Effect of Cu Doping on Synthesis, Composition and Sensor Properties of In2O3 Nanostructures
Abstract
1. Introduction
2. Experiments Performed
3. Results and Discussion
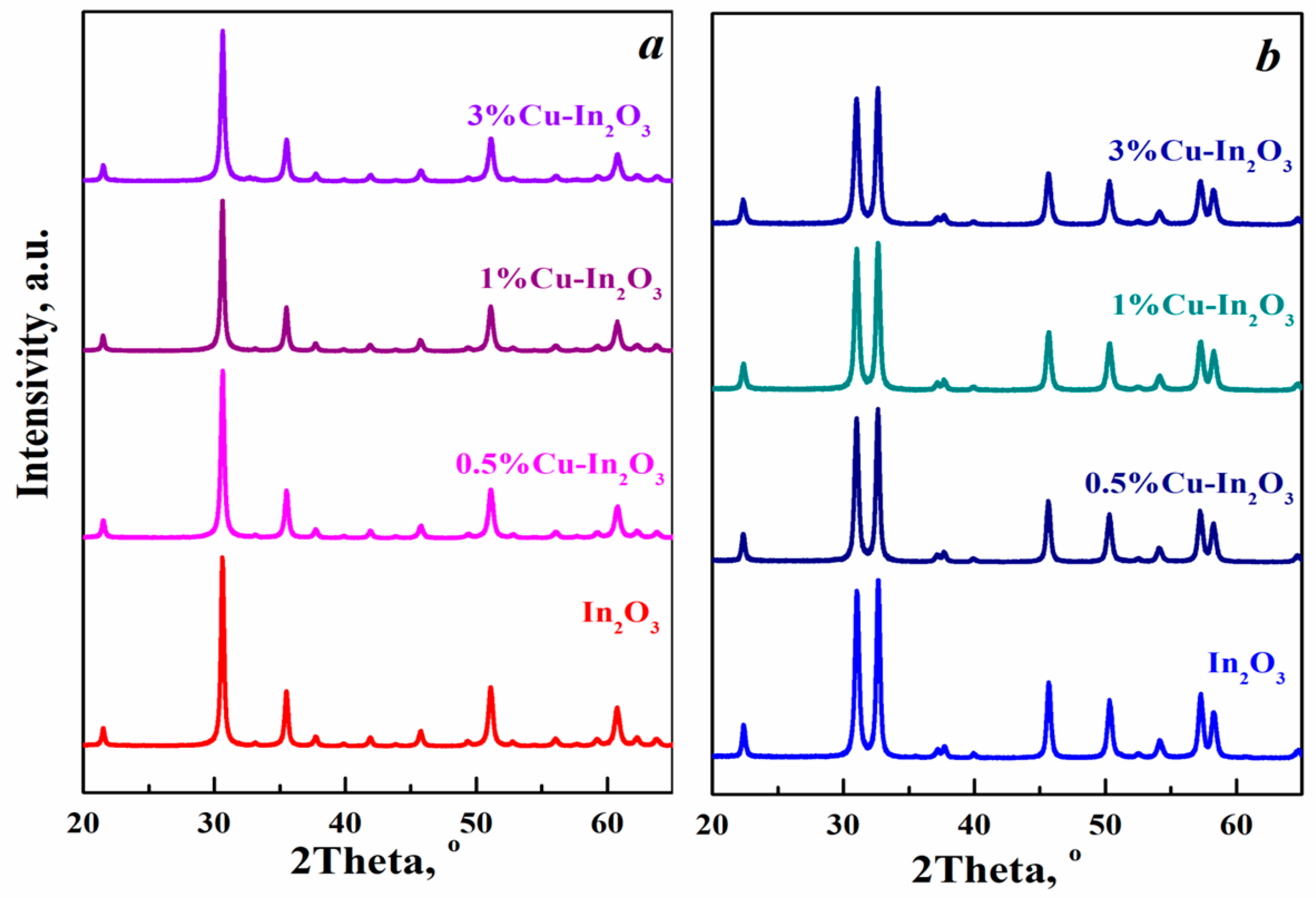
3.1. Structural Characteristics of Cu-Doped In2O3 Nanocomposites
3.2. Conductivity and Sensory Properties of CuO-In2O3 Composites in Detection of H2 and CO in Air
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaikh, T.; Jain, S. ZnO nanostructure-based gas sensors: Critical review based on their synthesis and morphology towards various oxidizing and reducing gases. Curr. Nanomater. 2023, 8, 336–360. [Google Scholar] [CrossRef]
- Gautam, D.; Gautam, Y.K.; Sharma, K.; Kumar, A.; Kumar, A.; Srivastava, V.; Singh, B.P. Recent developments in SnO2 nanostructures inspired hydrogen gas sensors. Int. J. Hydrogen Energy 2024, 81, 313–345. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Innovations in WO3 gas sensors: Nanostructure engineering, functionalization, and future perspectives. Heliyon 2024, 10, e27740. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, X.; Sun, X.F.; Shao, X.; Wang, H.Y. Strategies for improving the sensing performance of In2O3-based gas sensors for ethanol detection. J. Alloys Compd. 2023, 963, 171190. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Synthesis, structural and sensor properties of nanosized mixed oxides based on In2O3 particles. Int. J. Mol. Sci. 2023, 24, 1570. [Google Scholar] [CrossRef]
- Morulane, K.L.; Swart, H.C.; Motaung, D.E. A review on topical advancement and challenges of indium oxide based gas sensors: Future outlooks. J. Environ. Chem. Eng. 2024, 12, 112144. [Google Scholar] [CrossRef]
- Chen, F.; Yang, M.; Wang, X.; Song, Y.; Guo, L.; Xie, N.; Lu, G. Template-free synthesis of cubic-rhombohedral-In2O3 flower for ppb level acetone detection. Sens. Actuators B Chem. 2019, 290, 459–466. [Google Scholar] [CrossRef]
- Liang, T.T.; Kim, D.S.; Yoon, J.W.; Yu, Y.T. Rapid synthesis of rhombohedral In2O3 nanoparticles via a microwave-assisted hydrothermal pathway and their application for conductometric ethanol sensing. Sens. Actuators B Chem. 2021, 346, 130578. [Google Scholar] [CrossRef]
- Cao, E.; Wu, L.; Zhang, Y.; Sun, L.; Yu, Z.; Nie, Z. Hydrothermal synthesis of cubic-rhombohedral-In2O3 microspheres with superior acetone sensing performance. Appl. Surf. Sci. 2023, 613, 156045. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Ikim, M.I.; Ilegbusi, O.J.; Gromov, V.F.; Gerasimov, G.N. Effect of nanoparticle interaction on structural, conducting and sensing properties of mixed metal oxides. Chemosensors 2023, 11, 320. [Google Scholar] [CrossRef]
- Sharma, N.; Choudhury, S.P. Gas sensing using metal oxide semiconductor doped with rare earth elements: A review. Materials Sci. Eng. B 2024, 307. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gromov, V.F.; Gerasimov, G.N.; Spiridonova, E.Y.; Erofeeva, A.R.; Kurmangaleev, K.S.; Trakhtenberg, L.I. Structure, conductivity, and sensor properties of nanosized ZnO-In2O3 composites: Influence of synthesis method. Micromachines 2023, 14, 1685. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, S.; Din, S.T.U.; Shahid, A.; Amu-Darko, J.N.O.; Wang, M.; Qiao, G. A review on In2O3 nanostructures for gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112538. [Google Scholar] [CrossRef]
- Long, H.; Li, Y.; Chai, K.; Zeng, W. Metal oxide semiconductor-based core-shell nanostructures for chemiresistive gas sensing: A review. Sens. Actuators B Chem. 2024, 417, 136183. [Google Scholar] [CrossRef]
- Shi, C.; Hou, X.; Guo, R.; Zhang, W.; Zhou, Y. Starfish-like Zn doped In2O3 dendritic structure for superior triethylamine sensing by the facile co-precipitation method. Mater. Res. Bull. 2024, 173, 112668. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Wang, X.; Li, Y.; Zhang, B.; Sun, G.; Wang, Y. The multiple sensitization effects in La-doped In2O3 porous nanotubes enabling highly sensitive and selective detection of formaldehyde at low temperature. J. Alloys Compd. 2025, 1021, 179741. [Google Scholar] [CrossRef]
- Wei, D.; Jiang, W.; Gao, H.; Chuai, X.; Liu, F.; Liu, F.; Lu, G. Facile synthesis of La-doped In2O3 hollow microspheres and enhanced hydrogen sulfide sensing characteristics. Sens. Actuators B Chem. 2018, 276, 413–420. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Chen, Q.; Qu, J.; Guo, Y.; Zhou, K.; Ma, X. Co ions doping enhances n-butanol sensing performance of In2O3 nanospheres. Sens. Actuators B Chem. 2025, 424, 136898. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, X.; Sun, G.; Cao, J.; Wang, Y. The effects of Co doping on the gas sensing performance of In2O3 porous nanospheres. Sens. Actuators B Chem. 2024, 403, 135155. [Google Scholar] [CrossRef]
- Yong, P.; Wang, S.; Zhang, X.; Pan, H.; Shen, S. MOFs-derived Co-doped In2O3 hollow hexagonal cylinder for selective detection of ethanol. Chem. Phys. Lett. 2022, 795, 139517. [Google Scholar] [CrossRef]
- Wu, W.J.; Xu, J.C.; Hong, B.; Li, J.; Zeng, Y.X.; Peng, X.L.; Wang, X.Q. Highly-enhanced gas-sensing performance of metal-doped In2O3 microtubes from acceptor doping and double surface adsorption. Mater. Sci. Eng. B 2025, 311, 117784. [Google Scholar] [CrossRef]
- Yan, P.; Hou, W.; Wang, M.; Li, Y.; Ge, C.; Zhang, Z.; Bai, L. Light-activated 3DOM Zn-doped In2O3 for room-temperature ppb-level NO2 detection. Sens. Actuators B Chem. 2025, 426, 137002. [Google Scholar] [CrossRef]
- Kulkarni, S.C.; Salunke, V.T.; Naeem, S.; Patil, A.V. Enhanced structural, optical, and gas sensing properties of Zn-doped In2O3 nanomaterial synthesized via sol-gel technique. Ceram. Int. 2025, 51, 12253. [Google Scholar] [CrossRef]
- Ye, F.; Cai, X.M.; Zhong, X.; Tian, X.Q.; Jing, S.Y.; Huang, L.B.; Liang, G.X. The electrical and optical properties of Cu-doped In2O3 thin films. Thin Solid Films 2014, 556, 44–47. [Google Scholar] [CrossRef]
- Hu, X.; Tian, L.; Sun, H.; Wang, B.; Gao, Y.; Sun, P.; Lu, G. Highly enhanced NO2 sensing performances of Cu-doped In2O3 hierarchical flowers. Sens. Actuators B Chem. 2015, 221, 297–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, S.; Wang, M.; Liu, S.; Liu, G.; Meng, X.; Qiao, G. Electrospun Cu-doped In2O3 hollow nanofibers with enhanced H2S gas sensing performance. J. Adv. Ceram. 2022, 11, 427–442. [Google Scholar] [CrossRef]
- Lemos, S.C.; Romeiro, F.C.; de Paula, L.F.; Gonçalves, R.F.; de Moura, A.P.; Ferrer, M.M.; Lima, R.C. Effect of Er3+ ions on the phase formation and properties of In2O3 nanostructures crystallized upon microwave heating. J. Solid State Chem. 2017, 249, 58–63. [Google Scholar] [CrossRef]
- Lemos, S.C.S.; Nossol, E.; Ferrari, J.L.; Gomes, E.O.; Andres, J.; Gracia, L.; Lima, R.C. Joint theoretical and experimental study on the La doping process in In2O3: Phase transition and electrocatalytic activity. Inorg. Chem. 2019, 58, 11738–11750. [Google Scholar] [CrossRef]
- Li, P.; Cai, C.; Cheng, T.; Huang, Y. Hydrothermal synthesis and Cl2 sensing performance of porous-sheets-like In2O3 structures with phase transformation. RSC Adv. 2017, 7, 50760–50771. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Erofeeva, A.R.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Cobalt doped cubic and rhombohedral In2O3: The role of crystalline phase of indium oxide in sensor response to hydrogen. Chem. Phys. Lett. 2024, 845, 141321. [Google Scholar] [CrossRef]
- Roso, S.; Vilic, T.; Urakawa, A.; Llobet, E. Gas sensing properties of In2O3 cubes prepared by a hydrothermal method. Procedia Eng. 2016, 168, 247–250. [Google Scholar] [CrossRef]
- Van Tong, P.; Minh, L.H.; Van Duy, N.; Hung, C.M. Porous In2O3 nanorods fabricated by hydrothermal method for an effective CO gas sensor. Mater. Res. Bull. 2021, 137, 111179. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Ikim, M.I.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Chemical modification of impregnated SnO2-In2O3 nanocomposites due to interaction of sensor components. J. Alloys Compd. 2021, 883, 160817. [Google Scholar] [CrossRef]
- Muruganandham, M.; Lee, G.J.; Wu, J.J.; Levchuk, I.; Sillanpaa, M. By-product assisted hydrothermal synthesis of InOOH microflower composed of nanosheets. Mater. Lett. 2013, 98, 86–89. [Google Scholar] [CrossRef]
- Wu, L.; Cao, E.; Zhang, Y.; Sun, L.; Sun, B.; Yu, Z. La and Fe co-doped walnut-like cubic-rhombohedral-In2O3 for highly sensitive and selective detection of acetone vapor. Mater. Lett. 2023, 336, 133869. [Google Scholar] [CrossRef]
- Li, L.; Hong, D.; Liu, B.; Su, T.; Yang, X.; Yue, L.; Zhang, W. Porous Zn-doped In2O3 nanobelts for ppb level acetone sensing at low operating temperature. Sens. Actuators B Chem. 2025, 433, 137451. [Google Scholar] [CrossRef]
- Yan, T.; Wang, X.; Long, J.; Lin, H.; Yuan, R.; Dai, W.; Fu, X. Controlled preparation of In2O3, InOOH and In(OH)3 via a one-pot aqueous solvothermal route. New J. Chem. 2008, 32, 1843–1846. [Google Scholar] [CrossRef]
- Yu, D.; Yu, S.H.; Zhang, S.; Zuo, J.; Wang, D.; Qian, Y.T. Metastable hexagonal In2O3 nanofibers templated from InOOH nanofibers under ambient pressure. Adv. Funct. Mater. 2003, 13, 497–501. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Yang, Y. Zn-doped In2O3 nanostructures: Preparation, structure and gas-sensing properties. Cryst. Eng. Comm. 2012, 14, 1135–1142. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Kou, X.; Meng, F.; Chen, K.; Wang, T.; Sun, P.; Liu, F.; Lu, G. High-performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B Chem. 2020, 320, 128292. [Google Scholar] [CrossRef]
- Qin, W.; Lu, B.; Xu, X.; Shen, Y.; Meng, F. Metal organic framework-derived porous Ni-doped In2O3 for highly sensitive and selective detection to hydrogen at low temperature. Sens. Actuators B Chem. 2024, 417, 136123. [Google Scholar] [CrossRef]






| Sample | Crystalline Phase | 2θ, ° | Lattice Parameters, nm | Particle Size, nm |
|---|---|---|---|---|
| Hydrothermal samples (H2O) | ||||
| In2O3 | 100% c-In2O3 | 30.6024 (222) | a = b = c = 1.011161 | 34.5 |
| 0.5% Cu-In2O3 | 100% c-In2O3 | 30.6078 (222) | a = b = c = 1.010771 | 33.9 |
| 1% Cu-In2O3 | 100% c-In2O3 | 30.6097 (222) | a = b = c = 1.010664 | 30.7 |
| 3% Cu-In2O3 | 100% c-In2O3 | 30.6118 (222) | a = b = c = 1.010602 | 30.3 |
| Hydrothermal samples (C2H5OH) | ||||
| In2O3 | 100% rh-In2O3 | 31.0177(104) | a = b = 0.548671 c = 1.450863 | 24.6 |
| 0.5% Cu-In2O3 | 100% rh-In2O3 | 31.0194(104) | a = b = 0.548609c = 1.450558 | 24.5 |
| 1% Cu-In2O3 | 100% rh-In2O3 | 31.0198(104) | a = b = 0.548420c = 1.450442 | 23.6 |
| 3% Cu-In2O3 | 100% rh-In2O3 | 31.0208(104) | a = b = 0.548539c = 1.450134 | 21.4 |
| Sample | SBET, m2/g | Vmes, sm3/g | Smes, m2/g | dmes, nm | Vt, sm3/g |
|---|---|---|---|---|---|
| Hydrothermal samples (H2O) | |||||
| In2O3 | 15.1 | 0.040 | 12.1 | 3.9 | 0.043 |
| 0.5% Cu-In2O3 | 16.6 | 0.040 | 13.8 | 3.9 | 0.042 |
| 1% Cu-In2O3 | 20 | 0.047 | 14.9 | 3.5 | 0.053 |
| 3% Cu-In2O3 | 19.8 | 0.040 | 15.7 | 3.5 | 0.045 |
| Hydrothermal samples (C2H5OH) | |||||
| In2O3 | 23.1 | 0.070 | 16.3 | 3.2/19.3 | 0.074 |
| 0.5% Cu-In2O3 | 21.3 | 0.061 | 14.8 | 3.2/19.6 | 0.066 |
| 1% Cu-In2O3 | 23.2 | 0.062 | 15.6 | 3.2/18.9 | 0.068 |
| 3% Cu-97%In2O3 | 23.9 | 0.065 | 16.8 | 3.2/19 | 0.07 |
| Sample | OL, % | OV, % | OC, % |
|---|---|---|---|
| Hydrothermal samples (H2O) | |||
| In2O3 | 61.9 | 28.8 | 9.3 |
| 1% Cu-In2O3 | 55.1 | 34.3 | 10.6 |
| Hydrothermal samples (C2H5OH) | |||
| In2O3 | 62 | 31.8 | 6.2 |
| 1% Cu-In2O3 | 61.9 | 32.4 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikim, M.I.; Spiridonova, E.Y.; Ilegbusi, O.J.; Trakhtenberg, L.I. Effect of Cu Doping on Synthesis, Composition and Sensor Properties of In2O3 Nanostructures. Nanomaterials 2025, 15, 925. https://doi.org/10.3390/nano15120925
Ikim MI, Spiridonova EY, Ilegbusi OJ, Trakhtenberg LI. Effect of Cu Doping on Synthesis, Composition and Sensor Properties of In2O3 Nanostructures. Nanomaterials. 2025; 15(12):925. https://doi.org/10.3390/nano15120925
Chicago/Turabian StyleIkim, Mariya I., Elena Yu. Spiridonova, Olusegun Johnson Ilegbusi, and Leonid I. Trakhtenberg. 2025. "Effect of Cu Doping on Synthesis, Composition and Sensor Properties of In2O3 Nanostructures" Nanomaterials 15, no. 12: 925. https://doi.org/10.3390/nano15120925
APA StyleIkim, M. I., Spiridonova, E. Y., Ilegbusi, O. J., & Trakhtenberg, L. I. (2025). Effect of Cu Doping on Synthesis, Composition and Sensor Properties of In2O3 Nanostructures. Nanomaterials, 15(12), 925. https://doi.org/10.3390/nano15120925






