Impacts of Cerium Dioxide Nanoparticles on the Soil–Plant System and Their Potential Agricultural Applications
Abstract
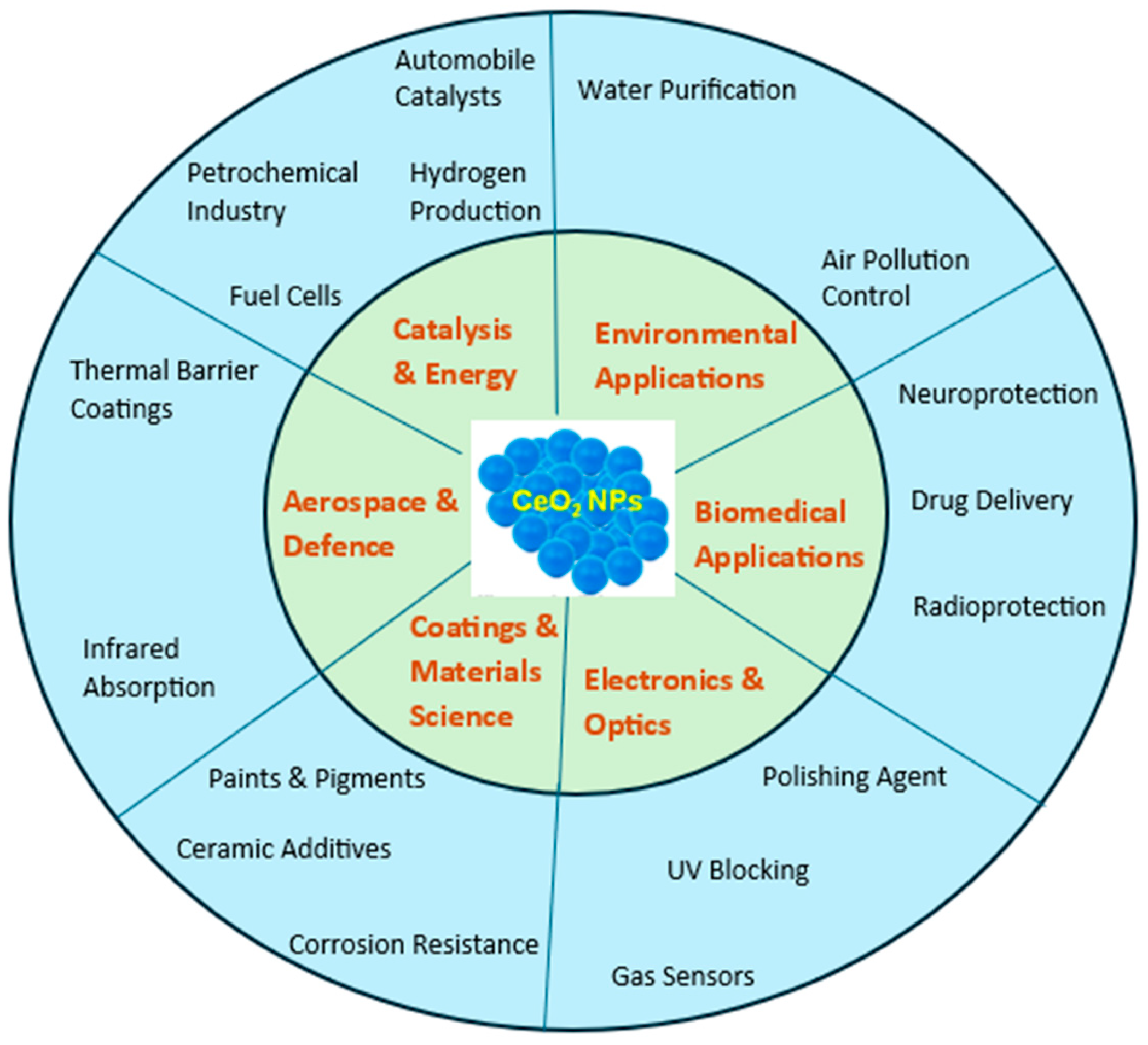
1. Introduction
1.1. Characteristics of CeO2-NPs
1.2. Environmental Release of CeO2-NPs
1.3. Environmental Fate of Nanoceria
2. Interaction and Transformations of CeO2-NPs in Soil
3. Impacts of CeO2-NPs on Soil Properties
3.1. Effects on Soil Chemistry
3.2. Effects on Soil Physical Properties
3.3. Effect on Soil Microbiology
3.4. Effects on Plant Microbial Symbiosis
4. CeO2-NP Plant Uptake, Translocation, and Transformation
5. Effects of CeO2-NPs on Crop Plants
5.1. Impacts on Seed Germination and Seedling Growth
5.2. Impacts on Crop Plant Physiology
5.3. Impacts on Plant Growth and Yield
5.4. Impacts on Crop Nutritional Quality
6. Applications of CeO2-NPs in Agriculture
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A. Toxicity of nanoparticles—Challenges and opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef] [PubMed]
- Collin, B.; Auffan, M.; Johnson, A.C.; Kaur, I.; Keller, A.A.; Lazareva, A.; Lead, J.R.; Ma, X.; Merrifield, R.C.; Svendsen, C.; et al. Environmental release, fate and ecotoxicological effects of manufactured ceria nanomaterials. Environ. Sci. Nano 2014, 1, 533–548. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Song, G.; Cheng, N.; Zhang, J.; Huang, H.; Yuan, Y.; He, X.; Luo, Y.; Huang, K. Nanoscale cerium oxide: Synthesis, biocatalytic mechanism, and applications. Catalysts 2021, 11, 1123. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, e1901556. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Particle Size Effect on the Properties of Cerium Oxide (CeO2) Nanoparticles Synthesized by Hydrothermal Method. Mech. Mater. Sci. Eng. J. 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Atran, A.A.; Ibrahim, F.A.; Hamdy, M.S. Functionalization and applications of the versatile CeO2 nanoparticles: A review. Inorg. Chem. Commun. 2024, 163, 112359. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A Phosphate-Dependent Shift in Redox State of Cerium Oxide Nanoparticles and Its Effects on Catalytic Properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef]
- Loddo, V.; Yurdakal, S.; Parrino, F. Economical aspects, toxicity, and environmental fate of cerium oxide. In Metal Oxides, Cerium Oxide (CeO2): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–373. ISBN 9780128156612. [Google Scholar] [CrossRef]
- Yadav, S.; Chamoli, S.; Kumar, P.; Maurya, P.K. Structural and functional insights in polysaccharides coated cerium oxide nanoparticles and their potential biomedical applications: A review. Int. J. Biol. Macromol. 2023, 246, 125673. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.; Traversa, E.; Ghibelli, L. Pharmacological Potential of Cerium Oxide Nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.; Sudipta, S.; Chandraiah, G. Nanoceria, the versatile nanoparticles: Promising biomedical applications. J. Control. Release 2021, 338, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.T.; Arai, Y. Environmental Geochemistry of Cerium: Applications and Toxicology of Cerium Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 1253–1278. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Pansambal, S.; Oza, R.; Borgave, S.; Chauhan, A.; Bardapurkar, P.; Vyas, S.; Ghotekar, S. Bioengineered cerium oxide (CeO2) nanoparticles and their diverse applications: A review. Appl Nanosci. 2023, 13, 6067–6092. [Google Scholar] [CrossRef]
- Ryabchikova, E. Nanomaterials editorial advances in nanomaterials in biomedicine. Nanomaterials 2021, 11, 118. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ali, A.M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, Z.; Fu, H.; White, J.C.; Lynch, I. Nanomaterial Transformation in the Soil–Plant System: Implications for Food Safety and Application in Agriculture. Small 2020, 16, 2000705. [Google Scholar] [CrossRef]
- Ayub, M.A.; Sohail, M.I.; Umair, M.; Rehman, M.Z.U.; Usman, M.; Sabir, M.; Rizwan, M.; Ali, S.; Ahmad, Z. Cerium oxide nanoparticles: Advances in synthesis, prospects and application in agro-ecosystem. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 209–250. [Google Scholar] [CrossRef]
- Li, X.; He, E.; Zhang, M.; Peijnenburg, W.; Song, L.; Cao, X.; Zhao, L.; Qiu, H. Interactions of CeO2 Nanoparticles with Natural Colloids and Electrolytes Impact Their Aggregation Kinetics and Colloidal Stability. J. Hazard. Mater. 2019, 386, 121973. [Google Scholar] [CrossRef]
- Dahle, J.; Livi, K.; Arai, Y. Effects of pH and Phosphate on CeO2 Nanoparticle Dissolution. Chemosphere 2014, 119, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, P.; Zhao, W.; Li, Y.; Jiang, Y.; Rui, Y.; Guo, Z.; Lynch, I. Interplay of Metal-Based Nanoparticles with Plant Rhizosphere Microenvironment: Implications for Nanosafety and Nano-Enabled Sustainable Agriculture. Environ. Sci. Nano 2023, 10, 372–392. [Google Scholar] [CrossRef]
- Shrivastava, M.; Srivastav, A.; Gandhi, S.; Rao, S.; Roychoudhury, A.; Kumar, A.; Singhal, R.K.; Jha, S.K.; Singh, S.D. Monitoring of Engineered Nanoparticles in Soil-Plant System: A Review. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100218. [Google Scholar] [CrossRef]
- Prakash, V.; Peralta-Videa, J.; Tripathi, D.K.; Ma, X.; Sharma, S. Recent Insights into the Impact, Fate and Transport of Cerium Oxide Nanoparticles in the Plant-Soil Continuum. Ecotoxicol. Environ. Saf. 2021, 221, 112403. [Google Scholar] [CrossRef]
- Pagano, L.; Rossi, R.; White, J.C.; Marmiroli, N.; Marmiroli, M. Nanomaterials biotransformation: In planta mechanisms of action. Environ. Pollut. 2023, 318, 120834. [Google Scholar] [CrossRef]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on Recent Developments in the Design, Application, and Impacts of Nanofertilizers in Agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- Xu, Z.; Long, X.; Jia, Y.; Zhao, D.; Pan, X. Occurrence, transport, and toxicity of nanomaterials in soil ecosystems: A review. Environ. Chem. Lett. 2022, 20, 3943–3969. [Google Scholar] [CrossRef]
- Loureiro, S.; Tourinho, P.S.; Cornelis, G.; Van Den Brink, N.W.; Díez-Ortiz, M.; Vázquez-Campos, S.; Pomar-Portillo, V.; Svendsen, C.; Van Gestel, C.A.M. Nanomaterials as Soil Pollutants. In Soil Pollution; Academic Press: Cambridge, MA, USA, 2018; pp. 161–190. ISBN 9780128498736. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef]
- Saenz, J.; Mohammadi, S.; Aleman, B.; Sapkota, P.; Ramirez, K.S.; Sharifan, H. Redox-Active Cerium Dioxide Nanoparticles for Mitigating Anthracene Contamination: Promising Solution to Polycyclic Aromatic Hydrocarbon Remediation in Stormwater-Affected Soils. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135657. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, K.; Chefetz, B.; Xing, B.; Lin, D. The pH and Concentration Dependent Interfacial Interaction and Heteroaggregation between Nanoparticulate Zero-Valent Iron and Clay Mineral Particles. Environ. Sci. Nano 2019, 6, 2129–2140. [Google Scholar] [CrossRef]
- Arenas, L.R.; Coustumer, P.L.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Removal Efficiency and Adsorption Mechanisms of CeO2 Nanoparticles onto Granular Activated Carbon Used in Drinking Water Treatment Plants. Sci. Total Environ. 2023, 856, 159261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Schwab, A.P.; White, J.C.; Ma, X. Impact of Nanoparticle Surface Properties on the Attachment of Cerium Oxide Nanoparticles to Sand and Kaolin. J. Environ. Qual. 2018, 47, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Peralta-Videa, J.R.; Trujillo-Reyes, J.; Sun, Y.; Barrios, A.C.; Niu, G.; Flores-Márgez, J.P.; Gardea-Torresdey, J.L. Soil Organic Matter Influences Cerium Translocation and Physiological Processes in Kidney Bean Plants Exposed to Cerium Oxide Nanoparticles. Sci. Total Environ. 2016, 569–570, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Musante, C.; White, J.; Schwab, A.; Wang, D.; Ebbs, S.; Ma, X. Bioavailability of Cerium Oxide Nanoparticles to Raphanus sativus L. in Two Soils. Plant Physiol. Biochem. 2017, 110, 185–193. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, H.S.; Shang, Y.M.; Lead, J.R.; Guo, Z.Z.; Hong, J.P. Response of Soil Bacterial Diversity, Predicted Functions, and Co-Occurrence Patterns to Nanoceria and Ionic Cerium Exposure. Microorganisms 2022, 10, 1982. [Google Scholar] [CrossRef]
- Pietrzak, M.; Skiba, E.; Wolf, W.M. Root-Applied Cerium Oxide Nanoparticles and Their Specific Effects on Plants: A Review. Int. J. Mol. Sci. 2024, 25, 4018. [Google Scholar] [CrossRef]
- Khan, F.; Lee, J.W.; Pham, D.N.T.; Khan, M.M.; Park, S.K.; Shin, I.S.; Kim, Y.M. Antibiofilm Action of ZnO, SnO2, and CeO2 Nanoparticles Towards Gram-Positive Biofilm Forming Pathogenic Bacteria. Recent Pat. Nanotechnol. 2020, 14, 239–249. [Google Scholar] [CrossRef]
- Khan, M.M.; Matussin, S.N.; Rahman, A. Recent Progress of Phytogenic Synthesis of ZnO, SnO2, and CeO2 Nanomaterials. Bioprocess Biosyst. Eng. 2022, 45, 619–645. [Google Scholar] [CrossRef]
- Hamidat, M.; Barakat, M.; Ortet, P.; Chanéac, C.; Rose, J.; Bottero, J.-Y.; Heulin, T.; Achouak, W.; Santaella, C. Design Defines the Effects of Nanoceria at a Low Dose on Soil Microbiota and the Potentiation of Impacts by Canola Plant. Environ. Sci. Technol. 2016, 50, 6892–6901. [Google Scholar] [CrossRef]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that Soil Properties and Organic Coating Drive the Phytoavailability of Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef]
- Jiao, C.; Dong, C.; Xie, C.; Luo, W.; Zhang, J.; Fan, S.; Liu, Y.; Ma, Y.; He, X.; Zhang, Z. Dissolution and Retention Process of CeO2 Nanoparticles in Soil with Dynamic Redox Conditions. Environ. Sci. Technol. 2021, 55, 14649–14657. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Bao, Y.; Guo, A.; Ma, J. Environmentally Relevant-Level CeO2 NP with Ferrous Amendment Alters Soil Bacterial Community Compositions and Metabolite Profiles in Rice-Planted Soils. J. Agric. Food Chem. 2020, 68, 8172–8184. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Zhang, Z.; Liu, S.; Ma, Y.; Zhang, P.; He, X.; Li, Y.; Zhang, J.; Li, H.; Rui, Y.; et al. Fate and Phytotoxicity of CeO2 Nanoparticles on Lettuce Cultured in the Potting Soil Environment. PLoS ONE 2015, 10, e0134261. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation, and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2018, 66, 6462–6473. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of Nanomaterials for Heavy Metal Removal from Water and Soil: A Review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
- Bi, X.; Zeng, C.; Westerhoff, P. Adsorption of Arsenic Ions Transforms Surface Reactivity of Engineered Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2020, 54, 9437–9444. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M.d.l.L. Impact on Some Soil Physical and Chemical Properties Caused by Metal and Metallic Oxide Engineered Nanoparticles: A Review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Trindade, T.; Duarte, A.C.; Pereira, E.; Koopmans, G.F.; Römkens, P.F.A.M. A framework to measure the availability of engineered nanoparticles in soils: Trends in soil tests and analytical tools. TrAC Trends Anal. Chem. 2016, 75, 129–140. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Rathore, I. Use of Nanoparticles in Moisture Retention and Soil Health Management. In Nanoscience and Soil-Water Interaction in Agroecosystem; CRC Press: Boca Raton, FL, USA, 2025; p. 1635. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B. Interaction of nanoceria with microorganisms. In Nanobiomaterials in Antimicrobial Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 419–450. [Google Scholar] [CrossRef]
- Modi, S.K.; Gaur, S.; Sengupta, M.; Singh, M.S. Mechanistic insights into nanoparticle surface-bacterial membrane interactions in overcoming antibiotic resistance. Front. Microbiol. 2023, 14, 1135579. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Morales, M.I.; Rico, C.M.; Hernandez-Viezcas, J.A.; Nunez, J.E.; Barrios, A.C.; Tafoya, A.; Flores-Marges, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013, 61, 6224–6230. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
- Tian, H.; Kah, M.; Kariman, K. Are nanoparticles a threat to mycorrhizal and rhizobial symbioses? A critical review. Front. Microbiol. 2019, 10, 1660. [Google Scholar] [CrossRef]
- Shahroz, M.; Ashiq, M.; Infal, M.; Riaz, A.; Chaudhry, Z.; Hassan, M.N.; Rana, B.A.; Arif, A.; Aslam, R.; Ulah, Q. Modulation of nitrogen fixation in legumes by nanoparticles: A biochemical perspective. Glob. Acad. J. Agric. Biosci. 2024, 6, 119–133. [Google Scholar] [CrossRef]
- Chhipa, H.; Gupta, A.K.; Sharma, R. Impact of nanoparticles on plants and its symbiotic microorganisms. In Nanomaterial-Plant Interactions, Nanoparticles and Plant-Microbe Interactions; Seena, S., Rai, A., Kumar, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 369–387. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F.R. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef]
- Ge, Y.; Priester, J.; Werfhorst, L.C.V.D.; Walker, S.L.; Roger, N.; Youn-Joo, A.; Joshua, S.; Jorge, G.T.; Patricia, H. Soybean Plants Modify Metal Oxide Nanoparticle Effects on Soil Bacterial Communities. Environ. Sci. Technol. 2014, 48, 13489–13496. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Zhang, Z.; He, X.; Li, Y.; Zhang, J.; Zheng, L.; Chu, S.; Yang, K.; Zhao, Y.; et al. Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology 2015, 9, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Reichman, J.R.; Slattery, M.R.; Johnson, M.G.; Andersen, C.P.; Harper, S.L. CeO2 nanoparticle dose and exposure modulate soybean development and plant-mediated responses in root-associated bacterial communities. Sci. Rep. 2024, 14, 10231. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered nanomaterials in soil: Their impact on soil microbiome and plant health. Plants 2021, 11, 109. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, A.; Singh, N.B.; Singh, A.; Singh, P. Plant-nanoceria interaction: Toxicity, accumulation, translocation, and biotransformation. S. Afr. J. Bot. 2019, 121, 239–247. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V. Nano-enabled agriculture for sustainable soil. Waste Manag. Bull. 2024, 2, 152–161. [Google Scholar] [CrossRef]
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Speciat. Bioavailab. 2018, 30, 123–134. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Wang, P.; Zhao, F.J. Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J. Hazard. Mater. 2017, 322, 292–300. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shixian, F.; Xuehan, W.; Zhiming, Z.; Xin, G.; Yong, Z. Effects of Nano-Cerium Oxide on Seed Germination and Seedling Growth of Vitex negundo. Phyton 2020, 89, 893–903. [Google Scholar] [CrossRef]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Zhang, J.Y. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, W.; Pei, H.; Chen, Y. The Impact of Cerium Oxide Nanoparticles on Tomato (Solanum lycopersicum L.) and Its Implications for Food Safety. Metallomics 2012, 4, 1105–1112. [Google Scholar] [CrossRef]
- Mattiello, A.; Pošćić, F.; Musetti, R.; Giordano, C.; Vischi, M.; Filippi, A.; Bertolini, A.; Marchiol, L. Evidences of genotoxicity and phytotoxicity in Hordeum vulgare exposed to CeO2 and TiO2 nanoparticles. Front. Plant Sci. 2015, 6, 1043. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Mujtaba Munir, M.A.; Ahmed, R.; Rehman, A. Biochar-assisted transformation of engineered-cerium oxide nanoparticles: Effect on wheat growth, photosynthetic traits and cerium accumulation. Ecotoxicol. Environ. Saf. 2020, 187, 109845. [Google Scholar] [CrossRef]
- Skiba, E.; Pietrzak, M.; Gapińska, M.; Wolf, W.M. Metal Homeostasis and Gas Exchange Dynamics in Pisum sativum L. Exposed to Cerium Oxide Nanoparticles. Int. J. Mol. Sci. 2020, 21, 8497. [Google Scholar] [CrossRef]
- Zhang, W.; Ebbs, S.D.; Musante, C.; White, J.C.; Gao, C.; Ma, X. Uptake and Accumulation of Bulk and Nanosized Cerium Oxide Particles and Ionic Cerium by Radish (Raphanus sativus L.). J. Agric. Food Chem. 2015, 63, 382–390. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Mukherjee, A.; Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential effects of CeO2 and ZnO nanoparticles on chlorophyll and secondary metabolities in alfalfa (Medicago sativa). Sci. Technol. J. 2015, 3, 7–13. [Google Scholar]
- Preetha, J.S.Y.; Sriram, D.; Premasudha, P.; Pudake, R.N.; Arun, M. Cerium oxide as a nanozyme for plant abiotic stress tolerance: An overview of the mechanisms. Plant Nano Biol. 2023, 6, 100049. [Google Scholar] [CrossRef]
- Corral-Diaz, B.; Peralta-Videa, J.R.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Morales, M.I.; Osuna-Avila, P.; Niu, G.; Hernandez- Viezcas, J.A.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L). Plant Physiol. Biochem. 2014, 84, 277–285. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Zhao, D.; Ding, Y.; Gao, L.; Su, X.; Song, K.; He, X. CeO2-NP priming enhances the seed vigor of alfalfa (Medicago sativa) under salt stress. Front. Plant Sci. 2024, 14, 1264698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, H.; Van Gestel, C.A.M.; Gong, B.; He, E. Impact of nanopesticide CuO-NPs and nanofertilizer CeO2-NPs on wheat Triticum aestivum under global warming scenarios. Chemosphere 2023, 328, 138576. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- Priester, J.H.; Moritz, S.C.; Espinosa, K.; Ge, Y.; Wang, Y.; Nisbet, R.M.; Schimel, J.P.; Goggi, S.; Torresdey, J.L.G.; Holden, P.A. Damage assessment for soybean cultivated in soil with either CeO2 or ZnO manufactured nanomaterials. Sci. Total Environ. 2017, 579, 1756–1768. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Li, M.; Guo, Z.; Ullah, S.; Rui, Y.; Lynch, I. Alleviation of nitrogen stress in rice (Oryza sativa) by ceria nanoparticles. Environ. Sci. Nano 2020, 7, 2930–2940. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Rossi, L.; Ebbs, S.D.; White, J.C. Multigenerational exposure to cerium oxide nanoparticles: Physiological and biochemical analysis reveals transmissible changes in rapid cycling Brassica rapa. NanoImpact 2016, 1, 46–54. [Google Scholar] [CrossRef]
- Conway, J.; Arielle, B.; Nicole, B.; Susan, M.; Keller, A. Environmental Stresses Increase Photosynthetic Disruption by Metal Oxide Nanomaterials in a Soil-Grown Plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea- Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Liu, S.; Wang, G.; Zhang, J.; He, X.; Zhang, J.; Rui, Y.; Zhang, Z. Phytotoxicity, uptake and transformation of nano-CeO2 in sand cultured romaine lettuce. Environ. Pollut. 2017, 220, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 332. [Google Scholar] [CrossRef]
- Tassi, E.; Giorgetti, L.; Morelli, E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Barbafieri, M. Physiological and biochemical responses of sunflower (Helianthus annuus L.) exposed to nano-CeO2 and excess boron: Modulation of boron phytotoxicity. Plant Physiol. Biochem. 2017, 110, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef]
- Majumdar, S.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Sahi, S.; Gardea-Torresdey, J.L. Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defence mechanisms. J. Hazard. Mater. 2014, 278, 279–287. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Skiba, E.; Pietrzak, M.; Glińska, S.; Wolf, W.M. The combined effect of ZnO and CeO2 nanoparticles on Pisum sativum L.: A photosynthesis and nutrients uptake study. Cells 2021, 10, 3105. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.P.; Ma, X. Mutual effects and in planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef]
- Pošćić, F.; Mattiello, A.; Fellet, G.; Miceli, F.; Marchiol, L. Effects of cerium and titanium oxide nanoparticles in soil on the nutrient composition of barley (Hordeum vulgare L.) kernels. Int. J. Environ. Res. Public Health 2016, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Dong, C.; Fan, S.; Jiao, C.; Song, Z.; Shen, J.; Zhao, Y.; Li, X.; Zhang, F.; Ma, Y.; et al. Effects of CeO2 Nanoparticles on Nutritional Quality of Two Crop Plants, Corn (Zea mays L.) and Soybean (Glycine max L.). Molecules 2023, 28, 1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar] [CrossRef]
- Xie, C.; Guo, Z.; Zhang, P.; Yang, J.; Zhang, J.; Ma, Y.; He, X.; Lynch, I.; Zhang, Z. Effect of CeO2 nanoparticles on plant growth and soil microcosm in a soil-plant interactive system. Environ. Pollut. 2022, 300, 118938. [Google Scholar] [CrossRef]
- Toksha, B.; Sonawale, V.; Vanarase, A.; Bornare, D.; Tonde, S.; Hazra, C.; Kundu, D.; Satdive, A.; Tayde, S.; Chatterjee, A. Nanofertilizers: A review on synthesis and impact of their use on crop yield and environment. Environ. Technol. Innov. 2021, 24, 101986. [Google Scholar] [CrossRef]
- Awad, S.M.; Hathout, T.A.; Farroh, K.Y. Pleotropic roles of biosynthesized cerium oxide nanoparticles on morphological, physiological and molecular aspects of Brassica napus. Egypt. J. Bot. 2023, 63, 765–786. [Google Scholar] [CrossRef]
- Adhikary, T.; Basak, P. The paradigm shift towards utilizing nanofertilizers paves a promising future in industrial agriculture and soilless cultivation. Hybrid Adv. 2024, 6, 100255. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, Y.; Fu, C.; Hu, J.; Chen, L.; Yan, J.; Khan, Z.; Wu, H.; Li, Z. CeO2 Nanoparticles Seed Priming Increases Salicylic Acid Level and ROS Scavenging Ability to Improve Rapeseed Salt Tolerance. Glob. Chall. 2022, 6, 2200025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adisa, I.O.; Rawat, S.; Pullagurala, V.L.R.; Dimkpa, C.O.; Elmer, W.H.; White, J.C.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Nutritional status of tomato (Solanum lycopersicum) fruit grown in Fusarium-infested soil: Impact of cerium oxide nanoparticles. J. Agric. Food Chem. 2020, 68, 1986–1997. [Google Scholar] [CrossRef]
- Baskar, V.; Safia, N.; Sree Preethy, K.; Dhivya, S.; Thiruvengadam, M.; Sathishkumar, R. A comparative study of phytotoxic effects of metal oxide (CuO, ZnO and NiO) nanoparticles on in-vitro grown Abelmoschus esculentus. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 155, 374–383. [Google Scholar] [CrossRef]
- Wohlmuth, J.; Tekielska, D.; Čechová, J.; Baránek, M. Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives. Plants 2022, 11, 2405. [Google Scholar] [CrossRef]


| Plant | Application Rate | Growth Media | Exposure Duration | Response | Reference |
|---|---|---|---|---|---|
| Triticum aestivum L. | 0, 125, 250, and 500 mg/kg | Soil media | 94 days | Improved plant growth, shoot biomass and grain yield | Rico et al. [97] |
| Lactuca sativa L. var. longifolia Lam. | 0–2000 mg/L | Sand | 21 days | Biomass reduction at dose above 2000 mg/kg | Zhang et al. [37,98] |
| Brassica rapa | 0–1000 mg/L | Soil mixture | 35 days | Reduced biomass and seed yield | Ma et al. [96] |
| Lactuca sativa | 1000 mg/kg | Potting soil | 30 days | Impeded plant growth and biomass | Gui et al. [46] |
| Hordeum. vulgare | 500 and 1000 mg/kg | Soil | 10 days | Reduced number of spikes, tillers, and leaf area | Marchiol et al. [99] |
| Solanum lycopersicum L. | (0.1–10 mg/L) | Potting mix | 70 days | Improved fruit yield and plant growth | Wang et al. [81] |
| Helianthus annuus L. | 0–800 mg/kg | Potting mix | 35 days | Altered biomass, oxidative stress | Tassi et al. [100] |
| Cucumis sativus | 0.2, 2, 20, 200 and 2000 mg/L | Hoagland solution | 14 days | No phytotoxicity | Ma et al. [67] |
| Coriandrum sativum L. | 62.5, 125, 250, 500 mg/kg | Soil | 30 days | Promotion of root and shoot growth at 125 mg/kg No effect on dry biomass | Morales et al. [59] |
| Cucumis sativus L. | 400, 800 mg/kg | Mixture of sandy soil, sand, and perlite | 53 days | No effect on biomass production, shoot length, and leaf area Decrease in fruit weight at 800 mg/kg | Zhao et al. [101] |
| Phaseolus vulgaris L. | 62.5, 125, 250, 500 mg/L | Hoagland solution | 15 days | Dose-dependent effects on biomass production | Majumdar et al. [102] |
| Arabidopsis thaliana (L.) Heynh | 100, 200, 500, 1000, 3000 mg/L | Agar plus Murashige & Skoog solution | 25 days | Increase in fresh biomass of roots and shoots at <500 mg/L Signs of toxicity at >1000 mg/L | Yang et al. [75] |
| Raphanus raphanistrum subsp. sativus (L.) | 10 mg/L | 25% Hoagland solution | 21 days | No effect on dry biomass No apparent adverse effect on the growth and development of plants | Zhang et al. [85] |
| Triticum aestivum (L.) | 100, 500, 1000, 2000 mg/L | 25% Hoagland solution | 20 days | Increased fresh biomass of roots and shoots at 100 and 500 mg/L; reduced at 1000 and 2000 mg/L Increase in shoot growth at 500 mg/L Decreased root elongation at 100, 1000, and 2000 mg/L | Abbas et al. [83] |
| Glycine max (L.) | 100 mg/kg | Soil under different moisture conditions | 21 days | Increased fresh and dry biomass of roots and shoots | Cao et al. [103] |
| Lathyrus oleraceus Lam. | 100, 200, 500 mg/L | Hoagland solution | 12 days | Decreased fresh biomass of roots and shoots at 500 mg/L No effect on dry biomass | Skiba et al. [84] and Skiba et al. [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukwattage, N.L.; Zhiyong, Z. Impacts of Cerium Dioxide Nanoparticles on the Soil–Plant System and Their Potential Agricultural Applications. Nanomaterials 2025, 15, 950. https://doi.org/10.3390/nano15120950
Ukwattage NL, Zhiyong Z. Impacts of Cerium Dioxide Nanoparticles on the Soil–Plant System and Their Potential Agricultural Applications. Nanomaterials. 2025; 15(12):950. https://doi.org/10.3390/nano15120950
Chicago/Turabian StyleUkwattage, Nadeesha L., and Zhang Zhiyong. 2025. "Impacts of Cerium Dioxide Nanoparticles on the Soil–Plant System and Their Potential Agricultural Applications" Nanomaterials 15, no. 12: 950. https://doi.org/10.3390/nano15120950
APA StyleUkwattage, N. L., & Zhiyong, Z. (2025). Impacts of Cerium Dioxide Nanoparticles on the Soil–Plant System and Their Potential Agricultural Applications. Nanomaterials, 15(12), 950. https://doi.org/10.3390/nano15120950







