Zn-URJC-12 Material Constituted of Two Different Organic Ligands for CO2 Valorization into Cyclic Carbonates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
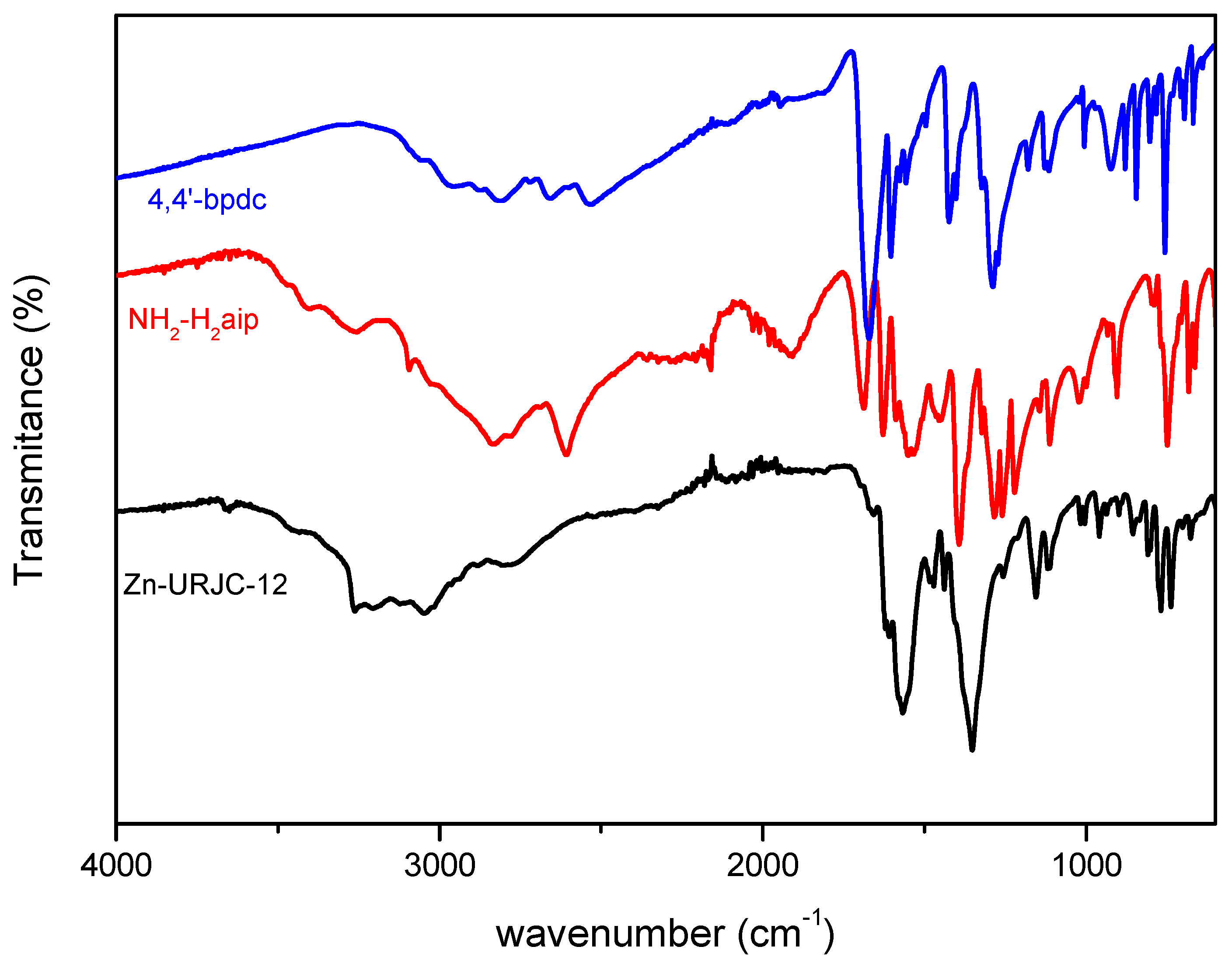
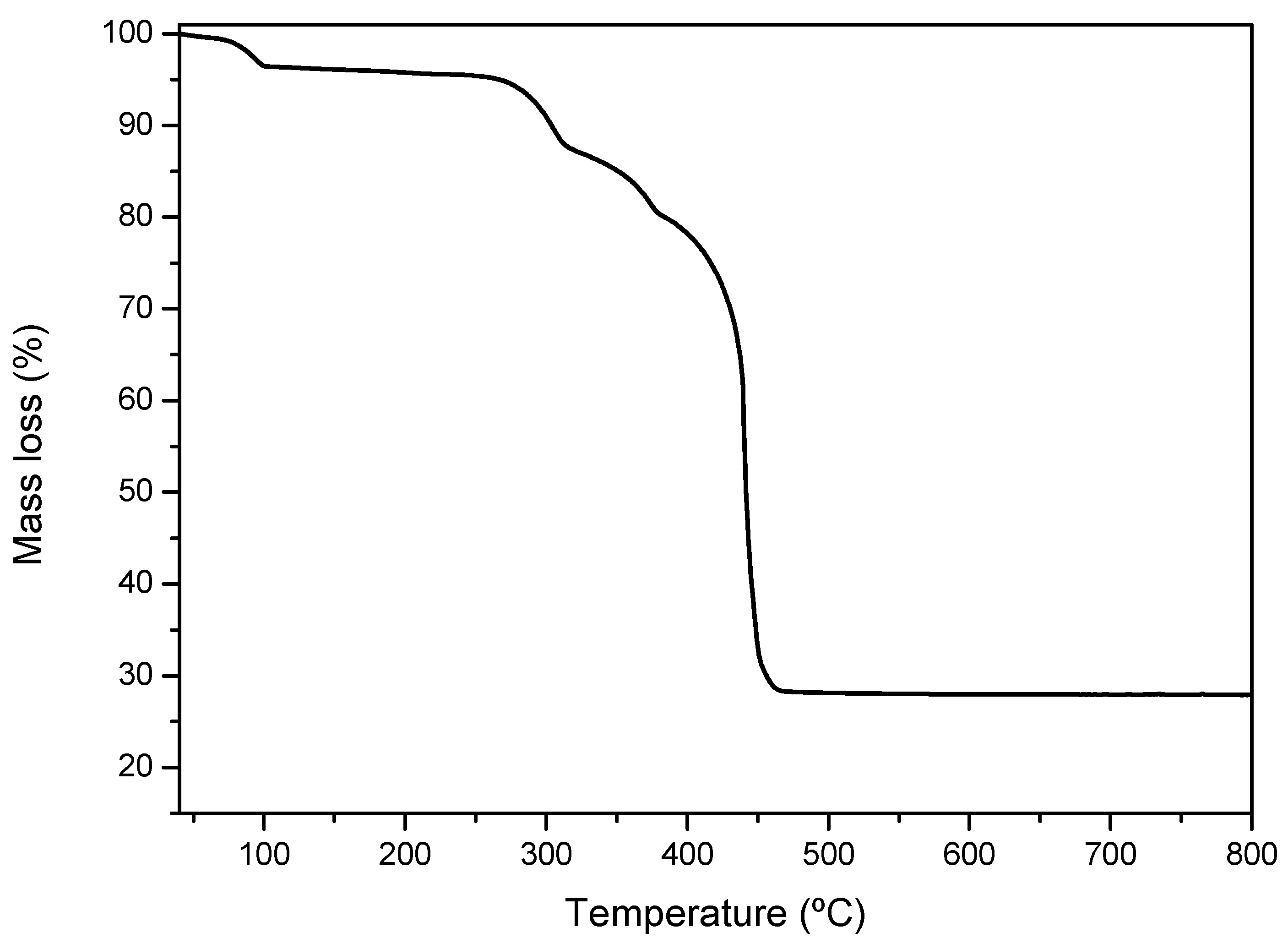
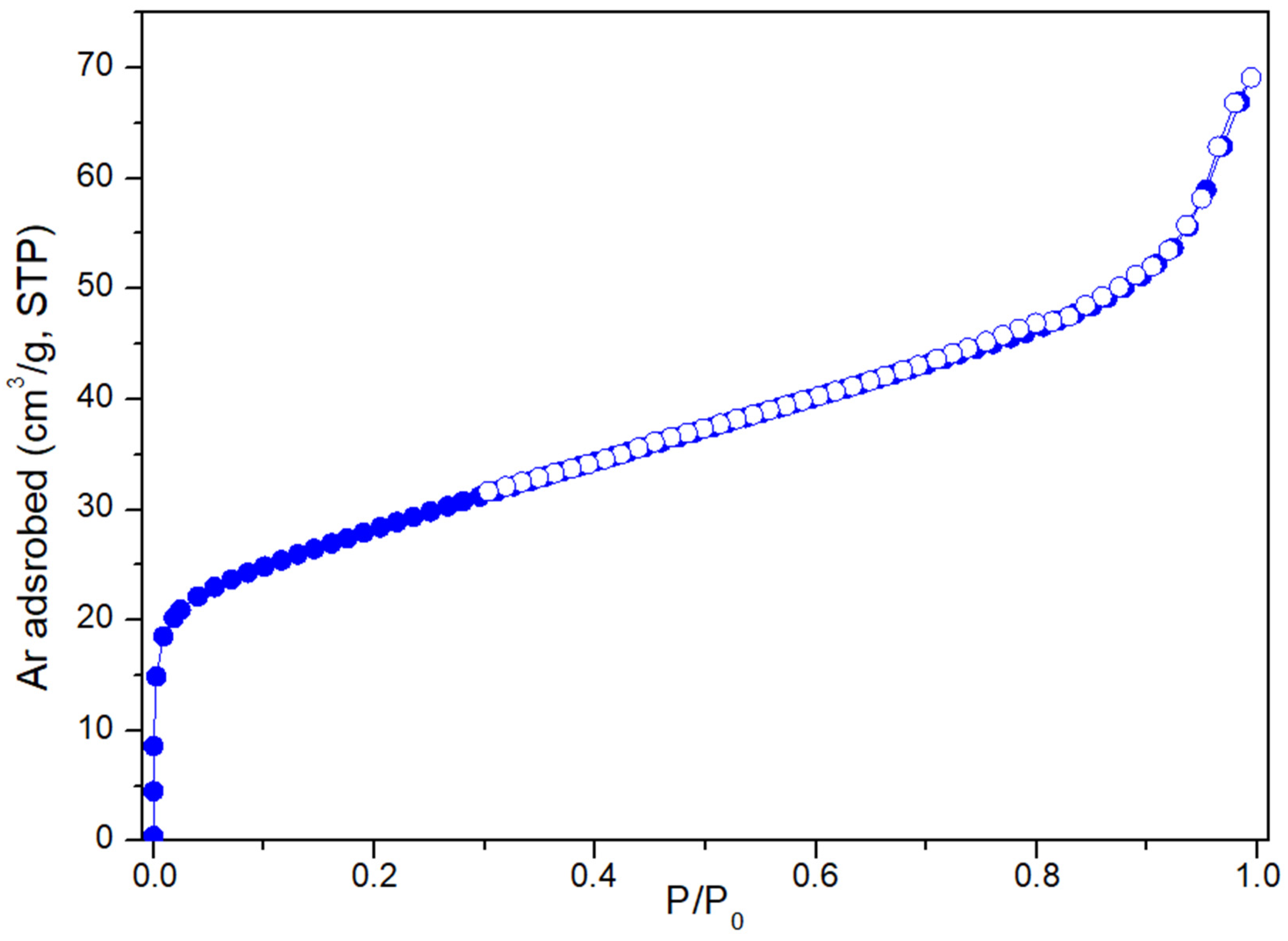
3.1. Catalyst Characterization
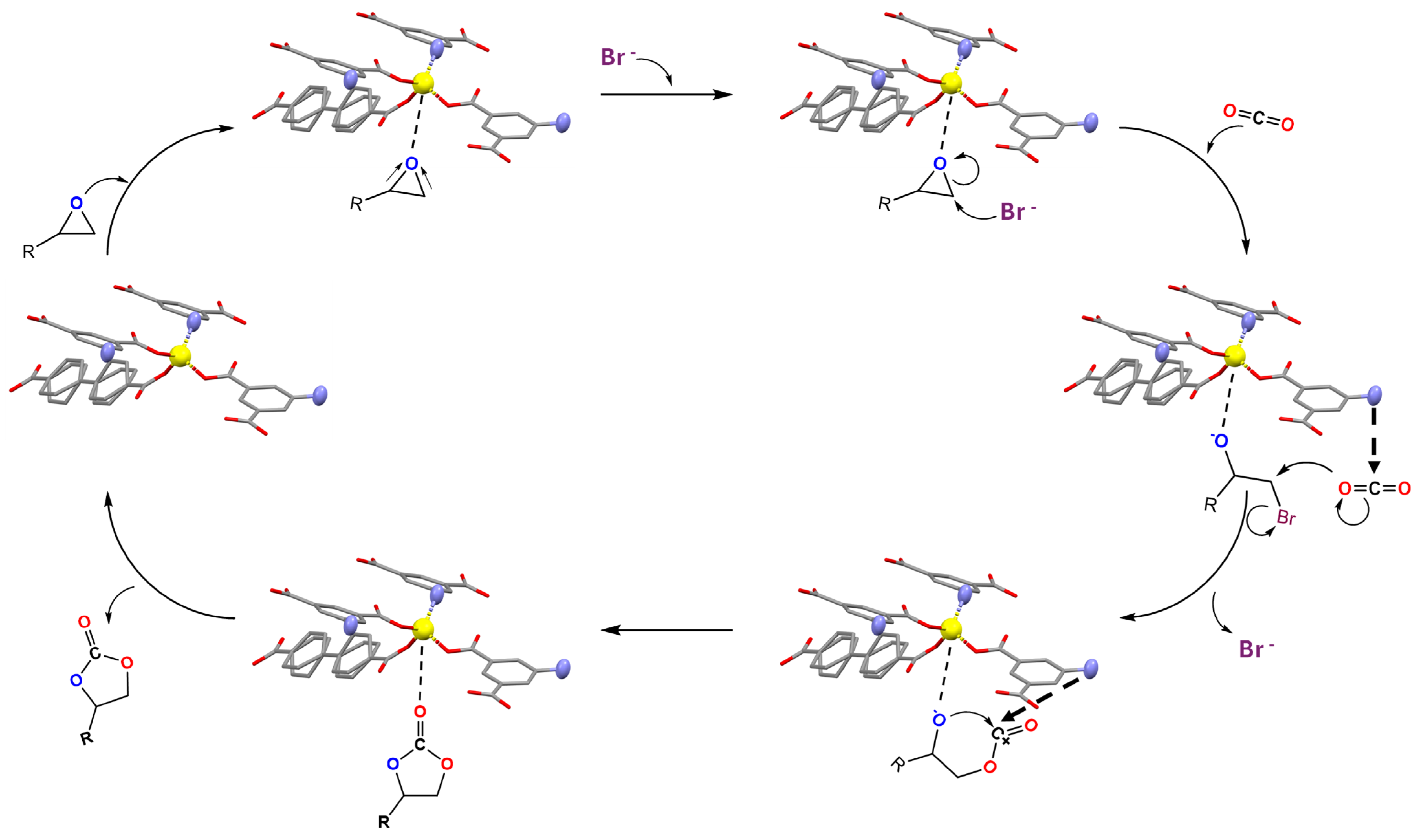
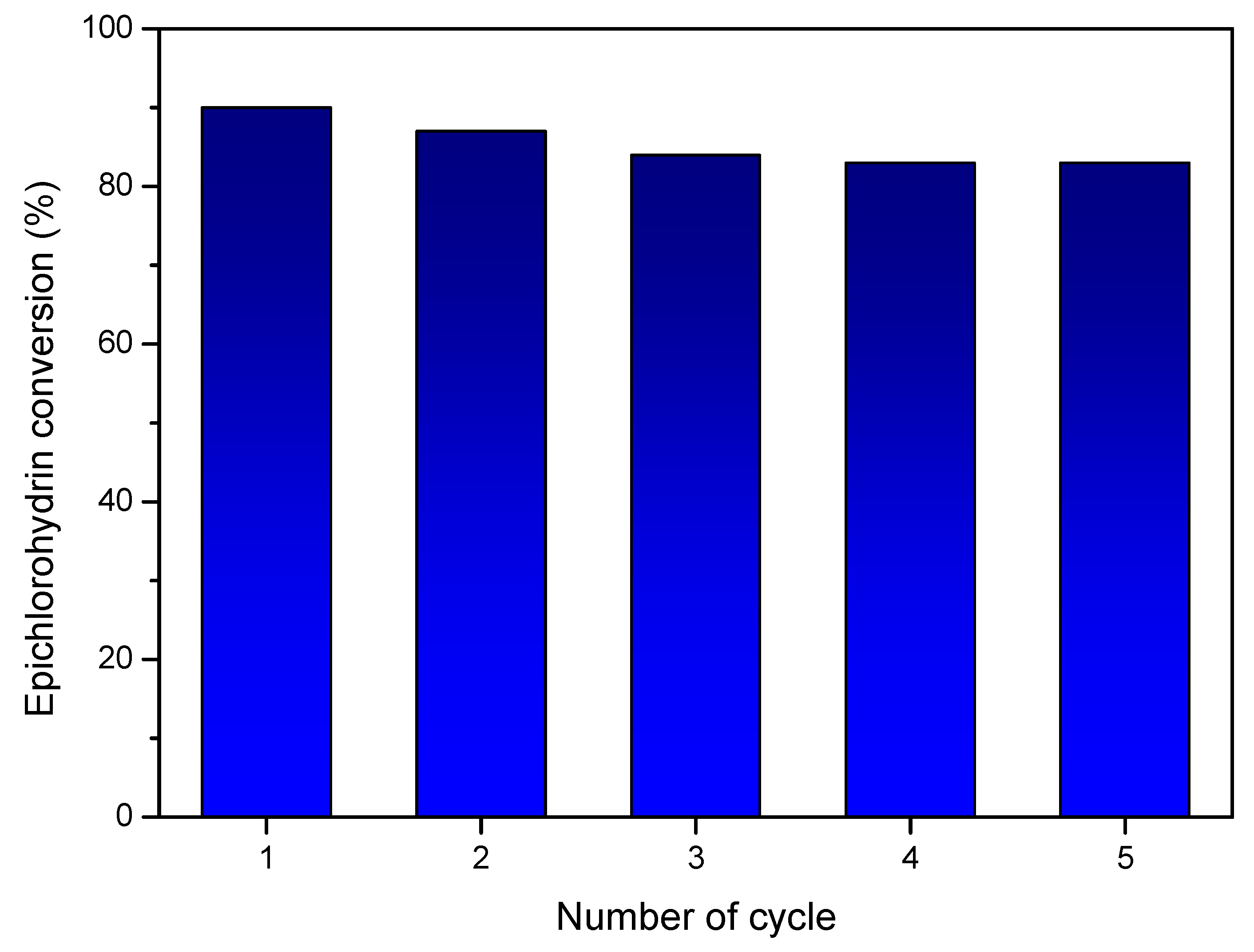
3.2. Catalytic Studies of Zn-URJC-12 for the Cycloaddition of CO2 and Epoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.G.; Zhang, G.Y.; Hu, T.L.; Bu, X.H. Metal-Organic Framework-Based Heterogeneous Catalysts for the Conversion of C1 Chemistry: CO, CO2 and CH4. Coord. Chem. Rev. 2019, 387, 79–120. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Kim, K.-H.; Dong, W.; Kim, S. A Novel Enhancement of Shape/Thermal Stability and Energy-Storage Capacity of Phase Change Materials through the Formation of Composites with 3D Porous (3,6)-Connected Metal–Organic Framework. Chem. Eng. J. 2020, 389, 124430. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Z.; Fang, Y.; Niu, J.; Yuan, W.; Li, L. A Novel Metal-Organic Framework Aerogel Based Hydrated Salt Composite Phase Change Material for Enhanced Solar Thermal Utilization. J. Energy Storage 2023, 58, 106354. [Google Scholar] [CrossRef]
- Tapiador, J.; Leo, P.; Calleja, G.; Orcajo, G. Open Zn-URJC-13 Efficient Catalyst for Mild CO2 Transformation Using Bulky Epoxides. Catal. Today 2024, 428, 114442. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Coronado, E. Magnetic Functionalities in MOFs: From the Framework to the Pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Samanta, P.; Let, S.; Mandal, W.; Dutta, S.; Ghosh, S.K. Luminescent Metal–Organic Frameworks (LMOFs) as Potential Probes for the Recognition of Cationic Water Pollutants. Inorg. Chem. Front. 2020, 7, 1801–1821. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-Organic Frameworks: Versatile Heterogeneous Catalysts for Efficient Catalytic Organic Transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A Review for Metal-Organic Frameworks (MOFs) Utilization in Capture and Conversion of Carbon Dioxide into Valuable Products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Rollin, P.; Soares, L.K.; Barcellos, A.M.; Araujo, D.R.; Lenardão, E.J.; Jacob, R.G.; Perin, G. Five-Membered Cyclic Carbonates: Versatility for Applications in Organic Synthesis, Pharmaceutical, and Materials Sciences. Appl. Sci. 2021, 11, 5024. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of Cyclic Carbonates from Epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Zhang, W.; Ping, R.; Lu, X.; Shi, H.; Liu, F.; Ma, J.; Liu, M. Rational Design of Lewis Acid-Base Bifunctional Nanopolymers with High Performance on CO2/Epoxide Cycloaddition without a Cocatalyst. Chem. Eng. J. 2023, 451, 138715. [Google Scholar] [CrossRef]
- Shiels, R.A.; Jones, C.W. Homogeneous and Heterogeneous 4-(N,N-Dialkylamino)Pyridines as Effective Single Component Catalysts in the Synthesis of Propylene Carbonate. J. Mol. Catal. A Chem. 2007, 261, 160–166. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, N.; Wei, W.; Sun, Y. Chemical Fixation of Carbon Dioxide to Propylene Carbonate over Amine-Functionalized Silica Catalysts. Catal. Today 2006, 115, 102–106. [Google Scholar] [CrossRef]
- Ramin, M.; Jutz, F.; Grunwaldt, J.D.; Baiker, A. Solventless Synthesis of Propylene Carbonate Catalysed by Chromium-Salen Complexes: Bridging Homogeneous and Heterogeneous Catalysis. J. Mol. Catal. A Chem. 2005, 242, 32–39. [Google Scholar] [CrossRef]
- Tapiador, J.; Leo, P.; Gándara, F.; Calleja, G.; Orcajo, G. Robust Cu-URJC-8 with Mixed Ligands for Mild CO2 Cycloaddition Reaction. J. CO2 Util. 2022, 64, 102166. [Google Scholar] [CrossRef]
- Tapiador, J.; García-Rojas, E.; Leo, P.; Martos, C.; Calleja, G.; Orcajo, G. Copper MOFs Performance in the Cycloaddition Reaction of CO2 and Epoxides. Microporous Mesoporous Mater. 2023, 361, 112741. [Google Scholar] [CrossRef]
- Tapiador, J.; Leo, P.; Rodríguez-Diéguez, A.; Choquesillo-Lazarte, D.; Calleja, G.; Orcajo, G. A Novel Zn-Based-MOF for Efficient CO2 Adsorption and Conversion under Mild Conditions. Catal. Today 2022, 390–391, 230–236. [Google Scholar] [CrossRef]
- Tapiador, J.; García-Rojas, E.; López-Patón, P.; Calleja, G.; Orcajo, G.; Martos, C.; Leo, P. Influence of Divalent Metal Ions on CO2 Valorization at Room Temperature by Isostructural MOF-74 Materials. J. Environ. Chem. Eng. 2023, 11, 109497. [Google Scholar] [CrossRef]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–Organic Frameworks for the Chemical Fixation of CO2 into Cyclic Carbonates. Coord. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Tran, Y.B.N.; Nguyen, P.T.K.; Luong, Q.T.; Nguyen, K.D. Series of M-MOF-184 (M = Mg, Co, Ni, Zn, Cu, Fe) Metal-Organic Frameworks for Catalysis Cycloaddition of CO2. Inorg. Chem. 2020, 59, 16747–16759. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Hu, Y.; Wang, B.; Sun, S.; Yang, X.; He, H. Predicting Metal-Organic Frameworks as Catalysts to Fix Carbon Dioxide to Cyclic Carbonate by Machine Learning. J. Mater. 2021, 7, 1029–1038. [Google Scholar] [CrossRef]
- Valverde-González, A.; Borrallo-Aniceto, M.C.; Díaz, U.; Maya, E.M.; Gándara, F.; Sánchez, F.; Iglesias, M. Nitrogen-Rich Cobalt (II) MOFs as Efficient Bifunctional Catalysts for Single or Tandem Oxidation and CO2 Conversion Reactions. J. CO2 Util. 2023, 67, 102298. [Google Scholar] [CrossRef]
- Shang, L.; Chen, X.-L.; Liu, L.; Cai, M.; Yan, R.-K.; Cui, H.-L.; Yang, H.; Wang, J.-J. Catalytic Performance of MOFs Containing Trinuclear Lanthanides Clusters in the Cycladdition Reaction of CO2 and Epoxide. J. CO2 Util. 2022, 65, 102235. [Google Scholar] [CrossRef]
- Guo, F. A Novel 2D Cu(II)-MOF as a Heterogeneous Catalyst for the Cycloaddition Reaction of Epoxides and CO2 into Cyclic Carbonates. J. Mol. Struct. 2019, 1184, 557–561. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Maji, T.K. Multi-Dimensional Metal-Organic Frameworks Based on Mixed Linkers: Interplay between Structural Flexibility and Functionality. Coord. Chem. Rev. 2022, 469, 214645. [Google Scholar] [CrossRef]
- Haldar, R.; Maji, T.K. Metal–Organic Frameworks (MOFs) Based on Mixed Linker Systems: Structural Diversities towards Functional Materials. CrystEngComm 2013, 15, 9276. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Mixed-Metal or Mixed-Linker Metal Organic Frameworks as Heterogeneous Catalysts. Catal. Sci. Technol. 2016, 6, 5238–5261. [Google Scholar] [CrossRef]
- Kleist, W.; Jutz, F.; Maciejewski, M.; Baiker, A. Mixed-Linker Metal-Organic Frameworks as Catalysts for the Synthesis of Propylene Carbonate from Propylene Oxide and CO2. Eur. J. Inorg. Chem. 2009, 2009, 3552–3561. [Google Scholar] [CrossRef]
- Manna, K.; Zhang, T.; Greene, F.X.; Lin, W. Bipyridine- and Phenanthroline-Based Metal–Organic Frameworks for Highly Efficient and Tandem Catalytic Organic Transformations via Directed C–H Activation. J. Am. Chem. Soc. 2015, 137, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guo, L.; Wang, H.; Gu, J.; Yang, Y.; Kirillova, M.V.; Kirillov, A.M. Coordination Polymers Constructed from an Adaptable Pyridine-Dicarboxylic Acid Linker: Assembly, Diversity of Structures, and Catalysis. Inorg. Chem. 2022, 61, 17951–17962. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.N.; Tran, T.V.; Phan, N.T.S.; Truong, T. Efficient and Recyclable Cu2(BDC)2(BPY)-Catalyzed Oxidative Amidation of Terminal Alkynes: Role of Bipyridine Ligand. Catal. Sci. Technol. 2015, 5, 851–859. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. Application of Two Cobalt-Based Metal–Organic Frameworks as Oxidative Desulfurization Catalysts. Inorg. Chem. 2015, 54, 11269–11275. [Google Scholar] [CrossRef]
- Payra, S.; Roy, S. CO2 Cycloaddition Reaction at Ambient Temperature and Pressure over Metal Organic Framework Catalysts. MRS Commun. 2023, 13, 1309–1314. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; He, Y.; Ao, Q.; Deng, Q.; Zeng, Z.; Wang, H.; Deng, S. A Stable Zn-Based Metal–Organic Framework as an Efficient Catalyst for Carbon Dioxide Cycloaddition and Alcoholysis at Mild Conditions. Catal. Lett. 2020, 150, 1408–1417. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Yuan, H.-B.; Xu, X.-B.; Huang, R.-B. Influential Factors on Assembly of First-Row Transition Metal Coordination Polymers. Inorg. Chim. Acta 2013, 403, 53–62. [Google Scholar] [CrossRef]
- Kongshaug, K.O.; Fjellvåg, H. Design of Novel Bilayer Compounds of the CPO-8 Type Containing 1D Channels. Inorg. Chem. 2006, 45, 2424–2429. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Lee, J.-J.; Su, C.-H.; Tsai, M.-J.; Li, C.-Y.; Wu, J.-Y. From Lamellar Net to Bilayered-Lamella and to Porous Pillared-Bilayer: Reversible Crystal-to-Crystal Transformation, CO2 Adsorption, and Fluorescence Detection of Fe3+, Al3+, Cr3+, MnO4−, and Cr2O72− in Water. Dalton Trans. 2020, 49, 14201–14215. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, L.; Zhang, X.; Xu, P.; Sun, J. Facile One-Pot Synthesis of Zn/Mg-MOF-74 with Unsaturated Coordination Metal Centers for Efficient CO2 Adsorption and Conversion to Cyclic Carbonates. ACS Appl. Mater. Interfaces 2021, 13, 61334–61345. [Google Scholar] [CrossRef]
- Castro-Gómez, F.; Salassa, G.; Kleij, A.W.; Bo, C. A DFT Study on the Mechanism of the Cycloaddition Reaction of CO2 to Epoxides Catalyzed by Zn(Salphen) Complexes. Chem. A Eur. J. 2013, 19, 6289–6298. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Phang, H.S.; Li, Y.; Zhao, Y. Highly Effective Carbon Fixation via Catalytic Conversion of CO2 by an Acylamide-Containing Metal–Organic Framework. Chem. Mater. 2017, 29, 9256–9261. [Google Scholar] [CrossRef]
- Liang, J.; Xie, Y.-Q.; Wu, Q.; Wang, X.-Y.; Liu, T.-T.; Li, H.-F.; Huang, Y.-B.; Cao, R. Zinc Porphyrin/Imidazolium Integrated Multivariate Zirconium Metal–Organic Frameworks for Transformation of CO2 into Cyclic Carbonates. Inorg. Chem. 2018, 57, 2584–2593. [Google Scholar] [CrossRef]
- Miralda, C.M.; MacIas, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic Imidazole Framework-8 Catalysts in the Conversion of CO2 to Chloropropene Carbonate. ACS Catal. 2012, 2, 180–183. [Google Scholar] [CrossRef]
- Ren, Y.; Shi, Y.; Chen, J.; Yang, S.; Qi, C.; Jiang, H. Ni(Salphen)-Based Metal-Organic Framework for the Synthesis of Cyclic Carbonates by Cycloaddition of CO2 to Epoxides. RSC Adv. 2013, 3, 2167–2170. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Yang, X.; Zhao, L.; Wang, G. Functionalized IRMOF-3 as Efficient Heterogeneous Catalyst for the Synthesis of Cyclic Carbonates. J. Mol. Catal. A Chem. 2012, 361–362, 12–16. [Google Scholar] [CrossRef]
- Macias, E.E.; Ratnasamy, P.; Carreon, M.A. Catalytic Activity of Metal Organic Framework Cu3(BTC)2 in the Cycloaddition of CO2 to Epichlorohydrin Reaction. Catal. Today 2012, 198, 215–218. [Google Scholar] [CrossRef]
- Eskemech, A.; Chand, H.; Karmakar, A.; Krishnan, V.; Koner, R.R. Zn-MOF as a Single Catalyst with Dual Lewis Acidic and Basic Reaction Sites for CO2 Fixation. Inorg. Chem. 2024, 63, 3757–3768. [Google Scholar] [CrossRef] [PubMed]
- Khattak, Z.A.K.; Ahmad, N.; Younus, H.A.; Ullah, H.; Yu, B.; Munawar, K.S.; Ashfaq, M.; Yaseen, M.; Danish, M.; Al-Abri, M.; et al. Ambient Conversion of CO2 and Epoxides to Cyclic Carbonates Using 3D Amide-Functionalized MOFs. Catal. Sci. Technol. 2024, 14, 1888–1901. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.; Li, Y.; Yang, E.; Zhao, X.; Li, L. Hollow Zn−Co Based Zeolitic Imidazole Framework as a Robust Heterogeneous Catalyst for Enhanced CO2 Chemical Fixation. Chem. Asian J. 2019, 14, 4375–4382. [Google Scholar] [CrossRef]
- Xu, W.; Chen, H.; Jie, K.; Yang, Z.; Li, T.; Dai, S. Entropy-Driven Mechanochemical Synthesis of Polymetallic Zeolitic Imidazolate Frameworks for CO2 Fixation. Angew. Chem. Int. Ed. 2019, 58, 5018–5022. [Google Scholar] [CrossRef]



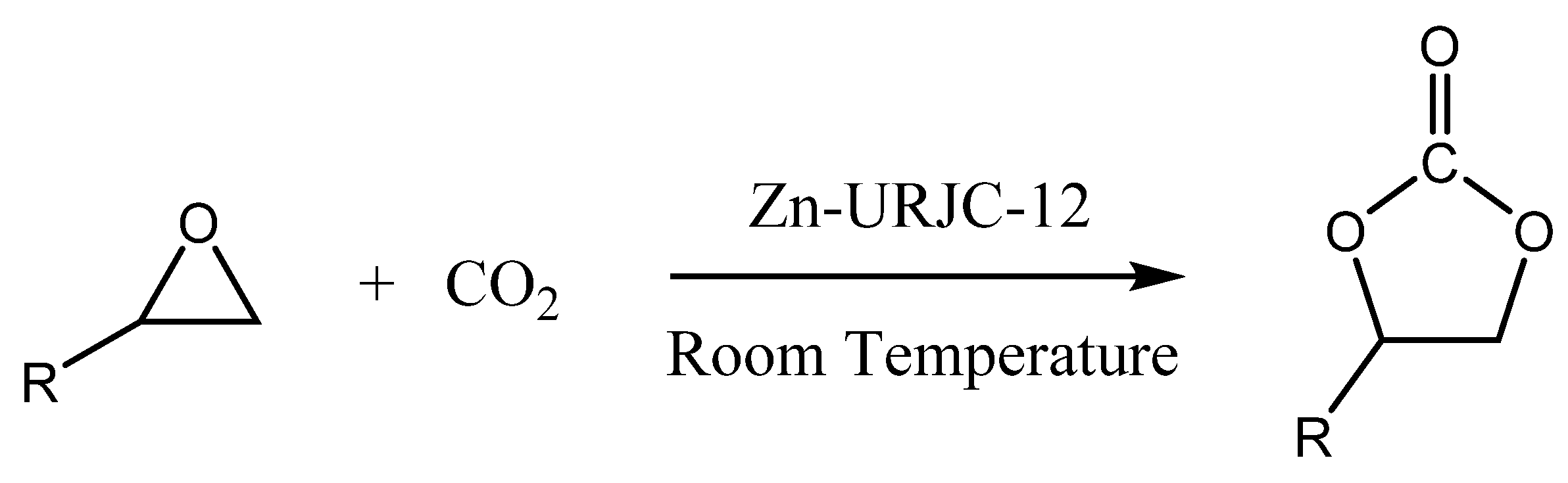
| Epoxide | Cyclic Carbonate | Conversion (%) a | Selectivity (%) b |
|---|---|---|---|
 |  | 100 | >99 |
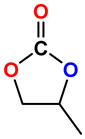
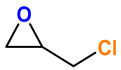 | 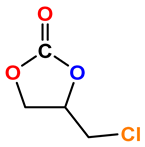 | 91 | >99 |
 | 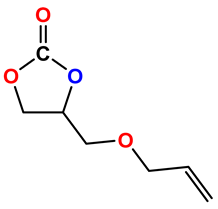 | 76 | >99 |
 |  | 59 | 64 |
| Material | Conversion (%) a | Selectivity (%) b |
|---|---|---|
| Zn-URJC-12 | 90 | >99 |
| Zn(NO3)2 | 80 | >99 |
| ZnBr2 | 33 | >99 |
| ZIF-8 | 76 | 95 |
| MOF | Pressure (Bar) | Temperature (°C) | Co-Catalyst/Time (h) | Yield (%) | Ref |
|---|---|---|---|---|---|
| Zn-URJC-12 | 12 | R.T. | TBAB/24 | 90 | This work |
| Cu-MOF-1 | 1 | R.T. | TBAB/48 | 88 | [47] |
| ZnTCPP | 1 | 140 | -/14 | 87 | [48] |
| ZIF-8 | 7 | 80 | -/4 | 44 | [49] |
| NR2-ZIF-8 | 7 | 80 | -/4 | 73 | |
| Ni(salphen)-MOF | 20 | 80 | TBAB/4 | 84 | [50] |
| F-IRMOF-3 | 20 | 140 | -/15 | 80 | [51] |
| HKUST-1 | 7 | 100 | -/4 | 34 | [52] |
| Zn-MOF | 1 | 70 | TBAB/24 | 98 | [53] |
| Zn-MOF | 1 | 40 | TBAB/2 | 100 | [54] |
| ZnCo-ZIF | 7 | 100 | -/4 | 99 | [55] |
| HE-ZIF | 10 | 100 | -/8 | 99 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapiador, J.; Leo, P.; Salcedo-Abraira, P.; Rodríguez-Diéguez, A.; Orcajo, G. Zn-URJC-12 Material Constituted of Two Different Organic Ligands for CO2 Valorization into Cyclic Carbonates. Nanomaterials 2025, 15, 1018. https://doi.org/10.3390/nano15131018
Tapiador J, Leo P, Salcedo-Abraira P, Rodríguez-Diéguez A, Orcajo G. Zn-URJC-12 Material Constituted of Two Different Organic Ligands for CO2 Valorization into Cyclic Carbonates. Nanomaterials. 2025; 15(13):1018. https://doi.org/10.3390/nano15131018
Chicago/Turabian StyleTapiador, Jesús, Pedro Leo, Pablo Salcedo-Abraira, Antonio Rodríguez-Diéguez, and Gisela Orcajo. 2025. "Zn-URJC-12 Material Constituted of Two Different Organic Ligands for CO2 Valorization into Cyclic Carbonates" Nanomaterials 15, no. 13: 1018. https://doi.org/10.3390/nano15131018
APA StyleTapiador, J., Leo, P., Salcedo-Abraira, P., Rodríguez-Diéguez, A., & Orcajo, G. (2025). Zn-URJC-12 Material Constituted of Two Different Organic Ligands for CO2 Valorization into Cyclic Carbonates. Nanomaterials, 15(13), 1018. https://doi.org/10.3390/nano15131018








