Density-Based Characterization of Microplastics via Cross-Halbach Magnetic Levitation
Abstract
1. Introduction
2. Materials and Methods
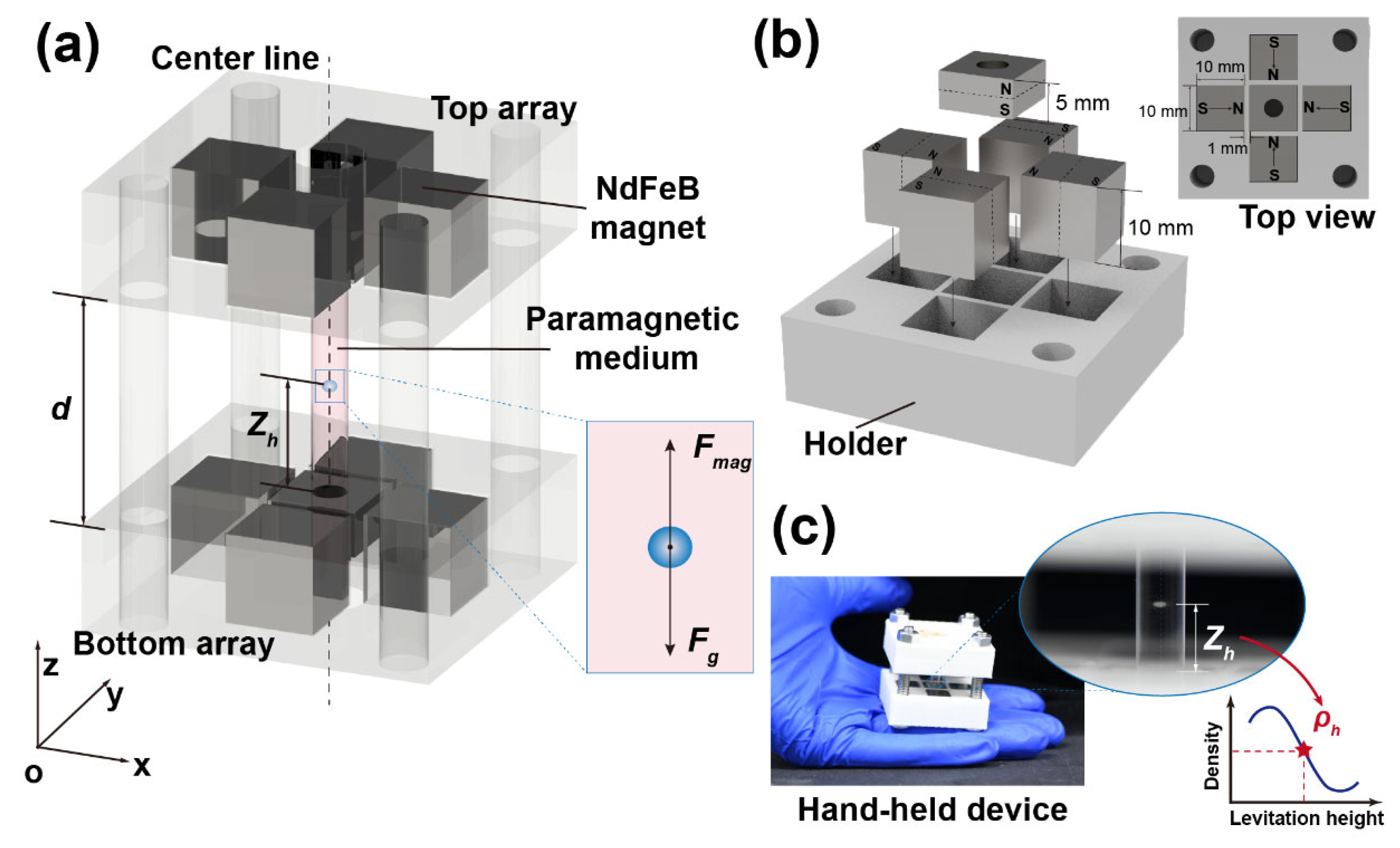
2.1. Design of the Cross-Halbach MagLev Device
2.2. Distribution of Equilibrium Position in Cross-Halbach MagLev
2.3. Methods for Density Measuring Process
3. Results and Discussion
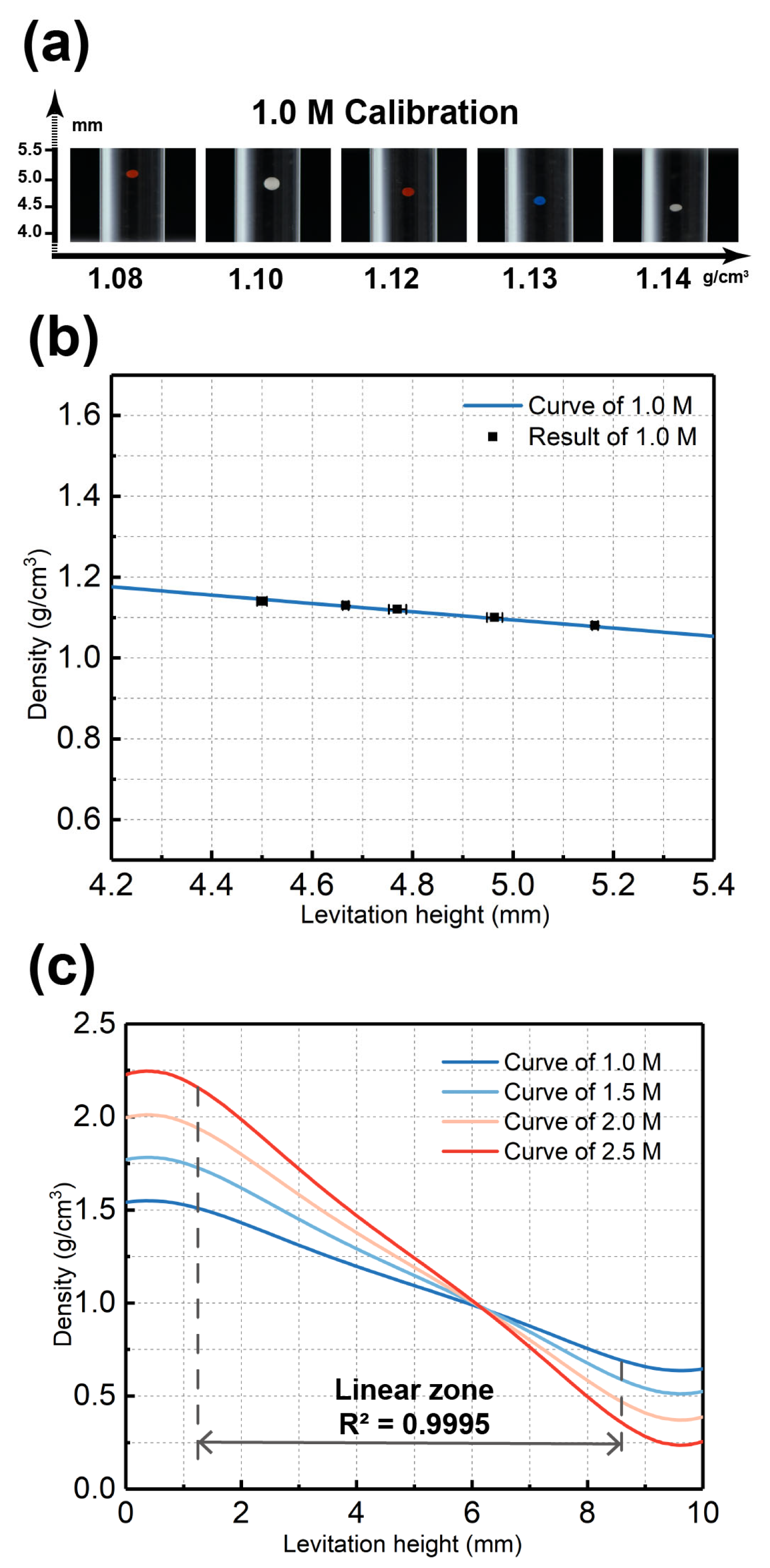
3.1. Verification and Calibration of the MagLev Device
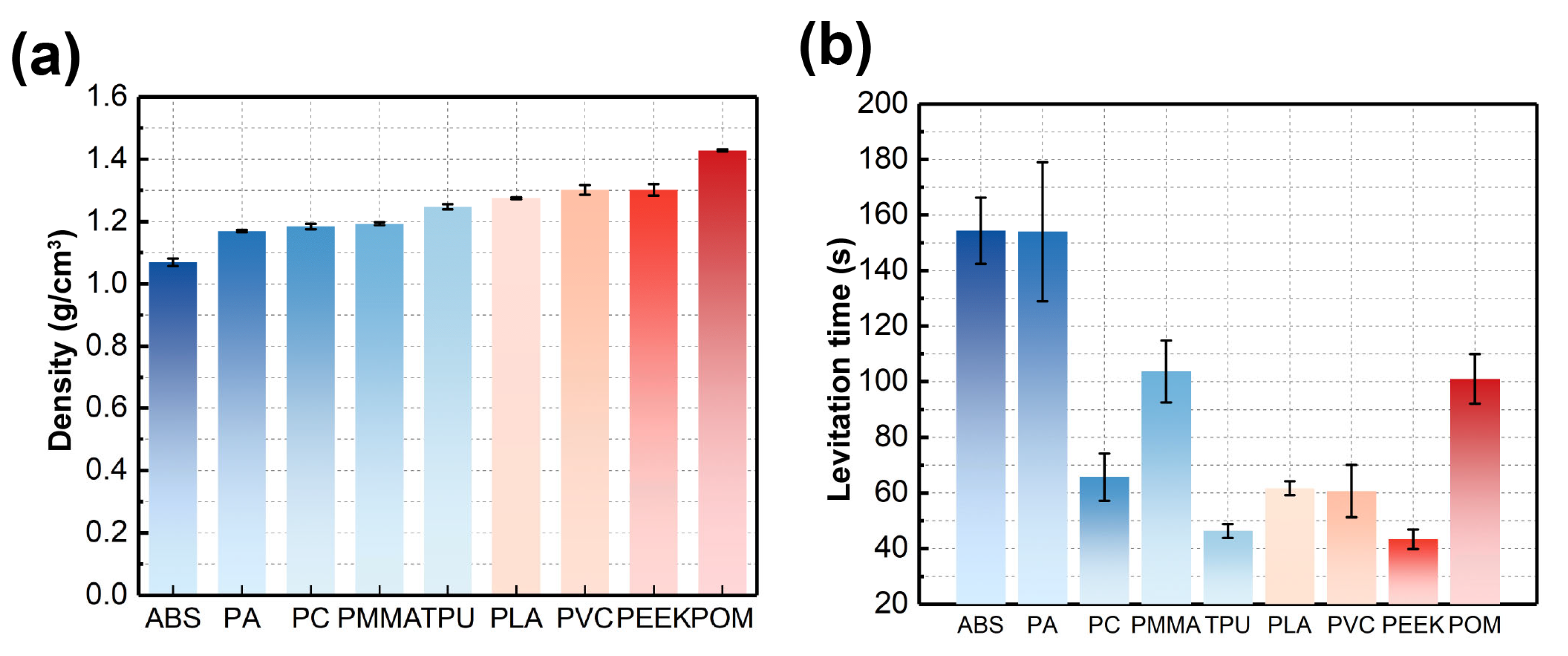
3.2. Density Characterization of Microplastics
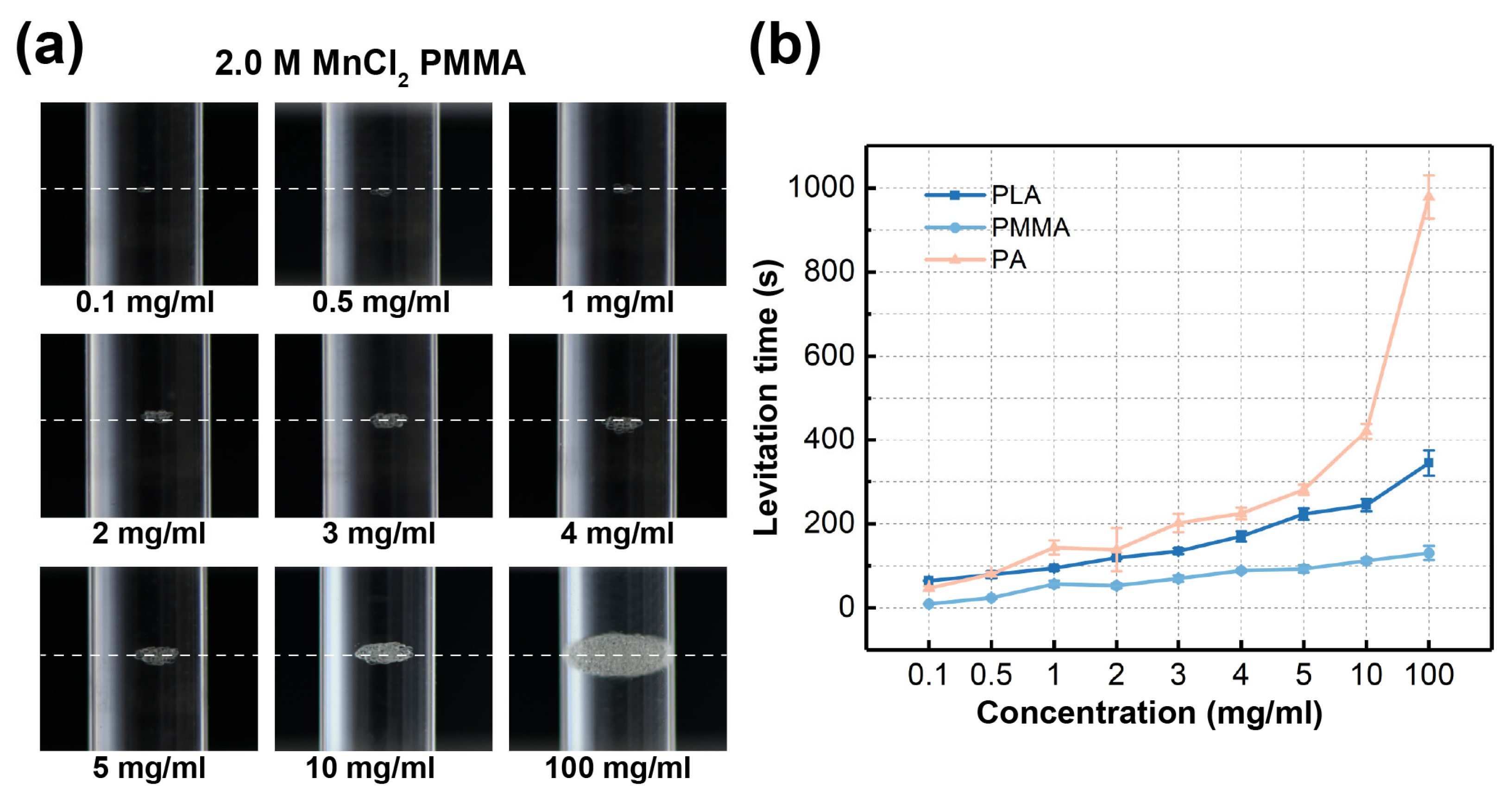
3.3. Density Measurement of Altering Concentrations
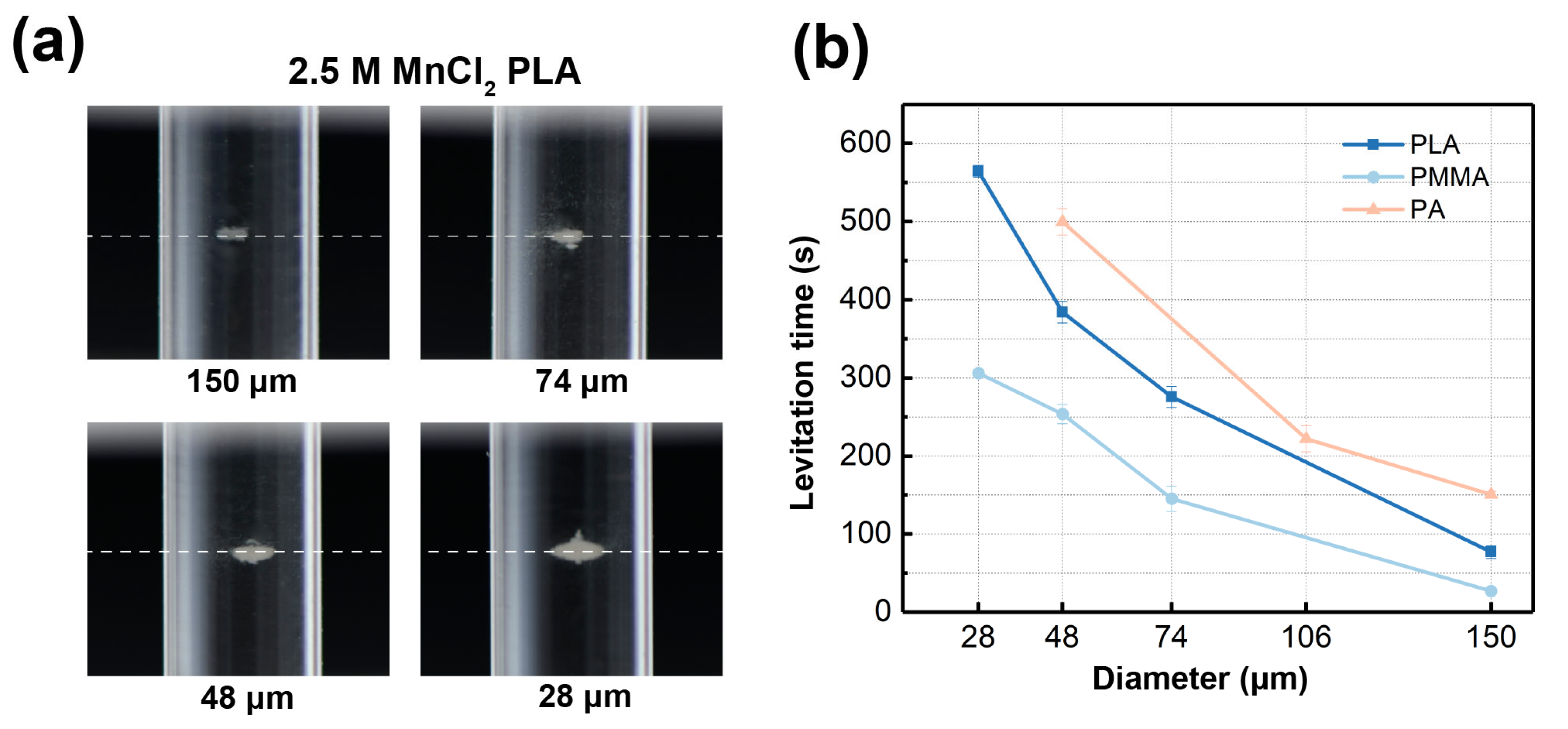
3.4. Density Measurement of Altering Particle Diameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research-What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Chiba, S.; Saito, H.; Fletcher, R.; Yogi, T.; Kayo, M.; Miyagi, S.; Ogido, M.; Fujikura, K. Human Footprint in the Abyss: 30 Year Records of Deep-Sea Plastic Debris. Mar. Policy 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of Atmospheric Transport for Microplastics Deposited in Remote Areas. Environ. Pollut. 2019, 254, 112953. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of Plastic Microbeads in Facial Scrubs and Their Estimated Emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The Impact of Debris on Marine Life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification Methods in Microplastic Analysis: A Review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-Term Aging and Degradation of Microplastic Particles: Comparing in Situ Oceanic and Experimental Weathering Patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Mirica, K.A.; Shevkoplyas, S.S.; Phillips, S.T.; Gupta, M.; Whitesides, G.M. Measuring Densities of Solids and Liquids Using Magnetic Levitation: Fundamentals. J. Am. Chem. Soc. 2009, 131, 10049–10058. [Google Scholar] [CrossRef]
- Ren, X.; Breadmore, M.C.; Maya, F. Bidimensional Dynamic Magnetic Levitation: Sequential Separation of Microplastics by Density and Size. Anal. Chem. 2024, 96, 3259–3266. [Google Scholar] [CrossRef]
- Ren, X.; Breadmore, M.C.; Maya, F. Biphasic Magnetic Levitation to Detect Organic Pollutants on Microplastics. Anal. Chem. 2022, 94, 9033–9039. [Google Scholar] [CrossRef]
- Bernstein, P.; Noudem, J. Superconducting Magnetic Levitation: Principle, Materials, Physics and Models. Supercond. Sci. Technol. 2020, 33, 033001. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, C.; Gu, F.; Wang, Y.; Fu, J.; Zhao, P. An Accurate and Versatile Density Measurement Device: Magnetic Levitation. Sens. Actuators B Chem. 2019, 295, 204–214. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, P.; Gu, F.; Zhang, X.; Xie, J.; He, Y.; Zhou, H.; Fu, J.; Turng, L.S. Axial-Circular Magnetic Levitation: A Three-Dimensional Density Measurement and Manipulation Approach. Anal. Chem. 2020, 92, 6925–6931. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xie, J.; Zhang, J.; Zhang, C.; Xia, N.; Fu, J. Evaluation of Polymer Injection Molded Parts via Density-Based Magnetic Levitation. J. Appl. Polym. Sci. 2020, 137, 48431. [Google Scholar] [CrossRef]
- Gao, Q.H.; Yan, G.; Zou, H.X.; Zhang, W.M.; Peng, Z.K.; Meng, G. Density-Based Measurement and Manipulation via Magnetic Levitation Enhanced by the Dual-Halbach Array. IEEE Sens. J. 2020, 20, 1730–1737. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, P.; Tang, D.; Xia, N.; Zhang, X.; Nie, J.; Gu, F.; Zhou, H.; Fu, J. Axial Magnetic Levitation: A High-Sensitive and Maneuverable Density-Based Analysis Device. Sens. Actuators B Chem. 2020, 304, 127362. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, P.; Zhang, J.; Zhou, H.; Fu, J.; Turng, L.S. Characterization of Polymer Materials Using Magnetic Levitation. J. Mater. Res. 2020, 35, 1182–1189. [Google Scholar] [CrossRef]
- Zhao, P.; Xie, J.; Gu, F.; Sharmin, N.; Hall, P.; Fu, J. Separation of Mixed Waste Plastics via Magnetic Levitation. Waste Manag. 2018, 76, 46–54. [Google Scholar] [CrossRef]
- Velazquez, S.; Ashkarran, A.A. Exploring the Limits of Magnetic Levitation: Submicron Particle Separation and Density Profiling. Mater. Res. Express 2025, 12, 056101. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Mahmoudi, M. Magnetic Levitation of Nanoscale Materials: The Critical Role of Effective Density. J. Phys. D Appl. Phys. 2024, 57, 065001. [Google Scholar] [CrossRef]
- Abrahamsson, C.K.; Nagarkar, A.; Fink, M.J.; Preston, D.J.; Ge, S.; Bozenko, J.S.; Whitesides, G.M. Analysis of Powders Containing Illicit Drugs Using Magnetic Levitation. Angew. Chem. 2020, 132, 884–891. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, D.; Cao, M.; Gu, F.; Cai, X.; Liu, X.; Cheng, Z.; Hall, P.; Fu, J.; Zhao, P. A Novel MagLev-Based Separation Approach for Heavy Metal Recycling. Resour. Conserv. Recycl. 2021, 174, 105769. [Google Scholar] [CrossRef]
- Navi, M.; Abbasi, N.; Jeyhani, M.; Gnyawali, V.; Tsai, S.S.H. Microfluidic Diamagnetic Water-in-Water Droplets: A Biocompatible Cell Encapsulation and Manipulation Platform. Lab. Chip 2018, 18, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Grover, W.H.; Bryan, A.K.; Diez-Silva, M.; Suresh, S.; Higgins, J.M.; Manalis, S.R. Measuring Single-Cell Density. Proc. Natl. Acad. Sci. USA 2011, 108, 10992–10996. [Google Scholar] [CrossRef]
- Yaman, S.; Anil-Inevi, M.; Ozcivici, E.; Tekin, H.C. Magnetic Force-Based Microfluidic Techniques for Cellular and Tissue Bioengineering. Front. Bioeng. Biotechnol. 2018, 6, 192. [Google Scholar] [CrossRef]
- Lyu, C.; Tang, D.; Zhang, C.; Xie, J.; Zhang, Q.; Nie, J.; He, Y.; Fu, J.; Wang, J.; Zhao, P. Magnetic Levitation for Non-Contact Manipulation and Measurement of Cells. Sens. Actuators B Chem. 2023, 385, 133692. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Gharibi, H.; Zeki, D.A.; Radu, I.; Khalighinejad, F.; Keyhanian, K.; Abrahamsson, C.K.; Ionete, C.; Saei, A.A.; Mahmoudi, M. Multi-Omics Analysis of Magnetically Levitated Plasma Biomolecules. Biosens. Bioelectron. 2022, 220, 114862. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Olfatbakhsh, T.; Ramezankhani, M.; Crist, R.C.; Berrettini, W.H.; Milani, A.S.; Pakpour, S.; Mahmoudi, M. Evolving Magnetically Levitated Plasma Proteins Detects Opioid Use Disorder as a Model Disease. Adv. Healthc. Mater. 2020, 9, 1901608. [Google Scholar] [CrossRef]
- Lyu, C.; Zhang, C.; Zhang, B.; Ni, X.; Fu, J.; Zhao, P. Cross-Halbach Magnetic Levitation Configuration for Density-Based Measurement, Separation and Detection. Sens. Actuators B Chem. 2025, 422, 136578. [Google Scholar] [CrossRef]
- Hilton, J.E.; McMurry, S.M. An Adjustable Linear Halbach Array. J. Magn. Magn. Mater. 2012, 324, 2051–2056. [Google Scholar] [CrossRef]
- Ge, S.; Nemiroski, A.; Mirica, K.A.; Mace, C.R.; Hennek, J.W.; Kumar, A.A.; Whitesides, G.M. Magnetic Levitation in Chemistry, Materials Science, and Biochemistry. Angew. Chem. Int. Ed. 2020, 59, 17810–17855. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Mamiya, M. Interaction Forces Between Nonmagnetic Particles in the Magnetized Magnetic Fluid. J. Magn. Magn. Mater. 1987, 65, 207–210. [Google Scholar] [CrossRef]
- Rauwerdink, A.M.; Weaver, J.B. Measurement of Molecular Binding Using the Brownian Motion of Magnetic Nanoparticle Probes. Appl. Phys. Lett. 2010, 96, 033702. [Google Scholar] [CrossRef]


| Concentration of MnCl2 (mol/L) | 1.5 | 2.0 | 2.5 | 3.0 |
| × 10−4 (cm3/mol) | 2.77 | 3.79 | 4.74 | 5.49 |
| (g/cm3) | 1.148 | 1.192 | 1.242 | 1.281 |
| Material | Medium | Density Value (g/cm3) | |
|---|---|---|---|
| Magnetic Levitation | Pycnometer | ||
| ABS | 1.5 M MnCl2 solution | 1.069 ± 0.012 | 1.069 ± 0.005 |
| PA | 1.5 M MnCl2 solution | 1.169 ± 0.002 | 1.168 ± 0.002 |
| PC | 1.5 M MnCl2 solution | 1.183 ± 0.008 | 1.183 ± 0.005 |
| PMMA | 1.5 M MnCl2 solution | 1.192 ± 0.005 | 1.190 ± 0.005 |
| TPU | 1.5 M MnCl2 solution | 1.247 ± 0.008 | 1.248 ± 0.003 |
| PLA | 2.5 M MnCl2 solution | 1.275 ± 0.002 | 1.275 ± 0.003 |
| PVC | 2.0 M MnCl2 solution | 1.301 ± 0.015 | 1.303 ± 0.005 |
| PEEK | 2.5 M MnCl2 solution | 1.301 ± 0.018 | 1.301 ± 0.004 |
| POM | 2.5 M MnCl2 solution | 1.428 ± 0.003 | 1.427 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, C.; Zhang, C.; Zhang, B.; Ni, X.; Wang, H.; Zhao, P. Density-Based Characterization of Microplastics via Cross-Halbach Magnetic Levitation. Nanomaterials 2025, 15, 941. https://doi.org/10.3390/nano15120941
Lyu C, Zhang C, Zhang B, Ni X, Wang H, Zhao P. Density-Based Characterization of Microplastics via Cross-Halbach Magnetic Levitation. Nanomaterials. 2025; 15(12):941. https://doi.org/10.3390/nano15120941
Chicago/Turabian StyleLyu, Chenxin, Chengqian Zhang, Baocai Zhang, Xuebin Ni, Hongchao Wang, and Peng Zhao. 2025. "Density-Based Characterization of Microplastics via Cross-Halbach Magnetic Levitation" Nanomaterials 15, no. 12: 941. https://doi.org/10.3390/nano15120941
APA StyleLyu, C., Zhang, C., Zhang, B., Ni, X., Wang, H., & Zhao, P. (2025). Density-Based Characterization of Microplastics via Cross-Halbach Magnetic Levitation. Nanomaterials, 15(12), 941. https://doi.org/10.3390/nano15120941









