Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare
Abstract
1. Introduction
1.1. Overview of Multimodal AI
1.2. Importance of AI in Biomedicine and Healthcare
1.3. Objectives of the Review
1.4. Materials and Methods
1.4.1. Databases Searched
- PubMed: For peer-reviewed articles on biomedical applications of AI, biomaterials development, and clinical healthcare innovations.
- IEEE Xplore: For technical research on AI algorithms such as convolutional neural networks (CNNs), recurrent neural networks (RNNs), and their deployment in biomedicine.
- Scopus: For a wide spectrum of research literature in materials science, biomedical engineering, and AI applications.
- Web of Science: For studies on the integration of AI technologies in healthcare and biomedical research.
1.4.2. Materials and Methods for AI Model Development
- Protein Data Bank (PDB): A critical resource for structural biology, used in training deep learning models like AlphaFold for accurate protein structure prediction. PDB provides experimentally validated protein and macromolecular structures, forming the backbone of structure-based AI applications.
- Medical Imaging Datasets: Publicly available databases such as The Cancer Imaging Archive (TCIA) and NIH Chest X-ray dataset were considered for studies utilizing AI in diagnostic imaging.
- Electronic Health Records (EHRs): Studies incorporating AI in patient stratification and clinical decision-making often utilized anonymized EHR datasets like MIMIC-III.
- Genomic Databases: Sources such as The Cancer Genome Atlas (TCGA) were used in multimodal AI models combining genetic, imaging, and clinical data.
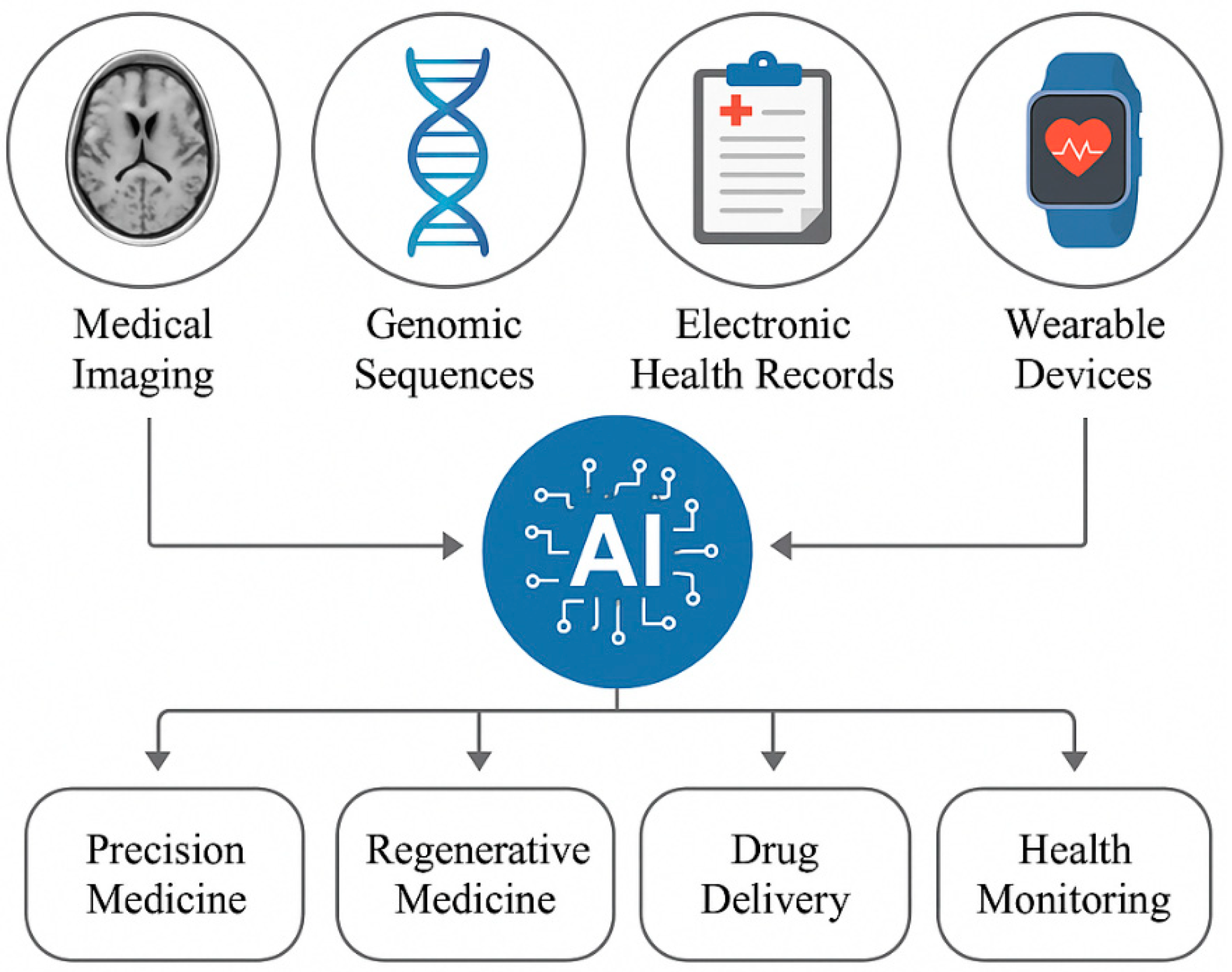
2. Role of Multimodal AI in Biomaterials Development
2.1. Combining Multimodal Data (Imaging, Genomic, and Clinical Data)
Leveraging Medical Imaging for Biomaterial Design
2.2. Harnessing Genomic Data for Personalized Biomaterials
Clinical Data Integration for Patient-Centric Biomaterial Design
2.3. AI-Driven Biomaterial Design for Tissue Engineering
- ➢
- Promote cellular infiltration and nutrient diffusion through optimized porosity;
- ➢
- Mimic tissue-specific mechanics (e.g., neural vs. musculoskeletal targets);
- ➢
- Maintain structural integrity during dynamic remodeling processes.
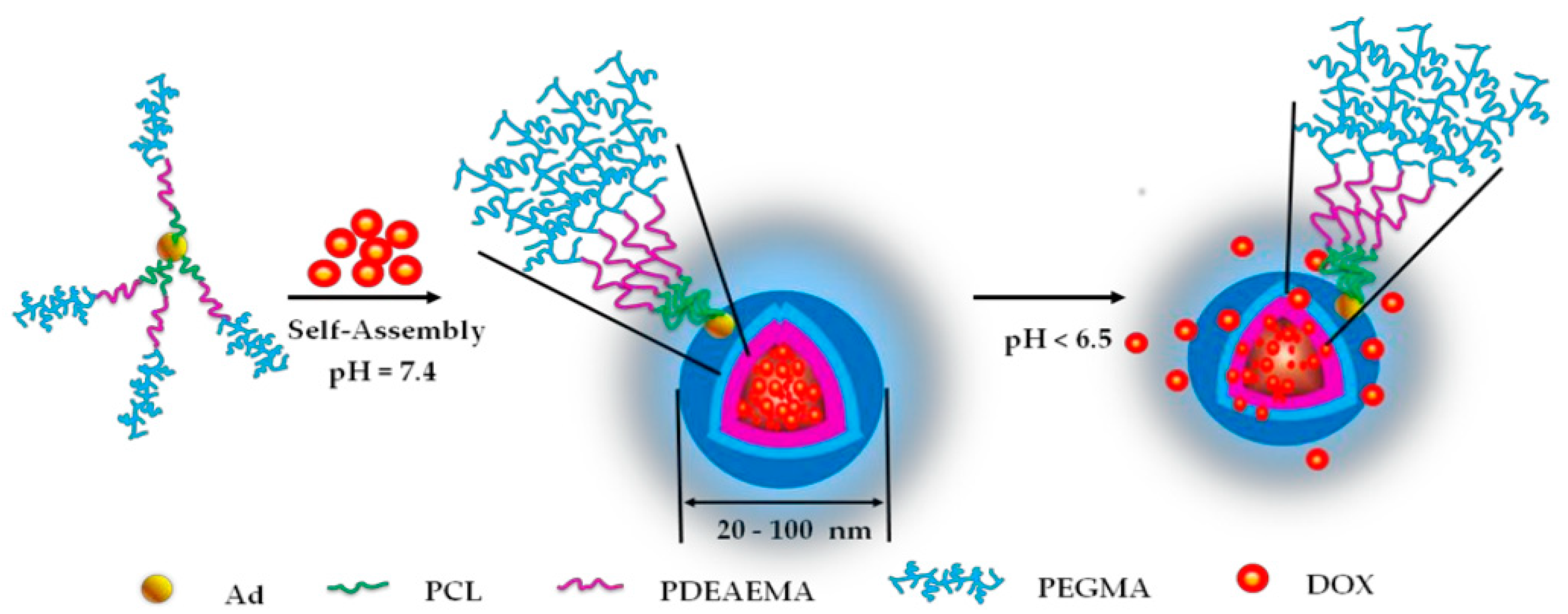
2.4. AI in Drug Delivery Systems
2.5. AI in Regenerative Medicine Applications
2.6. Multimodal AI in Biomaterials Science
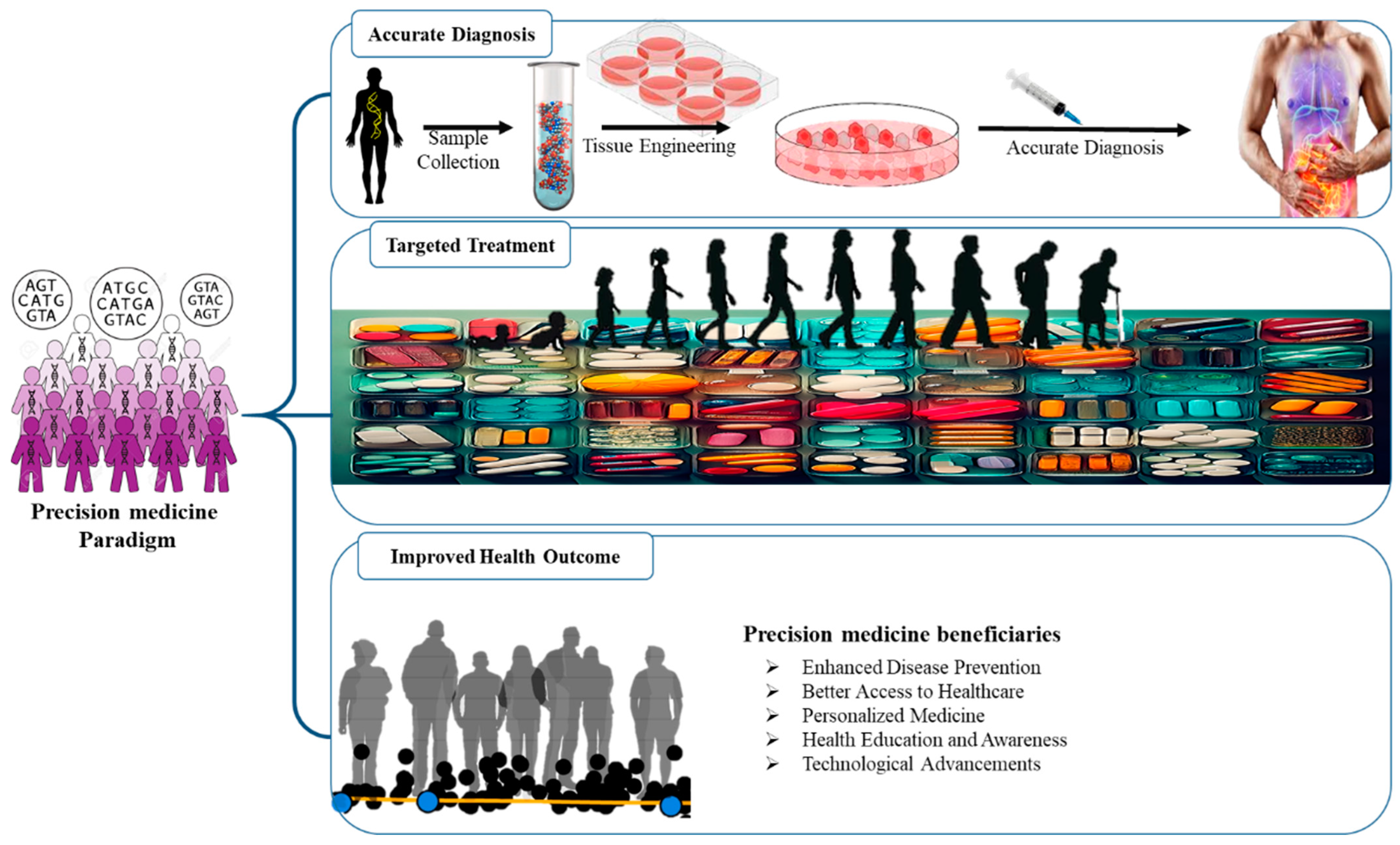
3. AI-Powered Diagnostics and Precision Medicine
3.1. Role of AI in Disease Diagnosis
3.2. AI-Driven Precision Medicine
3.3. Advanced Predictive Modeling in Healthcare Through Machine Learning
3.4. Multimodal Data Fusion for Enhanced Diagnostic Accuracy
4. Wearable Technologies and Real-Time Health Monitoring
4.1. AI Integration with Wearable Devices
4.2. AI-Enhanced Remote Patient Monitoring
4.3. Use of Multimodal Data for Continuous Health Surveillance
4.4. Impact of AI on Preventive Healthcare
5. Ethical, Regulatory, and Scalability Challenges
5.1. Ethical Concerns in AI Applications
5.2. Regulatory Frameworks for AI-Driven Systems
5.3. Challenges in Scaling AI Systems for Clinical Use
5.4. Addressing Data Privacy and Security
6. Future Opportunities for AI in Biomedicine
6.1. AI in Emerging Biotechnologies
6.2. Integration of AI with 3D Bioprinting
6.3. Role of AI in Personalized Therapeutic Design
6.4. Potential for AI in Drug Discovery
6.5. Advantages and Limitations of Multimodal AI in Biomaterials Science
6.6. Emerging Trends: Federated Learning, Digital Twins, and Clinical Translation
- Federated Learning for Privacy-Preserving AI: Federated learning is an emerging machine learning paradigm that enables model training across decentralized data sources without transferring sensitive patient data to a central server. This approach enhances data privacy and security while allowing AI models to learn from diverse healthcare datasets across institutions. It holds particular promise for collaborative biomaterials research and multi-center clinical studies, addressing data ownership concerns and complying with stringent privacy regulations.
- Integration with Digital Twins and 3D-Printed Biomaterials: Digital twins—virtual replicas of biological systems or patient-specific conditions—offer a powerful platform for simulating and optimizing biomaterial interactions, treatment responses, and implant integration. When combined with AI and 3D printing technologies, this integration enables the design of customized biomaterials tailored to individual patients’ anatomical and physiological profiles. AI-enhanced digital twins can predict outcomes of regenerative therapies or surgical implants, accelerating design iterations and improving therapeutic precision.
- Multimodal AI in Clinical Translation and Regulatory Approval: Multimodal AI systems that integrate clinical, molecular, and imaging data can streamline the clinical validation of new biomaterials and therapeutic devices. By providing more comprehensive and interpretable evidence of efficacy and safety, these systems can support regulatory submissions and facilitate approval pathways. Furthermore, explainable AI (XAI) techniques are increasingly being explored to meet transparency and accountability requirements in regulatory frameworks.
7. Conclusions
- Future Prospects for AI in Healthcare and Biomaterials
- Final Thoughts on Multimodal AI’s Impact
Funding
Conflicts of Interest
References
- Shaik, T.; Tao, X.; Li, L.; Xie, H.; Velásquez, J.D. A Survey of Multimodal Information Fusion for Smart Healthcare: Mapping the Journey from Data to Wisdom. Inf. Fusion 2024, 102, 102040. [Google Scholar] [CrossRef]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- van der Velden, B.H.M.; Kuijf, H.J.; Gilhuijs, K.G.A.; Viergever, M.A. Explainable Artificial Intelligence (XAI) in Deep Learning-Based Medical Image Analysis. Med. Image Anal. 2022, 79, 102470. [Google Scholar] [CrossRef]
- Hulsen, T. Literature Analysis of Artificial Intelligence in Biomedicine. Ann. Transl. Med. 2022, 10, 1284. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gao, C.; Wang, L. The Evolution of Artificial Intelligence in Biomedicine: Bibliometric Analysis. JMIR AI 2023, 2, e45770. [Google Scholar] [CrossRef]
- Nosrati, H.; Nosrati, M. Artificial Intelligence in Regenerative Medicine: Applications and Implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI Energized Hydrogel Design, Optimization and Application in Biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-Enabled Organoids: Construction, Analysis, and Application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Restrepo, J.C.; Dueñas, D.; Corredor, Z.; Liscano, Y. Advances in Genomic Data and Biomarkers: Revolutionizing NSCLC Diagnosis and Treatment. Cancers 2023, 15, 3474. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Lee, T.-H.; Sahoo, P.K. Smart Healthcare: Exploring the Internet of Medical Things with Ambient Intelligence. Electronics 2024, 13, 2309. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P. Challenges and Strategies for Wide-Scale Artificial Intelligence (AI) Deployment in Healthcare Practices: A Perspective for Healthcare Organizations. Artif. Intell. Med. 2024, 151, 102861. [Google Scholar] [CrossRef]
- Holzinger, A.; Keiblinger, K.; Holub, P.; Zatloukal, K.; Müller, H. AI for Life: Trends in Artificial Intelligence for Biotechnology. N. Biotechnol. 2023, 74, 16–24. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Zhu, Z.; Zhao, L.; Wang, H.; Song, C.; Chen, Y.; Zhao, Q.; Yang, J.; Pei, Y. A Comprehensive Review on Synergy of Multi-Modal Data and AI Technologies in Medical Diagnosis. Bioengineering 2024, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Soenksen, L.R.; Ma, Y.; Zeng, C.; Boussioux, L.; Villalobos Carballo, K.; Na, L.; Wiberg, H.M.; Li, M.L.; Fuentes, I.; Bertsimas, D. Integrated Multimodal Artificial Intelligence Framework for Healthcare Applications. npj Digit. Med. 2022, 5, 149. [Google Scholar] [CrossRef]
- Luo, Y.-S.; Chen, Z.; Hsieh, N.-H.; Lin, T.-E. Chemical and Biological Assessments of Environmental Mixtures: A Review of Current Trends, Advances, and Future Perspectives. J. Hazard. Mater. 2022, 432, 128658. [Google Scholar] [CrossRef]
- Lorenzo, G.; Ahmed, S.R.; Hormuth, D.A.; Vaughn, B.; Kalpathy-Cramer, J.; Solorio, L.; Yankeelov, T.E.; Gomez, H. Patient-Specific, Mechanistic Models of Tumor Growth Incorporating Artificial Intelligence and Big Data. Annu. Rev. Biomed. Eng. 2024, 26, 529–560. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef]
- Wei, H.; Bao, H.; Ruan, X. Perspective: Predicting and Optimizing Thermal Transport Properties with Machine Learning Methods. Energy AI 2022, 8, 100153. [Google Scholar] [CrossRef]
- Basu, B.; Gowtham, N.H.; Xiao, Y.; Kalidindi, S.R.; Leong, K.W. Biomaterialomics: Data Science-Driven Pathways to Develop Fourth-Generation Biomaterials. Acta Biomater. 2022, 143, 1–25. [Google Scholar] [CrossRef]
- Dangeti, A.; Bynagari, D.G.; Vydani, K. Revolutionizing Drug Formulation: Harnessing Artificial Intelligence and Machine Learning for Enhanced Stability, Formulation Optimization, and Accelerated Development. Int. J. Pharm. Sci. Med. 2023, 8, 18–29. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Torkashvand, M.; Bavisi, M.; Aldaghi, N.; Alizadeh, A. Recent Advances in Artificial Intelligent Strategies for Tissue Engineering and Regenerative Medicine. Ski. Res. Technol. 2024, 30, e70016. [Google Scholar] [CrossRef]
- Saragih, R.I.E. AI-Powered Education: Transforming Learning through Personalized and Scalable Solutions. Int. J. Inf. Syst. Innov. Technol. 2024, 3, 1–9. [Google Scholar] [CrossRef]
- Brown, B.N.; Haschak, M.J.; Nolfi, A.L.; Kulkarni, M. Moral and Ethical Issues in the Development of Biomaterials and Medical Products. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1509–1517. [Google Scholar]
- You, S.; Fan, Y.; Chen, Y.; Jiang, X.; Liu, W.; Zhou, X.; Zhang, J.; Zheng, J.; Yang, H.; Hou, X. Advancements and Prospects of Deep Learning in Biomaterials Evolution. Cell Rep. Phys. Sci. 2024, 5, 102116. [Google Scholar] [CrossRef]
- Rayed, M.E.; Islam, S.M.S.; Niha, S.I.; Jim, J.R.; Kabir, M.M.; Mridha, M.F. Deep Learning for Medical Image Segmentation: State-of-the-Art Advancements and Challenges. Inform. Med. Unlocked 2024, 47, 101504. [Google Scholar] [CrossRef]
- Fritz, B.; Fritz, J. Artificial Intelligence for MRI Diagnosis of Joints: A Scoping Review of the Current State-of-the-Art of Deep Learning-Based Approaches. Skeletal Radiol. 2022, 51, 315–329. [Google Scholar] [CrossRef]
- Frasca, M.; La Torre, D.; Repetto, M.; De Nicolò, V.; Pravettoni, G.; Cutica, I. Artificial Intelligence Applications to Genomic Data in Cancer Research: A Review of Recent Trends and Emerging Areas. Discov. Anal. 2024, 2, 10. [Google Scholar] [CrossRef]
- Vo, Q.D.; Saito, Y.; Ida, T.; Nakamura, K.; Yuasa, S. The Use of Artificial Intelligence in Induced Pluripotent Stem Cell-Based Technology over 10-Year Period: A Systematic Scoping Review. PLoS ONE 2024, 19, e0302537. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Faa, G.; Johri, A.M.; Mantella, L.E.; Fernandes, J.F.E.; Teji, J.S.; et al. Artificial Intelligence for Cardiovascular Disease Risk Assessment in Personalised Framework: A Scoping Review. eClinicalMedicine 2024, 73, 102660. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, K.S.; Sultana, A.; Madalageri, N.K.; Rangareddy, H. Artificial Intelligence’s Impact on Drug Discovery and Development From Bench to Bedside. Cureus 2023, 5, e47486. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal Biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for Bone-Tissue Engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Kolomenskaya, E.; Butova, V.; Poltavskiy, A.; Soldatov, A.; Butakova, M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines 2023, 12, 76. [Google Scholar] [CrossRef]
- Gokcekuyu, Y.; Ekinci, F.; Guzel, M.S.; Acici, K.; Aydin, S.; Asuroglu, T. Artificial Intelligence in Biomaterials: A Comprehensive Review. Appl. Sci. 2024, 14, 6590. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. Artificial Intelligence for Clinical Prediction: Exploring Key Domains and Essential Functions. Comput. Methods Programs Biomed. Update 2024, 5, 100148. [Google Scholar] [CrossRef]
- Timakova, A.; Ananev, V.; Fayzullin, A.; Makarov, V.; Ivanova, E.; Shekhter, A.; Timashev, P. Artificial Intelligence Assists in the Detection of Blood Vessels in Whole Slide Images: Practical Benefits for Oncological Pathology. Biomolecules 2023, 13, 1327. [Google Scholar] [CrossRef]
- Schork, N.J. Artificial Intelligence and Personalized Medicine. In Precision Medicine in Cancer Therapy; Springer: Berlin, Germany, 2019; pp. 265–283. [Google Scholar]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of Artificial Intelligence in Revolutionizing Drug Discovery. Fundam. Res. 2024, 5, 1273–1287. [Google Scholar] [CrossRef]
- Kapustina, O.; Burmakina, P.; Gubina, N.; Serov, N.; Vinogradov, V. User-Friendly and Industry-Integrated AI for Medicinal Chemists and Pharmaceuticals. Artif. Intell. Chem. 2024, 2, 100072. [Google Scholar] [CrossRef]
- Chou, W.-C.; Chen, Q.; Yuan, L.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. An Artificial Intelligence-Assisted Physiologically-Based Pharmacokinetic Model to Predict Nanoparticle Delivery to Tumors in Mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Integrating Artificial Intelligence for Drug Discovery in the Context of Revolutionizing Drug Delivery. Life 2024, 14, 233. [Google Scholar] [CrossRef]
- Suriyaamporn, P.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. The Artificial Intelligence-Powered New Era in Pharmaceutical Research and Development: A Review. AAPS PharmSciTech 2024, 25, 188. [Google Scholar] [CrossRef]
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial Intelligence (AI) and Big Data in Cancer and Precision Oncology. Comput. Struct. Biotechnol. J. 2020, 18, 2300–2311. [Google Scholar] [CrossRef]
- Das, K.P.; Chandra, J. Nanoparticles and Convergence of Artificial Intelligence for Targeted Drug Delivery for Cancer Therapy: Current Progress and Challenges. Front. Med. Technol. 2023, 4, 1067144. [Google Scholar] [CrossRef]
- Shadab, S.A.; Ansari, M.A.; Singh, N.; Verma, A.; Tripathi, P.; Mehrotra, R. Detection of Cancer from Histopathology Medical Image Data Using ML with CNN ResNet-50 Architecture. In Computational Intelligence in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 237–254. [Google Scholar]
- Verma, P.; Sahu, S.K.; Awasthi, V.K. Deep Neural Network With Feature Optimization Technique for Classification of Coronary Artery Disease. In Handbook of Research on Computer Vision and Image Processing in the Deep Learning Era; IGI Global: Hershey, PA, USA, 2022; pp. 257–269. [Google Scholar]
- Guleria, P. NLP-Based Clinical Text Classification and Sentiment Analyses of Complex Medical Transcripts Using Transformer Model and Machine Learning Classifiers. Neural Comput. Appl. 2025, 37, 341–366. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Zhang, W.; Chen, W.; Zhou, Z.; Heidari, A.A.; Chen, H.; Xu, G. ICycle-GAN: Improved Cycle Generative Adversarial Networks for Liver Medical Image Generation. Biomed. Signal Process. Control 2024, 92, 106100. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly Accurate Protein Structure Prediction for the Human Proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.; Singhal, R.; Yadav, J.P. Revolutionizing Drug Discovery: The Impact of Artificial Intelligence on Advancements in Pharmacology and the Pharmaceutical Industry. Intell. Pharm. 2024, 2, 367–380. [Google Scholar] [CrossRef]
- Suriyaamporn, P.; Pamornpathomkul, B.; Wongprayoon, P.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. The Artificial Intelligence and Design of Experiment Assisted in the Development of Progesterone-Loaded Solid-Lipid Nanoparticles for Transdermal Drug Delivery. Pharmacia 2024, 71, 1–12. [Google Scholar] [CrossRef]
- Deori Leena Hujuri, C. Artificial Intelligence (AI): It’s Role in Drug Discovery and Novel Drug Delivery System. Int. J. Sci. Res. 2024, 13, 1426–1430. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Yüceer, M. Application of Artificial Intelligence in Modeling of the Doxorubicin Release Behavior of PH and Temperature Responsive Poly(NIPAAm-Co-AAc)-PEG IPN Hydrogel. J. Drug Deliv. Sci. Technol. 2020, 57, 101603. [Google Scholar] [CrossRef]
- Yang, H.; Guo, J.; Tong, R.; Yang, C.; Chen, J.-K. PH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery. Polymers 2018, 10, 443. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Ratna, H.V.K.; Jeyaraman, N.; Venkatesan, A.; Ramasubramanian, S.; Yadav, S. Leveraging Artificial Intelligence and Machine Learning in Regenerative Orthopedics: A Paradigm Shift in Patient Care. Cureus 2023, 15, e49756. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial Intelligence Aided Design of Tissue Engineering Scaffolds Employing Virtual Tomography and 3D Convolutional Neural Networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef]
- Virijević, K.; Živanović, M.N.; Nikolić, D.; Milivojević, N.; Pavić, J.; Morić, I.; Šenerović, L.; Dragačević, L.; Thurner, P.J.; Rufin, M.; et al. AI-Driven Optimization of PCL/PEG Electrospun Scaffolds for Enhanced In Vivo Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 22989–23002. [Google Scholar] [CrossRef]
- Rezić, I.; Somogyi Škoc, M. Computational Methodologies in Synthesis, Preparation and Application of Antimicrobial Polymers, Biomolecules, and Nanocomposites. Polymers 2024, 16, 2320. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.W.; Syed, S.A.; Raziq, M. Smart Biomaterials and AI-Driven Approaches for the Tissue Engineering Advancement. In Proceedings of the 2024 Global Conference on Wireless and Optical Technologies (GCWOT), Malaga, Spain, 25 September 2024; pp. 1–7. [Google Scholar]
- Maramraju, S.; Kowalczewski, A.; Kaza, A.; Liu, X.; Singaraju, J.P.; Albert, M.V.; Ma, Z.; Yang, H. AI-organoid Integrated Systems for Biomedical Studies and Applications. Bioeng. Transl. Med. 2024, 9, e10641. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour, R.; Bagherpour, G.; Mohammadi, P. Application of Artificial Intelligence in Tissue Engineering. Tissue Eng. Part B Rev. 2024, 31, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, A.; Hartmann, S.; Vollmer, M.; Shavlokhova, V.; Brands, R.C.; Kübler, A.; Wollborn, J.; Hassel, F.; Couillard-Despres, S.; Lang, G.; et al. Multimodal Artificial Intelligence-Based Pathogenomics Improves Survival Prediction in Oral Squamous Cell Carcinoma. Sci. Rep. 2024, 14, 5687. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K.; Vincent, P.M.D.R.; Ramu Krishnan, N. Advancing Genome Editing with Artificial Intelligence: Opportunities, Challenges, and Future Directions. Front. Bioeng. Biotechnol. 2024, 11, 1335901. [Google Scholar] [CrossRef]
- Aguilar-Gallardo, C.; Bonora-Centelles, A. Integrating Artificial Intelligence for Academic Advanced Therapy Medicinal Products: Challenges and Opportunities. Appl. Sci. 2024, 14, 1303. [Google Scholar] [CrossRef]
- Bekbolatova, M.; Mayer, J.; Ong, C.W.; Toma, M. Transformative Potential of AI in Healthcare: Definitions, Applications, and Navigating the Ethical Landscape and Public Perspectives. Healthcare 2024, 12, 125. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S. Integrating Artificial Intelligence to Biomedical Science: New Applications for Innovative Stem Cell Research and Drug Development. Technologies 2024, 12, 95. [Google Scholar] [CrossRef]
- Carini, C.; Seyhan, A.A. Tribulations and Future Opportunities for Artificial Intelligence in Precision Medicine. J. Transl. Med. 2024, 22, 411. [Google Scholar] [CrossRef]
- Boehm, K.M.; Khosravi, P.; Vanguri, R.; Gao, J.; Shah, S.P. Harnessing Multimodal Data Integration to Advance Precision Oncology. Nat. Rev. Cancer 2022, 22, 114–126. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. AI in Diagnostic Imaging: Revolutionising Accuracy and Efficiency. Comput. Methods Programs Biomed. Update 2024, 5, 100146. [Google Scholar] [CrossRef]
- Ahn, J.S.; Shin, S.; Yang, S.-A.; Park, E.K.; Kim, K.H.; Cho, S.I.; Ock, C.-Y.; Kim, S. Artificial Intelligence in Breast Cancer Diagnosis and Personalized Medicine. J. Breast Cancer 2023, 26, 405. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. AI Outperforms Radiologists in Mammographic Screening. Nat. Rev. Clin. Oncol. 2020, 17, 134. [Google Scholar] [CrossRef]
- Cellina, M.; Cacioppa, L.M.; Cè, M.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Pais, D.; Bausano, M.V.; Rossini, N.; Bruno, A.; et al. Artificial Intelligence in Lung Cancer Screening: The Future Is Now. Cancers 2023, 15, 4344. [Google Scholar] [CrossRef]
- Kang, J.S. Application of Artificial Intelligence-Based Digital Pathology in Biomedical Research. Biomed. Sci. Lett. 2023, 29, 53–57. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Khandwala, Y.S.; Vesal, S.; Shao, W.; Yang, Q.; Soerensen, S.J.C.; Fan, R.E.; Ghanouni, P.; Kunder, C.A.; Brooks, J.D.; et al. A Review of Artificial Intelligence in Prostate Cancer Detection on Imaging. Ther. Adv. Urol. 2022, 14, 175628722211287. [Google Scholar] [CrossRef]
- Abadir, A.P.; Ali, M.F.; Karnes, W.; Samarasena, J.B. Artificial Intelligence in Gastrointestinal Endoscopy. Clin. Endosc. 2020, 53, 132–141. [Google Scholar] [CrossRef]
- Karalis, V.D. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- Fischer, C.G.; Pallavajjala, A.; Jiang, L.; Anagnostou, V.; Tao, J.; Adams, E.; Eshleman, J.R.; Gocke, C.D.; Lin, M.-T.; Platz, E.A.; et al. Artificial Intelligence-Assisted Serial Analysis of Clinical Cancer Genomics Data Identifies Changing Treatment Recommendations and Therapeutic Targets. Clin. Cancer Res. 2022, 28, 2361–2372. [Google Scholar] [CrossRef]
- Mirkin, S.; Albensi, B.C. Should Artificial Intelligence Be Used in Conjunction with Neuroimaging in the Diagnosis of Alzheimer’s Disease? Front. Aging Neurosci. 2023, 15, 1094233. [Google Scholar] [CrossRef]
- Aberathne, I.; Kulasiri, D.; Samarasinghe, S. Detection of Alzheimer’s Disease Onset Using MRI and PET Neuroimaging: Longitudinal Data Analysis and Machine Learning. Neural Regen. Res. 2023, 18, 2134. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ejike Innocent Nwankwo; Ebube Victor Emeihe; Mojeed Dayo Ajegbile; Janet Aderonke Olaboye; Chukwudi Cosmos Maha AI in Personalized Medicine: Enhancing Drug Efficacy and Reducing Adverse Effects. Int. Med. Sci. Res. J. 2024, 4, 806–833. [CrossRef]
- Alsharif, F. Artificial Intelligence in Oncology: Applications, Challenges and Future Frontiers. Int. J. Pharm. Investig. 2024, 14, 647–656. [Google Scholar] [CrossRef]
- Takeshita, T.; Iwase, H.; Wu, R.; Ziazadeh, D.; Yan, L.; Takabe, K. Development of a Machine Learning-Based Prognostic Model for Hormone Receptor-Positive Breast Cancer Using Nine-Gene Expression Signature. World J. Oncol. 2023, 14, 406–422. [Google Scholar] [CrossRef]
- Kiser, K.J.; Fuller, C.D.; Reed, V.K. Artificial Intelligence in Radiation Oncology Treatment Planning: A Brief Overview. J. Med. Artif. Intell. 2019, 2, 9. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Payra, S.; Singh, S.K. Artificial Intelligence and Machine Learning in Pharmacological Research: Bridging the Gap Between Data and Drug Discovery. Cureus 2023, 15, e44359. [Google Scholar] [CrossRef]
- Silva, P.; Jacobs, D.; Kriak, J.; Abu-Baker, A.; Udeani, G.; Neal, G.; Ramos, K. Implementation of Pharmacogenomics and Artificial Intelligence Tools for Chronic Disease Management in Primary Care Setting. J. Pers. Med. 2021, 11, 443. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The Artificial Intelligence and Machine Learning in Lung Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 55. [Google Scholar] [CrossRef]
- Olawade, D.B.; Aderinto, N.; Olatunji, G.; Kokori, E.; David-Olawade, A.C.; Hadi, M. Advancements and Applications of Artificial Intelligence in Cardiology: Current Trends and Future Prospects. J. Med. Surgery Public Health 2024, 3, 100109. [Google Scholar] [CrossRef]
- Guo, R.; Tian, X.; Bazoukis, G.; Tse, G.; Hong, S.; Chen, K.; Liu, T. Application of Artificial Intelligence in the Diagnosis and Treatment of Cardiac Arrhythmia. Pacing Clin. Electrophysiol. 2024, 47, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Wada, O.Z.; Odetayo, A.; David-Olawade, A.C.; Asaolu, F.; Eberhardt, J. Enhancing Mental Health with Artificial Intelligence: Current Trends and Future Prospects. J. Med. Surgery Public Health 2024, 3, 100099. [Google Scholar] [CrossRef]
- Poirot, M.G.; Ruhe, H.G.; Mutsaerts, H.-J.M.M.; Maximov, I.I.; Groote, I.R.; Bjørnerud, A.; Marquering, H.A.; Reneman, L.; Caan, M.W.A. Treatment Response Prediction in Major Depressive Disorder Using Multimodal MRI and Clinical Data: Secondary Analysis of a Randomized Clinical Trial. Am. J. Psychiatry 2024, 181, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Nasarian, E.; Alizadehsani, R.; Acharya, U.R.; Tsui, K.-L. Designing Interpretable ML System to Enhance Trust in Healthcare: A Systematic Review to Proposed Responsible Clinician-AI-Collaboration Framework. Inf. Fusion 2024, 108, 102412. [Google Scholar] [CrossRef]
- Li, Y.-H.; Li, Y.-L.; Wei, M.-Y.; Li, G.-Y. Innovation and Challenges of Artificial Intelligence Technology in Personalized Healthcare. Sci. Rep. 2024, 14, 18994. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Zhang, Y.; Zhu, H. Medical Image Analysis Using Deep Learning Algorithms. Front. Public Health 2023, 11, 1273253. [Google Scholar] [CrossRef]
- Yu, M.-Y.; Son, Y.-J. Machine Learning–Based 30-Day Readmission Prediction Models for Patients with Heart Failure: A Systematic Review. Eur. J. Cardiovasc. Nurs. 2024, 23, 711–719. [Google Scholar] [CrossRef]
- Allen, A.; Iqbal, Z.; Green-Saxena, A.; Hurtado, M.; Hoffman, J.; Mao, Q.; Das, R. Prediction of Diabetic Kidney Disease with Machine Learning Algorithms, upon the Initial Diagnosis of Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2022, 10, e002560. [Google Scholar] [CrossRef]
- Cè, M.; Caloro, E.; Pellegrino, M.E.; Basile, M.; Sorce, A.; Fazzini, D.; Oliva, G.; Cellina, M. Artificial Intelligence in Breast Cancer Imaging: Risk Stratification, Lesion Detection and Classification, Treatment Planning and Prognosis—A Narrative Review. Explor. Target. Anti-tumor Ther. 2022, 3, 795–816. [Google Scholar] [CrossRef]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Mari Aparici, C.; et al. A Deep Learning Model to Predict a Diagnosis of Alzheimer Disease by Using 18 F-FDG PET of the Brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Vrudhula, A.; Kwan, A.C.; Ouyang, D.; Cheng, S. Machine Learning and Bias in Medical Imaging: Opportunities and Challenges. Circ. Cardiovasc. Imaging 2024, 17, e015495. [Google Scholar] [CrossRef]
- A., S.; R., S. A Systematic Review of Explainable Artificial Intelligence Models and Applications: Recent Developments and Future Trends. Decis. Anal. J. 2023, 7, 100230. [Google Scholar] [CrossRef]
- Yadav, N.; Pandey, S.; Gupta, A.; Dudani, P.; Gupta, S.; Rangarajan, K. Data Privacy in Healthcare: In the Era of Artificial Intelligence. Indian Dermatol. Online J. 2023, 14, 788–792. [Google Scholar] [CrossRef]
- Gu, X.; Sabrina, F.; Fan, Z.; Sohail, S. A Review of Privacy Enhancement Methods for Federated Learning in Healthcare Systems. Int. J. Environ. Res. Public Health 2023, 20, 6539. [Google Scholar] [CrossRef]
- Salvi, M.; Loh, H.W.; Seoni, S.; Barua, P.D.; García, S.; Molinari, F.; Acharya, U.R. Multi-Modality Approaches for Medical Support Systems: A Systematic Review of the Last Decade. Inf. Fusion 2024, 103, 102134. [Google Scholar] [CrossRef]
- Guo, J.; Fathi Kazerooni, A.; Toorens, E.; Akbari, H.; Yu, F.; Sako, C.; Mamourian, E.; Shinohara, R.T.; Koumenis, C.; Bagley, S.J.; et al. Integrating Imaging and Genomic Data for the Discovery of Distinct Glioblastoma Subtypes: A Joint Learning Approach. Sci. Rep. 2024, 14, 4922. [Google Scholar] [CrossRef]
- Saxena, S.; Jena, B.; Gupta, N.; Das, S.; Sarmah, D.; Bhattacharya, P.; Nath, T.; Paul, S.; Fouda, M.M.; Kalra, M.; et al. Role of Artificial Intelligence in Radiogenomics for Cancers in the Era of Precision Medicine. Cancers 2022, 14, 2860. [Google Scholar] [CrossRef]
- Tunali, I.; Gillies, R.J.; Schabath, M.B. Application of Radiomics and Artificial Intelligence for Lung Cancer Precision Medicine. Cold Spring Harb. Perspect. Med. 2021, 11, a039537. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, M.; Naseem, U.; Nezhadmoghadam, F.; Jatoi, M.A.; Gulliver, T.A.; Tamez-Peña, J.G. Breast Cancer Risk Prediction Using Machine Learning: A Systematic Review. Front. Oncol. 2024, 14, 1343627. [Google Scholar] [CrossRef]
- Alsubaie, M.G.; Luo, S.; Shaukat, K. Alzheimer’s Disease Detection Using Deep Learning on Neuroimaging: A Systematic Review. Mach. Learn. Knowl. Extr. 2024, 6, 464–505. [Google Scholar] [CrossRef]
- Medhi, D.; Kamidi, S.R.; Mamatha Sree, K.P.; Shaikh, S.; Rasheed, S.; Thengu Murichathil, A.H.; Nazir, Z. Artificial Intelligence and Its Role in Diagnosing Heart Failure: A Narrative Review. Cureus 2024, 16, e59661. [Google Scholar] [CrossRef] [PubMed]
- Chiarito, M.; Luceri, L.; Oliva, A.; Stefanini, G.; Condorelli, G. Artificial Intelligence and Cardiovascular Risk Prediction: All That Glitters Is Not Gold. Eur. Cardiol. Rev. 2022, 17, e29. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Pal, S.; Lee, S.-S. From Machine Learning to Deep Learning: Advances of the Recent Data-Driven Paradigm Shift in Medicine and Healthcare. Curr. Res. Biotechnol. 2024, 7, 100164. [Google Scholar] [CrossRef]
- Harishbhai Tilala, M.; Kumar Chenchala, P.; Choppadandi, A.; Kaur, J.; Naguri, S.; Saoji, R.; Devaguptapu, B. Ethical Considerations in the Use of Artificial Intelligence and Machine Learning in Health Care: A Comprehensive Review. Cureus 2024, 16, e62443. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable Artificial Intelligence Biosensor Networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Kang, H.S.; Exworthy, M. Wearing the Future—Wearables to Empower Users to Take Greater Responsibility for Their Health and Care: Scoping Review. JMIR mHealth uHealth 2022, 10, e35684. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR mHealth uHealth 2020, 8, e18907. [Google Scholar] [CrossRef]
- Neri, L.; Oberdier, M.T.; van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef]
- Chiang, P.-H.; Wong, M.; Dey, S. Using Wearables and Machine Learning to Enable Personalized Lifestyle Recommendations to Improve Blood Pressure. IEEE J. Transl. Eng. Health Med. 2021, 9, 2700513. [Google Scholar] [CrossRef]
- Ehizogie Paul Adeghe; Chioma Anthonia Okolo; Olumuyiwa Tolulope Ojeyinka A Review of Wearable Technology in Healthcare: Monitoring Patient Health and Enhancing Outcomes. Open Access Res. J. Multidiscip. Stud. 2024, 7, 142–148. [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The Application of Digital Technologies and Artificial Intelligence in Healthcare: An Overview on Nutrition Assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and Regulatory Challenges of AI Technologies in Healthcare: A Narrative Review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef] [PubMed]
- Junaid, S.B.; Imam, A.A.; Balogun, A.O.; De Silva, L.C.; Surakat, Y.A.; Kumar, G.; Abdulkarim, M.; Shuaibu, A.N.; Garba, A.; Sahalu, Y.; et al. Recent Advancements in Emerging Technologies for Healthcare Management Systems: A Survey. Healthcare 2022, 10, 1940. [Google Scholar] [CrossRef]
- Peyroteo, M.; Ferreira, I.A.; Elvas, L.B.; Ferreira, J.C.; Lapão, L.V. Remote Monitoring Systems for Patients With Chronic Diseases in Primary Health Care: Systematic Review. JMIR mHealth uHealth 2021, 9, e28285. [Google Scholar] [CrossRef]
- Kobe, E.A.; McVeigh, T.; Hameed, I.; Fudim, M. Heart Failure Remote Monitoring: A Review and Implementation How-To. J. Clin. Med. 2023, 12, 6200. [Google Scholar] [CrossRef]
- Barrett, M.; Boyne, J.; Brandts, J.; Brunner-La Rocca, H.-P.; De Maesschalck, L.; De Wit, K.; Dixon, L.; Eurlings, C.; Fitzsimons, D.; Golubnitschaja, O.; et al. Artificial Intelligence Supported Patient Self-Care in Chronic Heart Failure: A Paradigm Shift from Reactive to Predictive, Preventive and Personalised Care. EPMA J. 2019, 10, 445–464. [Google Scholar] [CrossRef]
- Krzesiński, P. Digital Health Technologies for Post-Discharge Care after Heart Failure Hospitalisation to Relieve Symptoms and Improve Clinical Outcomes. J. Clin. Med. 2023, 12, 2373. [Google Scholar] [CrossRef]
- Gala, D.; Behl, H.; Shah, M.; Makaryus, A.N. The Role of Artificial Intelligence in Improving Patient Outcomes and Future of Healthcare Delivery in Cardiology: A Narrative Review of the Literature. Healthcare 2024, 12, 481. [Google Scholar] [CrossRef]
- Serrano, L.P.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Eldaly, A.S.; Haider, C.R.; Felton, C.L.; Paulson, M.R.; Maniaci, M.J.; et al. Benefits and Challenges of Remote Patient Monitoring as Perceived by Health Care Practitioners: A Systematic Review. Perm. J. 2023, 27, 100–111. [Google Scholar] [CrossRef]
- Jaime, F.J.; Muñoz, A.; Rodríguez-Gómez, F.; Jerez-Calero, A. Strengthening Privacy and Data Security in Biomedical Microelectromechanical Systems by IoT Communication Security and Protection in Smart Healthcare. Sensors 2023, 23, 8944. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Singh, N.; Jonassen, Z.; Groom, L.L.; Alfaro Arias, V.; Mandal, S.; Schoenthaler, A.; Mann, D.; Nov, O.; Dove, G. Operational Implementation of Remote Patient Monitoring Within a Large Ambulatory Health System: Multimethod Qualitative Case Study. JMIR Hum. Factors 2023, 10, e45166. [Google Scholar] [CrossRef]
- Iqbal, J.; Cortés Jaimes, D.C.; Makineni, P.; Subramani, S.; Hemaida, S.; Thugu, T.R.; Butt, A.N.; Sikto, J.T.; Kaur, P.; Lak, M.A.; et al. Reimagining Healthcare: Unleashing the Power of Artificial Intelligence in Medicine. Cureus 2023, 15, e44658. [Google Scholar] [CrossRef] [PubMed]
- Fraiche, A.M.; Matlock, D.D.; Gabriel, W.; Rapley, F.-A.; Kramer, D.B. Patient and Provider Perspectives on Remote Monitoring of Pacemakers and Implantable Cardioverter-Defibrillators. Am. J. Cardiol. 2021, 149, 42–46. [Google Scholar] [CrossRef]
- Alhejaili, R.; Alomainy, A. The Use of Wearable Technology in Providing Assistive Solutions for Mental Well-Being. Sensors 2023, 23, 7378. [Google Scholar] [CrossRef]
- Wang, W.-H.; Hsu, W.-S. Integrating Artificial Intelligence and Wearable IoT System in Long-Term Care Environments. Sensors 2023, 23, 5913. [Google Scholar] [CrossRef]
- Salehi, C.; Kershaw, K.A.; Storer, B.; Newby, J.; Murphy, M.J. Multi-Modal Management of Severe Health Anxiety in Diabetes Mellitus Type 1 in a Medical Clinic Setting: Including the Use of Blended Care Cognitive Behavioural Therapy and Pharmacotherapy. Psychiatry Res. Case Rep. 2024, 3, 100209. [Google Scholar] [CrossRef]
- Kong, S.-Y.; Cho, M.-K. Effects of Continuous Glucose Monitoring on Glycemic Control in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 571. [Google Scholar] [CrossRef]
- Ansari, Y.; Mourad, O.; Qaraqe, K.; Serpedin, E. Deep Learning for ECG Arrhythmia Detection and Classification: An Overview of Progress for Period 2017–2023. Front. Physiol. 2023, 14, 1246746. [Google Scholar] [CrossRef]
- Hiraoka, D.; Inui, T.; Kawakami, E.; Oya, M.; Tsuji, A.; Honma, K.; Kawasaki, Y.; Ozawa, Y.; Shiko, Y.; Ueda, H.; et al. Diagnosis of Atrial Fibrillation Using Machine Learning With Wearable Devices After Cardiac Surgery: Algorithm Development Study. JMIR Form. Res. 2022, 6, e35396. [Google Scholar] [CrossRef]
- Watson, A.; Wilkinson, T.M.A. Digital Healthcare in COPD Management: A Narrative Review on the Advantages, Pitfalls, and Need for Further Research. Ther. Adv. Respir. Dis. 2022, 16, 175346662210754. [Google Scholar] [CrossRef] [PubMed]
- Almansouri, N.E.; Awe, M.; Rajavelu, S.; Jahnavi, K.; Shastry, R.; Hasan, A.; Hasan, H.; Lakkimsetti, M.; AlAbbasi, R.K.; Gutiérrez, B.C.; et al. Early Diagnosis of Cardiovascular Diseases in the Era of Artificial Intelligence: An In-Depth Review. Cureus 2024, 16, e55869. [Google Scholar] [CrossRef] [PubMed]
- Azodo, I.; Williams, R.; Sheikh, A.; Cresswell, K. Opportunities and Challenges Surrounding the Use of Data From Wearable Sensor Devices in Health Care: Qualitative Interview Study. J. Med. Internet Res. 2020, 22, e19542. [Google Scholar] [CrossRef] [PubMed]
- Roos, L.G.; Slavich, G.M. Wearable Technologies for Health Research: Opportunities, Limitations, and Practical and Conceptual Considerations. Brain. Behav. Immun. 2023, 113, 444–452. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Truong, N.; Sun, K.; Wang, S.; Guitton, F.; Guo, Y. Privacy Preservation in Federated Learning: An Insightful Survey from the GDPR Perspective. Comput. Secur. 2021, 110, 102402. [Google Scholar] [CrossRef]
- Yang, S.; Varghese, P.; Stephenson, E.; Tu, K.; Gronsbell, J. Machine Learning Approaches for Electronic Health Records Phenotyping: A Methodical Review. J. Am. Med. Inform. Assoc. 2023, 30, 367–381. [Google Scholar] [CrossRef]
- Adam, R.; Dell’Aquila, K.; Hodges, L.; Maldjian, T.; Duong, T.Q. Deep Learning Applications to Breast Cancer Detection by Magnetic Resonance Imaging: A Literature Review. Breast Cancer Res. 2023, 25, 87. [Google Scholar] [CrossRef]
- Adiwinata, R.; Tandarto, K.; Arifputra, J.; Waleleng, B.; Gosal, F.; Rotty, L.; Winarta, J.; Waleleng, A.; Simadibrata, P.; Simadibrata, M. The Impact of Artificial Intelligence in Improving Polyp and Adenoma Detection Rate During Colonoscopy: Systematic-Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2023, 24, 3655–3663. [Google Scholar] [CrossRef]
- Kumari, V.; Kumar, N.; Kumar, K.S.; Kumar, A.; Skandha, S.S.; Saxena, S.; Khanna, N.N.; Laird, J.R.; Singh, N.; Fouda, M.M.; et al. Deep Learning Paradigm and Its Bias for Coronary Artery Wall Segmentation in Intravascular Ultrasound Scans: A Closer Look. J. Cardiovasc. Dev. Dis. 2023, 10, 485. [Google Scholar] [CrossRef]
- Chang, Z.; Zhan, Z.; Zhao, Z.; You, Z.; Liu, Y.; Yan, Z.; Fu, Y.; Liang, W.; Zhao, L. Application of Artificial Intelligence in COVID-19 Medical Area: A Systematic Review. J. Thorac. Dis. 2021, 13, 7034–7053. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kataria, A.; Bhambri, P.; Pareek, P.K.; Puri, V. Artificial Intelligence in Personalized Health Services for Better Patient Care. In Revolutionizing Healthcare: AI Integration with IoT for Enhanced Patient Outcomes; Springer Nature: Cham, Switzerland, 2024; pp. 89–108. [Google Scholar]
- Quazi, S. Artificial Intelligence and Machine Learning in Precision and Genomic Medicine. Med. Oncol. 2022, 39, 120. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Takano, K.; Katahira, K.; Kimura, K. Use Patterns of Smartphone Apps and Wearable Devices Supporting Physical Activity and Exercise: Large-Scale Cross-Sectional Survey. JMIR mHealth uHealth 2023, 11, e49148. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Soltan, A.A.S.; Eyre, D.W.; Clifton, D.A. Algorithmic Fairness and Bias Mitigation for Clinical Machine Learning with Deep Reinforcement Learning. Nat. Mach. Intell. 2023, 5, 884–894. [Google Scholar] [CrossRef]
- Pool, J.; Akhlaghpour, S.; Fatehi, F.; Burton-Jones, A. A Systematic Analysis of Failures in Protecting Personal Health Data: A Scoping Review. Int. J. Inf. Manage. 2024, 74, 102719. [Google Scholar] [CrossRef]
- Huhn, S.; Axt, M.; Gunga, H.-C.; Maggioni, M.A.; Munga, S.; Obor, D.; Sié, A.; Boudo, V.; Bunker, A.; Sauerborn, R.; et al. The Impact of Wearable Technologies in Health Research: Scoping Review. JMIR mHealth uHealth 2022, 10, e34384. [Google Scholar] [CrossRef]
- Hossain, E.; Rana, R.; Higgins, N.; Soar, J.; Barua, P.D.; Pisani, A.R.; Turner, K. Natural Language Processing in Electronic Health Records in Relation to Healthcare Decision-Making: A Systematic Review. Comput. Biol. Med. 2023, 155, 106649. [Google Scholar] [CrossRef]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Mirzaei, T.; Dharanikota, S. Patients’ Perceptions Toward Human–Artificial Intelligence Interaction in Health Care: Experimental Study. J. Med. Internet Res. 2021, 23, e25856. [Google Scholar] [CrossRef]
- Satyanarayana Rao, K. Informed Consent: An Ethical Obligation or Legal Compulsion? J. Cutan. Aesthet. Surg. 2008, 1, 33. [Google Scholar] [CrossRef]
- Nazer, L.H.; Zatarah, R.; Waldrip, S.; Ke, J.X.C.; Moukheiber, M.; Khanna, A.K.; Hicklen, R.S.; Moukheiber, L.; Moukheiber, D.; Ma, H.; et al. Bias in Artificial Intelligence Algorithms and Recommendations for Mitigation. PLOS Digit. Health 2023, 2, e0000278. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Brooks, J.R.; Alford, C.C.; Chang, C.S.; Mueller, N.M.; Umscheid, C.A.; Bierman, A.S. Awareness of Racial and Ethnic Bias and Potential Solutions to Address Bias With Use of Health Care Algorithms. JAMA Health Forum 2023, 4, e231197. [Google Scholar] [CrossRef] [PubMed]
- Ueda, D.; Kakinuma, T.; Fujita, S.; Kamagata, K.; Fushimi, Y.; Ito, R.; Matsui, Y.; Nozaki, T.; Nakaura, T.; Fujima, N.; et al. Fairness of Artificial Intelligence in Healthcare: Review and Recommendations. Jpn. J. Radiol. 2024, 42, 3–15. [Google Scholar] [CrossRef]
- Cheong, B.C. Transparency and Accountability in AI Systems: Safeguarding Wellbeing in the Age of Algorithmic Decision-Making. Front. Hum. Dyn. 2024, 6, 1421273. [Google Scholar] [CrossRef]
- Metta, C.; Beretta, A.; Pellungrini, R.; Rinzivillo, S.; Giannotti, F. Towards Transparent Healthcare: Advancing Local Explanation Methods in Explainable Artificial Intelligence. Bioengineering 2024, 11, 369. [Google Scholar] [CrossRef]
- Bottomley, D.; Thaldar, D. Liability for Harm Caused by AI in Healthcare: An Overview of the Core Legal Concepts. Front. Pharmacol. 2023, 14, 1297353. [Google Scholar] [CrossRef]
- Enqvist, L. ‘Human Oversight’ in the EU Artificial Intelligence Act: What, When and by Whom? Law Innov. Technol. 2023, 15, 508–535. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Balaji, S.; Jeyaraman, N.; Yadav, S. Unraveling the Ethical Enigma: Artificial Intelligence in Healthcare. Cureus 2023, 15, e43262. [Google Scholar] [CrossRef]
- Khalid, N.; Qayyum, A.; Bilal, M.; Al-Fuqaha, A.; Qadir, J. Privacy-Preserving Artificial Intelligence in Healthcare: Techniques and Applications. Comput. Biol. Med. 2023, 158, 106848. [Google Scholar] [CrossRef]
- Lievevrouw, E.; Marelli, L.; Van Hoyweghen, I. The FDA’s Standard-Making Process for Medical Digital Health Technologies: Co-Producing Technological and Organizational Innovation. Biosocieties 2022, 17, 549–576. [Google Scholar] [CrossRef]
- Aboy, M.; Minssen, T.; Vayena, E. Navigating the EU AI Act: Implications for Regulated Digital Medical Products. npj Digit. Med. 2024, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.B.; Harvey, H.; Rubin, D.L.; Irani, N.; Tse, J.R.; Langlotz, C.P. Regulatory Frameworks for Development and Evaluation of Artificial Intelligence–Based Diagnostic Imaging Algorithms: Summary and Recommendations. J. Am. Coll. Radiol. 2021, 18, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zuchowski, L.C.; Zuchowski, M.L.; Nagel, E. A Trust Based Framework for the Envelopment of Medical AI. npj Digit. Med. 2024, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Lämmermann, L.; Hofmann, P.; Urbach, N. Managing Artificial Intelligence Applications in Healthcare: Promoting Information Processing among Stakeholders. Int. J. Inf. Manage. 2024, 75, 102728. [Google Scholar] [CrossRef]
- Hines, P.A.; Gonzalez-Quevedo, R.; Lambert, A.I.O.M.; Janssens, R.; Freischem, B.; Torren Edo, J.; Claassen, I.J.T.M.; Humphreys, A.J. Regulatory Science to 2025: An Analysis of Stakeholder Responses to the European Medicines Agency’s Strategy. Front. Med. 2020, 7, 508. [Google Scholar] [CrossRef]
- Derraz, B.; Breda, G.; Kaempf, C.; Baenke, F.; Cotte, F.; Reiche, K.; Köhl, U.; Kather, J.N.; Eskenazy, D.; Gilbert, S. New Regulatory Thinking Is Needed for AI-Based Personalised Drug and Cell Therapies in Precision Oncology. npj Precis. Oncol. 2024, 8, 23. [Google Scholar] [CrossRef]
- Zinchenko, V.V.; Arzamasov, K.M.; Chetverikov, S.F.; Maltsev, A.V.; Novik, V.P.; Akhmad, E.S.; Sharova, D.E.; Andreychenko, A.E.; Vladzymyrskyy, A.V.; Morozov, S.P. Methodology for Conducting Post-Marketing Surveillance of Software as a Medical Device Based on Artificial Intelligence Technologies. Sovrem. Tehnol. V Med. 2022, 14, 15. [Google Scholar] [CrossRef]
- Palaniappan, K.; Lin, E.Y.T.; Vogel, S. Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare 2024, 12, 562. [Google Scholar] [CrossRef]
- Aerts, A.; Bogdan-Martin, D. Leveraging Data and AI to Deliver on the Promise of Digital Health. Int. J. Med. Inform. 2021, 150, 104456. [Google Scholar] [CrossRef]
- Goyal, P.; Malviya, R. Challenges and Opportunities of Big Data Analytics in Healthcare. Health Care Sci. 2023, 2, 328–338. [Google Scholar] [CrossRef]
- Vorisek, C.N.; Lehne, M.; Klopfenstein, S.A.I.; Mayer, P.J.; Bartschke, A.; Haese, T.; Thun, S. Fast Healthcare Interoperability Resources (FHIR) for Interoperability in Health Research: Systematic Review. JMIR Med. Inform. 2022, 10, e35724. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Systematic Review of Data-Centric Approaches in Artificial Intelligence and Machine Learning. Data Sci. Manag. 2023, 6, 144–157. [Google Scholar] [CrossRef]
- Celi, L.A.; Cellini, J.; Charpignon, M.-L.; Dee, E.C.; Dernoncourt, F.; Eber, R.; Mitchell, W.G.; Moukheiber, L.; Schirmer, J.; Situ, J.; et al. Sources of Bias in Artificial Intelligence That Perpetuate Healthcare Disparities—A Global Review. PLOS Digit. Health 2022, 1, e0000022. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, M.; Zhao, H.; Peng, Y.; Huang, F.; Lu, Z. A Survey of Recent Methods for Addressing AI Fairness and Bias in Biomedicine. J. Biomed. Inform. 2024, 154, 104646. [Google Scholar] [CrossRef] [PubMed]
- Steidl, M.; Felderer, M.; Ramler, R. The Pipeline for the Continuous Development of Artificial Intelligence Models—Current State of Research and Practice. J. Syst. Softw. 2023, 199, 111615. [Google Scholar] [CrossRef]
- Haritha, P. Resham Lohani Impact of AI Technology Disruption on Turnover Intention of Employees in Digital Marketing. Int. Res. J. Adv. Eng. Manag. 2024, 2, 389–400. [Google Scholar] [CrossRef]
- Charow, R.; Jeyakumar, T.; Younus, S.; Dolatabadi, E.; Salhia, M.; Al-Mouaswas, D.; Anderson, M.; Balakumar, S.; Clare, M.; Dhalla, A.; et al. Artificial Intelligence Education Programs for Health Care Professionals: Scoping Review. JMIR Med. Educ. 2021, 7, e31043. [Google Scholar] [CrossRef]
- Helman, S.; Terry, M.A.; Pellathy, T.; Williams, A.; Dubrawski, A.; Clermont, G.; Pinsky, M.R.; Al-Zaiti, S.; Hravnak, M. Engaging Clinicians Early during the Development of a Graphical User Display of an Intelligent Alerting System at the Bedside. Int. J. Med. Inform. 2022, 159, 104643. [Google Scholar] [CrossRef]
- Haefner, N.; Parida, V.; Gassmann, O.; Wincent, J. Implementing and Scaling Artificial Intelligence: A Review, Framework, and Research Agenda. Technol. Forecast. Soc. Change 2023, 197, 122878. [Google Scholar] [CrossRef]
- khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef]
- Karpathakis, K.; Morley, J.; Floridi, L. A Justifiable Investment in AI for Healthcare: Aligning Ambition with Reality. SSRN Electron. J. 2024, 34, 38. [Google Scholar] [CrossRef]
- Farhud, D.D.; Zokaei, S. Ethical Issues of Artificial Intelligence in Medicine and Healthcare. Iran. J. Public Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Yelne, S.; Chaudhary, M.; Dod, K.; Sayyad, A.; Sharma, R. Harnessing the Power of AI: A Comprehensive Review of Its Impact and Challenges in Nursing Science and Healthcare. Cureus 2023, 15, e49252. [Google Scholar] [CrossRef]
- Akinrinmade, A.O.; Adebile, T.M.; Ezuma-Ebong, C.; Bolaji, K.; Ajufo, A.; Adigun, A.O.; Mohammad, M.; Dike, J.C.; Okobi, O.E. Artificial Intelligence in Healthcare: Perception and Reality. Cureus 2023, 15, e45594. [Google Scholar] [CrossRef]
- Panch, T.; Mattie, H.; Atun, R. Artificial Intelligence and Algorithmic Bias: Implications for Health Systems. J. Glob. Health 2019, 9, 020318. [Google Scholar] [CrossRef]
- Balasubramaniam, N.; Kauppinen, M.; Rannisto, A.; Hiekkanen, K.; Kujala, S. Transparency and Explainability of AI Systems: From Ethical Guidelines to Requirements. Inf. Softw. Technol. 2023, 159, 107197. [Google Scholar] [CrossRef]
- Batra Deep Manishkumar, P. Revolutionizing Healthcare Platforms: The Impact of AI on Patient Engagement and Treatment Efficacy. Int. J. Sci. Res. 2024, 13, 273–280. [Google Scholar] [CrossRef]
- Seh, A.H.; Zarour, M.; Alenezi, M.; Sarkar, A.K.; Agrawal, A.; Kumar, R.; Ahmad Khan, R. Healthcare Data Breaches: Insights and Implications. Healthcare 2020, 8, 133. [Google Scholar] [CrossRef]
- Xiang, D.; Cai, W. Privacy Protection and Secondary Use of Health Data: Strategies and Methods. Biomed Res. Int. 2021, 2021, 6967166. [Google Scholar] [CrossRef]
- Broekhuizen, T.; Dekker, H.; de Faria, P.; Firk, S.; Nguyen, D.K.; Sofka, W. AI for Managing Open Innovation: Opportunities, Challenges, and a Research Agenda. J. Bus. Res. 2023, 167, 114196. [Google Scholar] [CrossRef]
- Gim, N.; Wu, Y.; Blazes, M.; Lee, C.S.; Wang, R.K.; Lee, A.Y. A Clinician’s Guide to Sharing Data for AI in Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, M.; Martin, R.; Heider, D. Privacy-Preserving Decentralized Learning Methods for Biomedical Applications. Comput. Struct. Biotechnol. J. 2024, 23, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Aliyu, A.; Evans, M.; Luo, C. Health Care Cybersecurity Challenges and Solutions Under the Climate of COVID-19: Scoping Review. J. Med. Internet Res. 2021, 23, e21747. [Google Scholar] [CrossRef]
- Clarke, M.; Martin, K. Managing Cybersecurity Risk in Healthcare Settings. Healthc. Manag. Forum 2024, 37, 17–20. [Google Scholar] [CrossRef]
- Cichonski, P.; Millar, T.; Grance, T.; Scarfone, K. Computer Security Incident Handling Guide: Recommendations of the National Institute of Standards and Technology; NIST: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Murdoch, B. Privacy and Artificial Intelligence: Challenges for Protecting Health Information in a New Era. BMC Med. Ethics 2021, 22, 122. [Google Scholar] [CrossRef]
- Banerjee, S.; Alsop, P.; Jones, L.; Cardinal, R.N. Patient and Public Involvement to Build Trust in Artificial Intelligence: A Framework, Tools, and Case Studies. Patterns 2022, 3, 100506. [Google Scholar] [CrossRef]
- Beil, M.; Proft, I.; van Heerden, D.; Sviri, S.; van Heerden, P.V. Ethical Considerations about Artificial Intelligence for Prognostication in Intensive Care. Intensive Care Med. Exp. 2019, 7, 70. [Google Scholar] [CrossRef]
- Reddy, S. Navigating the AI Revolution: The Case for Precise Regulation in Health Care. J. Med. Internet Res. 2023, 25, e49989. [Google Scholar] [CrossRef]
- Moore, W.; Frye, S. Review of HIPAA, Part 1: History, Protected Health Information, and Privacy and Security Rules. J. Nucl. Med. Technol. 2019, 47, 269–272. [Google Scholar] [CrossRef]
- Jain, V.; Balakrishnan, A.; Beeram, D.; Najana, M.; Chintale, P. Leveraging Artificial Intelligence for Enhancing Regulatory Compliance in the Financial Sector. Int. J. Comput. Trends Technol. 2024, 72, 124–140. [Google Scholar] [CrossRef]
- King, M.R. The Future of AI in Medicine: A Perspective from a Chatbot. Ann. Biomed. Eng. 2023, 51, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Pranav, A.; Kohli, A.; Ghosh, S.; Singh, D. The Contribution of Artificial Intelligence to Drug Discovery: Current Progress and Prospects for the Future. In Microbial Data Intelligence and Computational Techniques for Sustainable Computing; Springer: Berlin, Germany, 2024; pp. 1–23. [Google Scholar]
- Snigdha, E.Z.; Hossain, M.D.R.; Mahabub, S. AI-Powered Healthcare Tracker Development: Advancing Real-Time Patient Monitoring and Predictive Analytics Through Data-Driven Intelligence. J. Comput. Sci. Technol. Stud. 2023, 5, 229–239. [Google Scholar] [CrossRef]
- Dias, R.; Torkamani, A. Artificial Intelligence in Clinical and Genomic Diagnostics. Genome Med. 2019, 11, 70. [Google Scholar] [CrossRef]
- Perez, K.; Wisniewski, D.; Ari, A.; Lee, K.; Lieneck, C.; Ramamonjiarivelo, Z. Investigation into Application of AI and Telemedicine in Rural Communities: A Systematic Literature Review. Healthcare 2025, 13, 324. [Google Scholar] [CrossRef]
- Abrishami, M.; Pourabdolah, F.; Dalvandi, B.; Dalvandi, R.; Sarrafan, N.; Noorizadeh, S.; Etezadinia, A.; Negargar, S. Robotic Assist and Virtual Surgical Planning in Orthognathic Surgery. Galen Med. J. 2024, 13, e3672. [Google Scholar] [CrossRef]
- Giri, A.; Ud Din, F. Role of Data as an Interface between Primary, Secondary and Tertiary Care: Evidence from Literature. Inform. Health 2025, 2, 63–72. [Google Scholar] [CrossRef]
- Vilhekar, R.S.; Rawekar, A. Artificial Intelligence in Genetics. Cureus 2024, 16, e52035. [Google Scholar] [CrossRef] [PubMed]
- Santorsola, M.; Lescai, F. The Promise of Explainable Deep Learning for Omics Data Analysis: Adding New Discovery Tools to AI. New Biotechnol. 2023, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khoshandam, M.; Soltaninejad, H.; Mousazadeh, M.; Hamidieh, A.A.; Hosseinkhani, S. Clinical Applications of the CRISPR/Cas9 Genome-Editing System: Delivery Options and Challenges in Precision Medicine. Genes Dis. 2024, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Skepu, A.; Kim, N.; Mkhabele, M.; Khanyile, R.; Molefi, T.; Mbatha, S.; Setlai, B.; Mulaudzi, T.; Mabongo, M.; et al. AI and Precision Oncology in Clinical Cancer Genomics: From Prevention to Targeted Cancer Therapies-an Outcomes Based Patient Care. Inform. Med. Unlocked 2022, 31, 100965. [Google Scholar] [CrossRef]
- Zafar, I.; Rafique, A.; Fazal, J.; Manzoor, M.; Ul Ain, Q.; Rayan, R.A. Genome and Gene Editing by Artificial Intelligence Programs. In Advanced AI Techniques and Applications in Bioinformatics; CRC Press: Boca Raton, FL, USA, 2021; pp. 165–188. [Google Scholar]
- Qiu, X.; Li, H.; Ver Steeg, G.; Godzik, A. Advances in AI for Protein Structure Prediction: Implications for Cancer Drug Discovery and Development. Biomolecules 2024, 14, 339. [Google Scholar] [CrossRef]
- Lawson, C.E.; Martí, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J.; et al. Machine Learning for Metabolic Engineering: A Review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. How Could Artificial Intelligence Be Used to Increase the Potential of Biorefineries in the near Future? A Review. Environ. Technol. Innov. 2023, 32, 103277. [Google Scholar] [CrossRef]
- Jänes, J.; Beltrao, P. Deep Learning for Protein Structure Prediction and Design—Progress and Applications. Mol. Syst. Biol. 2024, 20, 162–169. [Google Scholar] [CrossRef]
- AlQuraishi, M. Machine Learning in Protein Structure Prediction. Curr. Opin. Chem. Biol. 2021, 65, 1–8. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial Intelligence in Drug Discovery and Development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Gu, N. Application of Artificial Intelligence in the Exploration and Optimization of Biomedical Nanomaterials. Nano Biomed. Eng. 2023, 15, 342–353. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Amorim, M.J.B. Using Machine Learning to Make Nanomaterials Sustainable. Sci. Total Environ. 2023, 859, 160303. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, V.; Coogan, Á.; Botov, D.; Gromova, Y.; Ushakova, E.V.; Gun’ko, Y.K. Expanding the Horizons of Machine Learning in Nanomaterials to Chiral Nanostructures. Adv. Mater. 2024, 36, 2308912. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Perez, A.; van Tilborg, D.; van der Meel, R.; Grisoni, F.; Albertazzi, L. Machine Learning-Guided High Throughput Nanoparticle Design. Digit. Discov. 2024, 3, 1280–1291. [Google Scholar] [CrossRef]
- Villa Nova, M.; Lin, T.P.; Shanehsazzadeh, S.; Jain, K.; Ng, S.C.Y.; Wacker, R.; Chichakly, K.; Wacker, M.G. Nanomedicine Ex Machina: Between Model-Informed Development and Artificial Intelligence. Front. Digit. Health 2022, 4, 799341. [Google Scholar] [CrossRef]
- Ramesh, S.; Deep, A.; Tamayol, A.; Kamaraj, A.; Mahajan, C.; Madihally, S. Advancing 3D Bioprinting through Machine Learning and Artificial Intelligence. Bioprinting 2024, 38, e00331. [Google Scholar] [CrossRef]
- An, J.; Chua, C.K.; Mironov, V. Application of Machine Learning in 3D Bioprinting: Focus on Development of Big Data and Digital Twin. Int. J. Bioprinting 2024, 7, 342. [Google Scholar] [CrossRef]
- Filippi, M.; Mekkattu, M.; Katzschmann, R.K. Sustainable Biofabrication: From Bioprinting to AI-Driven Predictive Methods. Trends Biotechnol. 2024, 43, 290–303. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Y.; Fang, J.; Li, Q. Machine Learning in Biomaterials, Biomechanics/Mechanobiology, and Biofabrication: State of the Art and Perspective. Arch. Comput. Methods Eng. 2024, 31, 3699–3765. [Google Scholar] [CrossRef]
- Xue, C.; Kowshik, S.S.; Lteif, D.; Puducheri, S.; Jasodanand, V.H.; Zhou, O.T.; Walia, A.S.; Guney, O.B.; Zhang, J.D.; Pham, S.T.; et al. AI-Based Differential Diagnosis of Dementia Etiologies on Multimodal Data. Nat. Med. 2024, 30, 2977–2989. [Google Scholar] [CrossRef]
- Jin, L.; Zhai, X.; Wang, K.; Zhang, K.; Wu, D.; Nazir, A.; Jiang, J.; Liao, W.-H. Big Data, Machine Learning, and Digital Twin Assisted Additive Manufacturing: A Review. Mater. Des. 2024, 244, 113086. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Li, Z.; Hu, J.; Park, S.S.; Kim, K. Optimized 3D Bioprinting Technology Based on Machine Learning: A Review of Recent Trends and Advances. Micromachines 2022, 13, 363. [Google Scholar] [CrossRef]
- Wang, D.; Shen, Z.; Dong, X.; Fang, Q.; Wang, W.; Dong, X.; Xiong, G. Deep Reinforcement Learning for Dynamic Error Compensation in 3D Printing. In Proceedings of the 2023 IEEE 19th International Conference on Automation Science and Engineering (CASE), Auckland, New Zealand, 26–30 August 2023; pp. 1–7. [Google Scholar]
- Ning, H.; Zhou, T.; Joo, S.W. Machine Learning Boosts Three-Dimensional Bioprinting. Int. J. Bioprinting 2023, 9, 739. [Google Scholar] [CrossRef]
- Ng, W.L.; Goh, G.L.; Goh, G.D.; Ten, J.S.J.; Yeong, W.Y. Progress and Opportunities for Machine Learning in Materials and Processes of Additive Manufacturing. Adv. Mater. 2024, 36, 2310006. [Google Scholar] [CrossRef]
- Ma, L.; Yu, S.; Xu, X.; Moses Amadi, S.; Zhang, J.; Wang, Z. Application of Artificial Intelligence in 3D Printing Physical Organ Models. Mater. Today Bio 2023, 23, 100792. [Google Scholar] [CrossRef]
- Haupt, S.E.; Chapman, W.; Adams, S.V.; Kirkwood, C.; Hosking, J.S.; Robinson, N.H.; Lerch, S.; Subramanian, A.C. Towards Implementing Artificial Intelligence Post-Processing in Weather and Climate: Proposed Actions from the Oxford 2019 Workshop. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200091. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, B.; Huang, J. Recent Advances and Applications of Artificial Intelligence in 3D Bioprinting. Biophys. Rev. 2024, 5, 031301. [Google Scholar] [CrossRef]
- Bardini, R.; Di Carlo, S. Computational Methods for Biofabrication in Tissue Engineering and Regenerative Medicine—A Literature Review. Comput. Struct. Biotechnol. J. 2024, 23, 601–616. [Google Scholar] [CrossRef]
- Lancellotti, C.; Cancian, P.; Savevski, V.; Kotha, S.R.R.; Fraggetta, F.; Graziano, P.; Di Tommaso, L. Artificial Intelligence & Tissue Biomarkers: Advantages, Risks and Perspectives for Pathology. Cells 2021, 10, 787. [Google Scholar] [CrossRef]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Chen, X.; Xie, H.; Tao, X.; Wang, F.L.; Leng, M.; Lei, B. Artificial Intelligence and Multimodal Data Fusion for Smart Healthcare: Topic Modeling and Bibliometrics. Artif. Intell. Rev. 2024, 57, 91. [Google Scholar] [CrossRef]
- Ayala Solares, J.R.; Diletta Raimondi, F.E.; Zhu, Y.; Rahimian, F.; Canoy, D.; Tran, J.; Pinho Gomes, A.C.; Payberah, A.H.; Zottoli, M.; Nazarzadeh, M.; et al. Deep Learning for Electronic Health Records: A Comparative Review of Multiple Deep Neural Architectures. J. Biomed. Inform. 2020, 101, 103337. [Google Scholar] [CrossRef] [PubMed]
- Badwan, B.A.; Liaropoulos, G.; Kyrodimos, E.; Skaltsas, D.; Tsirigos, A.; Gorgoulis, V.G. Machine Learning Approaches to Predict Drug Efficacy and Toxicity in Oncology. Cell Rep. Methods 2023, 3, 100413. [Google Scholar] [CrossRef]
- Pun, F.W.; Ozerov, I.V.; Zhavoronkov, A. AI-Powered Therapeutic Target Discovery. Trends Pharmacol. Sci. 2023, 44, 561–572. [Google Scholar] [CrossRef]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial Intelligence in Clinical Medicine: Catalyzing a Sustainable Global Healthcare Paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef]
- Poalelungi, D.G.; Musat, C.L.; Fulga, A.; Neagu, M.; Neagu, A.I.; Piraianu, A.I.; Fulga, I. Advancing Patient Care: How Artificial Intelligence Is Transforming Healthcare. J. Pers. Med. 2023, 13, 1214. [Google Scholar] [CrossRef]
- Cai, L.; Chu, J.; Xu, J.; Meng, Y.; Lu, C.; Tang, X.; Wang, G.; Tian, G.; Yang, J. Machine Learning for Drug Repositioning: Recent Advances and Challenges. Curr. Res. Chem. Biol. 2023, 3, 100042. [Google Scholar] [CrossRef]
- Vatansever, S.; Schlessinger, A.; Wacker, D.; Kaniskan, H.Ü.; Jin, J.; Zhou, M.; Zhang, B. Artificial Intelligence and Machine Learning-aided Drug Discovery in Central Nervous System Diseases: State-of-the-arts and Future Directions. Med. Res. Rev. 2021, 41, 1427–1473. [Google Scholar] [CrossRef]
- Han, R.; Yoon, H.; Kim, G.; Lee, H.; Lee, Y. Revolutionizing Medicinal Chemistry: The Application of Artificial Intelligence (AI) in Early Drug Discovery. Pharmaceuticals 2023, 16, 1259. [Google Scholar] [CrossRef]
- Tsvetanov, F. Integrating AI Technologies into Remote Monitoring Patient Systems. In Proceedings of the International Conference on Electronics, Engineering Physics and Earth Science (EEPES 2024), Kavala, Greece, 19–21 June 2024; p. 54. [Google Scholar]
- Yang, S.; Kar, S. Application of Artificial Intelligence and Machine Learning in Early Detection of Adverse Drug Reactions (ADRs) and Drug-Induced Toxicity. Artif. Intell. Chem. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Yagi, M.; Yamanouchi, K.; Fujita, N.; Funao, H.; Ebata, S. Revolutionizing Spinal Care: Current Applications and Future Directions of Artificial Intelligence and Machine Learning. J. Clin. Med. 2023, 12, 4188. [Google Scholar] [CrossRef]
- Vijayan, R.S.K.; Kihlberg, J.; Cross, J.B.; Poongavanam, V. Enhancing Preclinical Drug Discovery with Artificial Intelligence. Drug Discov. Today 2022, 27, 967–984. [Google Scholar] [CrossRef]
- Coler, E.A.; Chen, W.; Melnik, A.V.; Morton, J.T.; Aksenov, A.A. Metabolomics in the Era of Artificial Intelligence. Microbiota Host 2024, 2, e230017. [Google Scholar] [CrossRef]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial Intelligence in Cancer Target Identification and Drug Discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
- Liu, B.; He, H.; Luo, H.; Zhang, T.; Jiang, J. Artificial Intelligence and Big Data Facilitated Targeted Drug Discovery. Stroke Vasc. Neurol. 2019, 4, 206–213. [Google Scholar] [CrossRef]
- DiNuzzo, M. How Artificial Intelligence Enables Modeling and Simulation of Biological Networks to Accelerate Drug Discovery. Front. Drug Discov. 2022, 2, 1019706. [Google Scholar] [CrossRef]
- Lu, M.; Yin, J.; Zhu, Q.; Lin, G.; Mou, M.; Liu, F.; Pan, Z.; You, N.; Lian, X.; Li, F.; et al. Artificial Intelligence in Pharmaceutical Sciences. Engineering 2023, 27, 37–69. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational Approaches Streamlining Drug Discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Gentile, F.; Yaacoub, J.C.; Gleave, J.; Fernandez, M.; Ton, A.-T.; Ban, F.; Stern, A.; Cherkasov, A. Artificial Intelligence–Enabled Virtual Screening of Ultra-Large Chemical Libraries with Deep Docking. Nat. Protoc. 2022, 17, 672–697. [Google Scholar] [CrossRef]
- Oliveira, T.; Silva, M.; Maia, E.; Silva, A.; Taranto, A. Virtual Screening Algorithms in Drug Discovery: A Review Focused on Machine and Deep Learning Methods. Drugs Drug Candidates 2023, 2, 311–334. [Google Scholar] [CrossRef]
- Anstine, D.M.; Isayev, O. Generative Models as an Emerging Paradigm in the Chemical Sciences. J. Am. Chem. Soc. 2023, 145, 8736–8750. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Luo, Y.; Kang, S.; Tang, J.; Lightstone, F.C.; Fang, E.F.; Cornell, W.; Nussinov, R.; Cheng, F. Deep Generative Molecular Design Reshapes Drug Discovery. Cell Rep. Med. 2022, 3, 100794. [Google Scholar] [CrossRef]
- Bou, A.; Thomas, M.; Dittert, S.; Navarro, C.; Majewski, M.; Wang, Y.; Patel, S.; Tresadern, G.; Ahmad, M.; Moens, V.; et al. ACEGEN: Reinforcement Learning of Generative Chemical Agents for Drug Discovery. J. Chem. Inf. Model. 2024, 64, 5900–5911. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.-C. Machine Learning and Artificial Intelligence in Toxicological Sciences. Toxicol. Sci. 2022, 189, 7–19. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Surya Wibowo, A.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Toxicity Prediction: Recent Advances, Challenges, and Future Perspectives. J. Chem. Inf. Model. 2023, 63, 2628–2643. [Google Scholar] [CrossRef]
- Askin, S.; Burkhalter, D.; Calado, G.; El Dakrouni, S. Artificial Intelligence Applied to Clinical Trials: Opportunities and Challenges. Health Technol. 2023, 13, 203–213. [Google Scholar] [CrossRef]
- Lu, X.; Chen, M.; Lu, Z.; Shi, X.; Liang, L. Artificial Intelligence Tools for Optimising Recruitment and Retention in Clinical Trials: A Scoping Review Protocol. BMJ Open 2024, 14, e080032. [Google Scholar] [CrossRef]
- Makutam, V.; Yashashwini Achanti, S.; Doostan, M. INTEGRATION OF ARTIFICIAL INTELLIGENCE IN ADAPTIVE TRIAL DESIGNS: ENHANCING EFFICIENCY AND PATIENT-CENTRIC OUTCOMES. Int. J. Adv. Res. 2024, 12, 205–215. [Google Scholar] [CrossRef]
- Pakhrin, S.C.; Shrestha, B.; Adhikari, B.; KC, D.B. Deep Learning-Based Advances in Protein Structure Prediction. Int. J. Mol. Sci. 2021, 22, 5553. [Google Scholar] [CrossRef]
- Suwardi, A.; Wang, F.; Xue, K.; Han, M.; Teo, P.; Wang, P.; Wang, S.; Liu, Y.; Ye, E.; Li, Z.; et al. Machine Learning-Driven Biomaterials Evolution. Adv. Mater. 2022, 34, 2102703. [Google Scholar] [CrossRef] [PubMed]
- Buehler, M.J. Agentic Deep Graph Reasoning Yields Self-Organizing Knowledge Networks. arXiv 2025, arXiv:2502.13025. [Google Scholar]
- Choi, J.; Lee, B. Quantitative Topic Analysis of Materials Science Literature Using Natural Language Processing. ACS Appl. Mater. Interfaces 2024, 16, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Ranganathan, R. A Generative Approach to Materials Discovery, Design, and Optimization. ACS Omega 2022, 7, 25958–25973. [Google Scholar] [CrossRef]
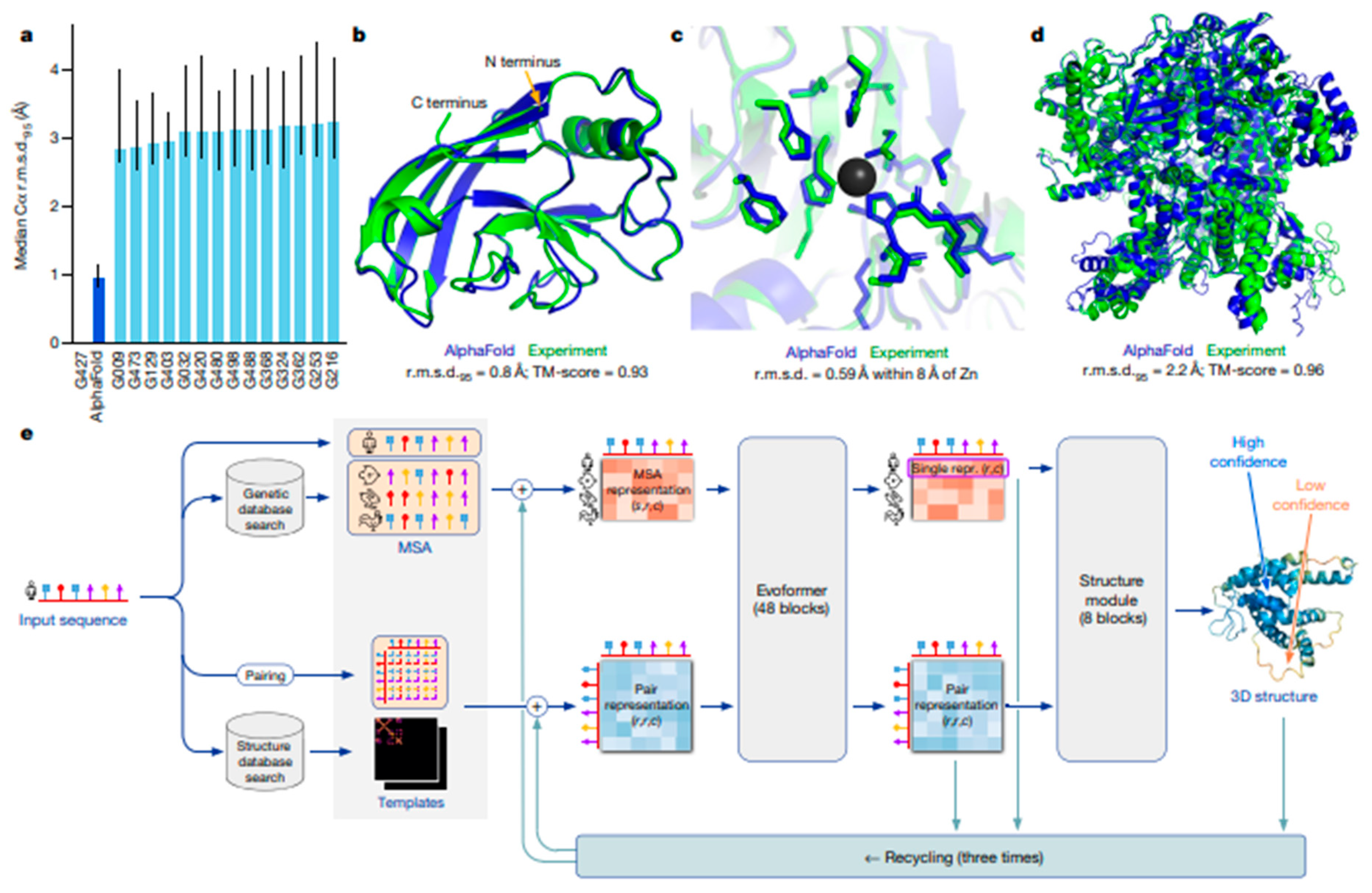
| Aspect | Traditional Biomaterials Development | Multimodal AI-Enhanced Biomaterials Development | Advantages of AI Integration | Ref |
|---|---|---|---|---|
| Data Utilization | Primarily relies on single-source data (e.g., biological assays) | Integrates diverse data sources (imaging, genomics, clinical data) | Provides holistic insights, improving biomaterial specificity and efficacy | [18] |
| Material Design Approach | Generalized designs based on population data or trial-and-error methods | Patient-specific designs based on individual health data | Enables precision and personalization in biomaterial properties | [19] |
| Predictive Modeling | Limited predictive capability, often requiring extensive experimentation | Advanced AI-driven modeling (e.g., AlphaFold for protein structures) | Reduces time and cost by predicting outcomes accurately before physical testing | [20] |
| Optimization of Properties | Based on empirical adjustments and physical testing iterations | AI analyzes complex relationships for optimal property tuning | Achieves targeted material properties efficiently for specific medical applications | [21] |
| Interaction with Biological Systems | Determined through iterative biocompatibility testing | AI predicts compatibility with biological systems using multi-omics data | Enhances biocompatibility and reduces adverse reactions | [22] |
| Speed of Development | Slower due to reliance on experimental validation | Accelerated through rapid AI-driven simulations and predictions | Shortens time-to-market for new biomaterials | [23] |
| Application in Regenerative Medicine | Limited personalization in grafts and scaffolds | AI customizes biomaterials to patient-specific regenerative needs | Promotes individualized tissue regeneration with higher success rates | [24] |
| Scalability and Adaptability | Challenging to scale personalized solutions | AI streamlines scalability by optimizing designs for diverse needs | Facilitates adaptable, scalable solutions for diverse patient populations | [25] |
| Ethical and Regulatory Challenges | Established guidelines for biomaterial safety | Emerging concerns over AI transparency, data privacy | Calls for updated regulatory frameworks and ethical standards | [26] |
| Material Type | AI Tools/Methods Used | Biomedical Applications | Ref |
|---|---|---|---|
| Polymeric Scaffolds | Machine learning (ML) for structure–property prediction; Inverse design algorithms | Optimizing porosity, stiffness, and degradation rates for tissue-specific scaffolds (e.g., bone, neural, and musculoskeletal) | [35] |
| Hydrogels | Multimodal AI (imaging + molecular + clinical data fusion); Deep learning for ECM replication | Skin regeneration, wound healing, and extracellular matrix (ECM)-mimicking hydrogels | [37,38] |
| Bone Scaffolds | CT/MRI-based AI analysis (CNNs); Predictive modeling for porosity and mechanical strength | Patient-specific bone grafts with optimized pore architecture for osteogenesis | [36] |
| Cartilage Scaffolds | AI-driven cell-material interaction modeling; Predictive algorithms for chondrocyte behavior | Cartilage repair with enhanced cell adhesion and differentiation | [39,40] |
| Vascular Grafts | AI-based endothelial cell response prediction; Genetic algorithm-driven material optimization | Blood vessel regeneration with reduced thrombosis risk | [40] |
| Immunocompatible Biomaterials | Genomic data integration with ML; Immune response prediction models | Personalized implants with minimized rejection risks (e.g., skin grafts, and bone scaffolds) | [41,42] |
| AI Model | Medical Application | Accuracy (%) | Precision (%) | Recall (%) | Reference |
|---|---|---|---|---|---|
| CNN (ResNet-50) | Cancer Detection (Histopathology) | 94.5 | 93.8 | 92.7 | [50] |
| Deep Neural Network (DNN) | Cardiovascular Disease Prediction | 89.2 | 87.5 | 88.3 | [51] |
| Transformer-Based Model (BERT) | Medical Text Analysis (EHR Processing) | 96.1 | 95.3 | 94.9 | [52] |
| Generative Adversarial Networks (GANs) (CycleGAN) | Medical Image Enhancement | 92.8 | 91.2 | 90.7 | [53] |
| AlphaFold | Protein Structure Prediction | >92.4 | N/A | N/A | [54] |
| AI Model | Biomaterial Type | Application | Predicted Property (Accuracy %) | Biocompatibility (%) | Reference |
|---|---|---|---|---|---|
| ML-Based Model (SVM) | Hydrogel Scaffolds | Cartilage Regeneration | 91.7 | 95.2 | [24] |
| Deep Learning (CNN) | Nanocomposites | Bone Tissue Engineering | 88.4 | 93.8 | [36] |
| Bayesian Optimization | 3D-Printed Biomaterials | Patient-Specific Implants | 94.5 | 97.1 | [22] |
| Reinforcement Learning | Bioactive Coatings | Antimicrobial Surfaces | 90.3 | 92.6 | [63] |
| AI-Guided GANs | Polymer-Based Biomaterials | Drug Delivery Systems | 87.9 | 91.3 | [64] |
| Aspect | Current State | Advancements with Multimodal AI | Challenges and Considerations | Future Implications |
|---|---|---|---|---|
| Data Integration | Data often remains siloed across imaging, genomics, and health records | AI combines diverse data sources, allowing holistic analysis for personalized material design | Ensuring data privacy, interoperability, and regulatory compliance | Enables comprehensive patient profiles for precision medicine |
| Biomaterial Design | Traditional biomaterials are designed based on generalized requirements | AI optimizes patient-specific biomaterials for drug delivery, tissue engineering, and regenerative applications | Complexity in validating AI-driven designs and achieving regulatory approvals | Patient-specific biomaterials that interact optimally with biological systems |
| AI Tools (e.g., AlphaFold) | Limited predictive accuracy for complex protein structures and interactions | High-accuracy prediction of protein folding and interactions | Algorithmic limitations in handling diverse biological contexts and data complexities | Accelerates biomaterial development tailored for biological compatibility |
| Diagnostic Precision | Diagnostic tools often rely on isolated data sources, limiting precision | AI-enhanced diagnostics integrate imaging, molecular, and clinical data for higher accuracy | Potential bias in algorithms and difficulty in validating AI-driven diagnostics | More accurate, personalized diagnoses supporting tailored treatments |
| Wearable Health Monitoring | Basic health tracking with limited data analysis capabilities | AI enhances real-time health monitoring for proactive interventions | Data privacy risks and lack of regulatory frameworks for wearable health data | Personalized health insights and early intervention possibilities |
| Ethical and Regulatory Issues | Emerging ethical standards; limited regulation for AI in healthcare | AI demands transparent and ethical algorithmic decisions for responsible healthcare use | Addressing algorithmic bias, data handling, and ensuring informed consent | Ethical and regulatory frameworks evolve, supporting AI integration in healthcare |
| Potential for Personalized Medicine | Limited precision and scalability in current treatments | AI enables tailored approaches, enhancing treatment efficacy and patient outcomes | Scalability, cost, and access barriers for AI-driven healthcare solutions | Transformative shift to data-driven, patient-centered healthcare systems |
| Opportunity Area | Current State | Future Potential with AI Integration | Impact on Biomedicine | Ref |
|---|---|---|---|---|
| Personalized Medicine | Limited to general population models | AI enables individualized treatments based on genetic, clinical, and lifestyle data | Enhances treatment efficacy and minimizes adverse reactions | [10] |
| Drug Discovery and Development | Time-intensive, costly with high attrition rates | AI accelerates drug discovery through predictive modeling and molecule screening | Reduces development time and cost, improving drug accessibility | [217] |
| Predictive Diagnostics | Diagnostics primarily based on isolated tests | Multimodal AI integrates diverse data for highly accurate, early diagnosis | Supports early intervention and improves prognosis | [16] |
| Tissue Engineering and Regenerative Medicine | Largely dependent on generalized biomaterials | AI designs patient-specific biomaterials for enhanced tissue compatibility | Improves patient outcomes and supports complex tissue repair | [66] |
| Wearable Health Monitoring | Limited to basic tracking (e.g., heart rate, steps) | AI-powered wearables analyze real-time data for personalized health insights | Facilitates proactive health management and early detection | [218] |
| Genomic Analysis and Precision Genomics | Analysis focused on specific genes or markers | AI analyzes entire genomes, identifying complex genetic interactions | Enables precise identification of genetic risk factors and mutations | [219] |
| Remote and Telemedicine Applications | Limited real-time patient analysis | AI enables real-time remote monitoring, diagnostic support, and patient triaging | Expands healthcare access, especially in underserved areas | [220] |
| Ethical and Regulatory Frameworks | Initial standards in place | AI-driven healthcare prompts the development of advanced ethical and regulatory models | Ensures responsible use, transparency, and patient trust | [126] |
| AI-Augmented Surgical Procedures | Limited AI assistance, primarily robotic arms | Future AI systems can assist in complex surgeries through real-time guidance | Enhances surgical precision and reduces risk of complications | [221] |
| Health Data Management | Fragmented data management across systems | AI consolidates data for seamless integration and patient care continuity | Improves care coordination and data-driven decision-making | [222] |
| AI Model | Application in Biomaterials Science | Strengths | Limitations | |
|---|---|---|---|---|
| Deep Learning (DL) (CNNs, RNNs) | Predicting biomaterial properties, image-based tissue analysis | High accuracy in feature extraction and pattern recognition | Requires large datasets; potential overfitting; lack of interpretability | [37] |
| AlphaFold (Deep Learning-Based Structural Prediction) | Protein-material interaction modeling, bioactive scaffold design | Highly accurate protein structure prediction; aids in rational biomaterial design | Computationally intensive; limited to protein-centric applications | [284] |
| Machine Learning (ML) (Random Forest, SVM, Decision Trees) | Biomaterial classification, mechanical property prediction | Efficient with small datasets; interpretable models | Performance depends on dataset quality; may lack deep feature extraction | [285] |
| Reinforcement Learning (RL) | Self-optimizing biomaterial formulations, drug delivery system design | Learns from trial-and-error; adaptive optimization | Requires extensive computational resources; long training times | [286] |
| Natural Language Processing (NLP) | Literature-based discovery of novel biomaterials | Automates knowledge extraction from vast scientific data | Limited understanding of contextual nuances in biomedical data | [287] |
| Bayesian Networks | Risk assessment in biomaterial biocompatibility and toxicity prediction | Handles uncertainty well; suitable for probabilistic modeling | Requires prior domain knowledge for accurate results | [22] |
| Generative Adversarial Networks (GANs) | Designing new biomaterials with optimized properties | Generates novel materials with desired characteristics | Training instability; potential for generating unrealistic structures | [288] |
| Hybrid AI Models (Combining DL, ML, and Statistical Methods) | Multimodal AI for patient-specific biomaterial optimization | Integrates diverse data sources for better decision-making | Complexity in integration; challenges in model validation | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. https://doi.org/10.3390/nano15120895
Parvin N, Joo SW, Jung JH, Mandal TK. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials. 2025; 15(12):895. https://doi.org/10.3390/nano15120895
Chicago/Turabian StyleParvin, Nargish, Sang Woo Joo, Jae Hak Jung, and Tapas K. Mandal. 2025. "Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare" Nanomaterials 15, no. 12: 895. https://doi.org/10.3390/nano15120895
APA StyleParvin, N., Joo, S. W., Jung, J. H., & Mandal, T. K. (2025). Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials, 15(12), 895. https://doi.org/10.3390/nano15120895








