Rational Construction of Nano-Scaled FeOOH/NiFe-LDH for Efficient Water Splitting
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
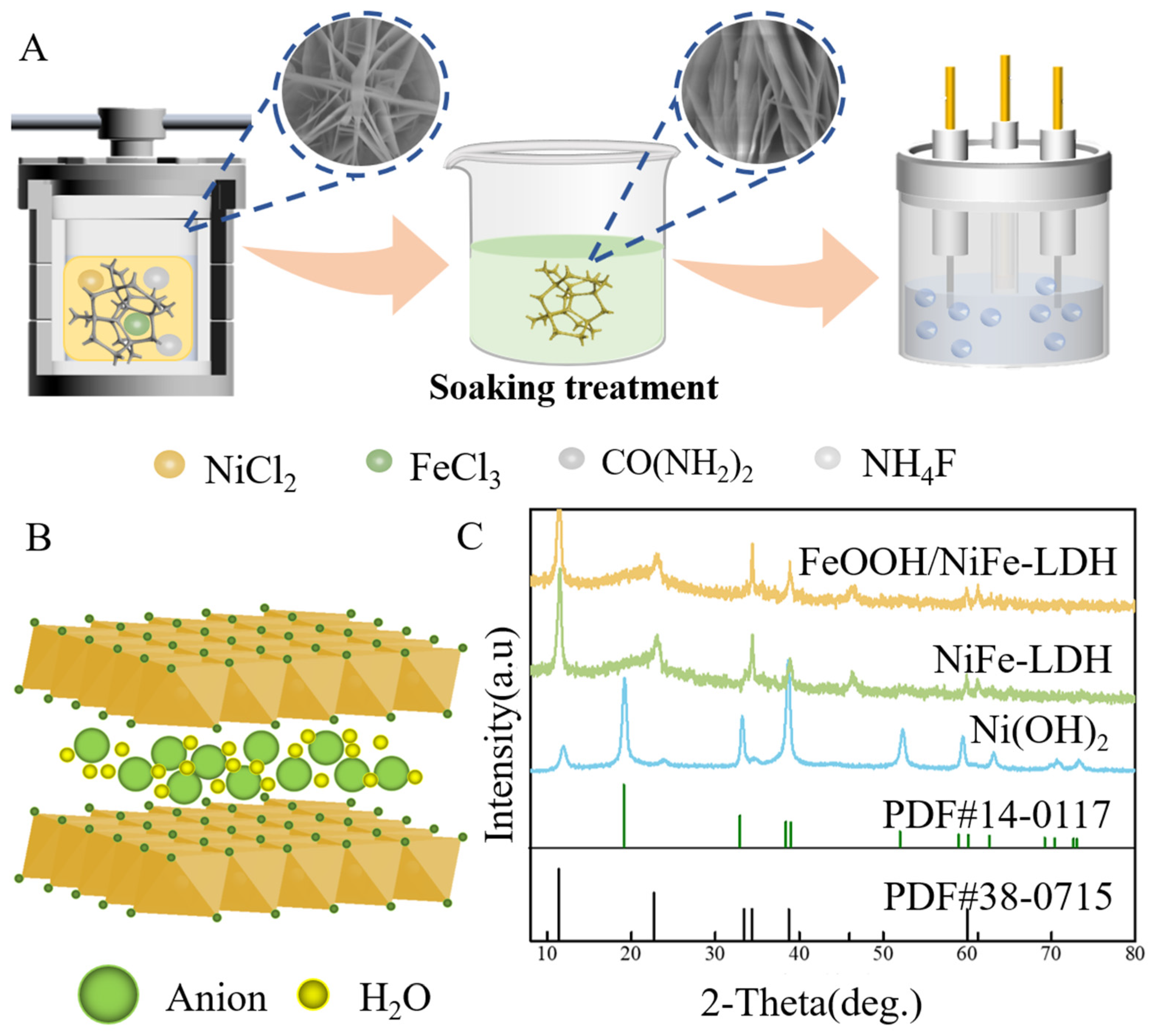
2.2. Preparation of Ultrathin Ni-Fe LDH
2.3. Preparation of FeOOH/NiFe-LDH
2.4. Physical Characterizations
2.5. Electrochemical Measurements
3. Results and Discussion
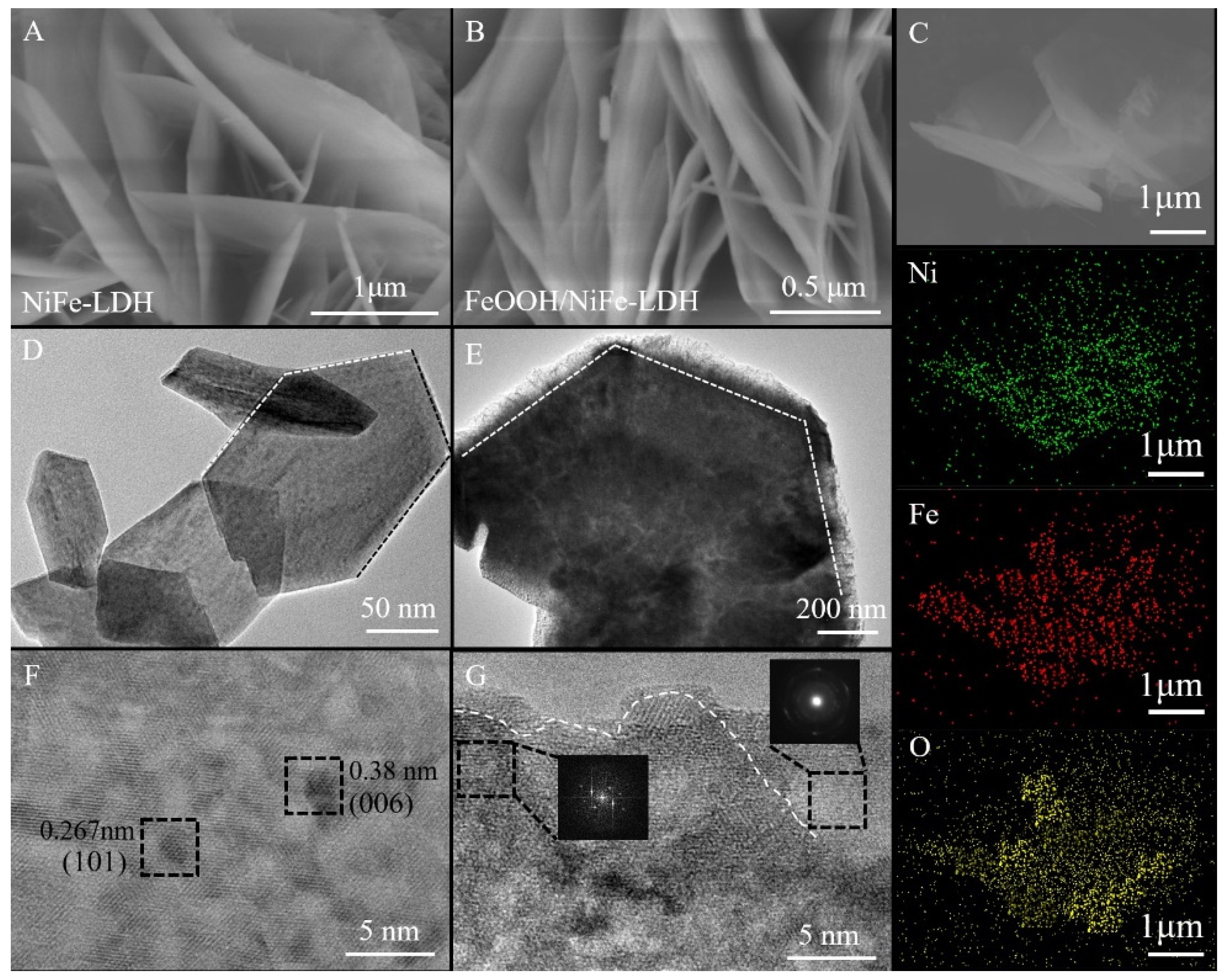
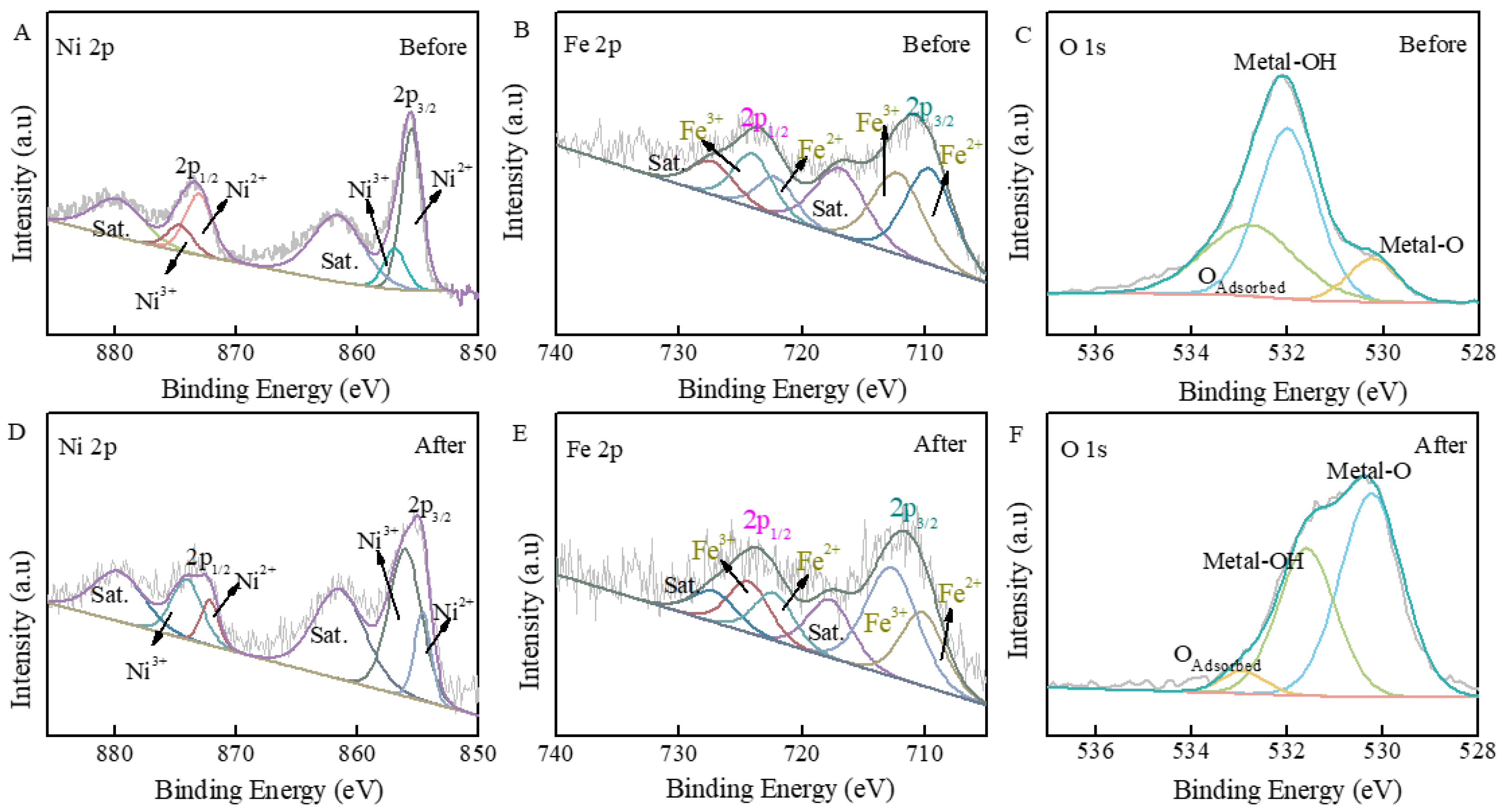
3.1. Physicochemical Properties of FeOOH/NiFe-LDH
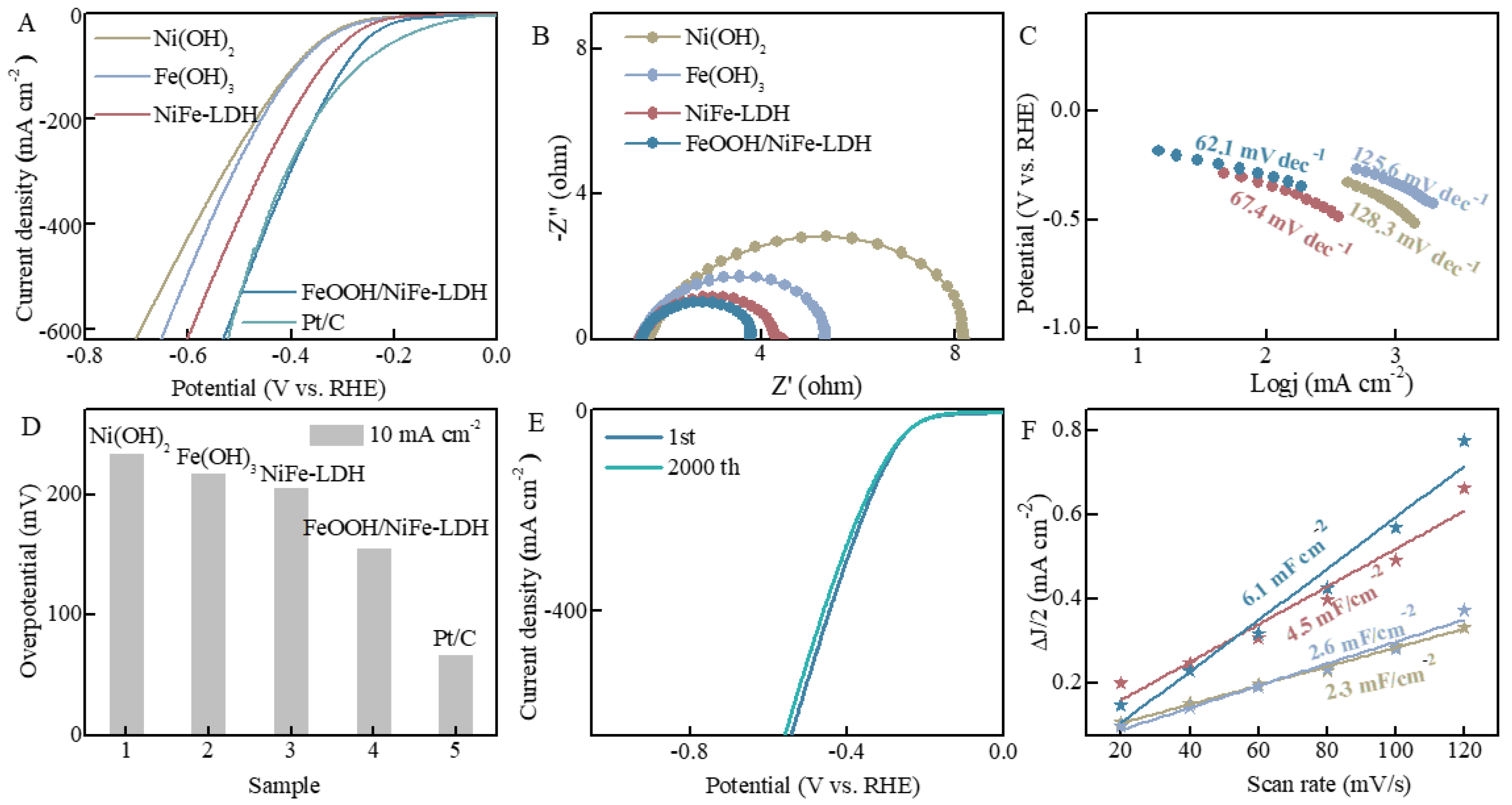
3.2. HER Measurements
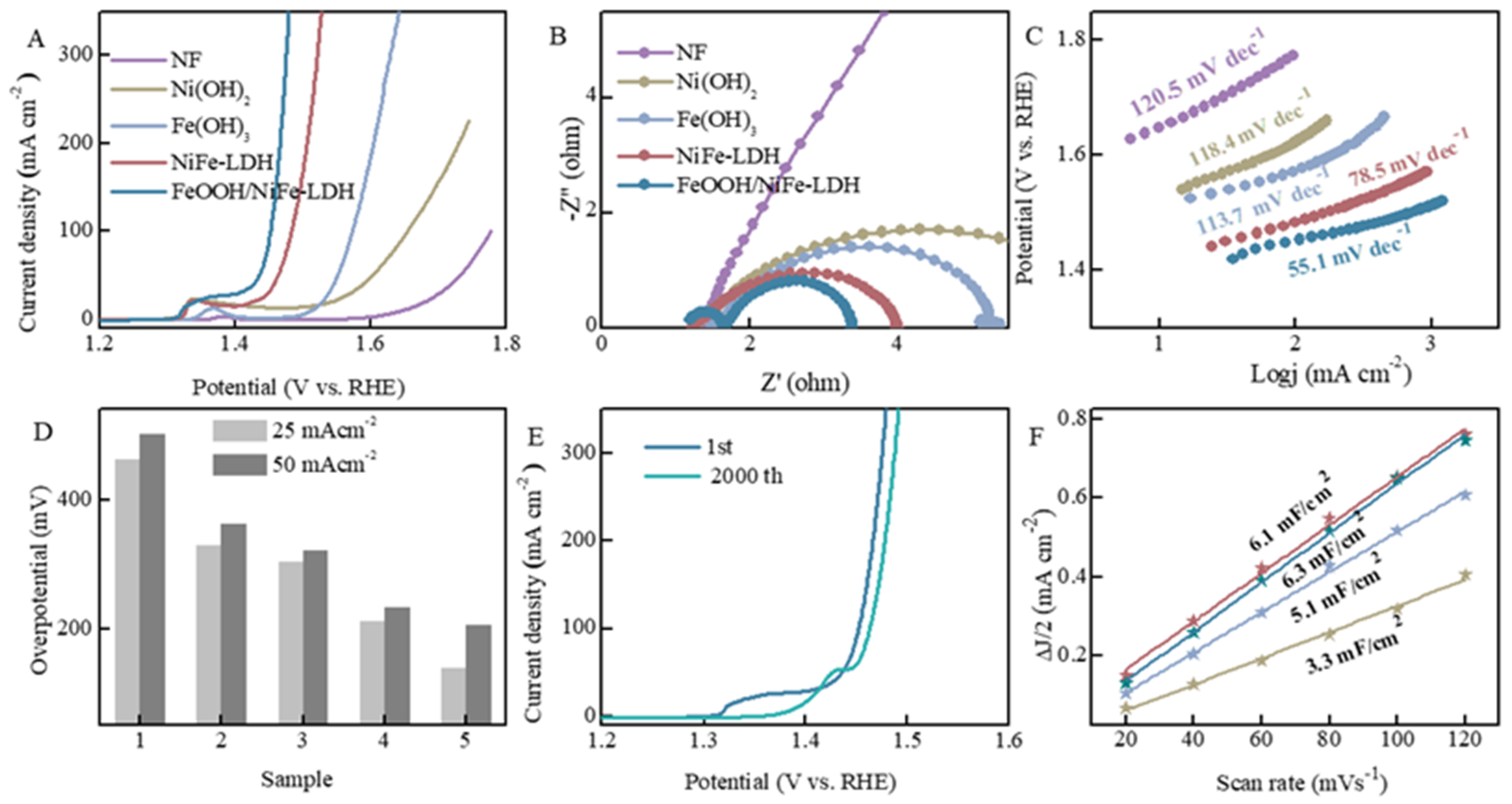
3.3. OER Measurements
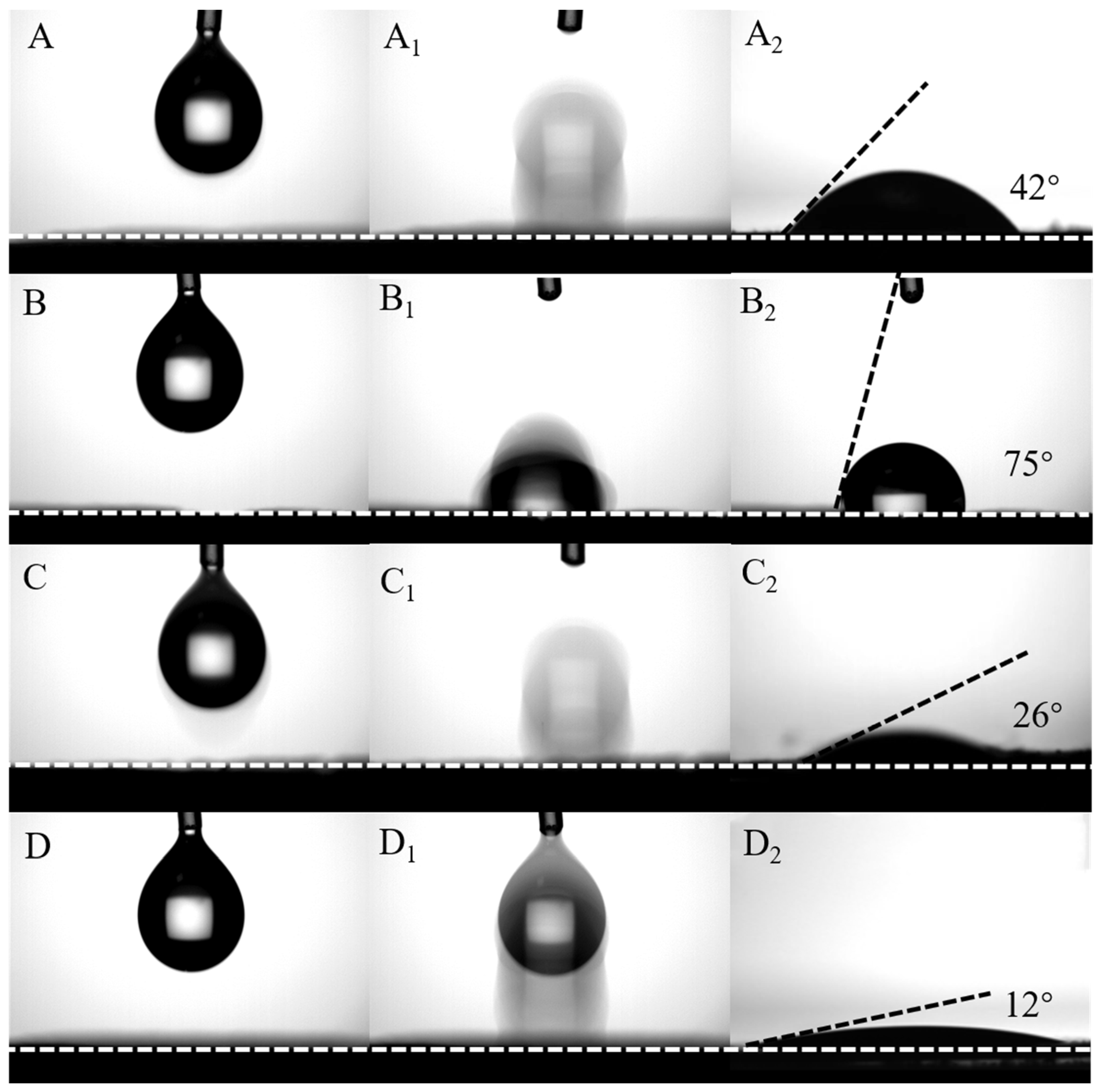
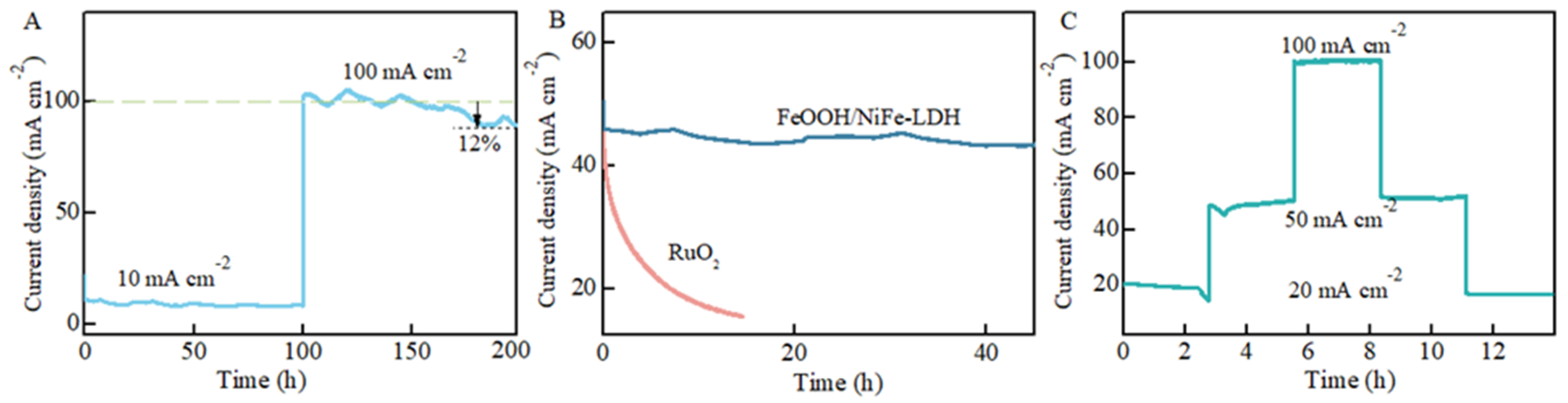
3.4. Hydrophilicity and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ai, L.; Zeng, Y. Hierarchical porous NiO architectures as highly recyclable adsorbents for effective removal of organic dye from aqueous solution. Chem. Eng. J. 2013, 215–216, 269–278. [Google Scholar] [CrossRef]
- Bonomo, M.; Naponiello, G.; Dini, D. Oxidative dissolution of NiO in aqueous electrolyte: An impedance study. J. Electroanal. Chem. 2018, 816, 205–214. [Google Scholar] [CrossRef]
- Che, Y.; Wang, K.; Wang, C.; Weng, B.; Chen, S.; Meng, S. Lattice Match-enabled Zn3In2S6@CdS S-Scheme Heterojunction with S Covalent Bond Bridge for Simultaneous H2O2 Photosynthesis and H2 Production. J. Mater. Sci. Technol. 2025, 243, 228–236. [Google Scholar] [CrossRef]
- Jin, C.; Zhai, P.; Wei, Y.; Chen, Q.; Wang, X.; Yang, W.; Xiao, J.; He, Q.; Liu, Q.; Gong, Y. Ni(OH)2 Templated Synthesis of Ultrathin Ni3S2 Nanosheets as Bifunctional Electrocatalyst for Overall Water Splitting. Small 2021, 33, 2102097. [Google Scholar] [CrossRef]
- Chen, C.; Kong, X. Enhanced Oxygen Evolution Reaction for Single Atomic Co Catalyst via Support Modification: A Density Functional Theory Design Predication. Inorg. Chem. 2018, 20, 13020–13026. [Google Scholar] [CrossRef]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient photocatalytic H2 evolution, CO2 reduction and N2 fixation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B Environ. 2021, 285, 119789. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, X.; Jiang, K.; Wang, Q.; Wang, X.; Shahzad, M.; Yin, F.; Xu, B.; Hua, Q.; Liu, H. Sub-3 nm Pt3Ni nanoparticles for urea-assisted water splitting. Adv. Compos. Hybrid Mater. 2025, 8, 182. [Google Scholar] [CrossRef]
- Fan, X.; Sun, R.; Zhu, Y.; Zhang, S.; Gou, L.; Lu, L.; Li, D. Controllable 3D Porous Ni Current Collector Coupled with Surface Phosphorization Enhances Na Storage of Ni3S2 Nanosheet Arrays. Small 2022, 8, 2106161. [Google Scholar] [CrossRef]
- Liu, G.X.; Guo, Y. Fabric-like rhodium-nickel-tungsten oxide nanosheets for highly-efficient electrocatalytic H2 generation in an alkaline electrolyte. J. Colloid Interface Sci. 2024, 659, 895–904. [Google Scholar] [CrossRef]
- Yang, H.; Li, F.; Wu, X.; Zhang, P.; Li, W.; Cao, S.; Shan, Y.; Sun, L. Improving the performance of water splitting electrodes by composite plating with nano-SiO2. Electrochim. Acta 2018, 281, 60–68. [Google Scholar] [CrossRef]
- Huang, Y.; Chong, X.-D.; Liu, C.-C.; Liang, Y.; Zhang, B. Boosting Hydrogen Production by Anodic Oxidation of Primary Amines over a NiSe Nanorod Electrode. Angew. Chem. Int. Ed. 2018, 57, 13163–13166. [Google Scholar] [CrossRef]
- Yi, Y.Z.; Wei, C.; Hu, Y. Controlling the Cation Exsolution of Perovskite to Customize Heterostructure Active Site for Oxygen Evolution Reaction. ACS Appl. Mater. Inter. 2022, 14, 25638–25647. [Google Scholar]
- Yin, X.-C.; Sun, G.; Wang, L.-X.; Bai, L.; Wang, Y.-Z.; Du, Q.-H.; Shao, G.-J. 3D hierarchical network NiCo2S4 nanoflakes grown on Ni foam as efficient bifunctional electrocatalysts for both hydrogen and oxygen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2017, 42, 25267–25276. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.-P.; Zhao, G.-Q.; Lin, Y.; Yu, Z.-W.; Xu, X.; Wang, X.-L.; Liu, H.-K.; Sun, W.-P.; Dou, S.-X. Active-Site-Enriched Iron-Doped Nickel/Cobalt Hydroxide Nanosheets for Enhanced Oxygen Evolution Reaction. ACS Catal. 2018, 8, 5382–5390. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Li, J.; Yang, M.-J.; Fang, Z.-S.; Jian, J.-H.; Yu, D.-S.; Chen, X.-D.; Dai, L.-M. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Liu, J.-Y.; Chen, X.-M.; Zheng, X.-L.; Mao, J.; Liu, H.; Ma, T.; Li, L.; Wang, W.-C.; Du, X.-X. Engineering NiO/NiFe LDH Intersection to Bypass Scaling Relationship for Oxygen Evolution Reaction via Dynamic Tridimensional Adsorption of Intermediates. Adv. Mater. 2019, 11, 1804769. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, W.; Yu, X.; Zhang, H.; Li, Y.; Sun, X.; Wang, X.; Wang, H.; Wang, J.; Luo, J.; et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Zhu, W.; Yu, X.-Y.; Zhang, H.-C.; Li, Y.-J.; Sun, X.-M.; Wang, X.-W.; Wang, H.; Wang, J.-M.; Luo, J.; et al. Hierarchically porous carbon materials derived from MIL-88(Fe) for superior high-rate and long cycling-life sodium ions batteries. J. Electroanal. Chem. 2019, 852, 113525. [Google Scholar]
- Zhang, K.-J.; Wang, F.-F.; Li, X.-Y.; Wang, S.-X.; Wang, Y.-H.; Zha, Q.-Q.; Ni, Y.-H. Connected design of Ni foam-supported porous Ni and FeOOH/porous Ni electrocatalysts for overall water splitting in alkaline media. J. Alloys Compd. 2023, 942, 169014. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-Y.; Guo, Z.-L.; Fang, Y.; Tang, C.-C.; Miao, N.-H.; Sa, B.-S.; Zhou, J.; Sun, Z.-M. Establishing theoretical landscapes for identifying basal plane active sites in MBene toward multifunctional HER, OER, and ORR catalysts. J. Colloid Interface Sci. 2023, 652, 1954–1964. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.-M.; Xing, Y.-Y.; Li, D.; Jiang, D.-L.; Chen, M.; Shi, W.-D.; Yuan, S.-Q. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Yang, H.-M.; Ni, C.-M.; Gao, X.-N.; Lin, S.-H.; He, X.-Y.; Tian, L.; Li, Z. Constructing Built-in-Electric Field for Boosting Electrocatalytic Water Splitting. ChemSusChem 2024, 17, e202400977. [Google Scholar] [CrossRef]
- Xu, H.-W.; Zhu, J.-W.; Wang, P.-Y.; Chen, D.; Zhang, C.-T.; Xiao, M.-J.; Ma, Q.-L.; Bai, H.-W.; Qin, R.; Ma, J.-J.; et al. Fe–Co–P multi-heterostructure arrays for efficient electrocatalytic water splitting. J. Mater. Chem. A 2021, 9, 24677–24685. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, B.; Chen, H.; You, W.; Jiang, C.; Yu, J. Hierarchical flower-like nickel(II) oxide microspheres with high adsorption capacity of Congo red in water. J. Colloid Interface Sci. 2017, 504, 688–696. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Li, Q.; Chen, C.; Li, Y.-L.; Tao, K.; Han, L. Co3O4@CoNi-LDH core/shell nanosheet arrays for high-performance battery-type supercapacitors. Chem. Eng. J. 2018, 350, 551–558. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, C.-F.; Yang, X.-G.; Zou, G.; Wu, H.-J.; Xi, S.-Q. Flame-like Ni(OH)2 strongly promotes the dissociation of water and can be used to produce an excellent hybrid electrocatalyst for the hydrogen evolution reaction in alkaline media. Electrochem. Commun. 2018, 91, 66–70. [Google Scholar] [CrossRef]
- Zhao, S.-L.; Xiao, J.-Y.; Qiu, M.; Zhu, Z.-H. Recent progress on transition metal-decorated and heteroatom-doped porous carbon-based oxygen catalysts. Sci. Sin.-Phys. Mech. Astron. 2023, 9, 290017. [Google Scholar] [CrossRef]
- Guo, B.; Huo, H.; Zhuang, Q. Iron Oxyhydroxide: Structure and Applications in Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2300557. [Google Scholar] [CrossRef]
- Guo, M.-L.; Huang, Z.-J.; Huang, T.-J.; Lin, Z.-Q.; Wang, M.-Y.; Tu, J.-C.; Ding, L.; Geng, W.-C. Interface electronic coupling in FeOOH-Co9S8 heterostructure for efficient oxygen evolution reaction. Appl. Surf. Sci. 2023, 638, 158002. [Google Scholar] [CrossRef]
- Bodhankar, P.-M.; Sarawade, P.-B.; Singh, G.; Vinu, A.; Dhawale, D.-S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Sun, L.; Zeng, Y.; Li, J.; Wang, H.; Hua, Q.; Lu, S. Enhancing water electrolysis through interfacial design of nickel foam. Langmuir 2025, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lu, S.; Wang, H.; Khalil, M.; Hua, Q.; Qi, X. In situ electrodeposition self-supported bimetallic sulfides for urea electrooxidation. ChemCatChem 2025, 48, e202500298. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, X.; Zeng, Y.; Hua, Q.; Wang, X.; Liu, Y.; Liu, H. Triphenylamine-substituted Ni(II) porphyrins for urea electro-oxidation. Inorg. Chem. 2024, 63, 20929–20934. [Google Scholar] [CrossRef]
- Li, K.-L.; Liu, X.-Y.; Zheng, T.-X.; Jiang, D.-B.; Zhou, Z.; Liu, C.-Q.I.; Zhang, Y.-X.; Losic, D.-S. Tuning MnO2 to FeOOH replicas with bio-template 3D morphology as electrodes for high performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 136–147. [Google Scholar] [CrossRef]
- Li, X.-M.; Hao, X.-G.; Wang, Z.-D.; Abudula, A.; Guan, G.-Q. In-situ intercalation of NiFe LDH materials: An efficient approach to improve electrocatalytic activity and stability for water splitting. J. Power Sources 2017, 347, 193–200. [Google Scholar] [CrossRef]
- Li, X.; Kou, Z.; Xi, S.; Chen, C.; Liang, H.-F. Porous NiCo2S4/FeOOH nanowire arrays with rich sulfide/hydroxide interfaces enable high OER activity. Nano Energy 2020, 78, 105230. [Google Scholar] [CrossRef]
- Li, Z.; Sun, L.; Zhang, Y.; Han, Y.; Zhuang, W.; Tian, L.; Tan, W. Coupled and decoupled electrochemical water splitting for boosting hydrogen evolution: A review and perspective. Coord. Chem. Rev. 2024, 510, 215837. [Google Scholar] [CrossRef]
- Liu, F.-L.; Wang, F.-Z.; Hao, X.-W.; Fan, Z.-F.; Tan, J.-Y. Effects of foam cathode electrode structure on alkaline water electrolysis for hydrogen production. Chem. Eng. Sci. 2024, 298, 120307. [Google Scholar] [CrossRef]
- Liu, H.Z.Q.-L.; Xu, J. Facile Preparation of Amorphous Fe-Co-Ni Hydroxide Arrays: A Highly Efficient Integrated Electrode for Water Oxidation. Inorg. Chem. 2018, 57, 15610–15617. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-H.; Zhang, B.; Li, D. Partial-sacrificial-template Synthesis of Fe/Ni Phosphides on Ni Foam: A Strongly Stabilized and Efficient Catalyst for Electrochemical Water Splitting. Electrochim. Acta 2017, 242, 260–267. [Google Scholar] [CrossRef]
- Yang, M.-S.; Ding, J.-Y.; Wang, Z.-W. NiMo-based alloy and its sulfides for energy-saving hydrogen production via sulfion oxidation assisted alkaline seawater splitting. Chin. Chem. Lett. 2025, in press. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, T.; Yu, X.; Xia, Y.; Niu, J.; Tao, Y.; Pan, J.; Chang, K.; He, J.; Liang, Y. Decreased energy barrier and improved interfacial kinetics for efficient photoelectrochemical water splitting using La-engineered LDHs coupled with BiVO4. Inorg. Chem. Front. 2025, 12, 1482–1492. [Google Scholar] [CrossRef]
- Ding, J.; Peng, Z.; Wang, Z.; Zeng, C.; Feng, Y.; Yang, M.; Hu, G.; Luo, J.; Liu, X. Phosphorus–tungsten dual-doping boosts acidic overall seawater splitting performance over RuOx nanocrystals. J. Mater. Chem. A. 2024, 12, 28023–28031. [Google Scholar] [CrossRef]
- Yue, C.-Z.; Zhang, X.-N.; Yin, J.; Zhou, H.-W.; Liu, K.; Liu, X. Highly efficient FeS2@FeOOH core-shell water oxidation electrocatalyst formed by surface reconstruction of FeS2 microspheres supported on Ni foam. Appl. Catal. B Environ. 2023, 339, 123171. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Zhang, X.; Cao, L.; Yang, D.; Kim, J.H.; Feng, L. Controllable Conversion from Single-Crystal Nanorods to Polycrystalline Nanosheets of NiCoV-LTH for Oxygen Evolution Reactionat Large Current Density. ACS Sustain. Chem. Eng. 2023, 8, 16091–16096. [Google Scholar] [CrossRef]
- Sarfraz, B.; Bashir, I.; Rauf, A. CuS/NiFe-LDH/NF as a Bifunctional Electrocatalyst for Hydrogen Evolution (HER) and Oxygen Evolution Reactions (OER). Fuel 2023, 337, 127253. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, K.; Huang, J.; Hui, C.; Li, X.; Feng, L. A review of modulation strategies for improving catalytic performance of transition metal sulfide self-supported electrodes for Hydrogen evolution reaction. Dalton Trans. 2024, 53, 3959–3969. [Google Scholar] [CrossRef]
- Sun, F.-C.; You, Z.-H. Bi-Functional Fe3O4/Au/CoFe-LDH Sandwich-Structured Electrocatalyst for Asymmet rical Electrolyzer with Low Operation Voltage. Small 2021, 46, e2103307. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Yu, Y.; Wang, F.; Wu, X.; Wang, X.; Mao, W.; Li, J.; Huang, W.; Chen, Q.; et al. Core-shell structured NiTe@FeOOH nanoarrays for efficient overall seawater splitting. Chem. Eng. J. 2023, 474, 145568. [Google Scholar] [CrossRef]
- Han, X.; Lin, Z.; He, X.; Cui, L.; Lu, D. The construction of defective FeCo-LDHs by in-situ polyaniline curved strategy as a desirable bifunctional electrocatalyst for OER and HER. Int. J. Hydrogen Energy 2020, 51, 26989. [Google Scholar] [CrossRef]
- Abed, S.A.J.; Laverdure, L.; Abdellah, A.; O’Brien, C.P.; Cole, K.; Sobrinho, P.; Sinton, D.; Higgins, D.; Mosey, N.J.; Thorpe, S.J.; et al. In Situ Formation of Nano Ni-Co Oxyhydroxide Enables Water Oxidation Electrocatalysts Durable at High Current Densities. Adv. Mater. 2021, 45, e2103812. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, J.; Wang, P.; Chen, D.; Zhang, C.; Xiao, M.; Ma, Q.; Bai, H.; Qin, R.; Ma, J.; et al. Fe–Co–P multi-heterostructure arrays for efficient electrocatalytic water splitting. J. Mater. Chem. A 2021, 43, 24677. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Kong, X. Ru-O-Cu center constructed by catalytic growth of Ru for efficient hydrogen evolution. Nano Energy 2023, 111, 108403. [Google Scholar] [CrossRef]
- Cheng, C.; Zheng, F.; Zhang, C.; Du, C.; Fang, Z.; Zhang, Z.; Chen, W. High-efficiency bifunctional electrocatalyst based on 3D freestanding Cu foam in situ armored CoNi alloy nanosheet arrays for overall water splitting. J. Power Sources 2019, 427, 184. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Hu, Y.; Cui, X.; Zhu, R.; Zhang, Y.; Yue, C.; Dan, J.G.; Cui, W.; Zhao, H.; et al. Metal-organic framework derived Fe-Co-CN/reduced graphene oxide for efficient HER and OER. Electrochim. Acta 2021, 365, 137384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Fu, X.; Wang, H.; Lu, S.; Li, B. Rational Construction of Nano-Scaled FeOOH/NiFe-LDH for Efficient Water Splitting. Nanomaterials 2025, 15, 949. https://doi.org/10.3390/nano15120949
Yu J, Fu X, Wang H, Lu S, Li B. Rational Construction of Nano-Scaled FeOOH/NiFe-LDH for Efficient Water Splitting. Nanomaterials. 2025; 15(12):949. https://doi.org/10.3390/nano15120949
Chicago/Turabian StyleYu, Juan, Xiubing Fu, Haoqi Wang, Shun Lu, and Bing Li. 2025. "Rational Construction of Nano-Scaled FeOOH/NiFe-LDH for Efficient Water Splitting" Nanomaterials 15, no. 12: 949. https://doi.org/10.3390/nano15120949
APA StyleYu, J., Fu, X., Wang, H., Lu, S., & Li, B. (2025). Rational Construction of Nano-Scaled FeOOH/NiFe-LDH for Efficient Water Splitting. Nanomaterials, 15(12), 949. https://doi.org/10.3390/nano15120949







