Machine-Learning-Guided Design of Nanostructured Metal Oxide Photoanodes for Photoelectrochemical Water Splitting: From Material Discovery to Performance Optimization
Abstract
1. Introduction
2. Fundamentals of Nanostructured Metal Oxide Photoanodes
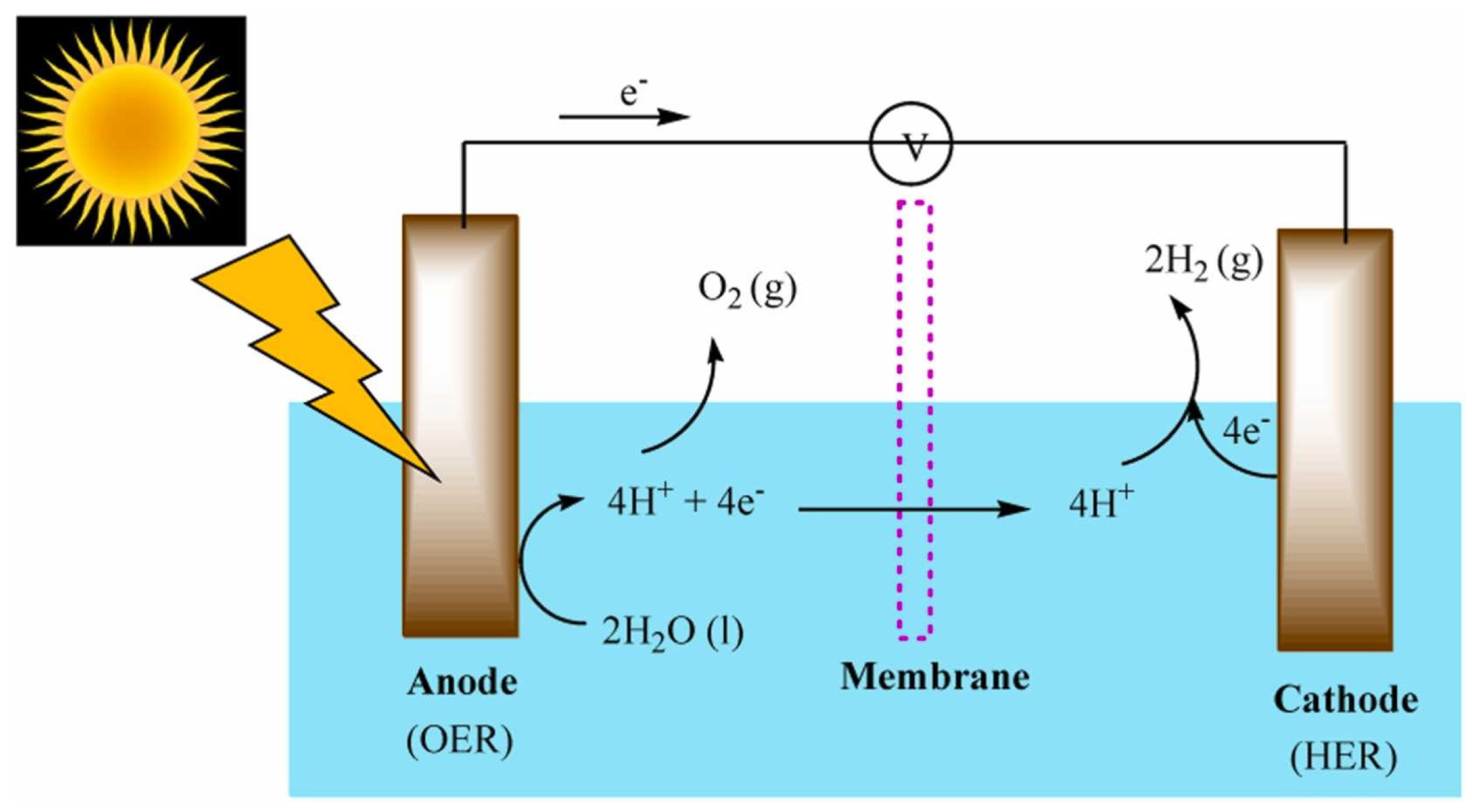
2.1. Operational Principles and Material Requirements
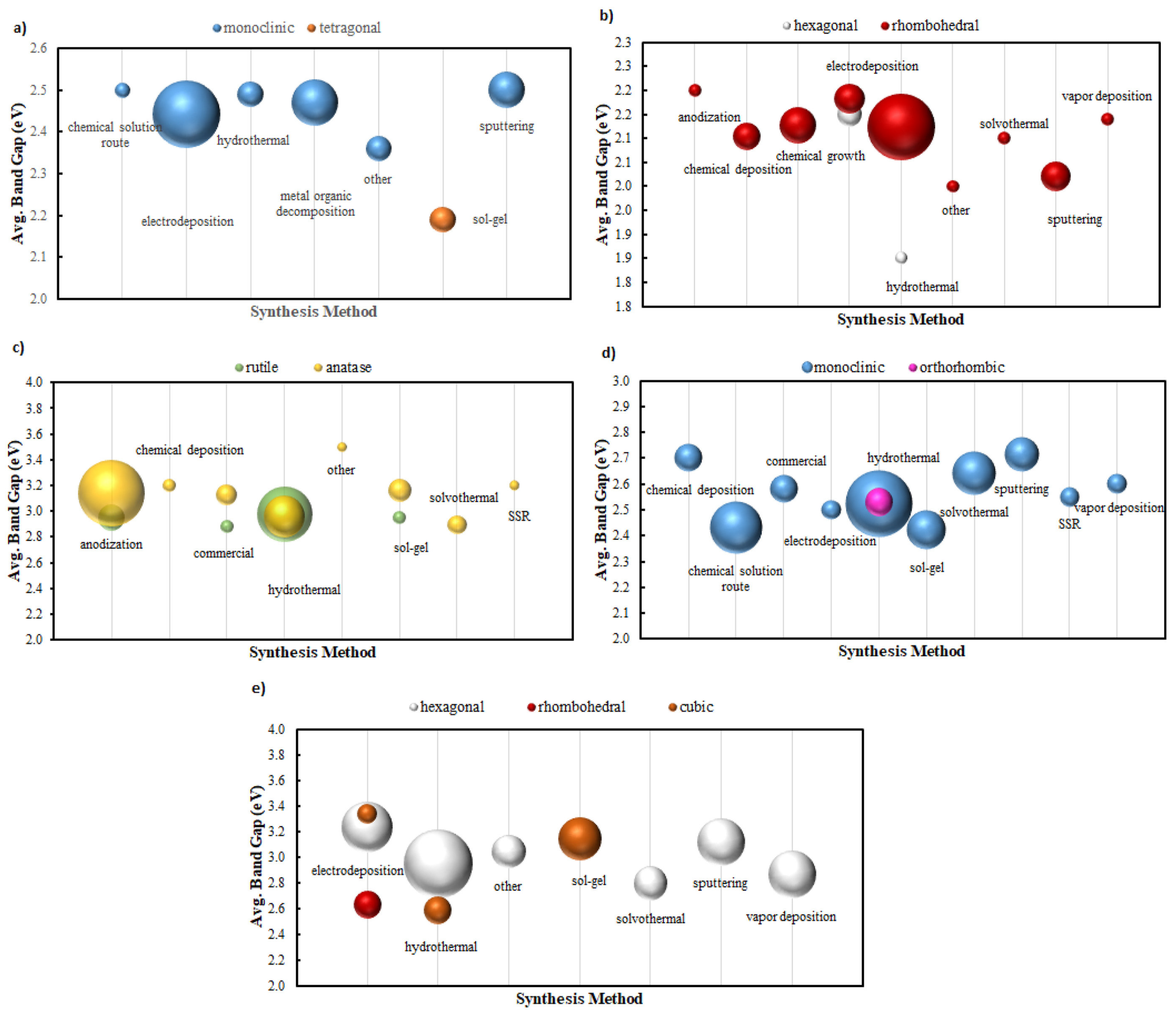
2.2. TiO2 (Titanium Dioxide)
2.3. α-Fe2O3 (Hematite)
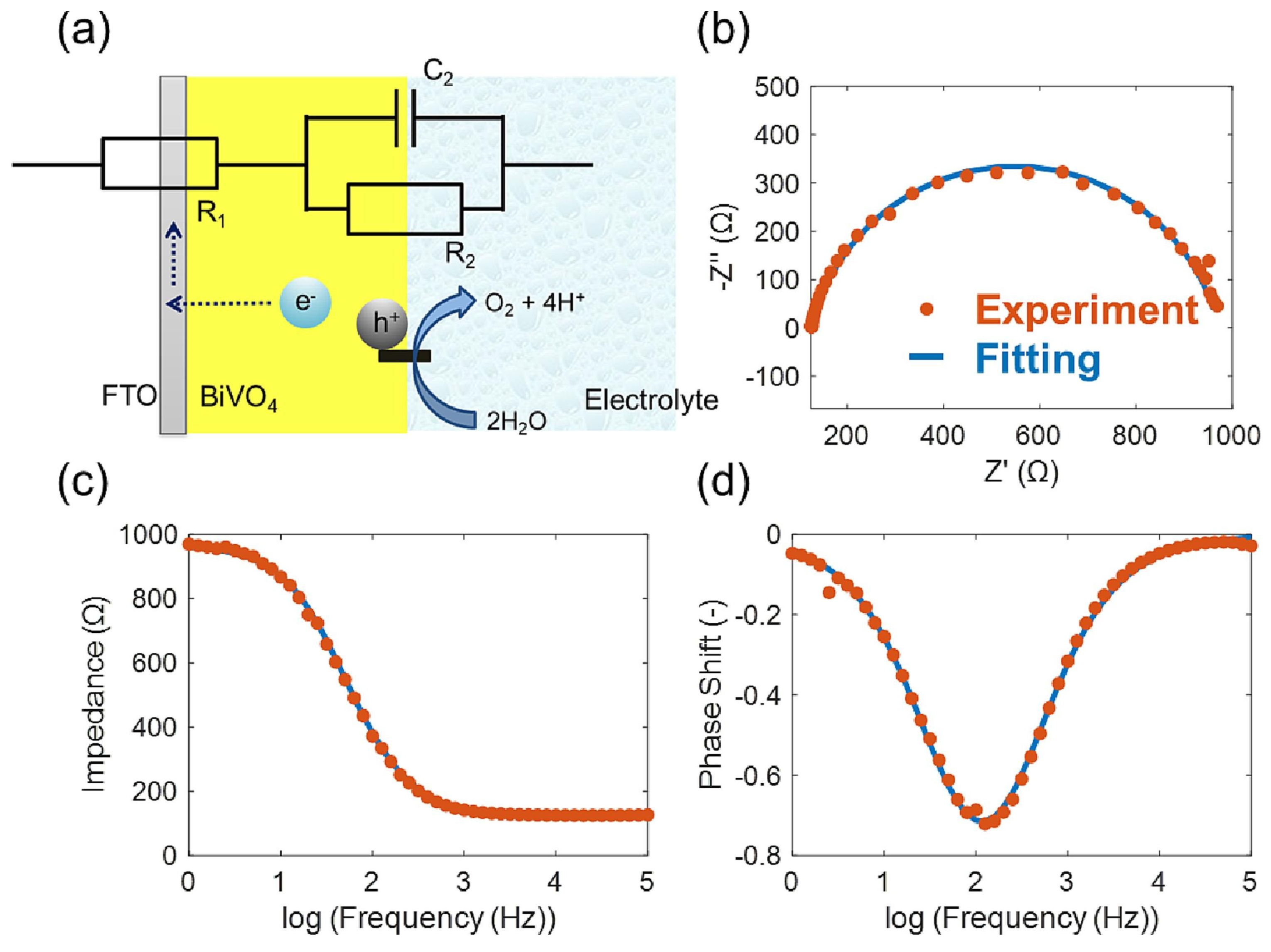
2.4. BiVO4 (Bismuth Vanadate)
2.5. WO3 (Tungsten Trioxide)
2.6. Other Noteworthy Oxide Photoanodes
3. Nanostructuring, Doping, and Interface Engineering—Conventional Approaches
3.1. Nanostructuring
3.2. Electronic Doping: From Single-Element to Co-Doping
3.3. Interface Engineering: Catalysts, Homo-Junctions, and Heterojunctions
4. Machine Learning in Materials Discovery for PEC Applications
4.1. High-Throughput Computing and Data Generation
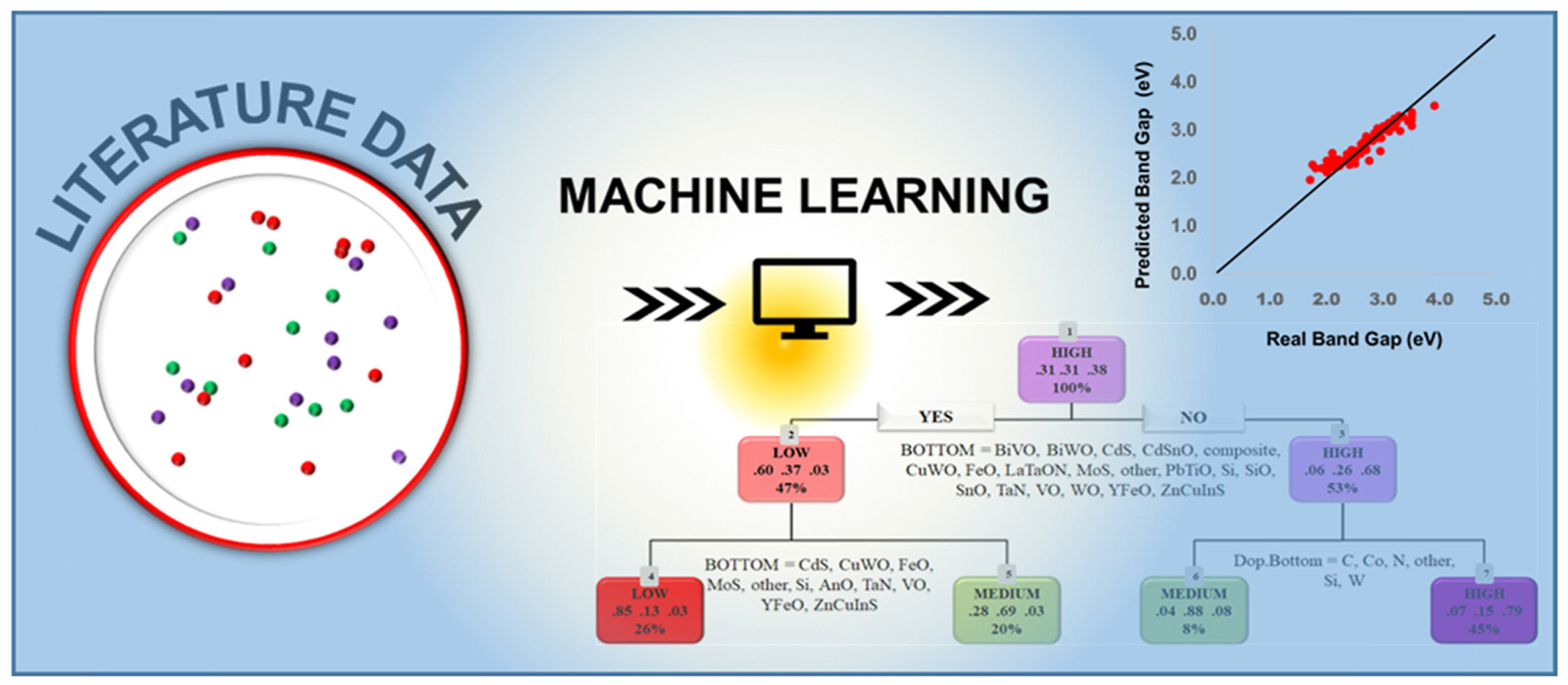
4.2. Predicting Material Properties Relevant to PEC
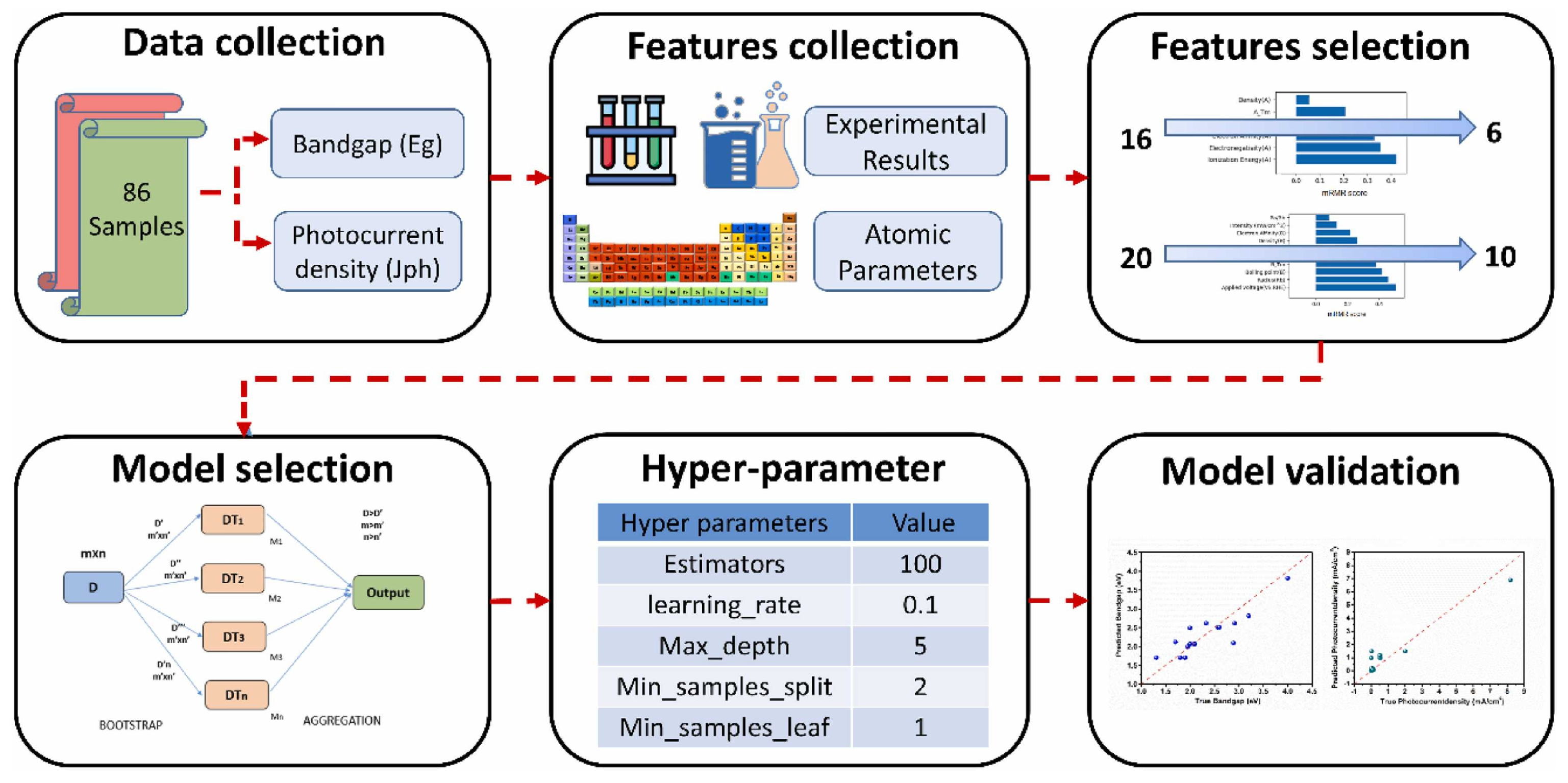
4.3. Combining Experimental Data and ML
4.4. Accelerating Discovery of New Compounds
4.5. Classification of ML Approaches in PEC Applications
5. ML-Guided Optimization of Material Properties (Doping, Morphology, Interfaces)
5.1. Dopant Selection and Composition Optimization
5.2. Morphology and Microstructure Optimization
5.3. Interface and Surface Optimization
5.4. Process Optimization and Upscaling
5.5. Considerations in Model Selection, Interpretability, and Uncertainty Quantification
6. Performance Prediction and Device-Level Integration
6.1. Comparative Evaluation of ML Models in PEC Applications
6.2. Predicting Photocurrent–Voltage Behavior
6.3. Device-Level Integration—Tandems and Modules
6.4. Stability and Lifetime Predictions
6.5. System-Level Optimization and Control
6.6. Example—Tandem Design Case Study
6.7. Experimental Integration and Feedback-Driven Model Refinement
7. Challenges and Future Outlook
7.1. Data Quality and Availability
7.2. Feature Selection and Interpretability
7.3. Generalization and Extrapolation
7.4. Integration with Experiment (Closing the Loop)
7.5. Scaling and Manufacturing Considerations
7.6. Emerging and Future Opportunities
- DFT + ML for reaction mechanisms: Applying ML to atomistic simulations (e.g., using neural networks to fit potential energy surfaces from DFT data) can allow simulation of complex surface reactions at lower cost. This could yield molecular-level understanding of water oxidation intermediates on photoanode surfaces. For instance, a ML-accelerated DFT study might map out how a dopant changes the binding energy of OH on a BiVO4 surface, explaining the catalytic effects observed.
- Inverse design via generative models: Generative adversarial networks (GANs) and other generative models can propose entirely new crystal structures or compositions optimized for target properties [65]. In the future, one might use a generative model to design a hypothetical oxide with a specific band structure and defect tolerance suitable for PEC operation—effectively inventing a new material on the computer that human experts might not conceive. Early attempts of GANs in material science have produced plausible new materials for batteries and photovoltaics; extending this to photoanodes is a matter of incorporating the right training data (perhaps from known photocatalysts).
- Knowledge incorporation: Expert knowledge and heuristics (like “d0 transition metal oxides tend to be good photoanodes” or “covalent oxyhydroxides have slow OER kinetics”) could be encoded as priors or constraints in ML models. This hybrid of expert systems and ML could lead to more efficient learning from smaller datasets and ensure that well-established principles guide the search, preventing the model from wasting time on chemically unreasonable candidates.
- Machine learning for co-catalyst design: We discussed catalysts in context of surfaces, but ML could also help design new water oxidation catalysts specifically tailored to photoanodes (for instance, ones that operate at lower overpotential or form ideal junctions with the semiconductor). Using datasets of electrocatalytic OER performance, ML models have started to identify descriptor-based trends. These can be applied to suggest catalysts that not only are active, but also chemically compatible with the photoanode (e.g., not leaching into it or blocking light). The ML model might learn, for example, that cobalt phosphates work well on BiVO4 because they passivate surface states without absorbing much light and then suggest analogous materials for other photoanodes.
- Cross-domain synergy: Combining data from photoelectrodes, photocatalytic powders, and electrocatalysts via transfer learning could create comprehensive models that understand the water splitting process in various forms. Lessons learned in one domain (e.g., stability trends in electrocatalysts) could inform predictions in another (stability of photoanodes).
7.7. Outlook
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green hydrogen for a sustainable future: A review of production methods, innovations, and applications. Int. J. Hydrogen Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Holmes-Gentle, I.; Tembhurne, S.; Suter, C.; Haussener, S. Kilowatt-scale solar hydrogen production system using a concentrated integrated photoelectrochemical device. Nat. Energy 2023, 8, 586–596. [Google Scholar] [CrossRef]
- Li, L.; Xu, H.; Zhang, Q.; Zhan, Z.; Liang, X.; Xing, J. Estimation methods of wetland carbon sink and factors influencing wetland carbon cycle: A review. Carbon Res. 2024, 3, 50. [Google Scholar] [CrossRef]
- Li, L.; Liang, T.; Zhao, M.; Lv, Y.; Song, Z.; Sheng, T.; Ma, F. A review on mycelial pellets as biological carriers: Wastewater treatment and recovery for resource and energy. Bioresour. Technol. 2022, 355, 127200. [Google Scholar] [CrossRef]
- Yang, J.W.; Ji, S.G.; Jeong, C.-S.; Kim, J.; Kwon, H.R.; Lee, T.H.; Lee, S.A.; Cheon, W.S.; Lee, S.; Lee, H.; et al. High-efficiency unbiased water splitting with photoanodes harnessing polycarbazole hole transport layers. Energy Environ. Sci. 2024, 17, 2541–2553. [Google Scholar] [CrossRef]
- Ba, K.; Li, H.; Zhang, K.; Lin, Y.; Zhu, W.; Xie, T. In situ formation of a Co-MOF/Ti–Fe2O3 photoanode for efficient photoelectrochemical water splitting. J. Mater. Chem. C 2024, 12, 17603–17610. [Google Scholar] [CrossRef]
- Qureshi, F.; Tahir, M. Photoelectrochemical water splitting with engineering aspects for hydrogen production: Recent advances, strategies and challenges. Int. J. Hydrogen Energy 2024, 69, 760–776. [Google Scholar] [CrossRef]
- Li, K.; Dong, W.J.; Mi, Z. Photoelectrochemical water splitting under concentrated sunlight: Best practices and protocols. Front. Energy Res. 2025, 13, 1550153. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, J.; Pihosh, Y.; Karmakar, K.; Zhang, B.; Domen, K.; Li, Y. Interface engineering for photoelectrochemical oxygen evolution reaction. Chem. Soc. Rev. 2025, 54, 1268–1317. [Google Scholar] [CrossRef]
- Schichtl, Z.G.; Carvalho, O.Q.; Tan, J.; Saund, S.S.; Ghoshal, D.; Wilder, L.M.; Gish, M.K.; Nielander, A.C.; Stevens, M.B.; Greenaway, A.L. Chemistry of Materials Underpinning Photoelectrochemical Solar Fuel Production. Chem. Rev. 2025, 125, 4768–4839. [Google Scholar] [CrossRef]
- Teh, I.J.; Putri, L.K.; Yaw, C.S.; Ng, W.C.; Chong, M.N.; Chai, S.-P. Engineering high-performance BiVO4 homo- and heterojunction Photoanodes for solar-driven Photoelectrochemical water splitting applications. Coord. Chem. Rev. 2025, 541, 216773. [Google Scholar] [CrossRef]
- Dong, G.; Yan, L.; Bi, Y. Advanced oxygen evolution reaction catalysts for solar-driven photoelectrochemical water splitting. J. Mater. Chem. A 2023, 11, 3888–3903. [Google Scholar] [CrossRef]
- Tu, J.; Li, J.; Pan, Z.; Zhu, X.; Ye, D.; Yang, Y.; Wang, H.; An, L.; Chen, R.; Liao, Q. Nitrogen-doped CdS/TiO2 nanorods heterojunction photoanode for efficient and stable photoelectrochemical water splitting. J. Power Sources 2025, 628, 235883. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-Light-Activated TiO2-Based Photocatalysts for the Inactivation of Pathogenic Bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Chauke, N.M.; Ngqalakwezi, A.; Raphulu, M. Transformative advancements in visible-light-activated titanium dioxide for industrial wastewater remediation. Int. J. Environ. Sci. Technol. 2025, 22, 8521–8552. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Pei, Y.; Han, W. Enhancing Photoelectrocatalytic Efficiency of BiVO4 Photoanodes by Crystal Orientation Control. Nanomaterials 2024, 14, 1870. [Google Scholar] [CrossRef]
- Wang, T.; Song, K.; Liu, H.; Li, H.; Zhang, Y.; Ren, W.; Zhang, R.; Li, K.; He, F.; Qin, Z.; et al. In-situ surface reconstruction of BiVO4/CuFe2O4 photoanode for efficient and robust solar water oxidation. Chem. Eng. J. 2025, 509, 161333. [Google Scholar] [CrossRef]
- Saqib, M.; Ali, S.; Khan, S.A.; Khan, A.M. The effect of simultaneous 3d transition bimetal doping (Ni/Mn, Co/Mn, Ni/Co) on the structural, optical and photocatalytic properties of ZnO based materials. Colloids Surf. A Physicochem. Eng. Asp. 2025, 724, 137459. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Ren, J.; Murdoch, B.J.; Lipton-Duffin, J.; Macleod, J.M.; Gómez, D.E.; van Embden, J.; Della Gaspera, E. Doping and Annealing Conditions Strongly Influence the Water Oxidation Performance of Hematite Photoanodes. ACS Appl. Mater. Interfaces 2025, 17, 32635–32645. [Google Scholar] [CrossRef]
- Chatterjee, P.; Piecha, D.; Kotarba, S.; Syrek, K.; Pisarek, M.; Sulka, G.D. Hydrothermal Surface Engineering of Anodic WO3 Photoelectrode by Simultaneous Iron Doping and Fe3O4/FeWO4 Formation. ACS Appl. Mater. Interfaces 2025, 17, 30284–30296. [Google Scholar] [CrossRef] [PubMed]
- Milon, A.M.; Usha, D.; Ashwin, B.M.; Dennison, M.S. Integrating microwave-assisted green synthesis, DFT simulations, and biological activity evaluation of copper-doped zinc oxide nanoparticles. Sci. Rep. 2025, 15, 19348. [Google Scholar] [CrossRef]
- Tajima, M.; Nagai, Y.; Chen, S.; Pan, Z.; Katayama, K. A robust methodology for PEC performance analysis of photoanodes using machine learning and analytical data. Analyst 2024, 149, 4193–4207. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Hu, W.; Zhang, L. Data-driven optimization and machine learning analysis of compatible molecules for halide perovskite material. npj Comput. Mater. 2024, 10, 114. [Google Scholar] [CrossRef]
- Wang, D.; Zan, R.; Zhu, X.; Zhang, Y.; Wang, Y.; Gu, Y.; Li, Y. A machine learning-assisted study of the formation of oxygen vacancies in anatase titanium dioxide. RSC Adv. 2024, 14, 33198–33205. [Google Scholar] [CrossRef]
- Park, S.; Lee, N.; Park, J.O.; Park, J.; Heo, Y.S.; Lee, J. Exploring the Latent Chemical Space of Oxygen Vacancy Formation Energy by a Machine Learning Ensemble. ACS Mater. Lett. 2024, 6, 66–72. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Feng, T.; Xiong, F.; Wang, J.; Wang, Y.; Wang, Z. Tripartite interaction representation algorithm for crystal graph neural networks. Sci. Rep. 2024, 14, 24881. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Grossman, J. Crystal Graph Convolutional Neural Networks for Accurate and Interpretable Prediction of Material Properties. Phys. Rev. Lett. 2017, 120, 145301. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Zheng, L.; Hou, J.; Zheng, H.; Sun, S.; Wang, L. Machine Learning Guided Dopant Selection for Metal Oxide-Based Photoelectrochemical Water Splitting: The Case Study of Fe2O3 and CuO. Adv. Mater. 2022, 34, 2106776. [Google Scholar] [CrossRef]
- Li, X.; Che, Y.; Chen, L.; Liu, T.; Wang, K.; Liu, L.; Yang, H.; Pyzer-Knapp, E.O.; Cooper, A.I. Sequential closed-loop Bayesian optimization as a guide for organic molecular metallophotocatalyst formulation discovery. Nat. Chem. 2024, 16, 1286–1294. [Google Scholar] [CrossRef]
- Kumar, A.; Pant, K.K.; Upadhyayula, S.; Kodamana, H. Multiobjective Bayesian Optimization Framework for the Synthesis of Methanol from Syngas Using Interpretable Gaussian Process Models. ACS Omega 2023, 8, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Hoar, B.B.; Lu, S.; Liu, C. Machine learning-based inverse design for electrochemically controlled microscopic gradients of O2 and H2O2. Proc. Natl. Acad. Sci. USA 2022, 119, e2206321119. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Bobadilla, A.V.; Schmitt, V.; Maier, C.S.; Mensing, S.; Stodtmann, S. Practical guide to SHAP analysis: Explaining supervised machine learning model predictions in drug development. Clin. Transl. Sci. 2024, 17, e70056. [Google Scholar] [CrossRef]
- Wu, Y.; Sicard, B.; Gadsden, S.A. Physics-informed machine learning: A comprehensive review on applications in anomaly detection and condition monitoring. Expert Syst. Appl. 2024, 255, 124678. [Google Scholar] [CrossRef]
- Zhong, X.; Gallagher, B.; Liu, S.; Kailkhura, B.; Hiszpanski, A.; Han, T.Y.-J. Explainable machine learning in materials science. npj Comput. Mater. 2022, 8, 204. [Google Scholar] [CrossRef]
- Oviedo, F.; Ferres, J.L.; Buonassisi, T.; Butler, K.T. Interpretable and Explainable Machine Learning for Materials Science and Chemistry. Acc. Mater. Res. 2022, 3, 597–607. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Chen, T.-W.; Ramachandran, R.; Chen, S.-M.; Anushya, G.; Al-Sehemi, A.G.; Mariyappan, V.; Alargarsamy, S.; Alam, M.M.; Mahesh, T.C.; Kalimuthu, P.; et al. An overview of semiconductor electrode materials for photoelectrochemical water splitting and CO2 conversion. Int. J. Electrochem. Sci. 2024, 19, 100542. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Jha, B.K.; Chaule, S.; Jang, J.-H. Enhancing photocatalytic efficiency with hematite photoanodes: Principles, properties, and strategies for surface, bulk, and interface charge transfer improvement. Mater. Chem. Front. 2024, 8, 2197–2226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Byun, W.J.; Wang, X.; Shin, T.J.; Jeong, H.Y.; Han, H.; Li, C.; Lee, J.S. Gradient tantalum-doped hematite homojunction photoanode improves both photocurrents and turn-on voltage for solar water splitting. Nat. Commun. 2020, 11, 4622. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-Doped Hematite Nanostructures for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; Han, L.; Smets, A.H.M.; Zeman, M.; Dam, B.; van de Krol, R. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 2013, 4, 2195. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Zhang, B.; Bi, Y. High-performance and stable BiVO4 photoanodes for solar water splitting via phosphorus-oxygen bonded FeNi catalysts. Energy Environ. Sci. 2022, 15, 2867–2873. [Google Scholar] [CrossRef]
- Ma, Z.; Song, K.; Lin, W.; Gao, F.H.; Tang, B.; Yang, W. WO3/BiVO4 Type-II Heterojunction Arrays Decorated with Oxygen-deficient ZnO Passivation Layer: A Highly Efficient and Stable Photoanode. ACS Appl. Mater. Interfaces 2018, 11, 889–897. [Google Scholar] [CrossRef]
- Sharma, L.; Minhová Macounová, K.; Nebel, R.; Krtil, P. The Impact of Composition on the Photoelectrochemical Performance of Molybdenum-Modified Tungsten Oxide in Acidic Media. Electrocatalysis 2025, 16, 451–461. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Noh, Y.-G.; Seo, H. Engineering band edge properties of WO3 with respect to photoelectrochemical water splitting potentials via a generalized doping protocol of first-row transition metal ions. Appl. Surf. Sci. 2020, 509, 145253. [Google Scholar] [CrossRef]
- Wang, F.; Valentin, C.; Pacchioni, G. Doping of WO3 for Photocatalytic Water Splitting: Hints from Density Functional Theory. J. Phys. Chem. C 2012, 116, 8901–8909. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef]
- Nomellini, C.; Polo, A.; Mesa, C.; Pastor, E.; Marra, G.; Grigioni, I.; Dozzi, M.; Giménez, S.; Selli, E. Improved Photoelectrochemical Performance of WO3/BiVO4 Heterojunction Photoanodes via WO3 Nanostructuring. ACS Appl. Mater. Interfaces 2023, 15, 52436–52447. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Lee, S.A.; Suh, J.M.; Hong, S.-P.; Jang, H.W. Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Appl. Sci. 2018, 8, 1765. [Google Scholar] [CrossRef]
- Thirumalaisamy, L.; Wei, Z.; Davies, K.R.; Allan, M.G.; McGettrick, J.; Watson, T.; Kuehnel, M.F.; Pitchaimuthu, S. Dual Shield: Bifurcated Coating Analysis of Multilayered WO3/BiVO4/TiO2/NiOOH Photoanodes for Sustainable Solar-to-Hydrogen Generation from Challenging Waters. ACS Sustain. Chem. Eng. 2024, 12, 3044–3060. [Google Scholar] [CrossRef]
- Abdullah, R.; Jalil, A.A.; Asmadi, M.; Hassan, N.S.; Bahari, M.B.; Alhassan, M.; Izzudin, N.M.; Sawal, M.H.; Saravanan, R.; Karimi-Maleh, H. Recent advances in zinc oxide-based photoanodes for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2025, 107, 183–207. [Google Scholar] [CrossRef]
- El ouardi, M.; Idrissi, A.E.; Ahsaine, H.A.; BaQais, A.; Saadi, M.; Arab, M. Current advances on nanostructured oxide photoelectrocatalysts for water splitting: A comprehensive review. Surf. Interfaces 2024, 45, 103850. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.; Xu, X.; Zhou, W.; Shao, Z. Perovskite Oxide Based Electrodes for High-Performance Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, S.; Wu, H.; Wang, Z.; Xu, S.; Wei, M.; Duan, X.; Su, J.; Cui, J. Rational design of nanostructured BiVO4/FeOx photoanode coupling with 2D Co(OH)2 cocatalyst for enhanced photoelectrochemical water splitting. Mater. Sci. Semicond. Process. 2024, 170, 107952. [Google Scholar] [CrossRef]
- Idei, T.; Nagai, Y.; Pan, Z.; Katayama, K. Identification of the Contributing Factors to the Photoelectric Conversion Efficiency for Hematite Photoanodes by Using Machine Learning. ACS Appl. Mater. Interfaces 2023, 15, 55644–55651. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Batra, R.; Song, L.; Ramprasad, R. Emerging materials intelligence ecosystems propelled by machine learning. Nat. Rev. Mater. 2021, 6, 655–678. [Google Scholar] [CrossRef]
- Mai, H.; Le, T.C.; Chen, D.; Winkler, D.A.; Caruso, R.A. Machine Learning for Electrocatalyst and Photocatalyst Design and Discovery. Chem. Rev. 2022, 122, 13478–13515. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nagai, Y.; Pan, Z.; Katayama, K. Convolutional neural network prediction of the photocurrent–voltage curve directly from scanning electron microscopy images. J. Mater. Chem. A 2023, 11, 22522–22532. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Tran, K.; Ulissi, Z.W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1, 696–703. [Google Scholar] [CrossRef]
- Pilania, G.; Mannodi-Kanakkithodi, A.; Uberuaga, B.P.; Ramprasad, R.; Gubernatis, J.E.; Lookman, T. Machine learning bandgaps of double perovskites. Sci. Rep. 2016, 6, 19375. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Dey, S.; Pattanayak, P.; Singh, T. Design of ternary metal oxides for photoelectrochemical water splitting using machine learning techniques. J. Environ. Chem. Eng. 2025, 13, 115260. [Google Scholar] [CrossRef]
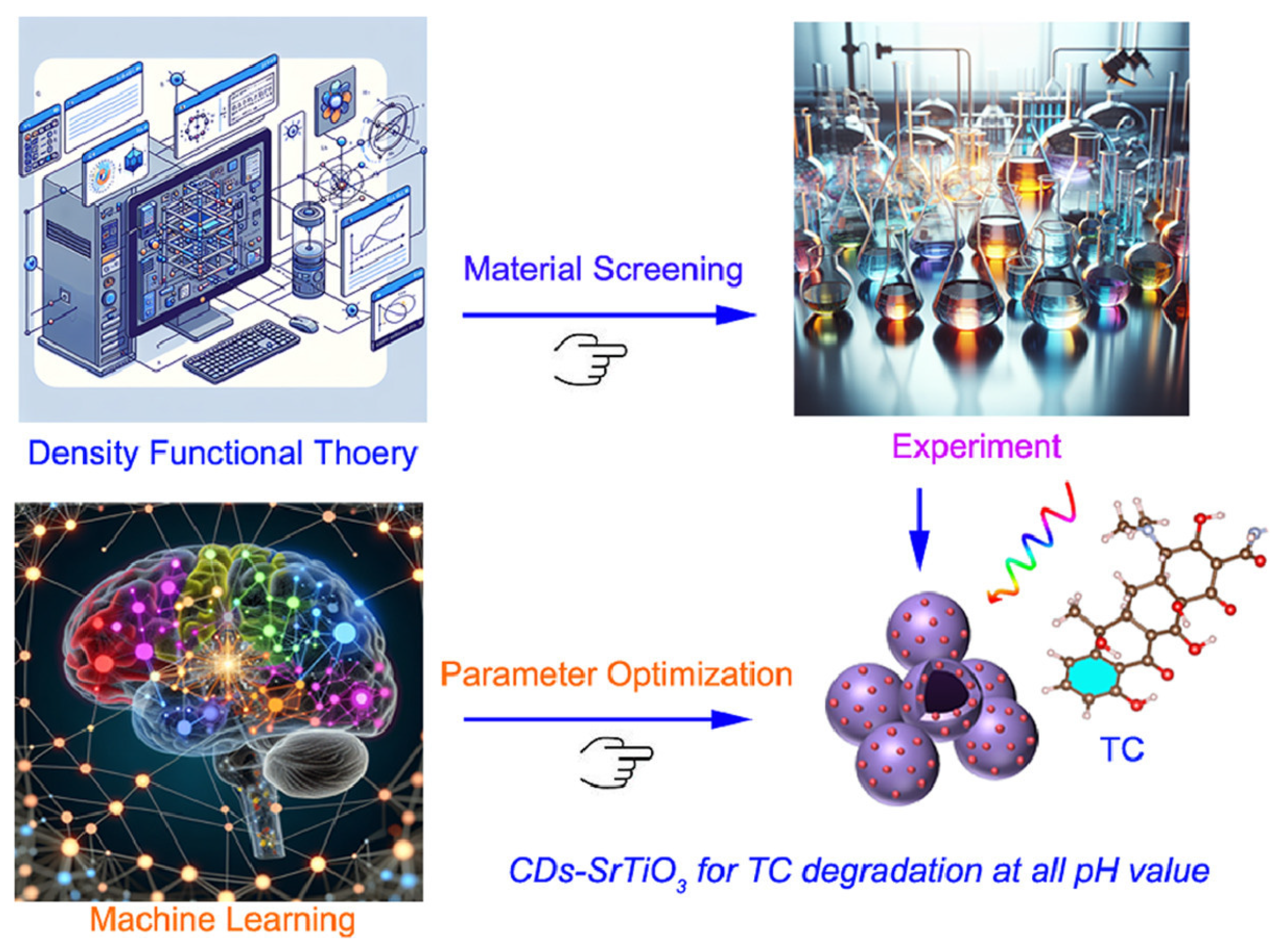
- Wang, L.; Yang, T.; Wei, M.; Guan, R.; Wei, W.; Jiang, J. Machine learning and DFT dual-guidance of carbon dots implanted SrTiO3 hollow nanosphere for efficient all-pH-value photocatalysis. J. Mater. Sci. Technol. 2025, 217, 169–181. [Google Scholar] [CrossRef]
- Oral, B.; Can, E.; Yildirim, R. Analysis of photoelectrochemical water splitting using machine learning. Int. J. Hydrogen Energy 2022, 47, 19633–19654. [Google Scholar] [CrossRef]
- Sunderraj, N.; Dhanushkodi, S.R.; Chidambaram, R.K.; Węglowski, B.; Skrzyniowska, D.; Schmid, M.; Fowler, M.W. Development of Semi-Empirical and Machine Learning Models for Photoelectrochemical Cells. Energies 2024, 17, 5313. [Google Scholar] [CrossRef]
- MacLeod, B.P.; Parlane, F.G.L.; Morrissey, T.D.; Häse, F.; Roch, L.M.; Dettelbach, K.E.; Moreira, R.; Yunker, L.P.E.; Rooney, M.B.; Deeth, J.R.; et al. Self-driving laboratory for accelerated discovery of thin-film materials. Sci. Adv. 2020, 6, eaaz8867. [Google Scholar] [CrossRef]
- Gutkowski, R.; Khare, C.; Conzuelo, F.; Kayran, Y.U.; Ludwig, A.; Schuhmann, W. Unraveling compositional effects on the light-induced oxygen evolution in Bi(V–Mo–X)O4 material libraries. Energy Environ. Sci. 2017, 10, 1213–1221. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhang, J.Y.; Liaw, P.K.; Yang, T. Machine Learning-Based Computational Design Methods for High-Entropy Alloys. High Entropy Alloys Mater. 2025. [Google Scholar] [CrossRef]
- Nasejje, S.; Mukhokosi, E.P.; Diale, M.; Velauthapillai, D. Device architectures for photoelectrochemical water splitting based on hematite: A review. Discov. Mater. 2024, 4, 44. [Google Scholar] [CrossRef]
- Corby, S.; Rao, R.R.; Steier, L.; Durrant, J.R. The kinetics of metal oxide photoanodes from charge generation to catalysis. Nat. Rev. Mater. 2021, 6, 1136–1155. [Google Scholar] [CrossRef]
- Shoda, H.; Pan, Z.; Sohn, W.Y.; Katayama, K. Insight into Charge Transport Behaviors in Hematite Photoanodes via Potential-Dependent Impedance Spectroscopy and Machine Learning. ACS Appl. Mater. Interfaces. 2025, 17, 7830–7837. [Google Scholar] [CrossRef]
- Idei, T.; Nagai, Y.; Pan, Z.; Katayama, K. Tailoring Hematite Photoanodes for Improved PEC Performance: The Role of Alcohol Species Revealed by SHAP Analysis. ACS Omega 2024, 9, 44837–44845. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yang, W.; Gao, J.; Ke, S.; Zhao, Z.; Wen, G. Construction of active sites within hematite via S and Cu co-doping for boosting peroxymonosulfate activation toward ciprofloxacin degradation. J. Environ. Chem. Eng. 2025, 13, 117301. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Li, J.; Liu, X.; Ma, Y.; Yao, Y.; Zhang, J.; Pan, L. Machine learning-accelerated exploration on element doping-triggering material performance improvement for energy conversion and storage applications. J. Mater. Chem. A 2025, 13, 17197–17213. [Google Scholar] [CrossRef]
- Xue, D.; Balachandran, P.V.; Hogden, J.; Theiler, J.; Xue, D.; Lookman, T. Accelerated search for materials with targeted properties by adaptive design. Nat. Commun. 2016, 7, 11241. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nagai, Y.; Pan, Z.; Katayama, K. Identification of dominant factors contributing to photocurrent density of BiVO4 photoanodes using Machine learning. J. Photochem. Photobiol. A Chem. 2023, 440, 114651. [Google Scholar] [CrossRef]
- Rumman, A.H.; Sahriar, M.A.; Islam, M.T.; Shorowordi, K.M.; Carbonara, J.; Broderick, S.; Ahmed, S. Data-driven design for enhanced efficiency of Sn-based perovskite solar cells using machine learning. APL Mach. Learn. 2023, 1, 046117. [Google Scholar] [CrossRef]
- Okunaka, S.; Hitomi, Y.; Tokudome, H. Green fabrication of nanoporous BiVO4 films on ITO substrates for photoelectrochemical water-oxidation. RSC Adv. 2018, 8, 31575–31580. [Google Scholar] [CrossRef]
- Xiao, B.-C.; Lin, L.-Y.; Hong, J.-Y.; Lin, H.-S.; Song, Y.-T. Synthesis of a monoclinic BiVO4 nanorod array as the photocatalyst for efficient photoelectrochemical water oxidation. RSC Adv. 2017, 7, 7547–7554. [Google Scholar] [CrossRef]
- Kondo, R.; Yamakawa, S.; Masuoka, Y.; Tajima, S.; Asahi, R. Microstructure recognition using convolutional neural networks for prediction of ionic conductivity in ceramics. Acta Mater. 2017, 141, 29–38. [Google Scholar] [CrossRef]
- Zhang, L.; Bing, Q.; Qin, H.; Yu, L.; Li, H.; Deng, D. Artificial intelligence for catalyst design and synthesis. Matter 2025, 8, 102138. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, H.; Zhang, B.; Yan, D.; Xiang, X. Surface fluorination of BiVO4 for the photoelectrochemical oxidation of glycerol to formic acid. Nat. Commun. 2024, 15, 8155. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Jiang, Y.; Ding, Y.; Jia, B.; Liu, R.; Li, P.; Yang, W.; Xia, L.; Sun, L.; Zhang, B. Surface Reconstruction and Passivation of BiVO4 Photoanodes Depending on the “Structure Breaker” Cs+. JACS Au 2023, 3, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Beker, W.; Siek, M.; Kim, J.; Lee, H.S.; Hyeon, T.; Grzybowski, B.A. Active learning guides discovery of a champion four-metal perovskite oxide for oxygen evolution electrocatalysis. Nat. Mater. 2024, 23, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Liu, J.; Hua, K.; Wang, X.; Zhang, X.; Shao, M.; Chen, Y.; Chen, J. Leveraging data mining, active learning, and domain adaptation for efficient discovery of advanced oxygen evolution electrocatalysts. Sci. Adv. 2025, 11, eadr9038. [Google Scholar] [CrossRef]
- Sakaushi, K.; Hoisang, W.; Tamura, R. Human–Machine Collaboration for Accelerated Discovery of Promising Oxygen Evolution Electrocatalysts with On-Demand Elements. ACS Cent. Sci. 2023, 9, 2216–2224. [Google Scholar] [CrossRef]
- Löffler, T.; Ludwig, A.; Rossmeisl, J.; Schuhmann, W. What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew. Chem. Int. Ed. 2021, 60, 26894–26903. [Google Scholar] [CrossRef]
- Peng, J.; Damewood, J.K.; Karaguesian, J.; Gómez-Bombarelli, R.; Shao-Horn, Y. Navigating multimetallic catalyst space with Bayesian optimization. Joule 2021, 5, 3069–3071. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Sahu, N.; Azad, C.; Kumar, U. Study and prediction of photocurrent density with external validation using machine learning models. Int. J. Hydrogen Energy 2024, 92, 1335–1355. [Google Scholar] [CrossRef]
- Wahab, H.; Heil, J.; Tyrrell, A.S.; Muller, T.; Ackerman, J.; Kotthoff, L.; Johnson, P.A. Optimization of structural and electrical properties of graphene-based TiO2 thin film device using Bayesian machine-learning approach. Ceram. Int. 2024, 50, 9114–9124. [Google Scholar] [CrossRef]
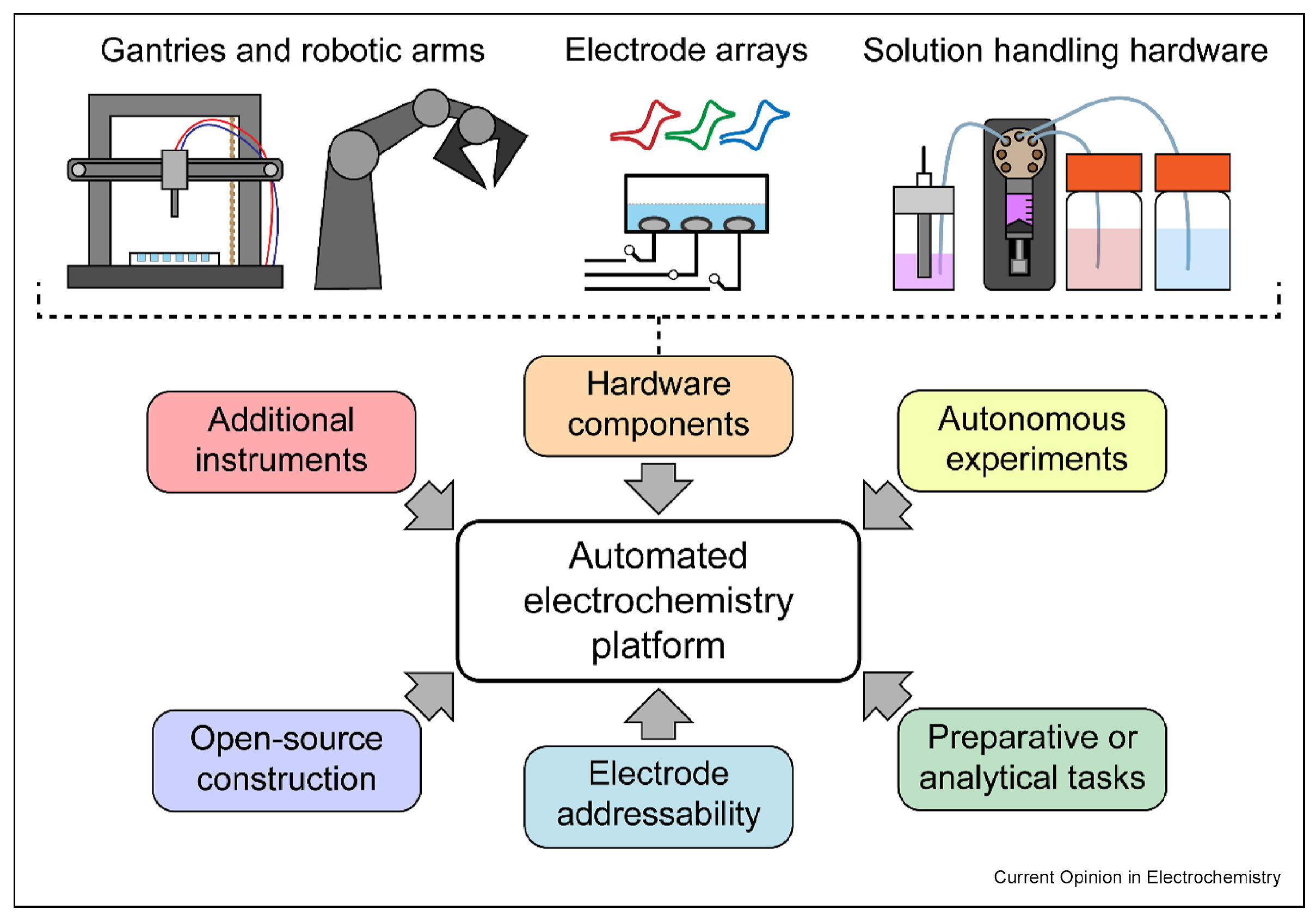
- Kormányos, A.; Jenewein, K.J.; Cherevko, S. High-throughput workflows in the service of (photo)electrocatalysis research. Trends Chem. 2022, 4, 475–478. [Google Scholar] [CrossRef]
- Pence, M.A.; Hazen, G.; Rodríguez-López, J. The emergence of automation in electrochemistry. Curr. Opin. Electrochem. 2025, 51, 101679. [Google Scholar] [CrossRef]
- Diaby, M.; Alimi, A.; Bardaoui, A.; Santos, D.M.F.; Chtourou, R.; Ben Assaker, I. Correlation between the Experimental and Theoretical Photoelectrochemical Response of a WO3 Electrode for Efficient Water Splitting through the Implementation of an Artificial Neural Network. Sustainability 2023, 15, 11751. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Chao, L.; Chen, Y.; Huang, W.; Chen, G. Perovskite solar cells empowered by machine learning. J. Energy Chem. 2025, 109, 403–437. [Google Scholar] [CrossRef]
- Haussener, S.; Hu, S.; Xiang, C.; Weber, A.Z.; Lewis, N.S. Simulations of the irradiation and temperature dependence of the efficiency of tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 2013, 6, 3605–3618. [Google Scholar] [CrossRef]
- Vilanova, A.; Lopes, T.; Spenke, C.; Wullenkord, M.; Mendes, A. Optimized photoelectrochemical tandem cell for solar water splitting. Energy Storage Mater. 2018, 13, 175–188. [Google Scholar] [CrossRef]
- Seger, B.; Castelli, I.E.; Vesborg, P.C.K.; Jacobsen, K.W.; Hansen, O.; Chorkendorff, I. 2-Photon tandem device for water splitting: Comparing photocathode first versus photoanode first designs. Energy Environ. Sci. 2014, 7, 2397–2413. [Google Scholar] [CrossRef]
- Hu, S.; Xiang, C.; Haussener, S.; Berger, A.D.; Lewis, N.S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 2013, 6, 2984–2993. [Google Scholar] [CrossRef]
- Xu, P.; Ji, X.; Li, M.; Lu, W. Small data machine learning in materials science. npj Comput. Mater. 2023, 9, 42. [Google Scholar] [CrossRef]
- Meredig, B.; Agrawal, A.; Kirklin, S.; Saal, J.E.; Doak, J.W.; Thompson, A.; Zhang, K.; Choudhary, A.; Wolverton, C. Combinatorial screening for new materials in unconstrained composition space with machine learning. Phys. Rev. B 2014, 89, 094104. [Google Scholar] [CrossRef]
| ML Approach Type | Representative Algorithms | Application Stage |
|---|---|---|
| Supervised Learning | Random Forests, SVM, Neural Networks | Property prediction, dopant selection, J–V modeling |
| Unsupervised Learning | K-means, PCA, Clustering methods | Data pattern discovery, material classification |
| Bayesian Optimization | Gaussian Process Regression + Acquisition Functions | Composition/morphology optimization |
| Physics-Informed ML | Physics-constrained Neural Networks, Hybrid DFT-ML | Predictive modeling with physical priors |
| Image-based Learning | CNN, Vision Transformers | Morphology-property correlation from SEM images |
| ML Type | Input Features | Dataset Size | Target Property | Validation Method | Performance Metrics |
|---|---|---|---|---|---|
| CNN (image-based) | SEM images | ~100 samples | Full J–V curve | Train/test split | R2 ≈ 0.95 |
| ANN (structured data) | Doping level, bath pH, catalyst presence | 297 samples | Photocurrent @ V_bias | K-fold CV (k = 5) | MAE ≈ 0.03 mA·cm−2 |
| Random Forest | Dopant ion radius, formation energy | 85 dopants | ΔPhotocurrent (Fe2O3) | Train/test split | R2 ≈ 0.89 |
| Decision Tree Classifier | Bandgap, effective mass, DFT features | ~1000 compounds | Activity classification | Accuracy metrics | Accuracy ≈ 81% |
| Random Forest | Literature-derived structure/morphology | 10,000+ records | Bandgap classification | Confusion matrix | Accuracy ≈ 85% |
| ANN | Annealing temp, thickness, pH | ~150 samples | Photocurrent profile | Bayesian optimization | Relative Error < 0.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Yu, S.; Meng, B.; Ju, Y.; Wang, S.; Wang, Y. Machine-Learning-Guided Design of Nanostructured Metal Oxide Photoanodes for Photoelectrochemical Water Splitting: From Material Discovery to Performance Optimization. Nanomaterials 2025, 15, 948. https://doi.org/10.3390/nano15120948
Liang X, Yu S, Meng B, Ju Y, Wang S, Wang Y. Machine-Learning-Guided Design of Nanostructured Metal Oxide Photoanodes for Photoelectrochemical Water Splitting: From Material Discovery to Performance Optimization. Nanomaterials. 2025; 15(12):948. https://doi.org/10.3390/nano15120948
Chicago/Turabian StyleLiang, Xiongwei, Shaopeng Yu, Bo Meng, Yongfu Ju, Shuai Wang, and Yingning Wang. 2025. "Machine-Learning-Guided Design of Nanostructured Metal Oxide Photoanodes for Photoelectrochemical Water Splitting: From Material Discovery to Performance Optimization" Nanomaterials 15, no. 12: 948. https://doi.org/10.3390/nano15120948
APA StyleLiang, X., Yu, S., Meng, B., Ju, Y., Wang, S., & Wang, Y. (2025). Machine-Learning-Guided Design of Nanostructured Metal Oxide Photoanodes for Photoelectrochemical Water Splitting: From Material Discovery to Performance Optimization. Nanomaterials, 15(12), 948. https://doi.org/10.3390/nano15120948






