The Design of the Ni3N/Nb4N5 Heterostructure as Bifunctional Adsorption/Electrocatalytic Materials for Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
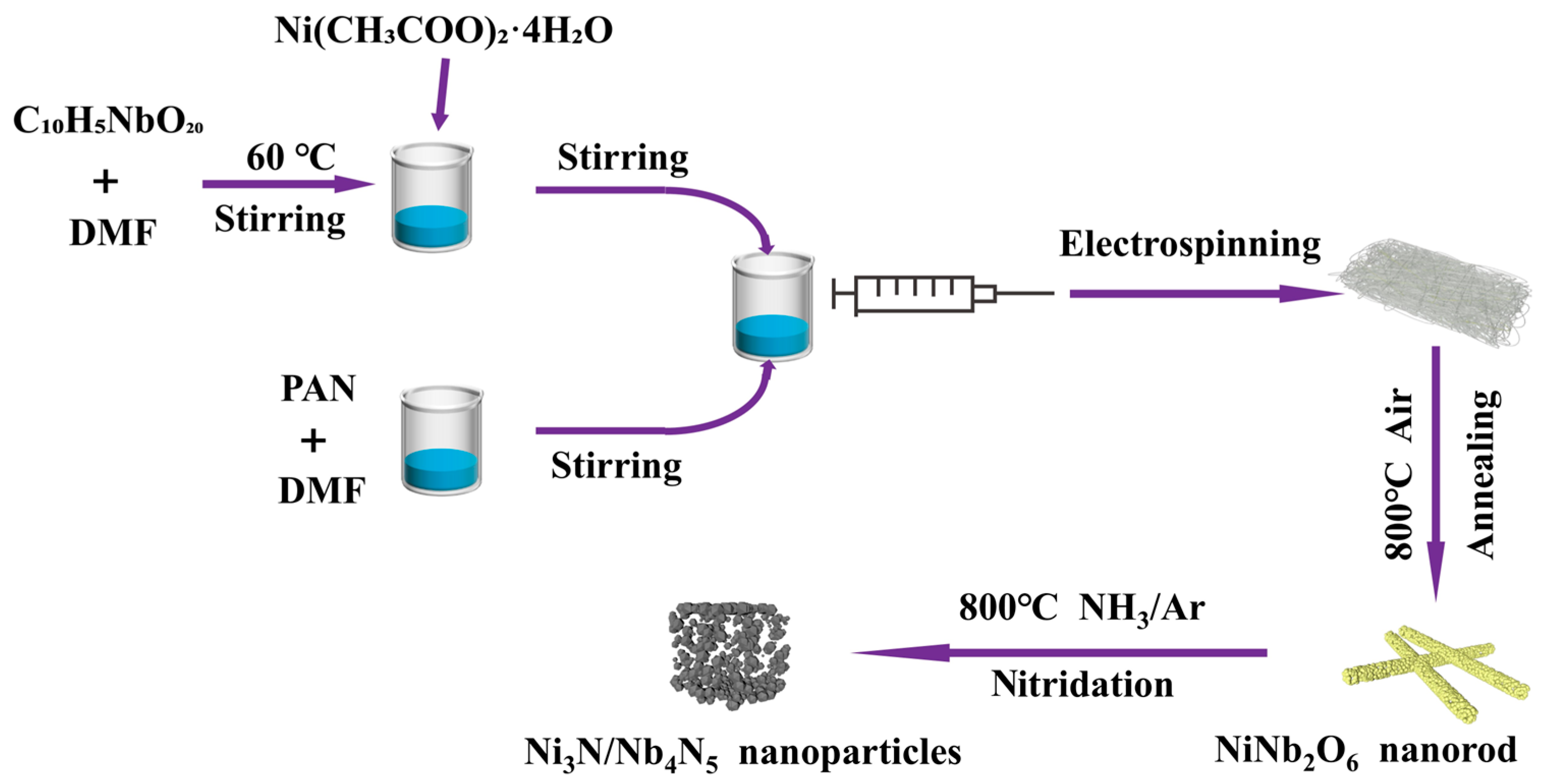
2.2. Fabrication of Ni₃N/Nb₄N₅ Heterostructure
2.3. Preparation of Modified Separators
2.4. Visualized Adsorption of Polysulfides
2.5. Fabrication and Characterization of Symmetric Cells
2.6. Experimental Procedure for Li₂S Nucleation Studies
2.7. Material Characterization
2.8. Material Preparation and Electrochemical Characterization
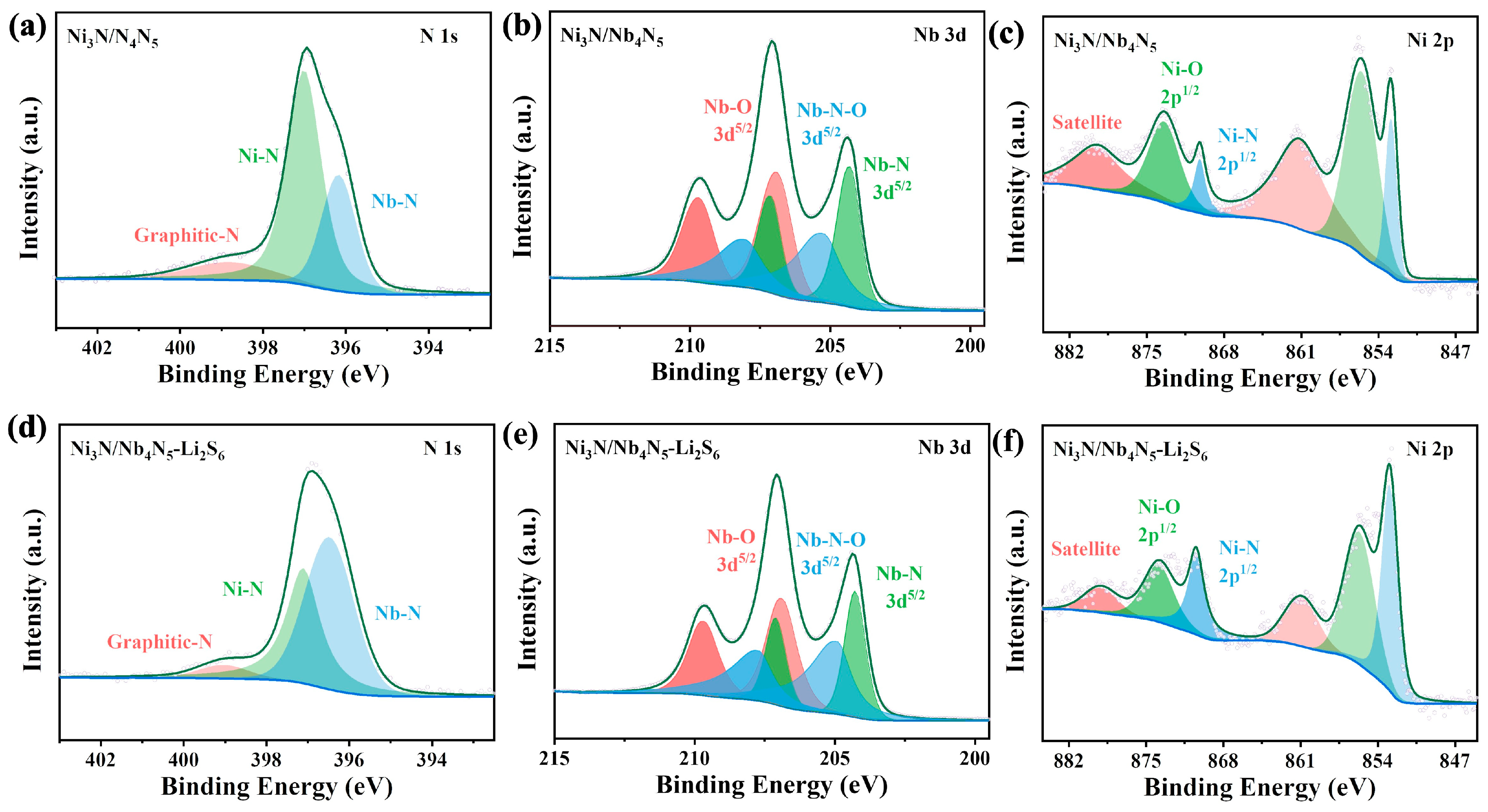
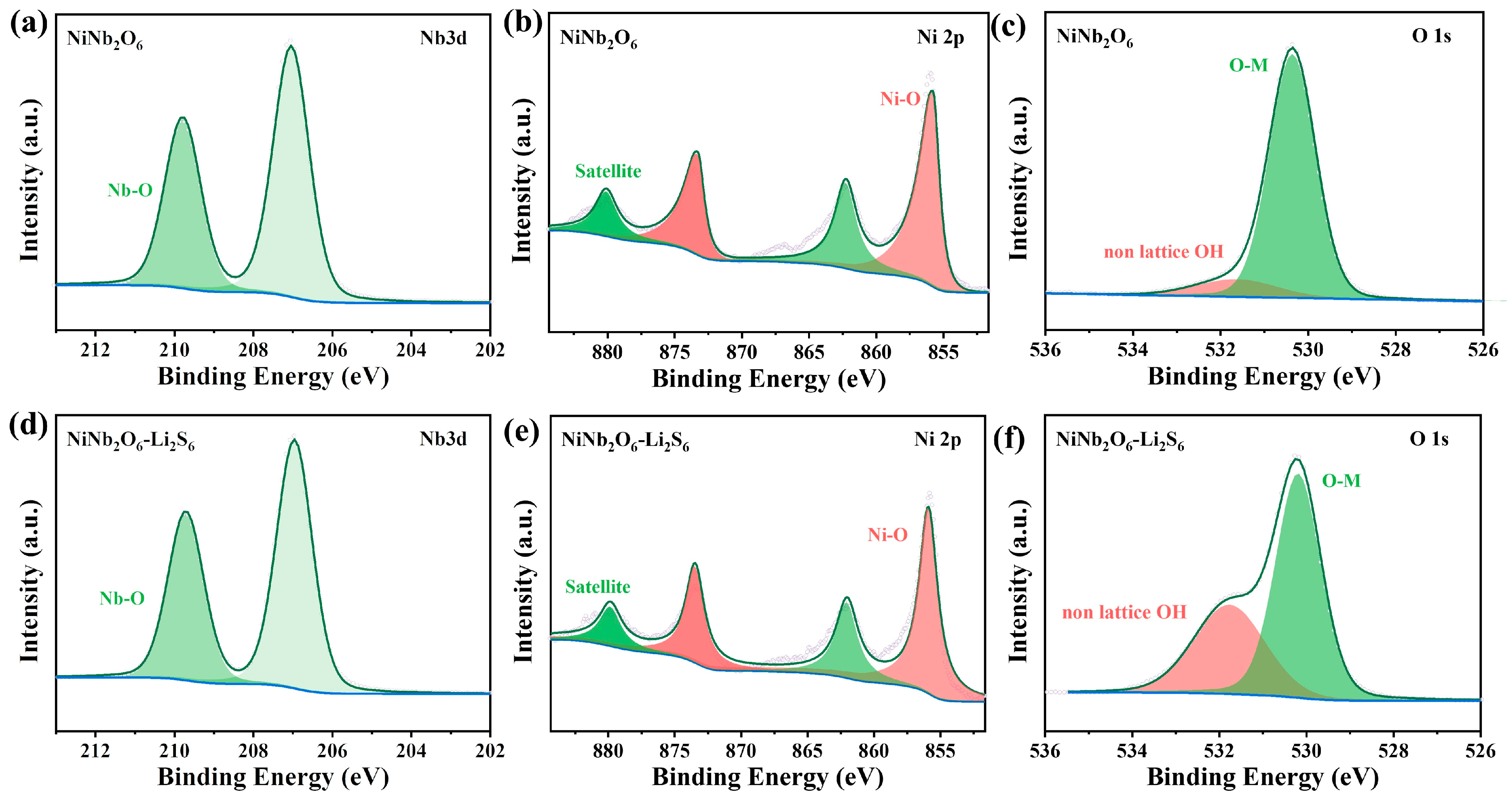
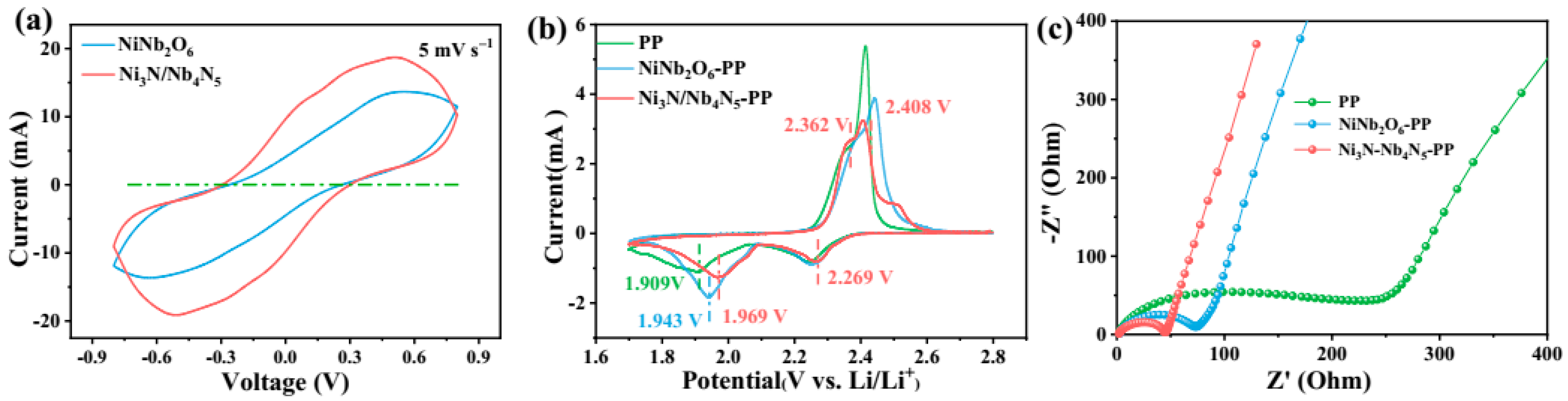
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Lu, J. Metal–air batteries: Will they be the future electrochemical energy storage device of choice. ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, X.; Wang, D.W. A review on system and materials for aqueous flexible metal–air batteries. Carbon Energy 2023, 5, e284. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. An overview of metal-air batteries, current progress, and future perspectives. J. Energy Storage 2022, 56, 106075. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, A.; Asif, M.; Shim, J.; Park, G. Exploring innovative trends and advancements in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2024, 170, 113288. [Google Scholar] [CrossRef]
- Zhang, C.; Biendicho, J.J.; Zhang, T.; Du, R.; Li, J.; Yang, X.; Arbiol, J.; Zhou, Y.; Morante, J.R.; Cabot, A. Combined high catalytic activity and efficient polar tubular nanostructure in urchin-like metallic NiCo2Se4 for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2019, 29, 1903842. [Google Scholar]
- Peng, H.; Huang, J.; Cheng, X.; Zhang, Q. Review on High-Loading and High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, W.; Li, J.; Xu, Z.; Guan, L. Encapsulating MWNTs into hollow porous carbon nanotubes: A tube-in-tube carbon nanostructure for high-performance lithium-sulfur batteries. Adv. Mater. 2014, 26, 5113–5118. [Google Scholar] [CrossRef]
- Kim, S.C.; Gao, X.; Liao, S.L.; Su, H.; Chen, Y.; Zhang, W.; Greenburg, L.; Pan, J.; Zheng, X.; Ye, Y.; et al. Solvation-property relationship of lithium-sulphur battery electrolytes. Nat. Commun. 2024, 15, 1268. [Google Scholar] [CrossRef]
- Guo, D.; Yuan, M.; Zheng, X.; Li, M.; Nan, C.; Sun, G.; Huang, X.; Li, H. Ultralong cycle life enabled by in situ growth of CoMo1-xP/Mo heterostructure for lithium-sulfur batteries. J. Energy Chem. 2022, 73, 5–12. [Google Scholar] [CrossRef]
- Preefer, M.B.; Oschmann, B.; Hawker, C.J.; Seshadri, R.; Wudi, F. High sulfur content material with stable cycling in lithium-sulfur batteries. Angew. Chem. Int. Ed. 2017, 129, 15314–15318. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Chen, J.; Wu, H.; Lu, S.; Wang, K.; Li, H.; Harris, C.J.; Xi, K.; Kumar, R.V.; et al. Enhancing catalytic activity of titanium oxide in lithium-sulfur batteries by band engineering. Adv. Energy Mater. 2019, 9, 1900953. [Google Scholar] [CrossRef]
- Du, M.; Geng, P.; Pei, C.; Jiang, X.; Shan, Y.; Hu, W.; Ni, L.; Pang, H. High-entropy Prussian blue analogues and their oxide family as sulfur hosts for lithium-sulfur batteries. Angew. Chem. Int. Ed. 2022, 61, e202209350. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.A.; Yuan, X.; Chen, Y.; Hu, J.; Mu, Q.; Ma, Y.; Zhao, X.; Miao, L.; Ahn, J.H.; Peng, Y.; et al. Anchoring MOF-derived CoS2 on sulfurized polyacrylonitrile nanofibers for high areal capacity lithium-sulfur batteries. J. Mater. Chem. A 2020, 8, 1298–1306. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Li, M.; Yuan, Y.; Zhao, Y.; Zhang, S.; Zhong, C.; Zhu, J.; Lu, J.; Zhang, H. Rational design of a Ni3N0.85 electrocatalyst to accelerate polysulfide conversion in lithium-sulfur batteries. ACS Nano 2020, 14, 6673–6682. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiao, J.; Liu, D.; He, Y.; Yang, Y.; Sun, D.; Pan, H.; Fnag, F.; Wu, R. High-entropy metal nitride embedded in concave porous carbon enabling polysulfide conversion in lithium-sulfur batteries. Small 2024, 20, 2405148. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.L.; Hu, C.; Liu, W.; Ng, M.F.; Sullivan, M.B.; Ying, J.Y. 3D carbonaceous nanostructured transition metal nitride, carbonitride and carbide as polysulfide regulators for lithium-sulfur batteries. Nano Energy 2022, 102, 107659. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Liu, W.; Liu, Y.; Lin, X.; Li, Y.; Li, P.; Huang, Z.; Feng, X.; Yu, L.; et al. TiO2 and Co nanoparticle-decorated carbon polyhedra as efficient sulfur host for high-performance lithium-sulfur batteries. Small 2019, 15, 1804533. [Google Scholar] [CrossRef]
- Liu, Y.; Chatterjee, A.; Rusch, P.; Wu, C.; Nan, P.; Peng, M.; Bettels, F.; Li, T.; Ma, C.; Zhang, C.; et al. Monodisperse molybdenum nanoparticles as highly efficient electrocatalysts for Li-S batteries. ACS Nano 2021, 15, 15047–15056. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Zhu, D.; Chen, D.; Lu, M.; Cao, J.; Wang, Y.; Huang, W.; Shao, Y.; Tu, H.; et al. Ultrafine Co3Se4 nanoparticles in nitrogen-doped 3D carbon matrix for high-stable and long-cycle-life lithium sulfur batteries. Adv. Energy Mater. 2020, 10, 1904273. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Hu, R.; Gu, J.; Cui, Y.; Li, B.; Chen, W.; Liu, C.; Shang, J.; Yang, S. Catalytic conversion of polysulfides on single atom zinc implanted MXene toward high-rate lithium-sulfur batteries. Adv. Funct. Mater. 2020, 30, 2002471. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Gu, S.; Yuan, H.; Jiang, F.; Li, Y.; Tan, W.; Long, Q.; Chen, J.; Xu, Z.; et al. N, S-coordinated Co single atomic catalyst boosting adsorption and conversion of lithium polysulfides for lithium-sulfur batteries. Small 2022, 18, 2204707. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, R.; Zhong, N.; Yin, S.; Ma, G.; Liang, X. Size-dependent cobalt catalyst for lithium sulfur batteries: From single atoms to nanoclusters and nanoparticles. Small Methods 2021, 5, 2100571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Fu, Q.; Xue, Y.; Cui, Z. The size-dependence of electrochemical thermodynamics of metal nanoparticles electrodes in theory and experiment. J. Electrochem. Soc. 2017, 164, 828–835. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Wan, F.; Niu, Z.; Wang, Y.; Zhang, Q.; Chen, J. Enhanced electrochemical kinetics and polysulfide traps of indium nitride for highly stable lithium-sulfur batteries. ACS Nano 2018, 12, 9578–9586. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Z.; Li, G.; Cheng, S.; Li, S.; Li, J.; Gao, R.; Li, M.; Sy, S.; Deng, Y.; et al. Revealing the rapid electrocatalytic behavior of ultrafine amorphous defective Nb2O5-x nanocluster toward superior Li-S performance. ACS Nano 2020, 14, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, X.; Li, B.; Shu, H.; Luo, L.; Xia, W.; Chen, M.; Zeng, P.; Yang, X.; Gao, P.; et al. NiMoO4 nanosheets anchored on N-S doped carbon clothes with hierarchical structure as a bidirectional catalyst toward accelerating polysulfides conversion for Li-S Battery. Adv. Funct. Mater. 2021, 31, 2101285. [Google Scholar] [CrossRef]
- Tian, D.; Song, X.; Wang, M.; Wu, X.; Qiu, Y.; Guan, B.; Xu, X.; Fang, L.; Zhang, N.; Sun, K. MoN supported on graphene as a bifunctional interlayer for advanced Li-S batteries. Adv. Energy Mater. 2019, 9, 1901940. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Dai, R.Q.; Zhu, S.; Zhou, L.Z.; Xu, Q.J.; Min, Y.L. Bimetallic nitride modified separator constructs internal electric field for high-performance lithium-sulfur battery. Chem. Eng. J. 2022, 429, 132454. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Cheng, C.; Yan, T.; Gao, W.; Mao, J.; Dai, K.; Zhang, L. Boosting polysulfide redox conversion of Li-S batteries by one-step-synthesized Co-Mo bimetallic nitride. J. Energy Chem. 2021, 61, 336–346. [Google Scholar] [CrossRef]
- He, J.R.; Chen, Y.F.; Manthiram, A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li-S batteries. Energy Environ. Sci. 2018, 11, 2560–2568. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Xie, J.; Fan, L.; Wu, J.; Jiang, R.; Jin, Z. Polar Co9S8 anchored on pyrrole-modified graphene with in situ growth of CNTs as multifunctional self-supporting medium for efficient lithium-sulfur batteries. Chem. Eng. J. 2023, 451, 138370. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.; Hong, X.; Xie, K.; Ma, Z.; Zheng, C.; Xiong, S. Monodispersed FeS2 electrocatalyst anchored to nitrogen-doped carbon host for lithium-sulfur batteries. Adv. Funct. Mater. 2022, 32, 2205471. [Google Scholar]
- Jiang, X.; Zhang, S.; Zou, B.; Li, G.; Yang, S.; Zhao, Y.; Lian, J.; Li, H.; Ji, H. Electrospun CoSe@ NC nanofiber membrane as an effective polysulfides adsorption-catalysis interlayer for Li-S batteries. Chem. Eng. J. 2022, 430, 131911. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Li, X.Y.; Li, B.Q.; Huang, J.Q. Lithium-sulfur batteries: An organodiselenide comediator to facilitate sulfur redox kinetics in lithium-sulfur batteries. Adv. Mater. 2021, 33, 2170100. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhang, C.; Ma, X.; Jin, Q.; Li, L.; Zhang, Z.; Zhang, X.; Wu, L. Strengthened dipole-dipole interaction on high-entropy oxide electrocatalysts for high-rate and excellently stable lithium-sulfur batteries. J. Energy Chem. 2025, 105, 292–301. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kang, J.; Paul, B.J.; Song, J.; Kim, J. Simple synthesis and particle size effects of TiO2 nanoparticle anodes for rechargeable lithium ion batteries. Electrochim. Acta 2013, 90, 112–118. [Google Scholar] [CrossRef]
- Jung, S.K.; Hwang, I.; Chang, D.; Park, K.Y.; Kim, S.J.; Seong, W.M.; Eum, D.; Park, J.; Kim, B.; Kim, J.; et al. Nanoscale phenomena in lithium-ion batteries. Chem. Rev. 2020, 120, 6684–6737. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.; Wang, S.; Wang, Z.; Li, M.; Bai, Y.; An, Q.; Xu, H.; Wu, F.; et al. Co-construction of sulfur vacancies and heterojunctions in tungsten disulfide to induce fast electronic/ionic diffusion kinetics for sodium-ion batteries. Adv. Mater. 2020, 32, e2005802. [Google Scholar] [CrossRef]
- Li, B.; Su, Q.; Yu, L.; Zhang, J.; Du, G.; Wang, D.; Han, D.; Zhang, M.; Ding, S.; Xu, B. Tuning the band structure of MoS2 via Co9S8@MoS2 core-shell structure to boost catalytic activity for lithium-sulfur batteries. ACS Nano 2020, 14, 17285–17294. [Google Scholar] [CrossRef]
- Zhou, T.; Lv, W.; Li, J.; Zhou, G.; Zhao, Y.; Fan, S.; Liu, B.; Li, B.; Kang, F.; Yang, Q.H. Twinborn TiO2-TiN heterostructures enabling smooth trapping-diffusion-conversion of polysulfides towards ultralong life lithium-sulfur batteries. Energy Environ. Sci. 2017, 10, 1694–1703. [Google Scholar] [CrossRef]
- Ren, Y.; Zhai, Q.; Wang, B.; Hu, L.; Ma, Y.; Dai, Y.; Tang, S.; Meng, X. Synergistic adsorption-electrocatalysis of 2D/2D heterostructure toward high performance Li-S batteries. Chem. Eng. J. 2022, 439, 135535. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@NC heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium-sulfur batteries. ACS Nano 2021, 15, 7114–7130. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Shang, W.; Zhang, X.; Li, Y.; Qiao, S.; Zhong, Y.; Deng, X.; Shi, X.; Zhang, Q.; Hao, C.; et al. Facile synthesis of heterostructured MoS2-MoO3 nanosheets with active electrocatalytic sites for high-performance lithium-sulfur batteries. ACS Nano 2021, 15, 20478–20488. [Google Scholar] [CrossRef] [PubMed]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials Aspects of nano-ionics. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Xu, Z.L.; Lin, S.; Onofrio, N.; Zhou, L.; Shi, F.; Lu, W.; Kang, K.; Zhang, Q.; Lau, S.P. Exceptional catalytic effects of black phosphorus quantum dots in shuttling-free lithium sulfur batteries. Nat. Commun. 2018, 9, 4164. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Wang, Y.; Chen, R.; Wu, Z.; Wang, S. LDHs derived nanoparticle-stacked metal nitride as interlayer for long-life lithium sulfur batteries. Sci. Bull. 2018, 63, 169–175. [Google Scholar] [CrossRef]
- ABhargav, J.R.; He, A.; Gupta, A. Manthiram, Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Lim, W.G.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in Li-S batteries: Enhanced redox kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef]
- Fan, S.; Huang, S.; Pam, M.E.; Chen, S.; Wu, Q.; Hu, J.; Wang, Y.; Ang, L.K.; Yan, C.; Shi, Y.; et al. Design multifunctional catalytic interface: Toward regulation of polysulfide and Li. Small 2019, 15, e1906132. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, S.; Jin, Y.; Zhu, H.; Zhang, Q.; Guo, J.; Li, X.; Dong, Z.; Yang, N.; Cong, Y. Spontaneous electrochemical reconstruction of NiNb2O6@C for high-rate lithium-ion batteries. Chem. Eng. J. 2024, 495, 153397. [Google Scholar] [CrossRef]
- Zhao, S.; Lian, J.; Zhang, S.; Cui, Y.; Li, G.; Wang, Y.; Li, H. Molten salt synthesis of submicron NiNb2O6 anode material with ultra-high rate performance for lithium-ion batteries. Chem. Eng. J. 2023, 461, 141997. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, H.; Liu, H.; Hu, Y.; Yuan, T.; Dai, S.; Liu, M.; Hu, H. Design of phase interface and defect in niobium-nickel oxide for ultrafast Li-ion storage. J. Mater. Sci. Technol. 2023, 147, 145–152. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Li, W.; Zheng, M.; Wang, J.; Ma, S.; Yin, G.; Zuo, P.; Sun, X. Immobilization and kinetic promotion of polysulfides by molybdenum carbide in lithium-sulfur batteries. Chem. Eng. J. 2021, 411, 128563. [Google Scholar] [CrossRef]
- Luo, Z.H.; Zheng, M.; Zhou, M.X.; Sheng, X.T.; Chen, X.L.; Shao, J.J.; Wang, T.S.; Zhou, G. 2D Nanochannel interlayer realizing high-performance lithium-sulfur batteries. Adv. Mater. 2025, 37, 2417321. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, Z.; Yan, X.; Wang, D.; Liu, J.; Guo, X.; Zhang, Z.; Li, G. In situ engineered ZnS-FeS heterostructure in N-doped carbon nanocages accelerating polysulfide redox kinetics for lithium sulfur batteries. J. Mater. Chem. A 2020, 8, 433–442. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulfide trap for utilizing pure sulfur cathodes in lithium-sulfur batteries. Adv. Mater. 2014, 26, 7352–7357. [Google Scholar] [CrossRef]
- Fan, F.Y.; Carter, W.C.; Chiang, Y.M. Mechanism and kinetics of Li2S precipitation in lithium-sulfur batteries. Adv. Mater. 2015, 27, 5203–5209. [Google Scholar] [CrossRef]
- Kong, Z.; Lin, Y.; Hu, J.; Wang, Y.; Zhan, L. Phosphorus doped hierarchical porous carbon nanosheet array as an electrocatalyst to enhance polysulfides anchoring and conversion. Chem. Eng. J. 2022, 436, 132719. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Von Lim, Y.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent advances in heterostructure engineering for lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Li, R.; Zhou, X.; Shen, H.; Yang, M.; Li, C. Conductive holey MoO2-Mo3N2 heterojunctions as job-synergistic cathode host with low surface area for high-loading Li-S batteries. ACS Nano 2019, 13, 10049–10061. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhou, X.; Zhang, B.; Zhao, Z.; Song, X.; Chen, K.; Wang, L.; Ma, Z.; Liu, J. The versatile establishment of charge storage in polymer solid electrolyte with enhanced charge transfer for LiF-rich SEI generation in lithium metal batteries. Angew. Chem. 2024, 136, e202320149. [Google Scholar] [CrossRef]




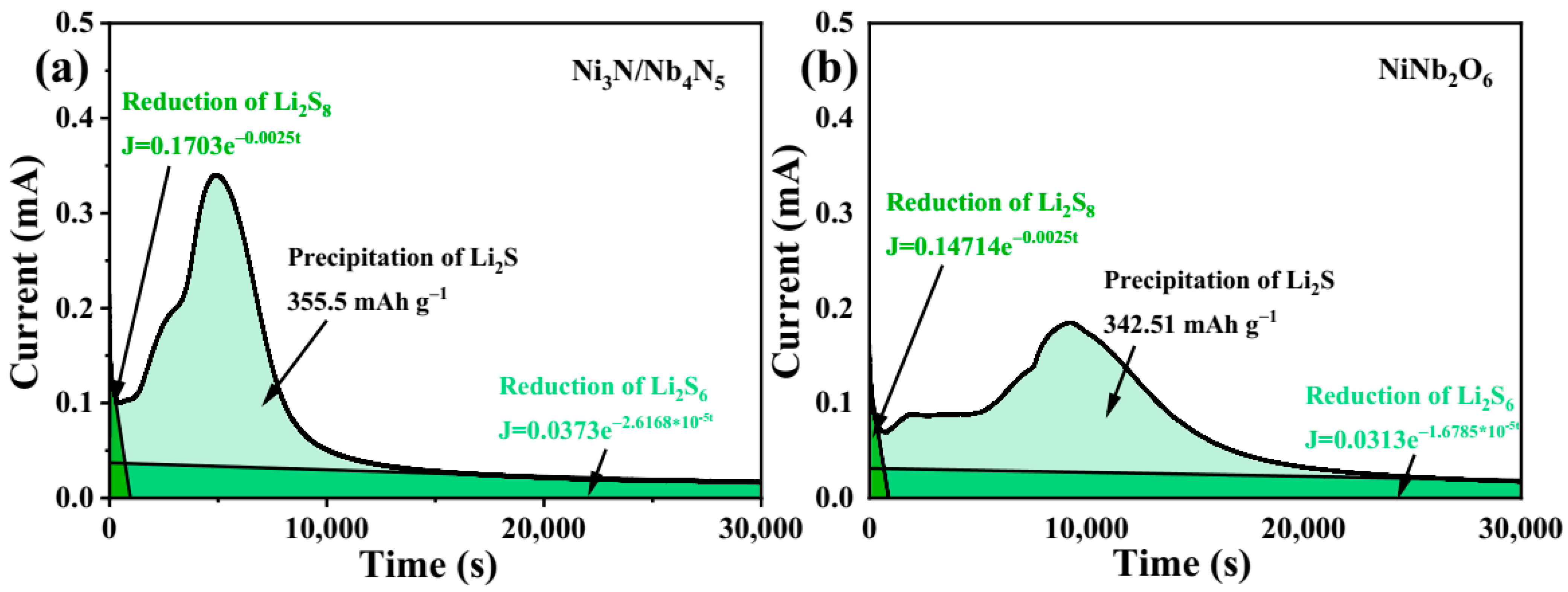
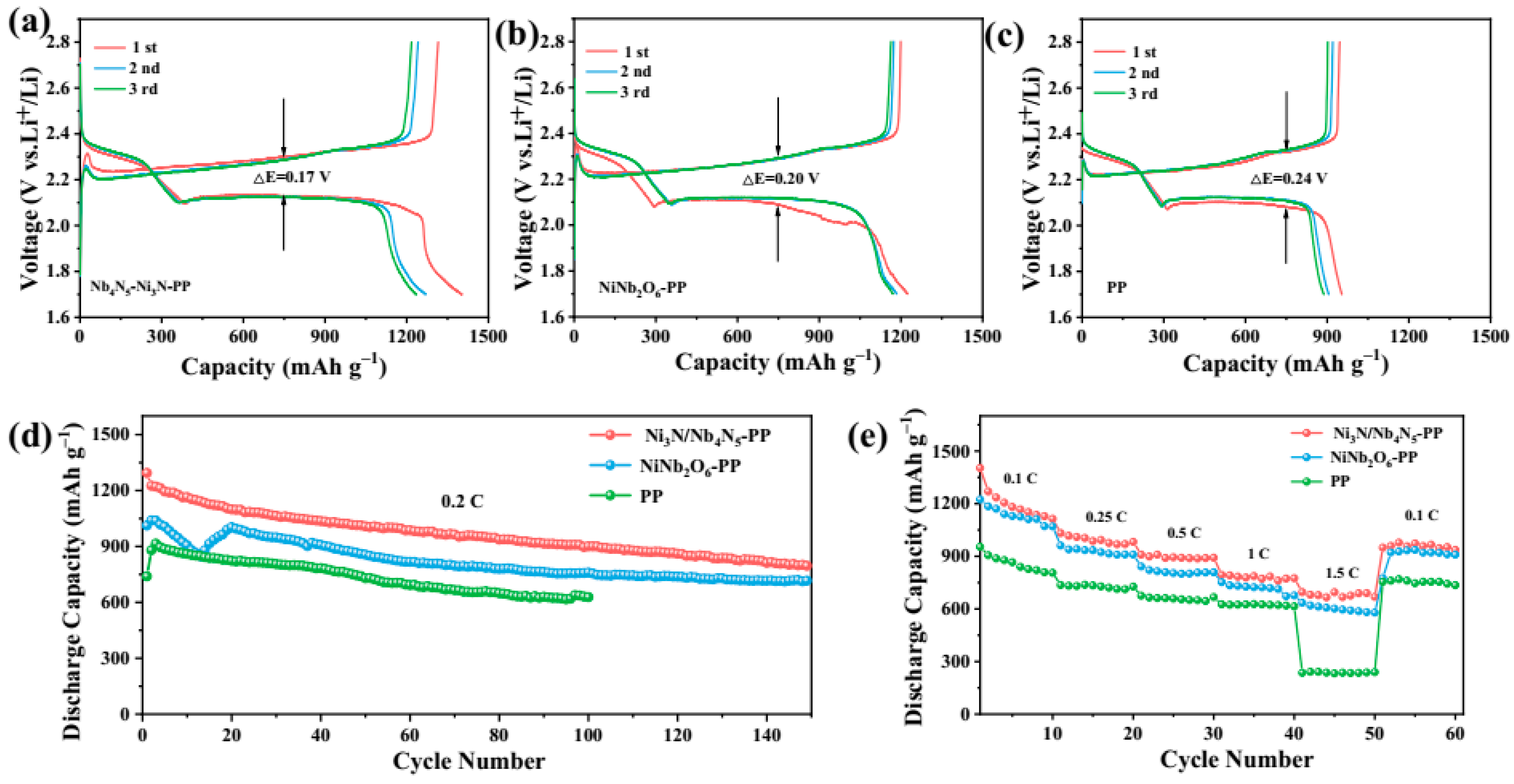
| Typology | Current Density | Number of Cycles | Capacity Decay Rate | Capacity/Retention Rate | ||
|---|---|---|---|---|---|---|
| 1 | Co9S8@MoS2/CNF | interlayer | 1.0 C | 400 | 0.09% | [39] |
| 2 | TiO2–TiN | interlayer | 0.3 C | 300 | / | 927 mAh g−1 [40] |
| 3 | Ti3C2Tx/Ni-Co MOF | modified separators | 0.2 C | 200 | 0.06% | 1100 mAh g−1/87.3% [41] |
| 4 | ZnS-SnS@NC | modified separators | 4.0 C | 2000 | 0.01% | 632 mAh g−1/74.9% [42] |
| 5 | MoS2-MoO3 | modified separators | 1.0 C | 600 | 0.01% | 92.00% [43] |
| 6 | This work | modified separators | 0.2 C | 200 | 0.25% | 796.2 mAh g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shang, W.; Zhang, S.; Xu, C.; Lian, J.; Li, G. The Design of the Ni3N/Nb4N5 Heterostructure as Bifunctional Adsorption/Electrocatalytic Materials for Lithium–Sulfur Batteries. Nanomaterials 2025, 15, 1015. https://doi.org/10.3390/nano15131015
Li X, Shang W, Zhang S, Xu C, Lian J, Li G. The Design of the Ni3N/Nb4N5 Heterostructure as Bifunctional Adsorption/Electrocatalytic Materials for Lithium–Sulfur Batteries. Nanomaterials. 2025; 15(13):1015. https://doi.org/10.3390/nano15131015
Chicago/Turabian StyleLi, Xialei, Wen Shang, Shan Zhang, Chun Xu, Jiabiao Lian, and Guochun Li. 2025. "The Design of the Ni3N/Nb4N5 Heterostructure as Bifunctional Adsorption/Electrocatalytic Materials for Lithium–Sulfur Batteries" Nanomaterials 15, no. 13: 1015. https://doi.org/10.3390/nano15131015
APA StyleLi, X., Shang, W., Zhang, S., Xu, C., Lian, J., & Li, G. (2025). The Design of the Ni3N/Nb4N5 Heterostructure as Bifunctional Adsorption/Electrocatalytic Materials for Lithium–Sulfur Batteries. Nanomaterials, 15(13), 1015. https://doi.org/10.3390/nano15131015






