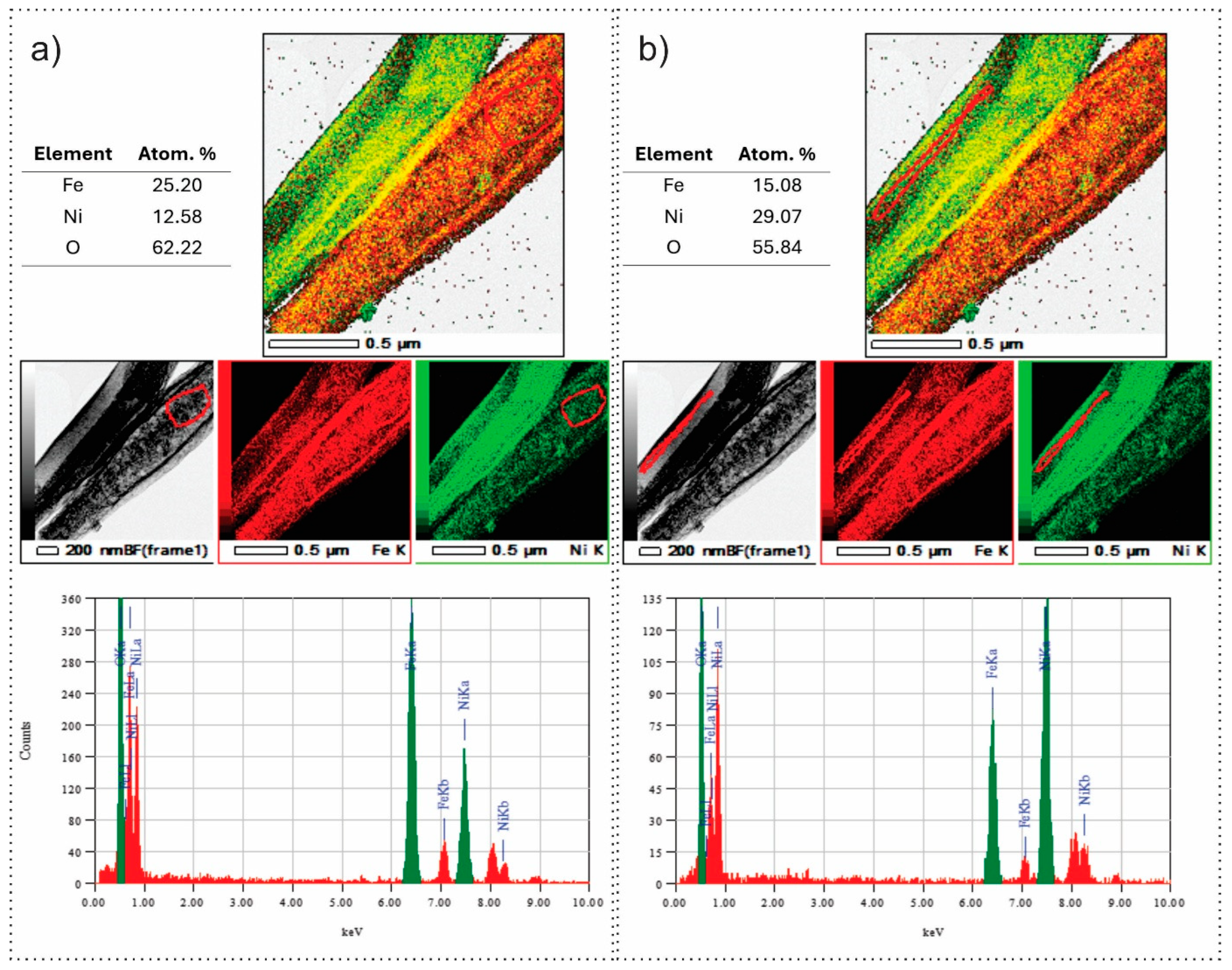
3.1. Role of the Shell Precursor to Fabrication of the Core–Shell Nanofibers
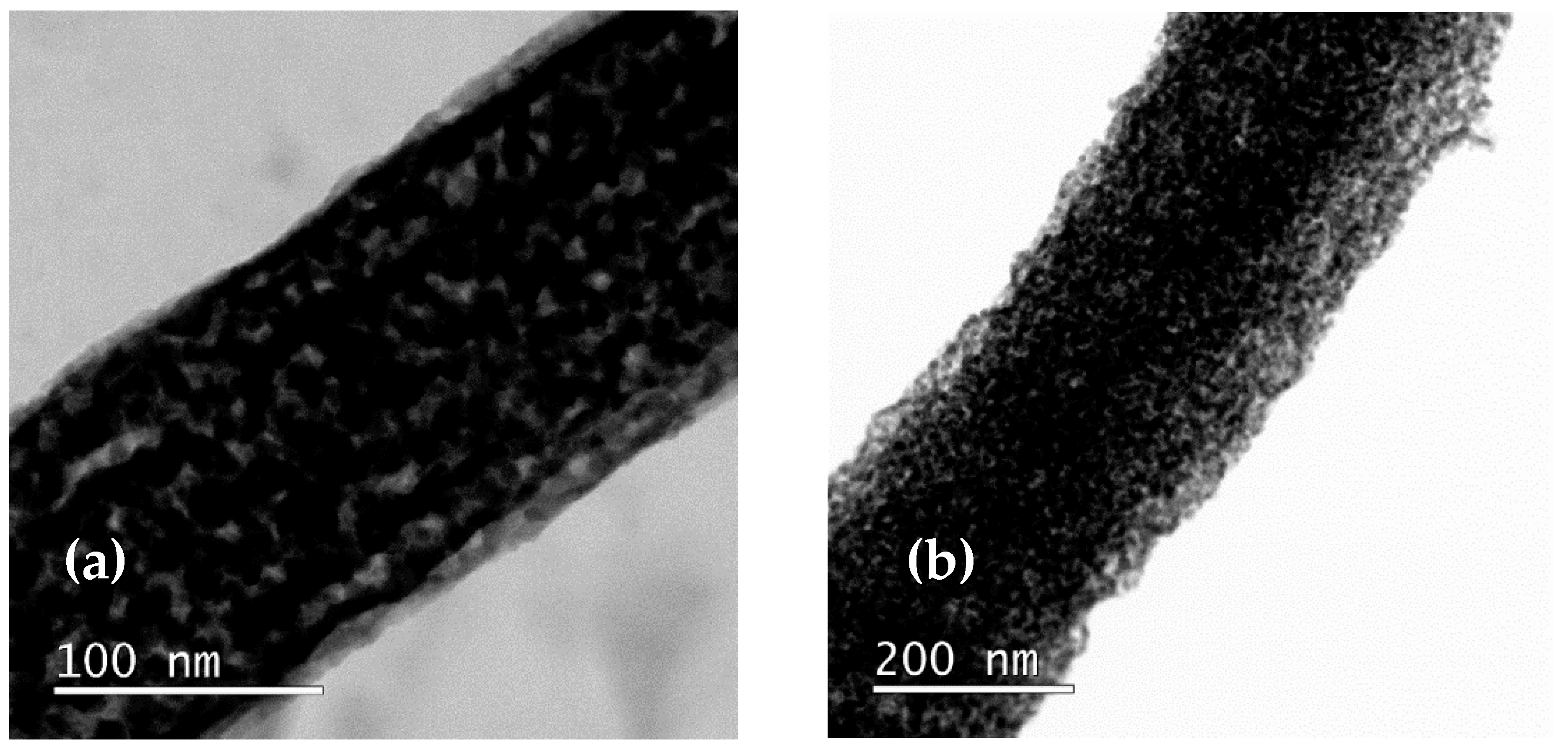
3.2. Optimization of Variation of Core Concentration in FeNi Core–Shell Nanofibers
3.2.1. Variation of the Fe Nitrate Concentration in the Core Solution
- FeNi35: PAN 7.55% Fe 5.5% (0.3 g Fe nitrate)/PVP 11.1% Ni 8.51% (0.5 g Ni acetate);
- FeNi45: PAN 7.41% Fe 7.23% (0.4 g Fe nitrate)/PVP 11.1% Ni 8.51% (0.5 g Ni acetate);
- FeNi55: PAN 7.28% Fe 8.89% (0.5 g Fe nitrate)/PVP 11.1% Ni 8.51% (0.5 g Ni acetate);
- FeNi65: PAN 7.15% Fe 10.4% (0.6 g Fe nitrate)/PVP 11.1% Ni 8.51% (0.5 g Ni acetate).
3.2.2. Variation of the Ni Acetate Concentration in the Shell Solution
- FeNi31: PAN 7.55% Fe 5.5% (0.3 g Fe nitrate)/PVP 11.7% Ni 2.27% (0.15 g Ni acetate);
- FeNi33: PAN 7.55% Fe 5.5% (0.3 g Fe nitrate)/PVP 11.4% Ni 5.29% (0.3 g Ni acetate);
- FeNi35: PAN 7.55% Fe 5.5% (0.3 g Fe nitrate)/PVP 11.1% Ni 8.51% (0.5 g Ni acetate).
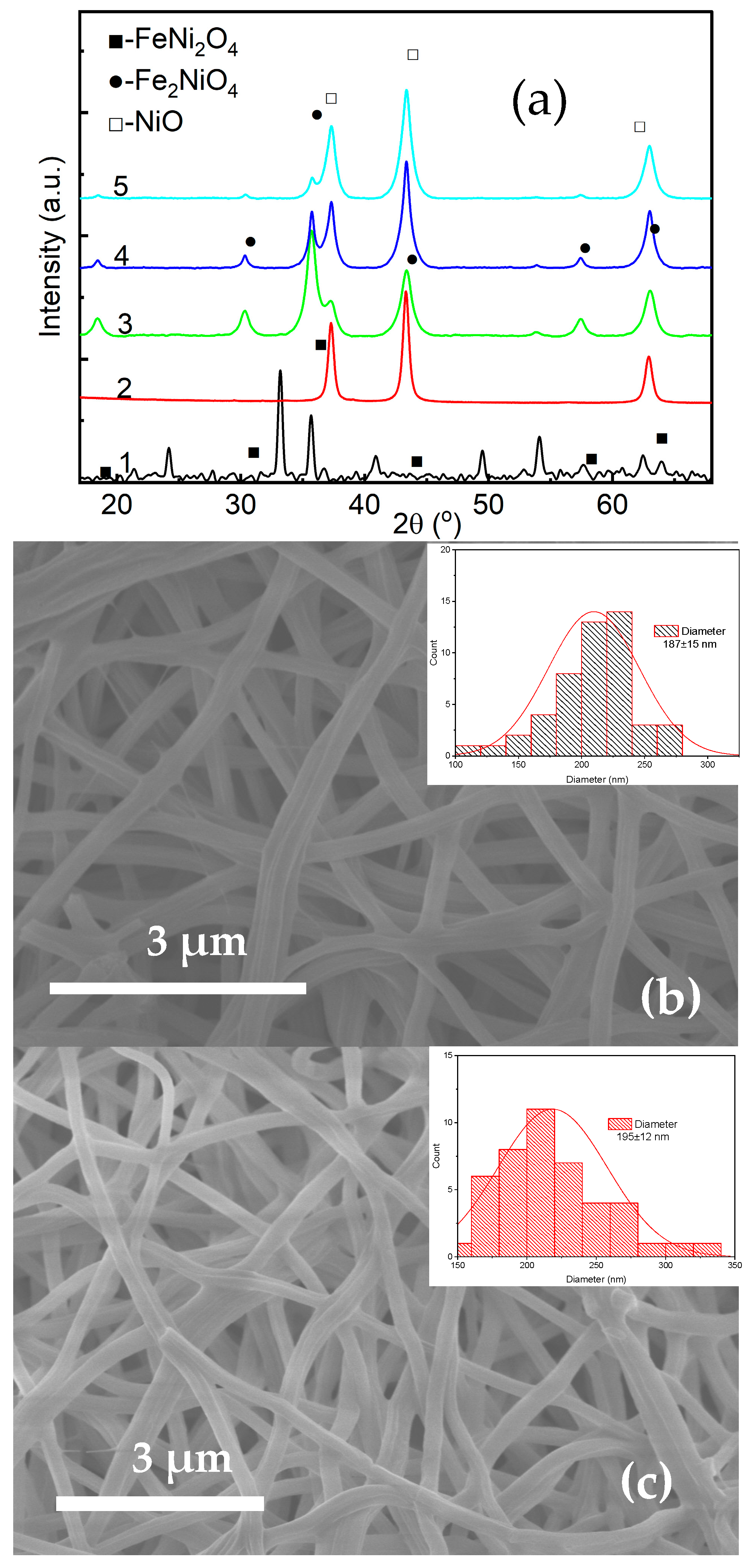
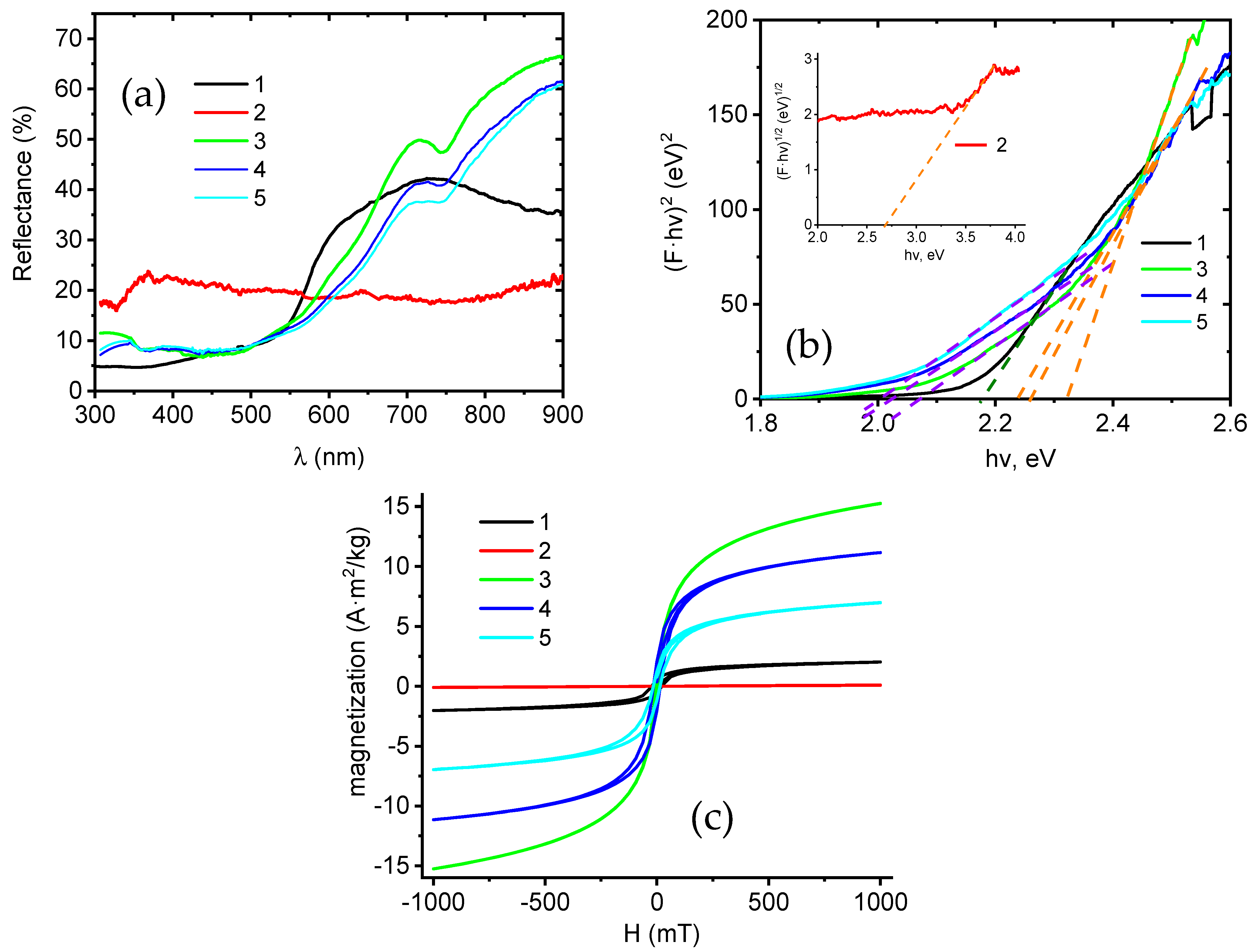
3.3. Structure, Optical, and Magnetic Properties of the Fe3O4 and NiO Core–Shell Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Chen, X.; Lin, X.; Yan, J.; Yu, D.G.; Liu, P.; Yang, H. Electrospun Multi-Chamber Core–Shell Nanofibers and Their Controlled Release Behaviors: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1954. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, S.; Yun, J.S. Electrospinning and Partial Etching Behaviors of Core–Shell Nanofibers Directly Electrospun on Mesh Substrates for Application in a Cover-Free Compact Air Filter. Nanomaterials 2024, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, N.; Alizadeh, S.; Majidi, J.; Saeed, M.; Ghadimi, T.; Tahermanesh, K.; Arabsorkhi-Mishabi, A.; Pezeshki-Modaress, M. Heat-Treated Alginate-Polycaprolactone Core-Shell Nanofibers by Emulsion Electrospinning Process for Biomedical Applications. Int. J. Biol. Macromol. 2024, 275, 133709. [Google Scholar] [CrossRef]
- Nair, K.G.; Vishnuraj, R.; Pullithadathil, B. Integrated Co-Axial Electrospinning for a Single-Step Production of 1D Aligned Bimetallic Carbon Fibers@AuNPs–PtNPs/NiNPs–PtNPs towards H2 Detection. Mater. Adv. 2022, 3, 443–455. [Google Scholar] [CrossRef]
- Sukumar, T.; Kadirvelu, K. Core-Shell Nanofibers With Fire Retardant Properties Prepared By A Co-Axial Electrospinning Technique. ChemistrySelect 2022, 7, e202201679. [Google Scholar] [CrossRef]
- Zhang, X.; Aravindan, V.; Kumar, P.S.; Liu, H.; Sundaramurthy, J.; Ramakrishna, S.; Madhavi, S. Synthesis of TiO2 Hollow Nanofibers by Co-Axial Electrospinning and Its Superior Lithium Storage Capability in Full-Cell Assembly with Olivine Phosphate. Nanoscale 2013, 5, 5973–5980. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panigrahi, G.K.; Dhal, J.P.; Sahoo, J.K.; Behera, A.K.; Panda, P.C.; Patel, P.; Mund, S.K.; Muduli, S.M.; Panda, L. Co-Axial Electrospun Hollow MgO Nanofibers for Efficient Removal of Fluoride Ions from Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129877. [Google Scholar] [CrossRef]
- Fu, L.; Xu, J.; Liu, Q.; Liu, C.; Fan, S.; Ramakrishna, S.; Tang, W. Gas Sensors Based on Co3O4/TiO2 Core-Shell Nanofibers Prepared by Coaxial Electrospinning for Breath Marker Acetone Detection. Ceram. Int. 2024, 50, 3443–3452. [Google Scholar] [CrossRef]
- Rafieipour, H.; Vaezi, M.R.; Kazemzadeh, A. Synthesis and Characterisation of Ceramic Core/Shell Nanofibres via Single Stage Co-Axial Electrospinning. Micro Nano Lett. 2016, 11, 707–711. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Hou, Y.; Wang, C.; Yang, Y.; Shang, J.; Cheng, X. Core-Shell Catalysts for the Elimination of Organic Contaminants in Aqueous Solution: A Review. Chem. Eng. J. 2023, 455, 140604. [Google Scholar] [CrossRef]
- Wang, I.; Liu, L.; Yu, S.; Lai, N.C.; Gao, Y.; Li, Z.; Liu, J.; Wang, W. Highly Sintering-Resistant Iron Oxide with a Hetero-Oxide Shell for Chemical Looping Water Splitting. Int. J. Hydrog. Energy 2024, 57, 438–449. [Google Scholar] [CrossRef]
- Sethy, P.P.; Pani, T.K.; Rout, S.; Sundaray, B. Structural and Magnetic Properties of Ni/C Core–Shell Nanofibers Prepared by One Step Co-Axial Electrospinning Method. J. Mater. Sci. Mater. Electron. 2023, 34, 807. [Google Scholar] [CrossRef]
- Peng, X.; Santulli, A.C.; Sutter, E.; Wong, S.S. Fabrication and Enhanced Photocatalytic Activity of Inorganic Core–Shell Nanofibers Produced by Coaxial Electrospinning. Chem. Sci. 2012, 3, 1262–1272. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 Core-Shell Nanofibers and Their Enhanced Gas Sensing Performance Based on Different Work Function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Wang, R.; Zhang, T. A Formaldehyde Sensor: Significant Role of p-n Heterojunction in Gas-Sensitive Core-Shell Nanofibers. Sens. Actuators B Chem. 2018, 258, 1230–1241. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.; Barsan, N. Design of Core-Shell Heterostructure Nanofibers with Different Work Function and Their Sensing Properties to Trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, J.; Tang, L.; Zeng, Z.; Zhu, B. A Novel Ex-Situ Method to Fabricate PH-Responsive Material Based on Core-Shell Fe3O4@SiO2 Nanoparticles for Multi-Functional Oil-Water Separation and Efficient Recycling. J. Env. Chem. Eng. 2024, 12, 112422. [Google Scholar] [CrossRef]
- Talebi, P.; Singh, H.; Rani, E.; Huttula, M.; Cao, W. Surface Plasmon-Driven Photocatalytic Activity of Ni@NiO/NiCO3 Core–Shell Nanostructures. RSC Adv. 2021, 11, 2733–2743. [Google Scholar] [CrossRef]
- Pham, H.L.; Nguyen, V.D.; Nguyen, V.K.; Le, T.H.P.; Ta, N.B.; Pham, D.C.; Tran, Q.T.; Dang, V.T. Rational Design of Magnetically Separable Core/Shell Fe3O4/ZnO Heterostructures for Enhanced Visible-Light Photodegradation Performance. RSC Adv. 2021, 11, 22317–22326. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Fe3O4/Carbon Composite Nanofiber Absorber with Enhanced Microwave Absorption Performance. Mater. Sci. Eng. B 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Kayaci, F.; Ozgit-Akgun, C.; Donmez, I.; Biyikli, N.; Uyar, T. Polymer-Inorganic Core-Shell Nanofibers by Electrospinning and Atomic Layer Deposition: Flexible Nylon-ZnO Core-Shell Nanofiber Mats and Their Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 6185–6194. [Google Scholar] [CrossRef] [PubMed]
- Lys, A.; Zabolotnii, V.; Čaplovičová, M.; Tepliakova, I.; Berzins, A.; Sahul, M.; Čaplovič, Ľ.; Pogrebnjak, A.; Iatsunskyi, I.; Viter, R. Core-Shell Nanofibers of ZnFe2O4/ZnO for Enhanced Visible-Light Photoelectrochemical Performance. J. Alloys Compd. 2024, 984, 173885. [Google Scholar] [CrossRef]
- Okpara, E.C.; Olatunde, O.C.; Wojuola, O.B.; Onwudiwe, D.C. Applications of Transition Metal Oxides and Chalcogenides and Their Composites in Water Treatment: A Review. Environ. Adv. 2023, 11, 100341. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A Review on Transition Metal Oxides Based Photocatalysts for Degradation of Synthetic Organic Pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Y.H.; Sun, P.K.; Liu, L.G.; Guo, C.; Wang, X.L.; Zhang, T.F.; Cong, Y.N.; Wei, Z.B. One-Step Synthesis of Double-Walled NiCo2O4 Hollow Nanotubes Using Hollow Fiber Templates Created by Airflow-Coaxial Electrospinning. Vacuum 2024, 228, 113526. [Google Scholar] [CrossRef]
- Sethy, P.P.; Sundaray, B. Magnetic Behavior of Fe3O4@C Nanofibers by a Facile Co-Axial Electrospinning Method. Nanotechnology 2023, 34, 445701. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.S.; Lee, B.S.; Yu, W.R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Xin, B.; Xu, Y. Coaxial Electrospinning: Jet Motion, Core-Shell Fiber Morphology, and Structure as a Function of Material Parameters. Ind. Eng. Chem. Res. 2020, 59, 6301–6308. [Google Scholar] [CrossRef]
- Sun, B.; Duan, B.; Yuan, X. Preparation of Core/Shell PVP/PLA Ultrafine Fibers by Coaxial Electrospinning. J. Appl. Polym. Sci. 2006, 102, 39–45. [Google Scholar] [CrossRef]
- Hieu, N.T.; Baik, S.J.; Jun, Y.; Lee, M.; Chung, O.H.; Park, J.S. Electrospun Coaxial Titanium Dioxide/Carbon Nanofibers for Use in Anodes of Dye-Sensitized Solar Cells. Electrochim. Acta 2014, 142, 144–151. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Wang, W.; Liu, Y.; Cui, B.; Guo, X. Facile Synthesis of Specific FeMnO3 Hollow Sphere/Graphene Composites and Their Superior Electrochemical Energy Storage Performances for Supercapacitor. J. Power Sources 2014, 248, 465–473. [Google Scholar] [CrossRef]
- Moura, J.V.B.; Pinheiro, G.S.; Freire, P.T.C.; Filho, J.M.; Saraiva, G.D.; Viana, B.C.; Luz-Lima, C. High-Pressure Raman Scattering on Fe2(MoO4)3 Microcrystals Obtained by a Hydrothermal Method. Vib. Spectrosc. 2016, 87, 88–93. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, Y.; Yan, J.M.; Jiang, Q. Regulating Fe2(MoO4)3 by Au Nanoparticles for Efficient N2 Electroreduction under Ambient Conditions. Adv. Energy Mater. 2021, 11, 2003701. [Google Scholar] [CrossRef]
- Khanam, S.; Zakaria, A.K.M.; Ahsan, M.H.; Datta, T.K.; Aktar, S.; Liba, S.I.; Hossain, S.; Das, A.K.; Kamal, I.; Yunus, S.M.; et al. Study of the Crystallographic and Magnetic Structure in the Nickel Substituted Cobalt Ferrites by Neutron Diffraction. Mater. Sci. Appl. 2015, 6, 332–342. [Google Scholar] [CrossRef][Green Version]
- El-Masry, M.M.; Ramadan, R. The Effect of CoFe2O4, CuFe2O4 and Cu/CoFe2O4 Nanoparticles on the Optical Properties and Piezoelectric Response of the PVDF Polymer. Appl. Phys. A Mater. Sci. Process 2022, 128, 110. [Google Scholar] [CrossRef]
- Meng, L.; Dong, J.; Chen, J.; Li, L.; Huang, Q.; Lu, J. Activation of Peracetic Acid by Spinel FeCo2O4 Nanoparticles for the Degradation of Sulfamethoxazole. Chem. Eng. J. 2023, 456, 141084. [Google Scholar] [CrossRef]
- Bertolucci, E.; Galletti, A.M.R.; Antonetti, C.; Marracci, M.; Tellini, B.; Piccinelli, F.; Visone, C. Chemical and Magnetic Properties Characterization of Magnetic Nanoparticles. In Proceedings of the 2015 IEEE Instrumentation and Measurement Technology Conference, Pisa, Italy, 11–14 May 2015; pp. 1492–1496. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Xu, J.; Lu, Y.; Liu, Y.; Qiu, K.; Zhang, Y.; Luo, Y. Solution Growth of NiO Nanosheets Supported on Ni Foam as High-Performance Electrodes for Supercapacitors. Nanoscale Res. Lett. 2014, 9, 424. [Google Scholar] [CrossRef]
- Ma, L.; Shi, X.; Zhang, X.; Li, L. Electrospinning of Polycaprolacton/Chitosan Core-Shell Nanofibers by a Stable Emulsion System. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123956. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Hou, J.; Zhang, W.; Xiang, C.; Li, L. Effect of the Electrical Conductivity of Core Solutions on the Morphology and Structure of Core–Shell CA-PCL/CS Nanofibers. New J. Chem. 2017, 41, 15072–15078. [Google Scholar] [CrossRef]
- Qayoom, M.; Shah, K.A.; Pandit, A.H.; Firdous, A.; Dar, G.N. Dielectric and Electrical Studies on Iron Oxide (α-Fe2O3) Nanoparticles Synthesized by Modified Solution Combustion Reaction for Microwave Applications. J. Electroceram. 2020, 45, 7–14. [Google Scholar] [CrossRef]
- Aytan, E.; Debnath, B.; Kargar, F.; Barlas, Y.; Lacerda, M.M.; Li, J.X.; Lake, R.K.; Shi, J.; Balandin, A.A. Spin-Phonon Coupling in Antiferromagnetic Nickel Oxide. Appl. Phys. Lett. 2017, 111, 252402. [Google Scholar] [CrossRef]
- Jaiswal, R.; Ranganath, K.V.S. Carbon Nanoparticles on Magnetite: A New Heterogeneous Catalyst for the Oxidation of 5-Hydroxymethylfurfural (5-HMF) to 2,5-Diformoylfuran (DFF). J. Inorg. Organomet. Polym. Mater. 2021, 31, 4504–4511. [Google Scholar] [CrossRef]
- Hai, N.H.; Phu, N.D.; Luong, N.H.; Chau, N.; Chinh, H.D.; Hoang, L.H.; Leslie-Pelecky, D.L. Mechanism for Sustainable Magnetic Nanoparticles under Ambient Conditions. J. Korean Phys. Soc. 2008, 52, 1327–1331. [Google Scholar] [CrossRef]
- Baghaie Yazdi, M.; Choi, K.Y.; Wulferding, D.; Lemmens, P.; Alff, L. Raman Study of the Verwey Transition in Magnetite Thin Films. New J. Phys. 2013, 15, 103032. [Google Scholar] [CrossRef]
- Gebretinsae, H.G.; Tsegay, M.G.; Nuru, Z.Y. Biosynthesis of Nickel Oxide (NiO) Nanoparticles from Cactus Plant Extract. Mater. Today Proc. 2021, 36, 566–570. [Google Scholar] [CrossRef]
- Caso, D.; Serrano, A.; Jaafar, M.; Prieto, P.; Kamra, A.; González-Ruano, C.; Aliev, F.G. Microwave Field-Induced Changes in Raman Modes and Magnetic Force Images of Antiferromagnetic NiO Films. Condens. Matter 2024, 9, 7. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Puust, L.; Grabis, J. Magnon and Phonon Excitations in Nanosized NiO. Latv. J. Phys. Tech. Sci. 2019, 56, 61–72. [Google Scholar] [CrossRef]
- Alhashem, Z.; Awada, C.; Ahmed, F.; Farha, A.H. Structural and Magnetic Properties Study of Fe2O3/NiO/Ni2FeO4 Nanocomposites. Crystals 2021, 11, 613. [Google Scholar] [CrossRef]
- Sedrati, C.; Alleg, S.; Boussafel, H.; Bendali Hacine, A. Structure and Magnetic Properties of Nickel Ferrites Synthesized by a Facile Co-Precipitation Method: Effect of the Fe/Ni Ratio. J. Mater. Sci. Mater. Electron. 2021, 32, 24548–24559. [Google Scholar] [CrossRef]
- Soam, A.; Kumar, R.; Thatoi, D.; Singh, M. Electrochemical Performance and Working Voltage Optimization of Nickel Ferrite/Graphene Composite Based Supercapacitor. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3325–3331. [Google Scholar] [CrossRef]
- Liu, D.; Li, D.; Yang, D. Size-Dependent Magnetic Properties of Branchlike Nickel Oxide Nanocrystals. AIP Adv. 2017, 7, 15028. [Google Scholar] [CrossRef]
- Sahu, B.; Panigrahi, U.K.; Chakravarty, S.; Hussain, S.; Mallick, P. Structural, Optical, and Magnetic Properties of NiO/NiFe2O4 Nanocomposites. Appl. Phys. A Mater. Sci. Process 2023, 129, 584. [Google Scholar] [CrossRef]
- Ai, Q.; Yuan, Z.; Huang, R.; Yang, C.; Jiang, G.; Xiong, J.; Huang, Z.; Yuan, S. One-Pot Co-Precipitation Synthesis of Fe3O4 Nanoparticles Embedded in 3D Carbonaceous Matrix as Anode for Lithium Ion Batteries. J. Mater. Sci. 2019, 54, 4212–4224. [Google Scholar] [CrossRef]
- Imran, M.; Coskun, H.; Khan, N.A.; Ouyang, J. Role of Annealing Temperature of Nickel Oxide (NiOx) as Hole Transport Layer in Work Function Alignment with Perovskite. Appl. Phys. A Mater. Sci. Process 2021, 127, 117. [Google Scholar] [CrossRef]
- Huang, W.; Ding, S.; Chen, Y.; Hao, W.; Lai, X.; Peng, J.; Tu, J.; Cao, Y.; Li, X. 3D NiO Hollow Sphere/Reduced Graphene Oxide Composite for High-Performance Glucose Biosensor. Sci. Rep. 2017, 7, 5220. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, M.; Zhang, Z.; An, Z.; Wang, Y.; Hu, X. Improved Electrocatalytic Methanol Oxidation of NiO Nanosheet Arrays with Synergistic High Surface Oxygen Vacancy and Ni3+/Ni2+ Ratio. Int. J. Hydrog. Energy 2023, 48, 27679–27685. [Google Scholar] [CrossRef]
- Jouini, K.; Raouafi, A.; Dridi, W.; Daoudi, M.; Mustapha, B.; Chtourou, R.; Hosni, F. Investigation of Gamma-Ray Irradiation Induced Phase Change from NiO to Ni2O3 for Enhancing Photocatalytic Performance. Optik 2019, 195, 163109. [Google Scholar] [CrossRef]
- Kawrani, S.; Boulos, M.; Bekheet, M.F.; Viter, R.; Nada, A.A.; Riedel, W.; Roualdes, S.; Cornu, D.; Bechelany, M. Segregation of Copper Oxide on Calcium Copper Titanate Surface Induced by Graphene Oxide for Water Splitting Applications. Appl. Surf. Sci. 2020, 516, 146051. [Google Scholar] [CrossRef]
- Choudhary, S.; Hasina, D.; Saini, M.; Ranjan, M.; Mohapatra, S. Facile Synthesis, Morphological, Structural, Photocatalytic and Optical Properties of ZnFe2O4-ZnO Hybrid Nanostructures. J. Alloys Compd. 2022, 895, 162723. [Google Scholar] [CrossRef]
- Tahir, D.; Ilyas, S.; Rahmat, R.; Heryanto, H.; Fahri, A.N.; Rahmi, M.H.; Abdullah, B.; Hong, C.C.; Kang, H.J. Enhanced Visible-Light Absorption of Fe2O3 Covered by Activated Carbon for Multifunctional Purposes: Tuning the Structural, Electronic, Optical, and Magnetic Properties. ACS Omega 2021, 6, 28334–28346. [Google Scholar] [CrossRef]
- Leão-Neto, V.S.; da Silva, A.C.; Camargo, L.P.; Da Silva Pelissari, M.R.; da Silva, P.R.C.; Parreira, P.S.; Segatelli, M.G.; Dall′Antonia, L.H. Fabrication of RGO/α-Fe2O3 Electrodes: Characterization and Use in Photoelectrocatalysis. J. Mater. Sci. Mater. Electron. 2020, 31, 16882–16897. [Google Scholar] [CrossRef]
- Delice, S.; Isik, M.; Gasanly, N.M. Temperature-Dependent Tuning of Band Gap of Fe3O4 Nanoparticles for Optoelectronic Applications. Chem. Phys. Lett. 2024, 840, 141139. [Google Scholar] [CrossRef]
- Haider, A.J.; Al-Anbari, R.; Sami, H.M.; Haider, M.J. Enhance Preparation and Characterization of Nickel-Oxide as Self-Cleaning Surfaces. Energy Procedia 2019, 157, 1328–1342. [Google Scholar] [CrossRef]
- Hashem, M.; Saion, E.; Al-Hada, N.M.; Kamari, H.M.; Shaari, A.H.; Talib, Z.A.; Paiman, S.B.; Kamarudeen, M.A. Fabrication and Characterization of Semiconductor Nickel Oxide (NiO) Nanoparticles Manufactured Using a Facile Thermal Treatment. Results Phys. 2016, 6, 1024–1030. [Google Scholar] [CrossRef]
- Hosny, N.M. Synthesis, Characterization and Optical Band Gap of NiO Nanoparticles Derived from Anthranilic Acid Precursors via a Thermal Decomposition Route. Polyhedron 2011, 30, 470–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, H.; Gang, C.; Guan, H.; Dong, C.; Yin, Z. Porous, Tremella-like NiFe2O4 with Ultrathin Nanosheets for Ppb-Level Toluene Detection. Crystals 2023, 13, 865. [Google Scholar] [CrossRef]
- Tong, S.K.; Chi, P.W.; Kung, S.H.; Wei, D.H. Tuning Bandgap and Surface Wettability of NiFe2O4 Driven by Phase Transition. Sci. Rep. 2018, 8, 1338. [Google Scholar] [CrossRef]
- Bhanuchandar, S.; Vinothkumar, G.; Arunkumar, P.; Sribalaji, M.; Keshri, A.K.; Babu, K.S. Unravelling the Role of Cationic Ni2+ Vacancies and Ni3+ Ions in Non-Stoichiometric NiO: Breakdown of Anti-Ferromagnetic Ordering and Large Exchange Bias. J. Mater. Sci. 2023, 58, 13136–13153. [Google Scholar] [CrossRef]
- Barwant, M.; Karande, V.; Basnet, P.; Kumar, D.; Sargazi, S.; Mirzaei, M.; Jabir, M.S.; Sanap, D.; Ghotekar, S. Insights into the Antioxidant and Anticancer Properties of Novel Biologically Synthesized NiO/Ni2O3 Nanoparticles Using Sargassum Tenerrimum. J. Solgel Sci. Technol. 2024, 111, 409–420. [Google Scholar] [CrossRef]



| Core–Shell Nanostructure | Application | Reference |
|---|---|---|
| SnO2/TiO2 nanofibers | Rhodamine B photocatalytic degradation | [13] |
| SnO2-WO3 nanofibers | Ethanol, toluene, acetone resistive sensors | [14] |
| Co3O4-ZnO nanofibers | Formaldehyde resistive sensor | [15] |
| In2O3−SnO2 nanofibers | Trimethylamine resistive sensor | [16] |
| Fe3O4@SiO2 nanofibers | pH sensor, oil–water separation | [17] |
| Ni@NiO/NiCO3 | Photocatalytic water splitting | [18] |
| Fe3O4/ZnO heterostructures | Rhodamine B photocatalytic degradation | [19] |
| Fe3O4@C nanofibers | Microwave absorption | [20] |
| Co3O4/TiO2 nanofibers | Acetone resistive sensor | [8] |
| Nylon–ZnO nanofibers | Photocatalytic degradation of Rhodamine B | [21] |
| Core | Shell | |
|---|---|---|
| FeNi31 | 174 ± 15 nm | 13 ± 7 nm |
| FeNi33 | 172 ± 12 nm | 23 ± 12 nm |
| FeNi35 | 178 ± 22 nm | 36 ± 15 nm |
| Sample | Ni3+ 2p3/2/Ni2+ 2p3/2 | Fe3+ 2p3/2/Fe2+ 2p3/2 | (O 1s 530 eV)/ (O 1s 531 eV) |
|---|---|---|---|
| FeNi31 | 3.2008 | 3.026882 | 0.37 |
| FeNi33 | 3.887043 | 2.988601 | 0.21 |
| FeNi35 | 3.667256 | 5.988984 | 0.205 |
| Sample | MS* (A m2/kg) | HC (kA/m) | HC (Oe) | χ (m3/kg) | χ (emu/(g·kOe)) |
|---|---|---|---|---|---|
| FeNi31 | 15.3 | 2.1 | 26.0 | 1.2 | 14.9 |
| FeNi33 | 11.2 | 12.1 | 152.1 | 0.8 | 10.1 |
| FeNi35 | 6.1 | 4.3 | 53.4 | 0.4 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viter, R.; Zabolotnii, V.; Sahul, M.; Čaplovičová, M.; Tepliakova, I.; Sints, V.; Fioravanti, A. Novel Core–Shell Metal Oxide Nanofibers with Advanced Optical and Magnetic Properties Deposited by Co-Axial Electrospinning. Nanomaterials 2025, 15, 1026. https://doi.org/10.3390/nano15131026
Viter R, Zabolotnii V, Sahul M, Čaplovičová M, Tepliakova I, Sints V, Fioravanti A. Novel Core–Shell Metal Oxide Nanofibers with Advanced Optical and Magnetic Properties Deposited by Co-Axial Electrospinning. Nanomaterials. 2025; 15(13):1026. https://doi.org/10.3390/nano15131026
Chicago/Turabian StyleViter, Roman, Viktor Zabolotnii, Martin Sahul, Mária Čaplovičová, Iryna Tepliakova, Viesturs Sints, and Ambra Fioravanti. 2025. "Novel Core–Shell Metal Oxide Nanofibers with Advanced Optical and Magnetic Properties Deposited by Co-Axial Electrospinning" Nanomaterials 15, no. 13: 1026. https://doi.org/10.3390/nano15131026
APA StyleViter, R., Zabolotnii, V., Sahul, M., Čaplovičová, M., Tepliakova, I., Sints, V., & Fioravanti, A. (2025). Novel Core–Shell Metal Oxide Nanofibers with Advanced Optical and Magnetic Properties Deposited by Co-Axial Electrospinning. Nanomaterials, 15(13), 1026. https://doi.org/10.3390/nano15131026








