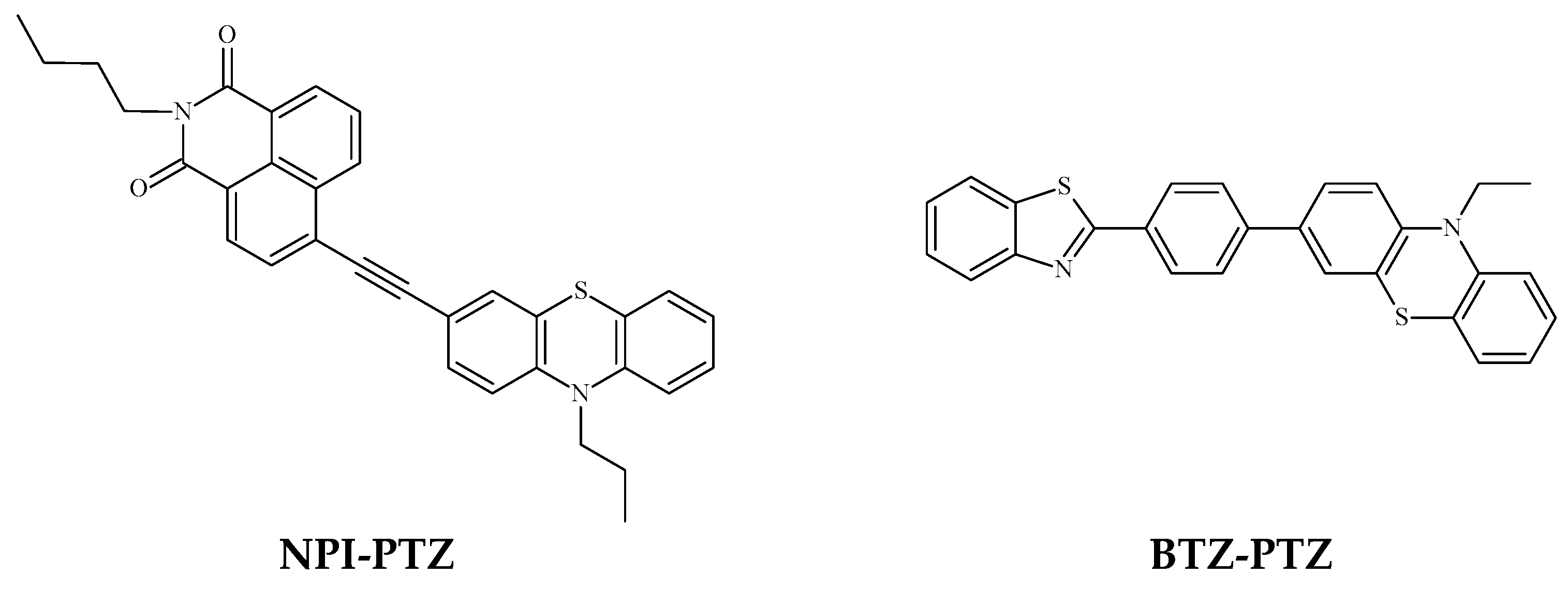
Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Mesenchymal/Stromal Stem Cell Culture
2.3. MTT Viability Assay and Phototoxicity Assay
2.4. Intracellular ROS Production
2.5. Trypan Blue Exclusion Assay
2.6. Fluorescence Microscopy Analysis
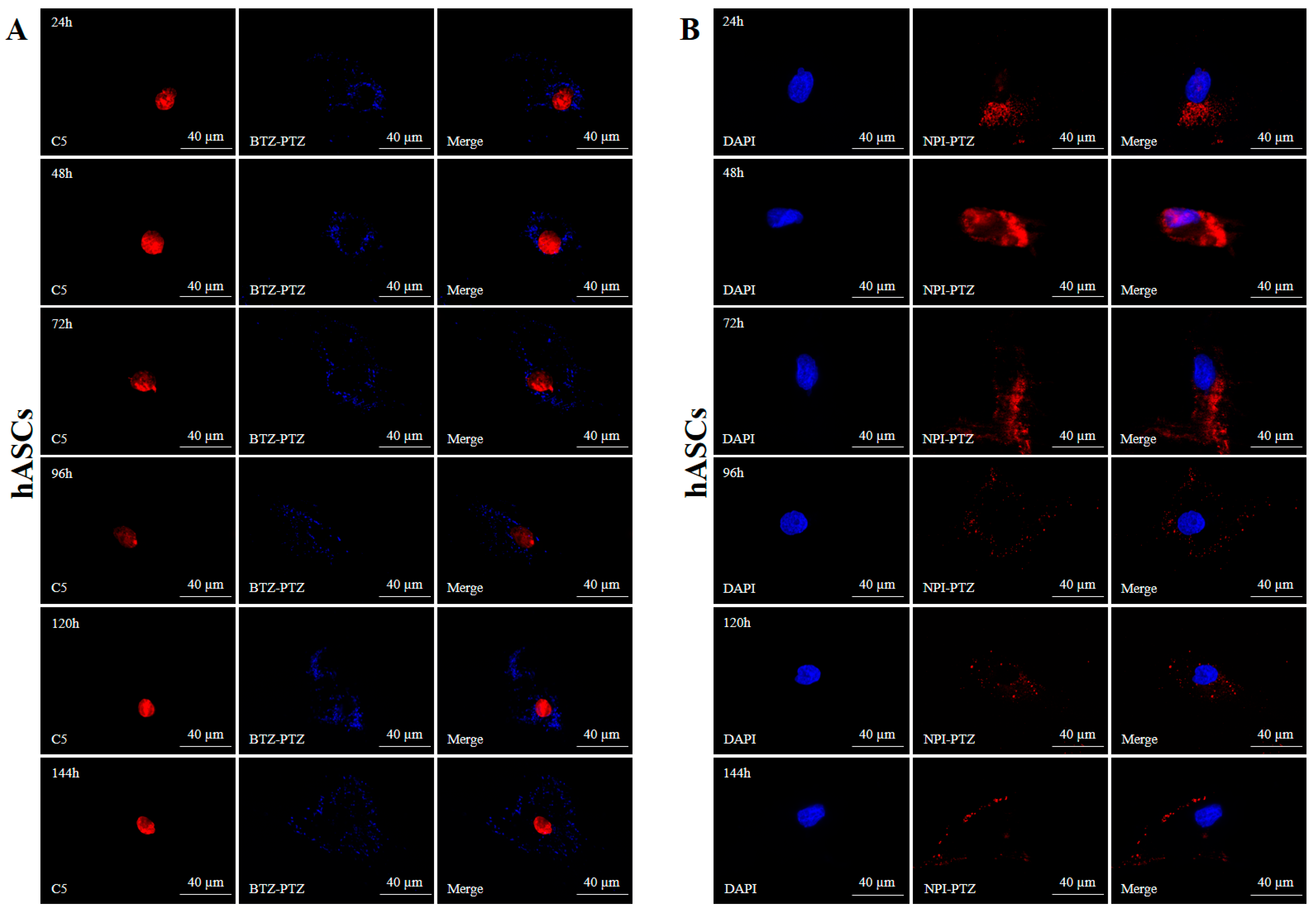
2.6.1. Fluorescence Intensity Quantification over Time
- CTCF = corrected total cell fluorescence;
- IntDen = cell fluorescence integrated density;
- A = area of the selected cell;
- BGmean = mean fluorescence of the background readings (calculated as the mean of five different ROIs).
2.6.2. Evaluation of Maximum Feret’s Diameter of Nuclei
2.7. Statistical Analysis
3. Results
3.1. Analysis of Cell Growth Inhibition and IC50 Determination of Phototoxic Compounds
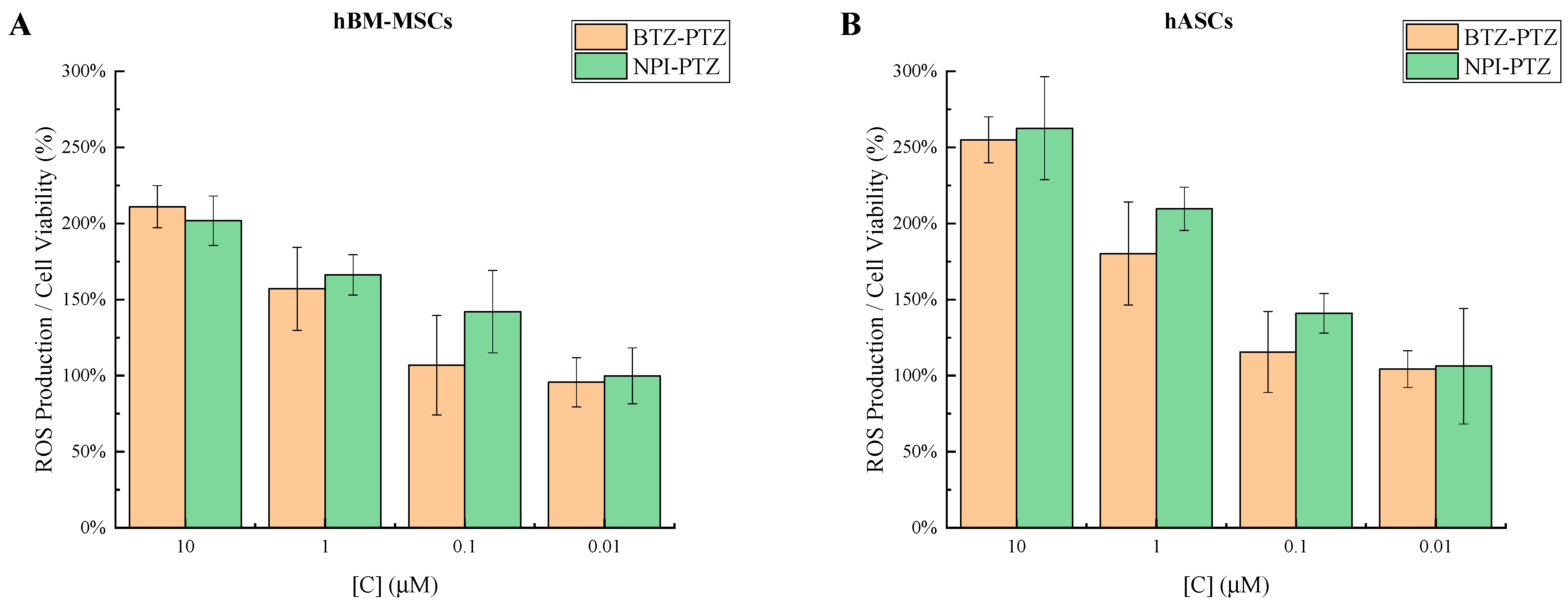
3.2. Evaluation of Intracellular ROS Production
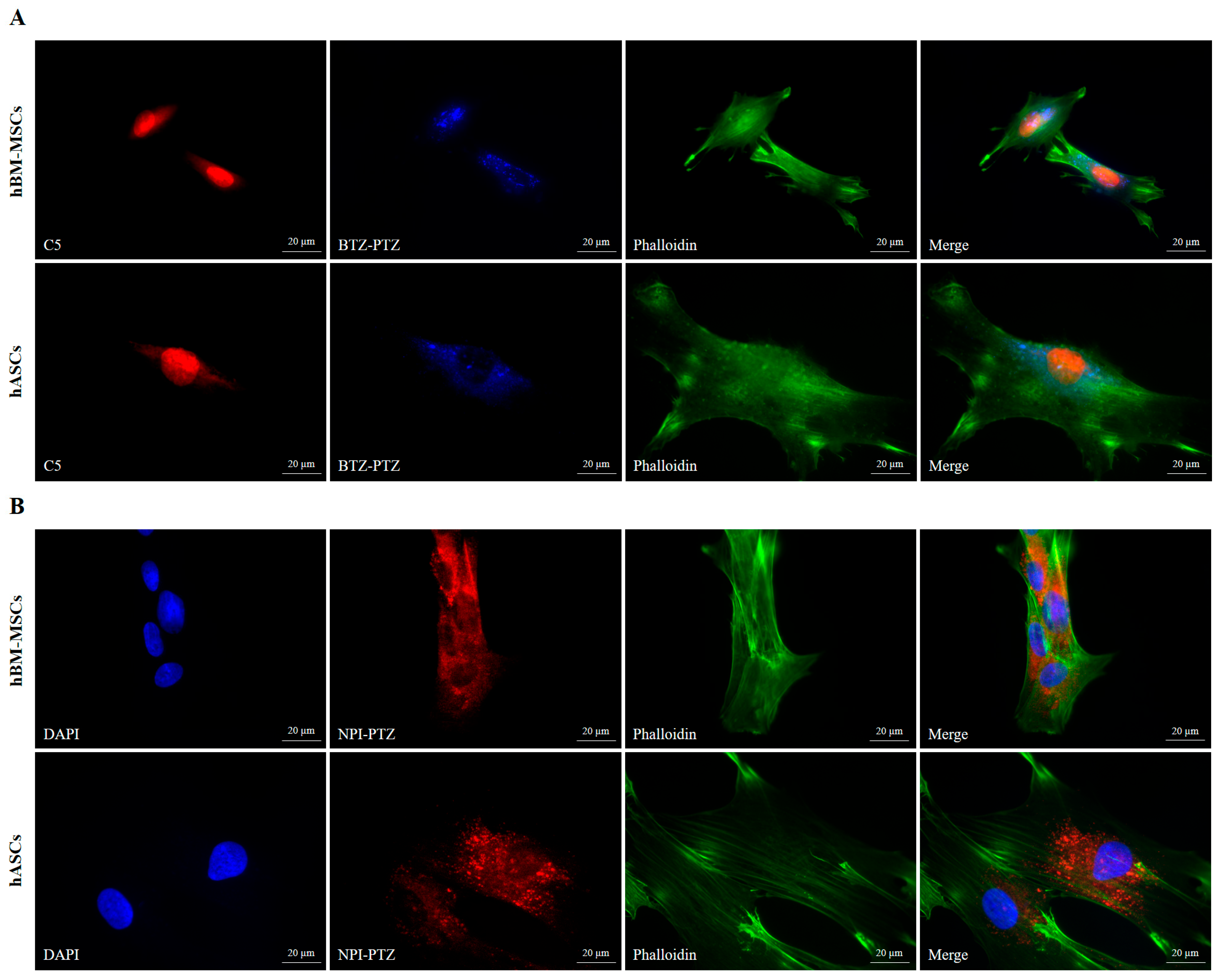
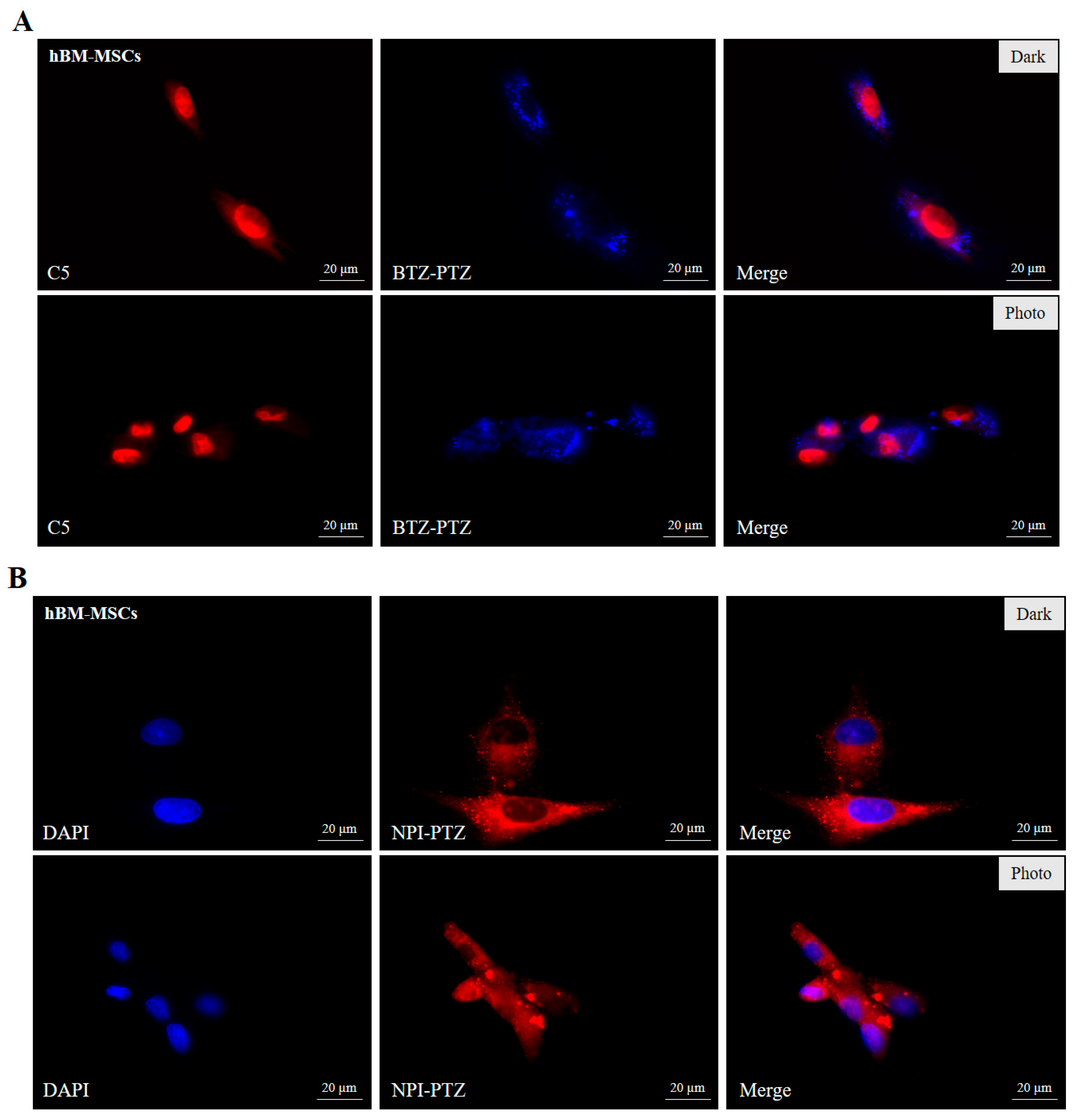
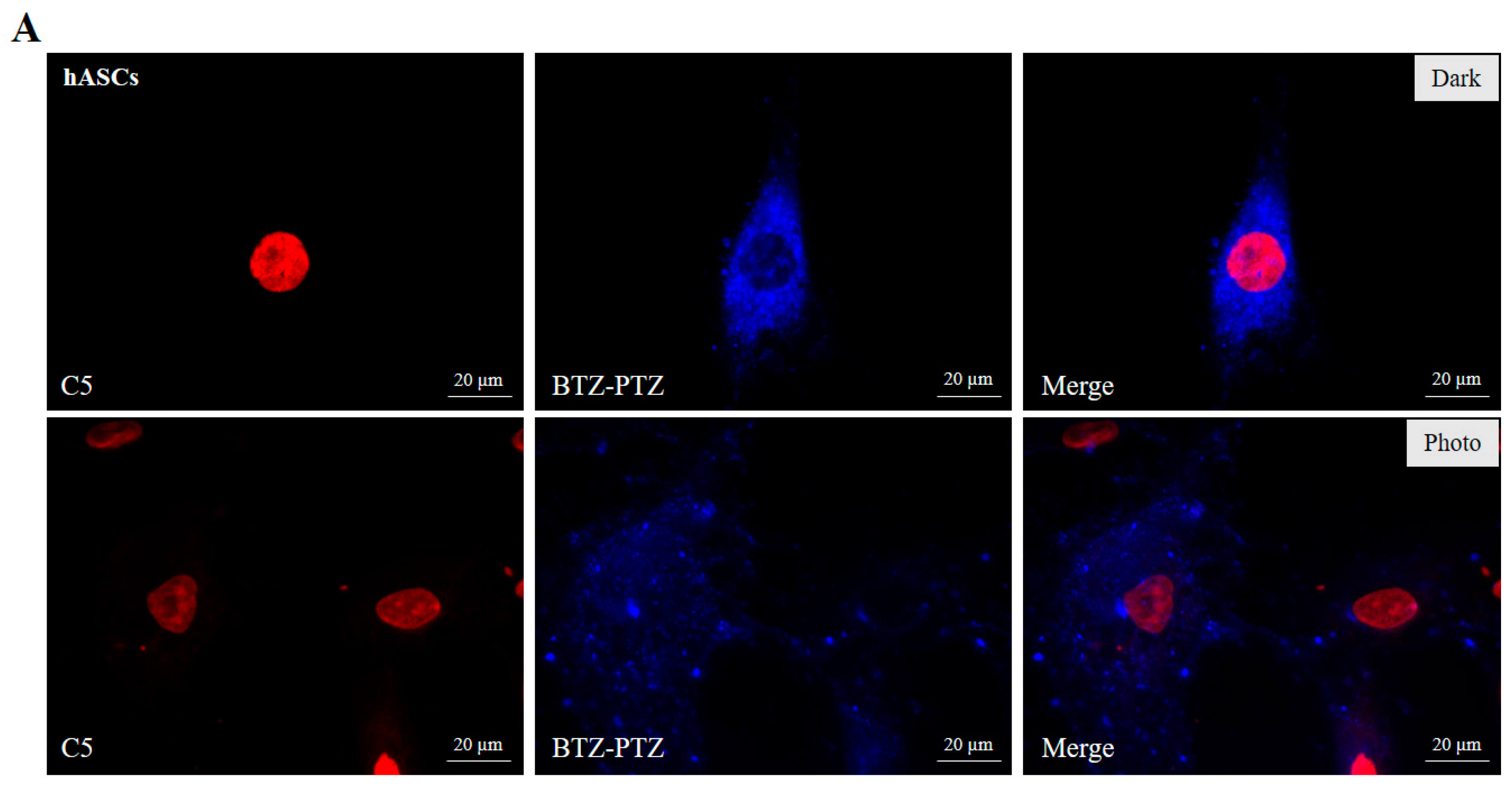
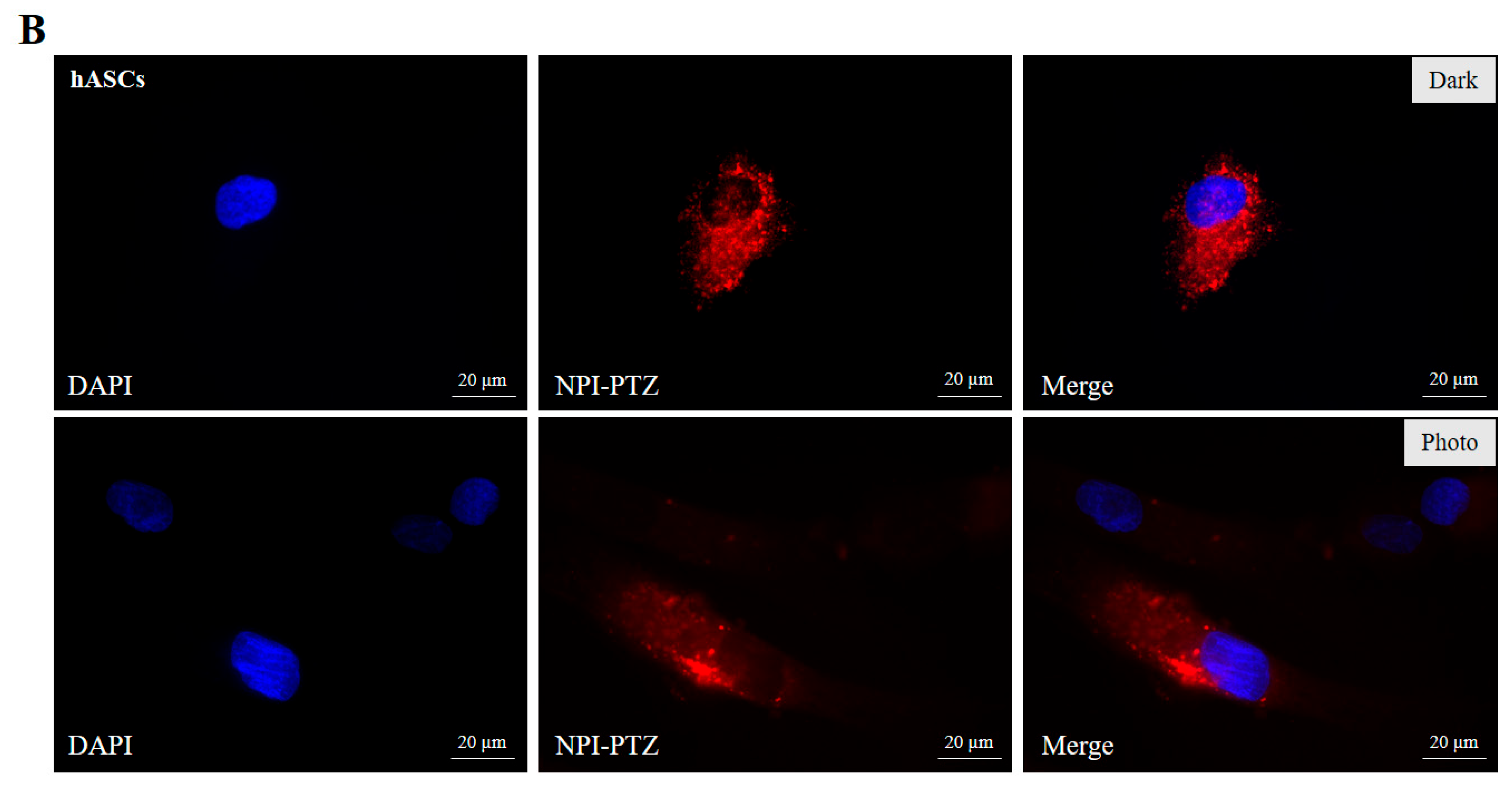
3.3. Fluorescence Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calzoni, E.; Bertoldi, A.; Cesaretti, A.; Alabed, H.B.R.; Cerrotti, G.; Pellegrino, R.M.; Buratta, S.; Urbanelli, L.; Emiliani, C. Aloe Extracellular Vesicles as Carriers of Photoinducible Metabolites Exhibiting Cellular Phototoxicity. Cells 2024, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, T.; Cesaretti, A.; Mancini, P.; Montegiove, N.; Calzoni, E.; Ekbote, A.; Misra, R.; Carlotti, B. Room-Temperature Phosphorescence and Cellular Phototoxicity Activated by Triplet Dynamics in Aggregates of Push–Pull Phenothiazine-Based Isomers. J. Phys. Chem. B 2023, 127, 1385–1398. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Meng, C.; Feng, F. Recent Advances for Enhanced Photodynamic Therapy: From New Mechanisms to Innovative Strategies. Chem. Sci. 2024, 15, 12234–12257. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Stanciu, S.G.; Peng, H.; Wu, A.; Yang, F. Photodynamic Therapy with NIR-II Probes: Review on State-of-the-Art Tools and Strategies. Mater. Horiz. 2024, 11, 5815–5842. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming Barriers in Photodynamic Therapy Harnessing Nano-Formulation Strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef] [PubMed]
- Oluwajembola, A.M.; Cleanclay, W.D.; Onyia, A.F.; Chikere, B.N.; Zakari, S.; Ndifreke, E.; De Campos, O.C. Photosensitizers in Photodynamic Therapy: An Advancement in Cancer Treatment. Results Chem. 2024, 10, 101715. [Google Scholar] [CrossRef]
- Fu, S.; Yang, R.; Zhang, L.; Liu, W.; Du, G.; Cao, Y.; Xu, Z.; Cui, H.; Kang, Y.; Xue, P. Biomimetic CoO@AuPt Nanozyme Responsive to Multiple Tumor Microenvironmental Clues for Augmenting Chemodynamic Therapy. Biomaterials 2020, 257, 120279. [Google Scholar] [CrossRef]
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion Nanoparticle-Mediated Photodynamic Therapy Induces Autophagy and Cholesterol Efflux of Macrophage-Derived Foam Cells via ROS Generation. Cell Death Dis. 2017, 8, e2864. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Kylychbekov, S.; Majidov, I.; Khuzhakulov, Z.; Er, A.Y.; Kitchens, C.; Banga, S.; Er, A.O. The Role of Efflux Pump Inhibitor in Enhancing Antimicrobial Efficiency of Ag NPs and MB as an Effective Photodynamic Therapy Agent. Photodiagnosis Photodyn. Ther. 2024, 47, 104212. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, L.; Ran, X.-Y.; Xu, J.-X.; Zhang, L.-N.; Kong, Q.-Q.; Yu, X.-Q.; Li, K. Molecular Engineering of D-π-A Conjugate with N-Heterocycle Purine for Enhanced ROS Generation and Photodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2400728. [Google Scholar] [CrossRef]
- Ni, J.; Yu, L.; Wang, Y.; Yang, T.; Bai, Y.; Zheng, B.; Liang, M.; Ye, X.; Quan, Y.-Y.; Lin, F.; et al. Win-Win Integration: A Mitochondria Targeted AIE Photosensitizer for Hypochlorite Detection and Type I & Type II Photodynamic Therapy. Anal. Chim. Acta 2024, 1320, 343035. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kenry; Hu, F. Targeted Antibacterial Photodynamic Therapy with Aggregation-Induced Emission Photosensitizers. Interdiscip. Med. 2024, 2, e20230038. [Google Scholar] [CrossRef]
- Cao, W.; Liu, B.; Xia, F.; Duan, M.; Hong, Y.; Niu, J.; Wang, L.; Liu, Y.; Li, C.; Cui, D. MnO 2 @Ce6-Loaded Mesenchymal Stem Cells as an “Oxygen-Laden Guided-Missile” for the Enhanced Photodynamic Therapy on Lung Cancer. Nanoscale 2020, 12, 3090–3102. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Yang, W.; Yue, H.; Lu, G.; Wang, W.; Deng, Y.; Ma, G.; Wei, W. Advances in Delivering Oxidative Modulators for Disease Therapy. Research 2022, 2022, 9897464. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Xie, X.; Song, T.; Yang, G.; Su, Q.; Li, T.; Li, S.; Wu, C.; You, F.; et al. Engineered Mesenchymal Stem Cells as a Biotherapy Platform for Targeted Photodynamic Immunotherapy of Breast Cancer. Adv. Healthc. Mater. 2022, 11, 2101375. [Google Scholar] [CrossRef]
- Li, X.; Kim, C.; Shin, J.M.; Lee, D.; Kim, G.; Chung, H.-M.; Hong, K.-S.; Yoon, J. Mesenchymal Stem Cell-Driven Activatable Photosensitizers for Precision Photodynamic Oncotherapy. Biomaterials 2018, 187, 18–26. [Google Scholar] [CrossRef]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte Chemotactic Protein-1 Secreted by Primary Breast Tumors Stimulates Migration of Mesenchymal Stem Cells. Clin. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Gaspar, V.M.; Monteiro, M.V.; Freitas, B.; Silva, N.J.O.; Mano, J.F. Screening of Dual Chemo-Photothermal Cellular Nanotherapies in Organotypic Breast Cancer 3D Spheroids. J. Control. Release 2021, 331, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Sasportas, L.S.; Kasmieh, R.; Wakimoto, H.; Hingtgen, S.; van de Water, J.A.J.M.; Mohapatra, G.; Figueiredo, J.L.; Martuza, R.L.; Weissleder, R.; Shah, K. Assessment of Therapeutic Efficacy and Fate of Engineered Human Mesenchymal Stem Cells for Cancer Therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4822–4827. [Google Scholar] [CrossRef]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.-C.; Passirani, C.; Sindji, L.; Schiller, P.; Montero-Menei, C.; Menei, P. Mesenchymal Stem Cells as Cellular Vehicles for Delivery of Nanoparticles to Brain Tumors. Biomaterials 2010, 31, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Bagó, J.R.; Alfonso-Pecchio, A.; Okolie, O.; Dumitru, R.; Rinkenbaugh, A.; Baldwin, A.S.; Miller, C.R.; Magness, S.T.; Hingtgen, S.D. Therapeutically Engineered Induced Neural Stem Cells Are Tumour-Homing and Inhibit Progression of Glioblastoma. Nat. Commun. 2016, 7, 10593. [Google Scholar] [CrossRef]
- Yao, S.; Li, X.; Liu, J.; Sun, Y.; Wang, Z.; Jiang, Y. Maximized Nanodrug-Loaded Mesenchymal Stem Cells by a Dual Drug-Loaded Mode for the Systemic Treatment of Metastatic Lung Cancer. Drug Deliv. 2017, 24, 1372–1383. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, S.; Guo, J.; Alahdal, M.; Cao, S.; Yang, Z.; Zhang, F.; Shen, Y.; Sun, M.; Mo, R.; et al. Targeted Delivery of Doxorubicin by Nano-Loaded Mesenchymal Stem Cells for Lung Melanoma Metastases Therapy. Sci. Rep. 2017, 7, 44758. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Guo, J.; He, Y.; Alahdal, M.; Tang, S.; Zhao, Y.; Yang, Z.; Gao, H.; Hu, W.; Jiang, H.; et al. Nano-Loaded Human Umbilical Cord Mesenchymal Stem Cells as Targeted Carriers of Doxorubicin for Breast Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 642–652. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk Factors in the Development of Stem Cell Therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Cesaretti, A.; Bianconi, T.; Coccimiglio, M.; Montegiove, N.; Rout, Y.; Gentili, P.L.; Misra, R.; Carlotti, B. Aggregation-Induced Emission in Phenothiazine-Based Fluorophores: An Insight into the Excited State and Aggregate Formation Mechanism. J. Phys. Chem. C 2022, 126, 10429–10440. [Google Scholar] [CrossRef]
- Rout, Y.; Montanari, C.; Pasciucco, E.; Misra, R.; Carlotti, B. Tuning the Fluorescence and the Intramolecular Charge Transfer of Phenothiazine Dipolar and Quadrupolar Derivatives by Oxygen Functionalization. J. Am. Chem. Soc. 2021, 143, 9933–9943. [Google Scholar] [CrossRef]
- Cesaretti, A.; Calzoni, E.; Montegiove, N.; Bianconi, T.; Alebardi, M.; La Serra, M.A.; Consiglio, G.; Fortuna, C.G.; Elisei, F.; Spalletti, A. Lighting-Up the Far-Red Fluorescence of RNA-Selective Dyes by Switching from Ortho to Para Position. Int. J. Mol. Sci. 2023, 24, 4812. [Google Scholar] [CrossRef] [PubMed]
- Argentati, C.; Morena, F.; Guidotti, G.; Soccio, M.; Lotti, N.; Martino, S. Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness. Cells 2023, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Calzoni, E.; Cordellini, M.; Emiliani, C.; D’Angelo, F.; Martino, S. Ex-Vivo Tissues Engineering Modeling for Reconstructive Surgery Using Human Adult Adipose Stem Cells and Polymeric Nanostructured Matrix. Nanomaterials 2016, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- The World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Participants; The World Medical Association: Ferney-Voltaire, France, 2024. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Emiliani, C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. J. Funct. Biomater. 2021, 12, 32. [Google Scholar] [CrossRef]
- Montanari, C.; Sheokand, M.; Cesaretti, A.; Calzoni, E.; Misra, R.; Carlotti, B. Ad-Hoc Functionalization of Phenothiazine Dioxide Derivatives to Achieve Blue Thermally Activated Delayed Fluorescence in Organic Nanoaggregates. J. Phys. Chem. C 2024, 128, 19688–19700. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Jakubowski, W.; Bartosz, G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: What does it measure? Cell Biol. Int. 2000, 24, 757–760. [Google Scholar] [CrossRef]
- Bonini, M.G.; Rota, C.; Tomasi, A.; Mason, R.P. The Oxidation of 2′,7′-Dichlorofluorescin to Reactive Oxygen Species: A Self-Fulfilling Prophesy? Free Radic. Biol. Med. 2006, 40, 968–975. [Google Scholar] [CrossRef]
- Cesaretti, A.; Mencaroni, L.; Bonaccorso, C.; Botti, V.; Calzoni, E.; Carlotti, B.; Fortuna, C.G.; Montegiove, N.; Spalletti, A.; Elisei, F. Amphiphilicity-Controlled Localization of Red Emitting Bicationic Fluorophores in Tumor Cells Acting as Bio-Probes and Anticancer Drugs. Molecules 2022, 27, 3713. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Pellegrino, R.M.; Emiliani, C. HexA-Enzyme Coated Polymer Nanoparticles for the Development of a Drug-Delivery System in the Treatment of Sandhoff Lysosomal Storage Disease. J. Funct. Biomater. 2022, 13, 37. [Google Scholar] [CrossRef]
- Bicchi, I.; Morena, F.; Argentati, C.; Nodari, L.R.; Emiliani, C.; Gelati, M.; Vescovi, A.L.; Martino, S. Storage of Mutant Human SOD1 in Non-Neural Cells from the Type-1 Amyotrophic Lateral Sclerosis ratG93A Model Correlated with the Lysosomes’ Dysfunction. Biomedicines 2021, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Billmann, C.; Schäffner, I.; Heppt, J.; Lie, D.C. Analysis of β-Catenin Signalling Activity Suggests Differential Regulation of Ontogenetically Distinct Dentate Granule Neuron Populations. Int. J. Dev. Neurosci. 2025, 85, e70009. [Google Scholar] [CrossRef]
- Chen, F.; Bai, M.; Cao, K.; Zhao, Y.; Wei, J.; Zhao, Y. Fabricating MnO2 Nanozymes as Intracellular Catalytic DNA Circuit Generators for Versatile Imaging of Base-Excision Repair in Living Cells. Adv. Funct. Mater. 2017, 27, 1702748. [Google Scholar] [CrossRef]
- McNulty, J.; Babu-Dokuburra, C.; Scattolon, J.; Zepeda-Velazquez, C.; Wesesky, M.A.; Caldwell, J.K.; Zheng, W.; Milosevic, J.; Kinchington, P.R.; Bloom, D.C.; et al. Truncated Ring-A Amaryllidaceae Alkaloid Modulates the Host Cell Integrated Stress Response, Exhibiting Antiviral Activity to HSV-1 and SARSCoV-2. Sci. Rep. 2023, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.B.; Starosta, R.T.; Pilar, E.F.S.; Kunz, J.D.; Tomedi, J.; Cerski, C.T.S.; Ruppenthal, R.D. Nuclear Morphometry and Chromatin Texture Changes in Hepatocellular Carcinoma Samples May Predict Outcomes of Liver Transplanted Patients. BMC Gastroenterol. 2022, 22, 189. [Google Scholar] [CrossRef]
- Katayama, A.; Toss, M.S.; Parkin, M.; Sano, T.; Oyama, T.; Quinn, C.M.; Ellis, I.O.; Rakha, E.A. Nuclear Morphology in Breast Lesions: Refining Its Assessment to Improve Diagnostic Concordance. Histopathology 2022, 80, 515–528. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Fan, Z. A Review: Small Organic Molecule Dual/Multi-Organelle-Targeted Fluorescent Probes. Talanta 2023, 259, 124529. [Google Scholar] [CrossRef]
- Chua, M.H.; Chin, K.L.O.; Loh, X.J.; Zhu, Q.; Xu, J. Aggregation-Induced Emission-Active Nanostructures: Beyond Biomedical Applications. ACS Nano 2023, 17, 1845–1878. [Google Scholar] [CrossRef]
- Zhao, H.; Li, N.; Ma, C.; Wei, Z.; Zeng, Q.; Zhang, K.; Zhao, N.; Tang, B.Z. An AIE Probe for Long-Term Plasma Membrane Imaging and Membrane-Targeted Photodynamic Therapy. Chin. Chem. Lett. 2023, 34, 107699. [Google Scholar] [CrossRef]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. Hydrogen Peroxide-Preconditioned Human Adipose-Derived Stem Cells Enhance the Recovery of Oligodendrocyte-Like Cells after Oxidative Stress-Induced Damage. Int. J. Mol. Sci. 2020, 21, 9513. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.; Yoon, J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Gani, M.; Xodo, L.E.; Rapozzi, V. Bystander Effect in Photosensitized Prostate Cancer Cells with a Different Grade of Malignancy: The Role of Nitric Oxide. Nitric Oxide 2022, 128, 25–36. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-Induced ROS Release in Plant and Animal Cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef]
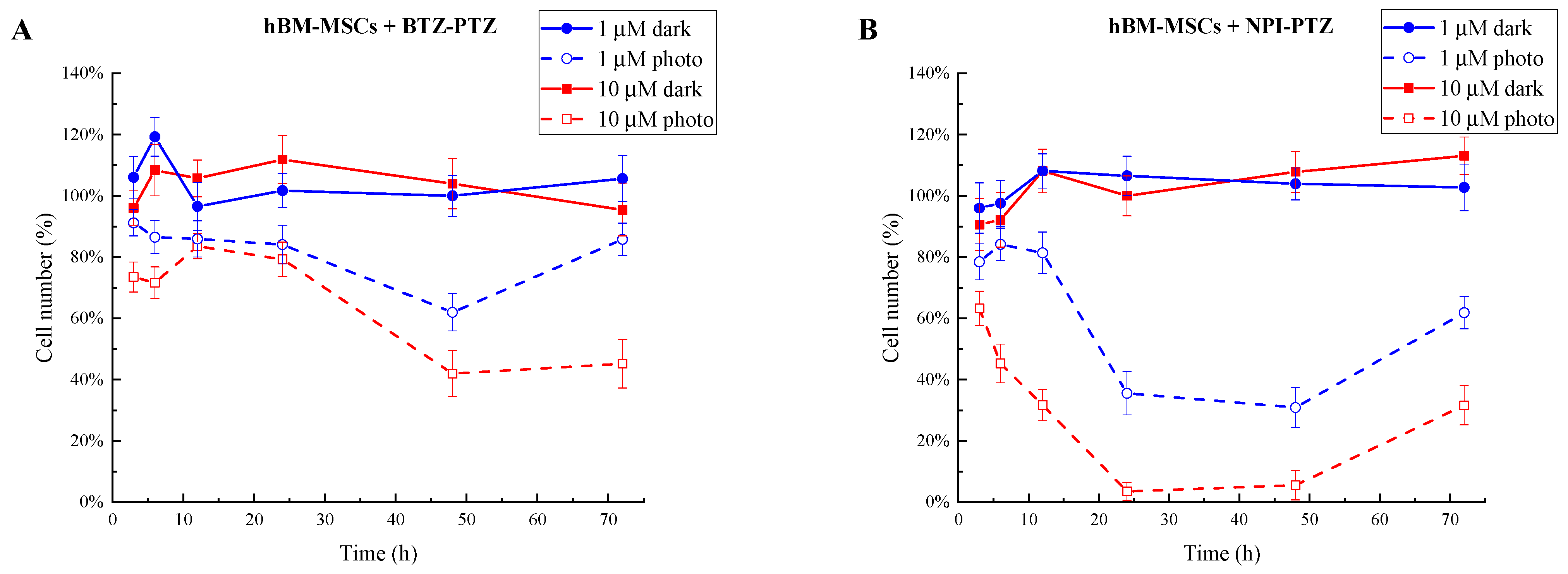
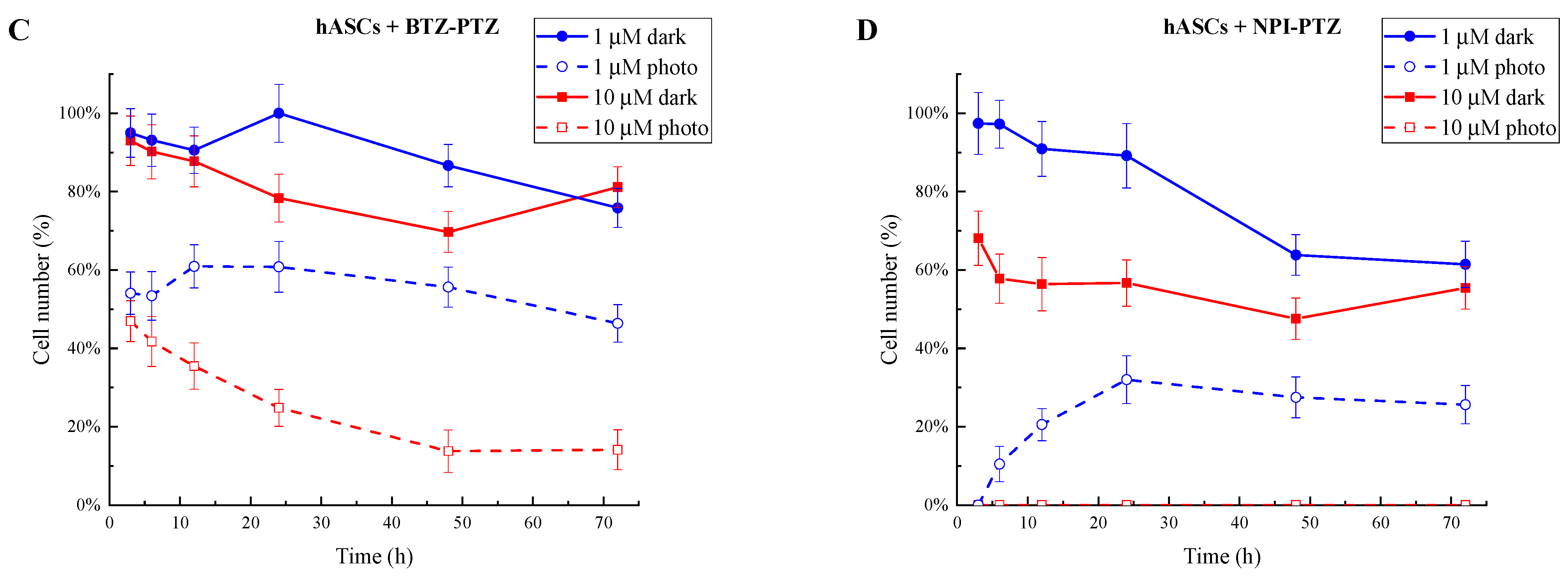

| Cells | IC50/µM | |||
|---|---|---|---|---|
| BTZ-PTZ | NPI-PTZ | |||
| 12′ 30″ | 25′ | 12′ 30″ | 25′ | |
| hBM-MSCs | - | 8.1 ± 0.9 | 10 ± 1 | 6.3 ± 0.6 |
| hASCs | >10 | 2.2 ± 0.2 | 6.6 ± 0.7 | 1.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzoni, E.; Cesaretti, A.; Montegiove, N.; Valicenti, M.L.; Morena, F.; Misra, R.; Carlotti, B.; Martino, S. Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy. Nanomaterials 2025, 15, 894. https://doi.org/10.3390/nano15120894
Calzoni E, Cesaretti A, Montegiove N, Valicenti ML, Morena F, Misra R, Carlotti B, Martino S. Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy. Nanomaterials. 2025; 15(12):894. https://doi.org/10.3390/nano15120894
Chicago/Turabian StyleCalzoni, Eleonora, Alessio Cesaretti, Nicolò Montegiove, Maria Luisa Valicenti, Francesco Morena, Rajneesh Misra, Benedetta Carlotti, and Sabata Martino. 2025. "Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy" Nanomaterials 15, no. 12: 894. https://doi.org/10.3390/nano15120894
APA StyleCalzoni, E., Cesaretti, A., Montegiove, N., Valicenti, M. L., Morena, F., Misra, R., Carlotti, B., & Martino, S. (2025). Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy. Nanomaterials, 15(12), 894. https://doi.org/10.3390/nano15120894









