Comparative Study of Free and Encapsulated Hypocrellin B on Photophysical-Chemical Properties, Cellular Uptake, Subcellular Distribution, and Phototoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Liposomal HB (HB@Lipo)
2.2. Preparation of HB-Loaded PLGA Nanoparticles (HB@PLGA)
2.3. Characterization
2.4. Cellular Uptake of HB, HB@Lipo and HB@PLGA Analyzed Using Flow Cytometry
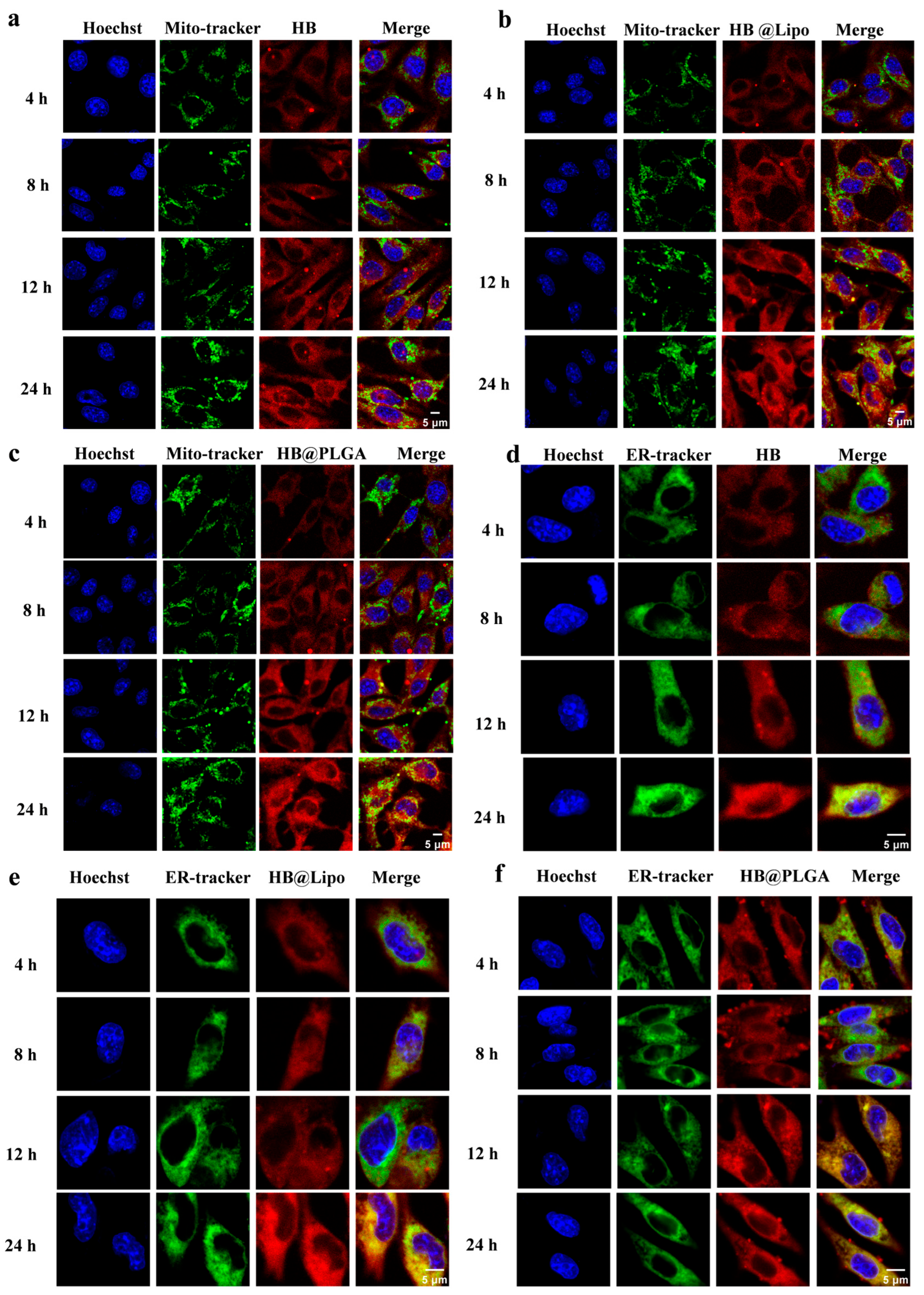
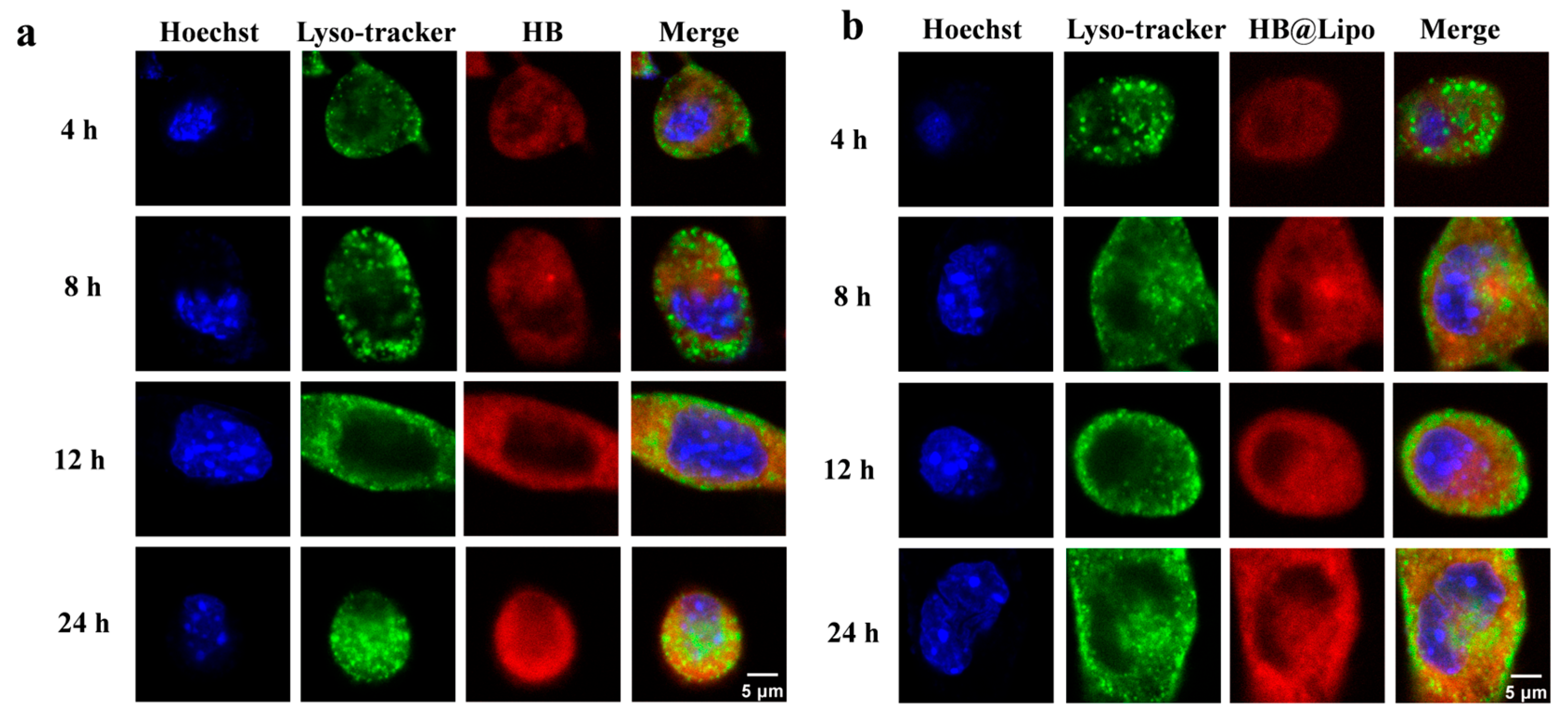
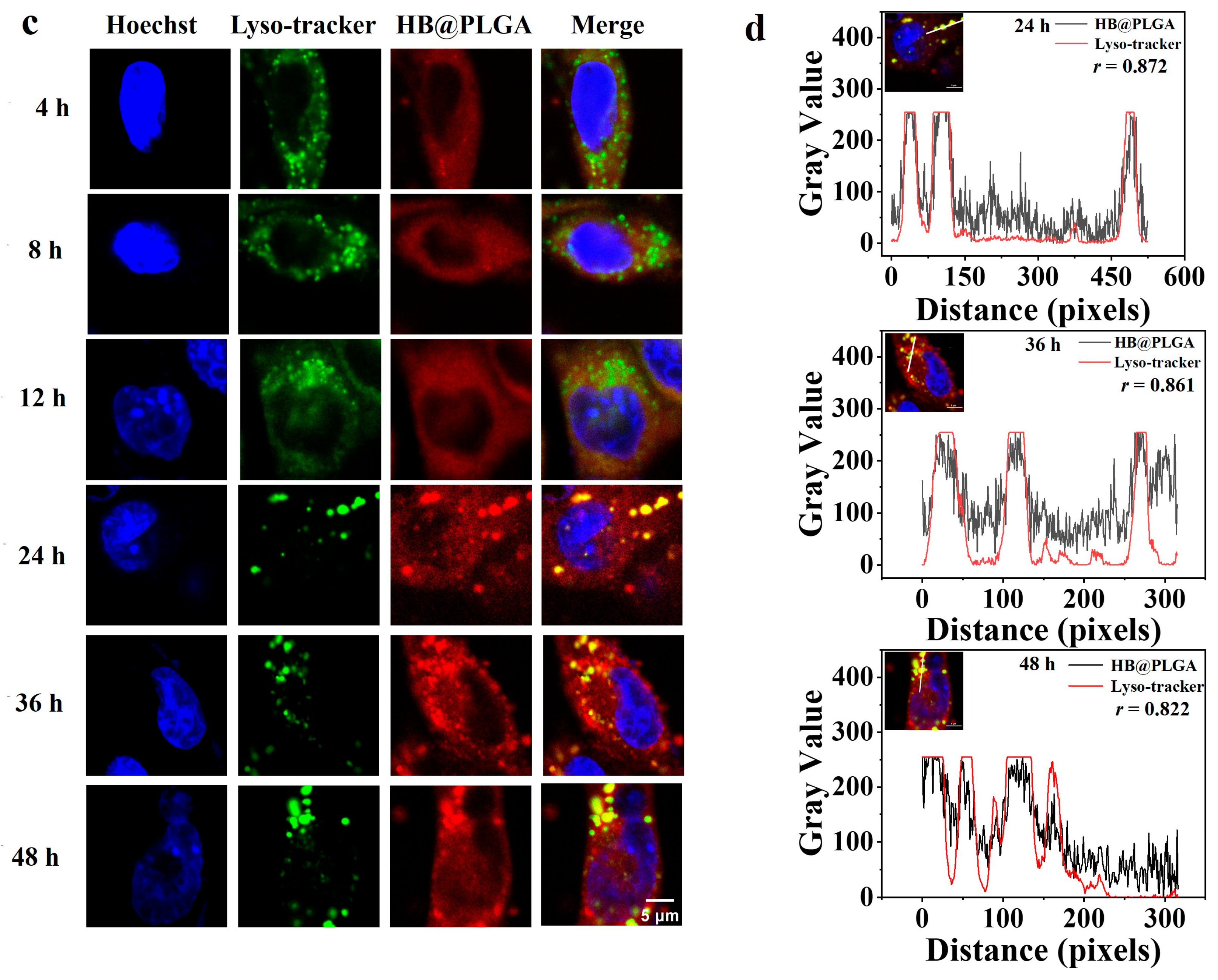
2.5. Subcellular Localization of HB, HB@Lipo, and HB@PLGA Observed Using Confocal Laser Scanning Microscopy
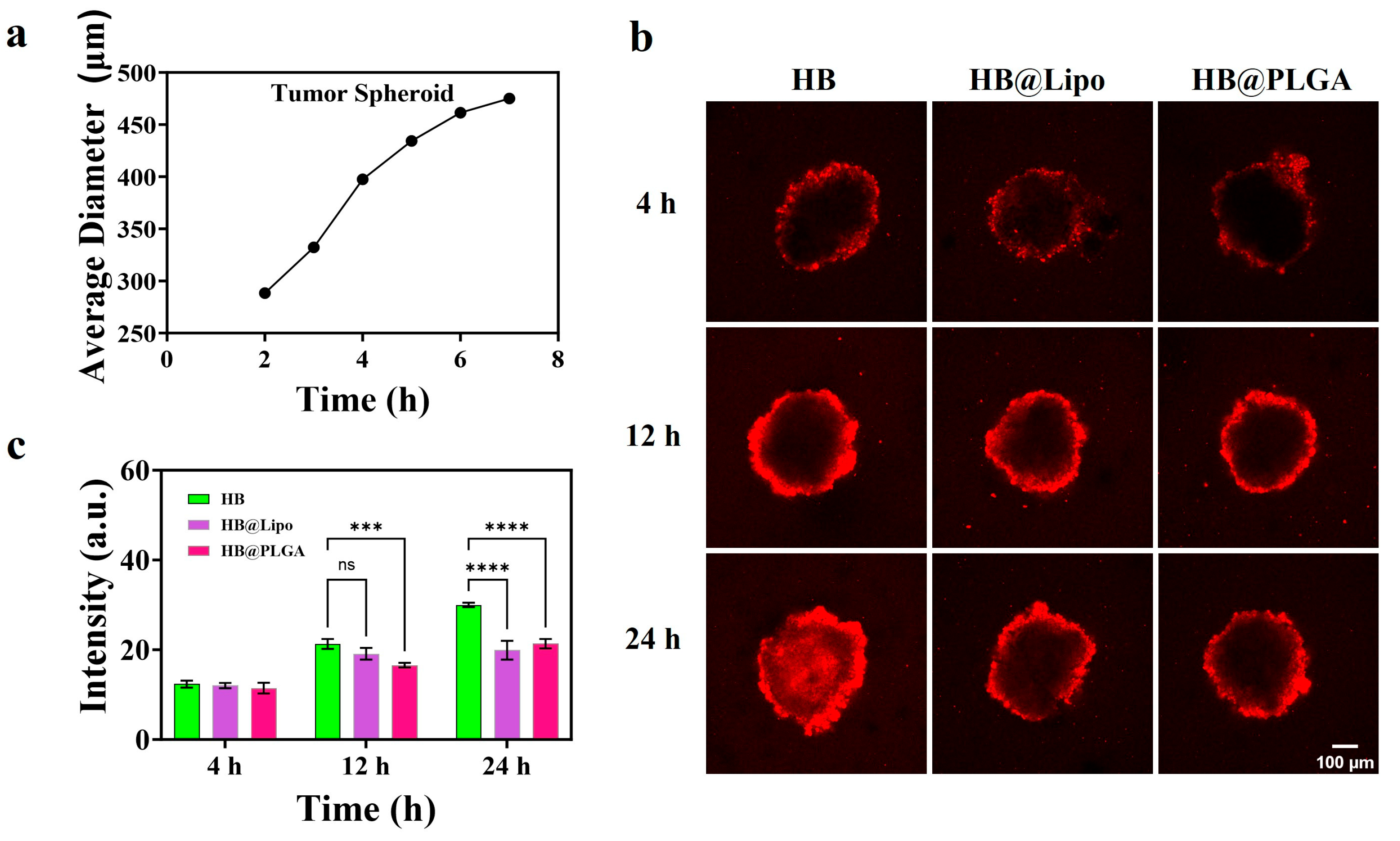
2.6. Culture of Tumor Spheroids
2.7. Distribution of HB, HB@Lipo or HB@PLGA in Tumor Spheroids
2.8. MTT Cell Viability Assay
2.9. ROS Detection Using DCFH-DA Staining
2.10. Statistical Analysis
3. Results
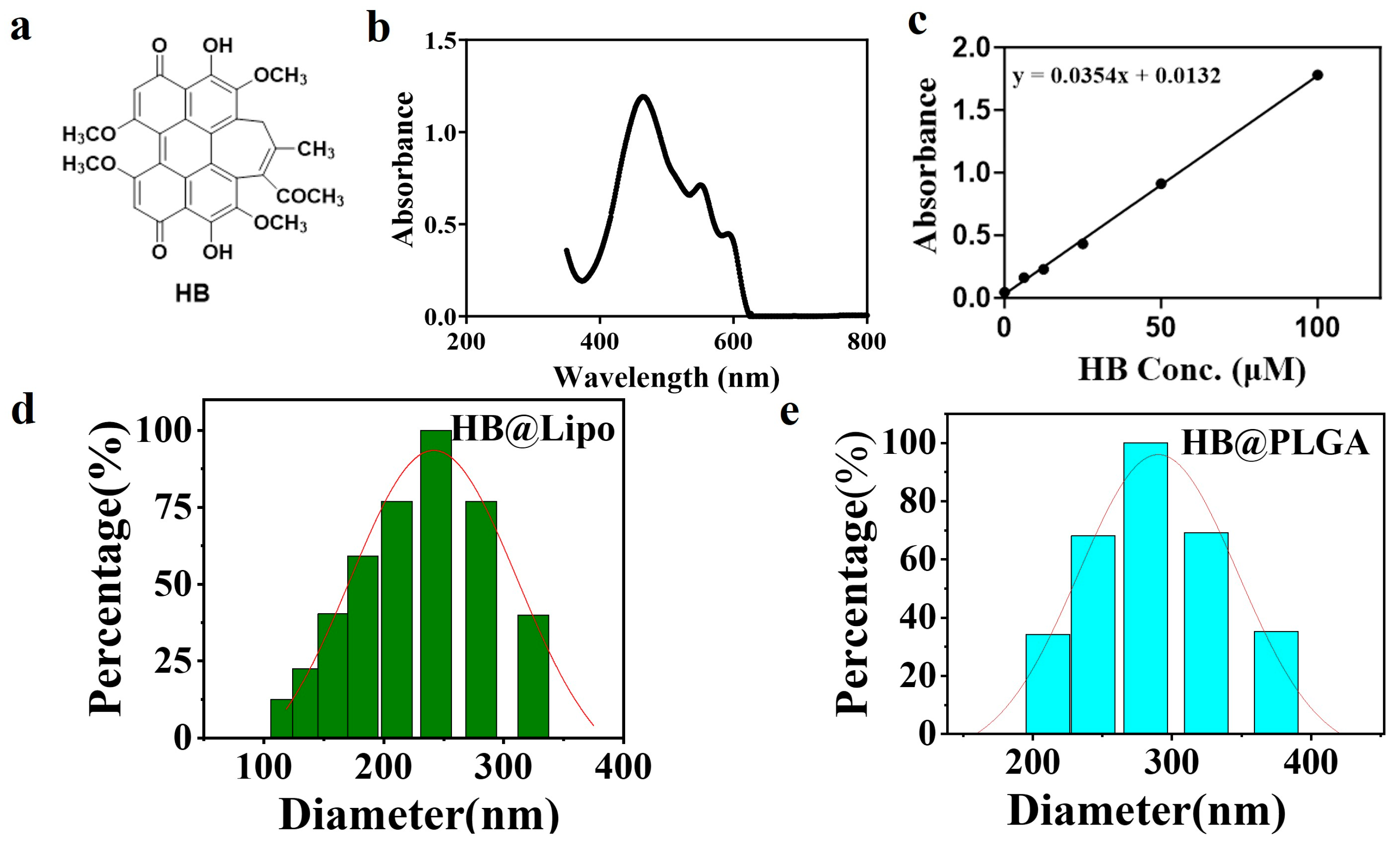
3.1. Preparation and Characterization of HB@Lipo
3.2. Preparation and Optimization of HB@PLGA
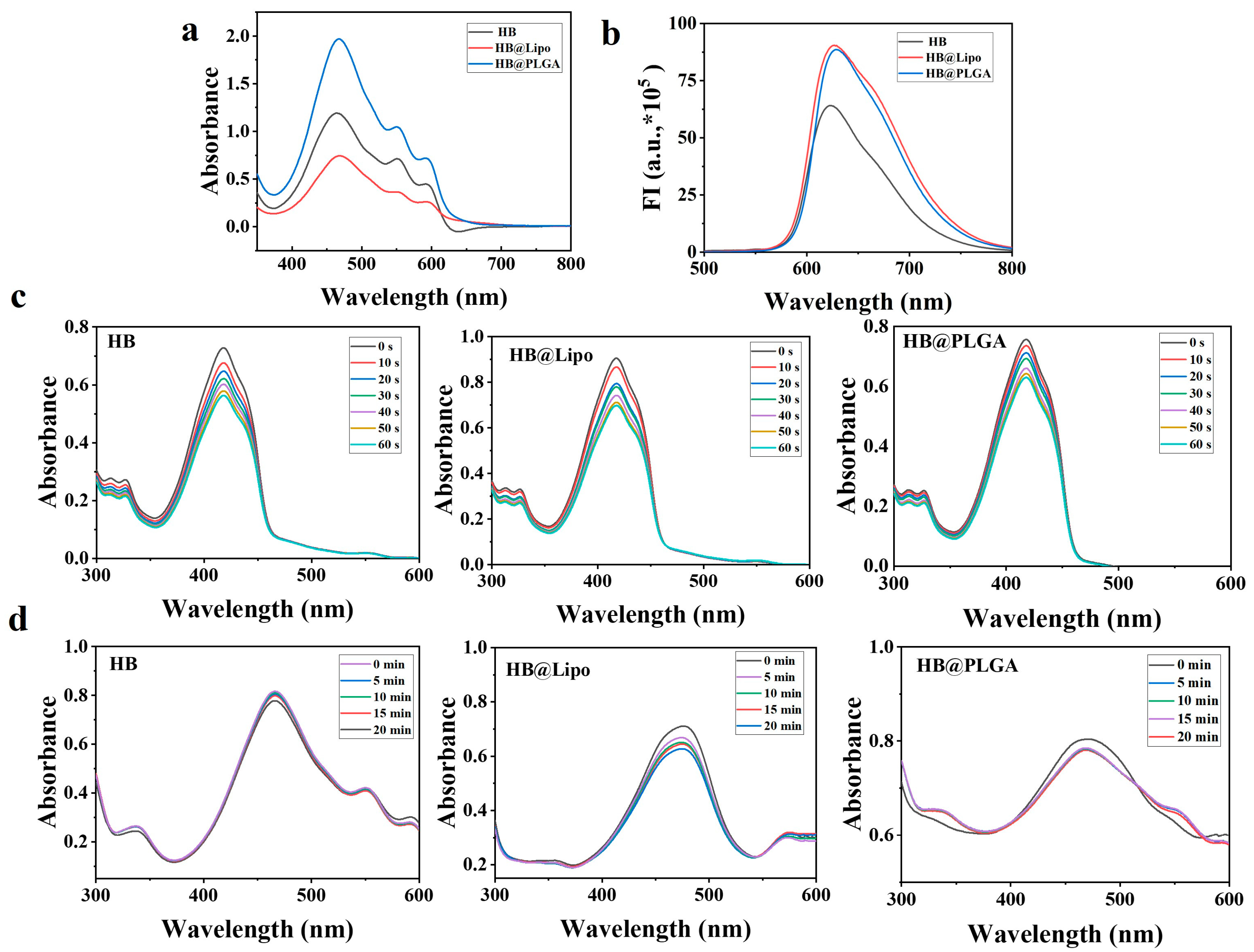
3.3. Photophysical-Chemical Properties of HB,HB@Lipo, and HB@PLGA
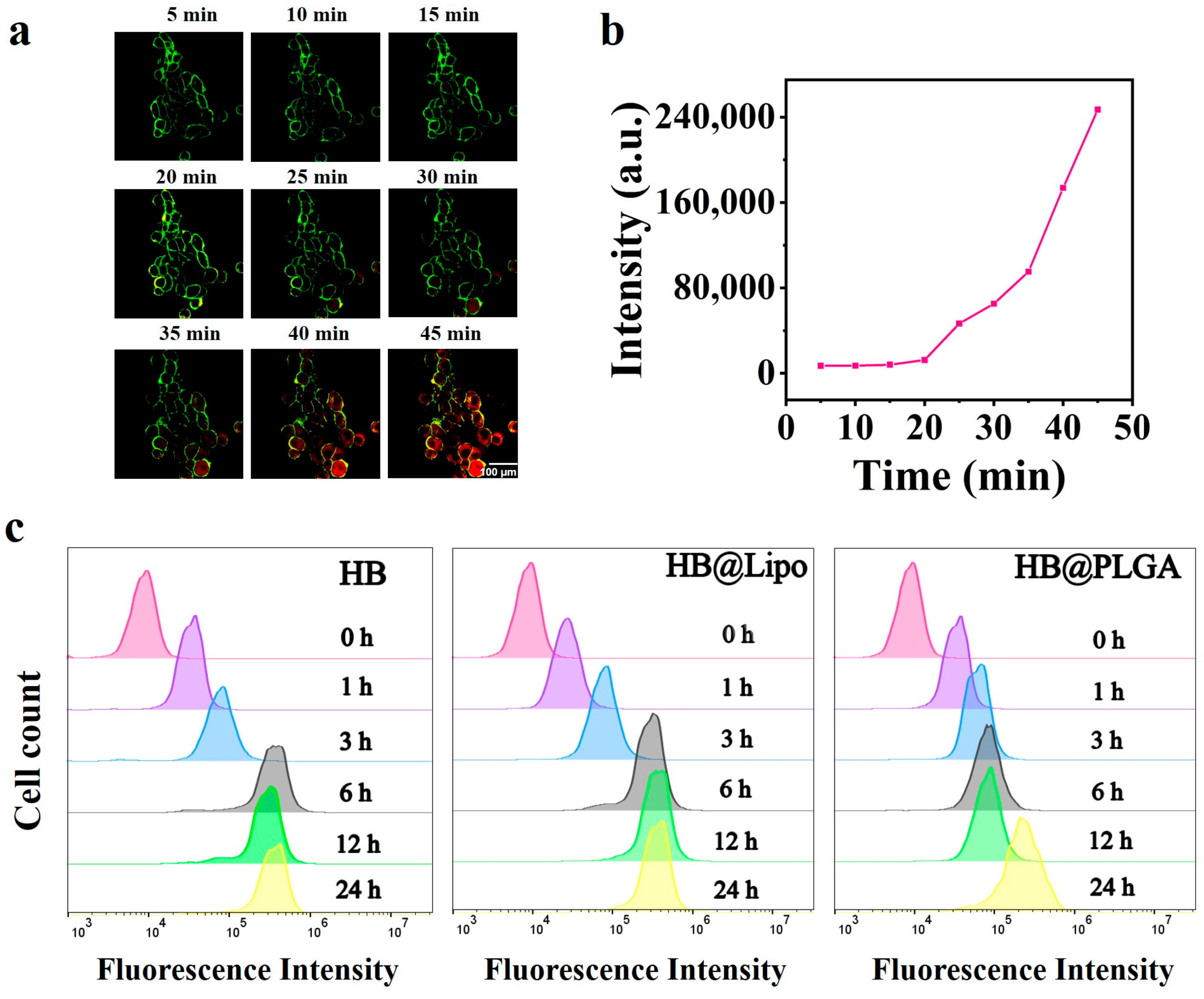
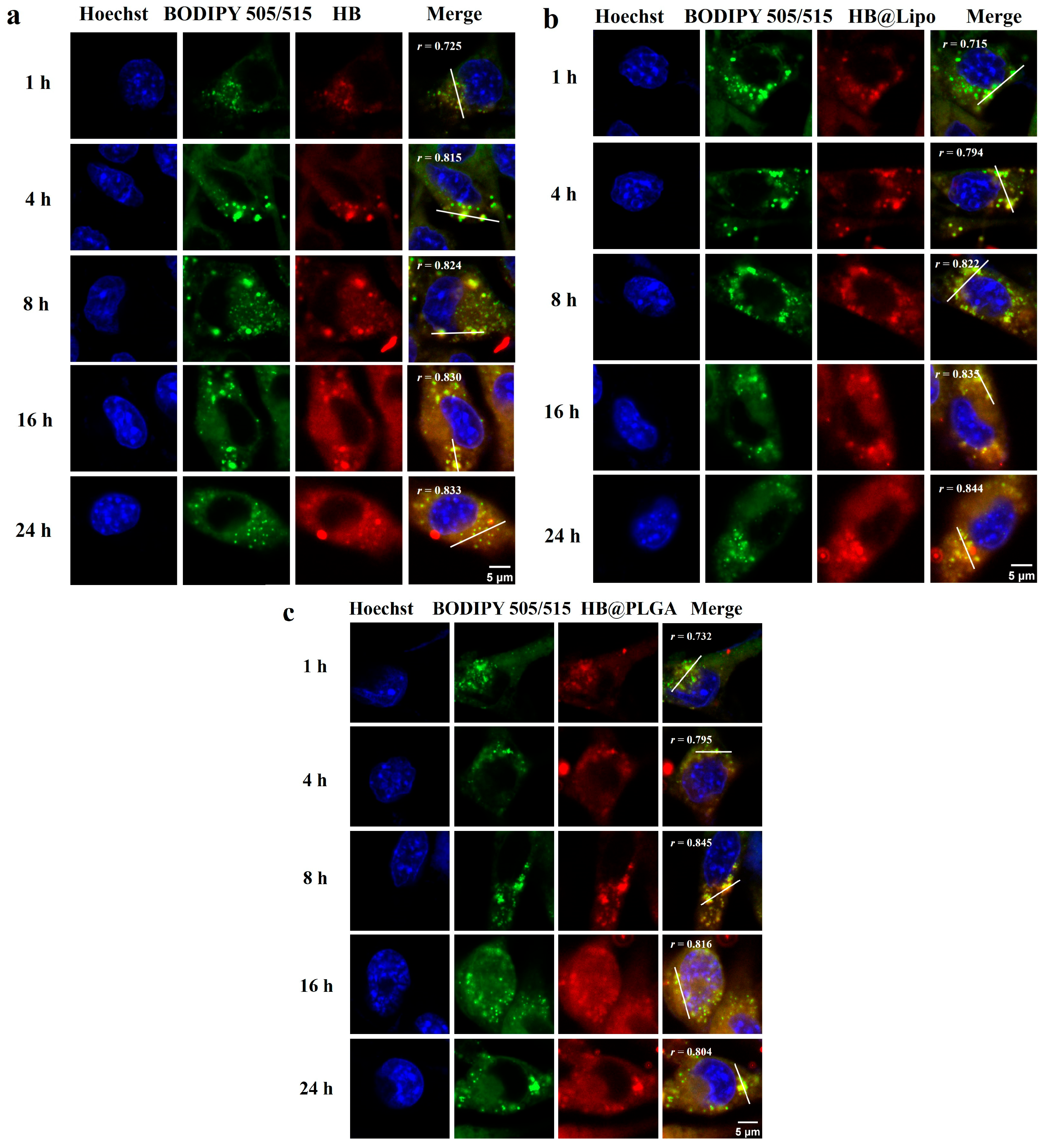
3.4. Cellular Uptake and Subcellular Localization of HB, HB@Lipo and HB@PLGA
3.5. Distribution of HB, HB@Lipo and HB@PLGA in 3D Tumor Spheroids
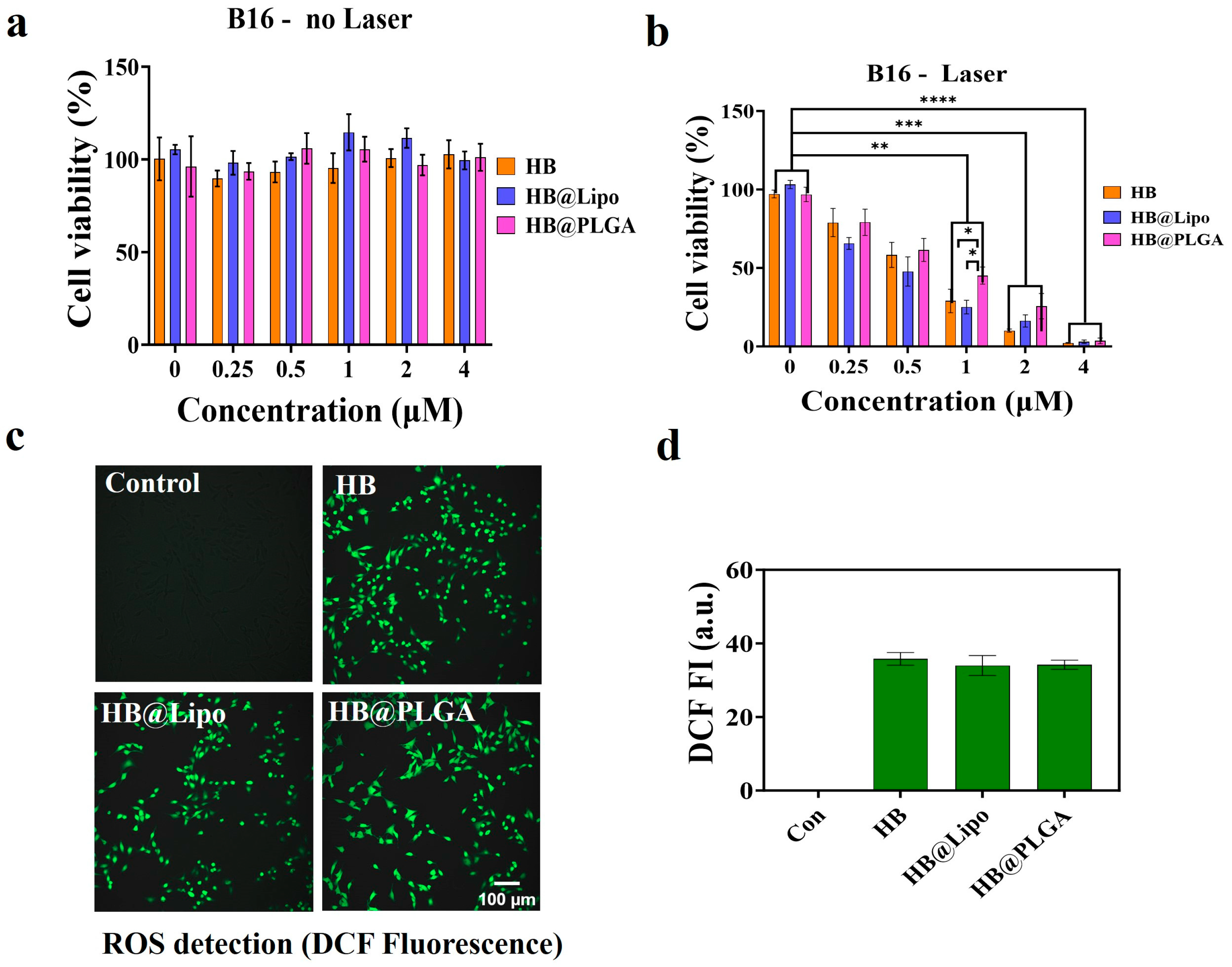
3.6. Photodynamic Effects of HB, HB@Lipo and HB@PLGA In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| HB | Hypocrellin B |
| ROS | Reactive oxygen species |
| HPD | Hematoporphyrin derivatives |
| PLGA | Poly(lactic-co-glycolic acid) |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| ER | Endoplasmic reticulum |
References
- Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Szpara, J.; Bartusik-Aebisher, D. Advances in Medicine: Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 8258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Duan, L.; Wang, A.; Fei, J.; Li, J. Insight into the Efficiency of Oxygen Introduced Photodynamic Therapy (PDT) and Deep PDT against Cancers with Various Assembled Nanocarriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1583. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Gomer, C.J.; Doiron, D.R.; White, L.; Jester, J.V.; Dunn, S.; Szirth, B.C.; Razum, N.J.; Murphree, A.L. Hematoporphyrin Derivative Photoradiation Induced Damage to Normal and Tumor Tissue of the Pigmented Rabbit Eye. Curr. Eye Res. 1984, 3, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C.; Bonnett, R.; Scourides, P.A. In Vivo Biological Activity of the Components of Haematoporphyrin Derivative. Br. J. Cancer 1982, 45, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of Photodynamic Therapy with Hypocrellin B on Apoptosis, Adhesion, and Migration of Cancer Cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Wei, G. Effects of Photodynamic Therapy Using Red LED-Light Combined with Hypocrellin B on Apoptotic Signaling in Cutaneous Squamous Cell Carcinoma A431 Cells. Photodiagn. Photodyn. Ther. 2023, 43, 103683. [Google Scholar] [CrossRef]
- Natesan, S.; Krishnaswami, V.; Ponnusamy, C.; Madiyalakan, M.; Woo, T.; Palanisamy, R. Hypocrellin B and Nano Silver Loaded Polymeric Nanoparticles: Enhanced Generation of Singlet Oxygen for Improved Photodynamic Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 935–946. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Wang, R.; Qu, J.; Tang, X.; Zhang, J.; Ou, A.; Li, Q.; Chen, G.; Zheng, C.; Muhitdinov, B.; Huang, Y. Lactoferrin-Modified Gambogic Acid Liposomes for Colorectal Cancer Treatment. Mol. Pharm. 2023, 20, 3925–3936. [Google Scholar] [CrossRef]
- Bigdeli, A.; Makhmalzadeh, B.S.; Feghhi, M.; SoleimaniBiatiani, E. Cationic Liposomes as Promising Vehicles for Timolol/Brimonidine Combination Ocular Delivery in Glaucoma: Formulation Development and in Vitro/in Vivo Evaluation. Drug Deliv. Transl. Res. 2023, 13, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. Hypericin-Based Photodynamic Therapy Induces Surface Exposure of Damage-Associated Molecular Patterns like HSP70 and Calreticulin. Cancer Immunol. Immunother. 2012, 61, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Paraptosis after ER Photodamage Initiated by m-Tetra (Hydroxyphenyl) Chlorin. Photochem. Photobiol. 2021, 97, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Liu, W.; Sha, J.; Yu, Z.; Wang, S.; Ren, H.; Zhang, W.; Lee, C.-S.; Wang, P. A Dual Organelle-Targeting Photosensitizer Based on Curcumin for Enhanced Photodynamic Therapy. J. Mater. Chem. B 2023, 11, 10836–10844. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Z.-C.; Chen, L.-J.; Liang, H.-F.; Lu, H.-W.; Chen, Q.; Liang, B.; Aji, A.; Dong, J.; Tian, Q.-W.; et al. Nuclear-Targeted Nanostrategy Regulates Spatiotemporal Communication for Dual Antitumor Immunity. Adv. Healthc. Mater. 2024, 13, e2302342. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, Y.; Peng, Q.; Lu, S.; Long, S.; Li, M.; Wang, H.; Lu, S.; Chen, X.; Fan, J.; et al. Lipid Droplet Targeting Type I Photosensitizer for Ferroptosis via Lipid Peroxidation Accumulation. Adv. Mater. 2024, 36, e2309711. [Google Scholar] [CrossRef]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of Polymer Micelles Affects Interactions with Tumor Cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ Entry into the Cell: Relevance to Drug Delivery. Cell Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Biodegradable Polymeric Micelles Composed of Doxorubicin Conjugated PLGA-PEG Block Copolymer. J. Control. Release 2001, 70, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kerdous, R.; Sureau, F.; Bour, A.; Bonneau, S. Release Kinetics of an Amphiphilic Photosensitizer by Block-Polymer Nanoparticles. Int. J. Pharm. 2015, 495, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol. Biol. 2023, 2622, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-Loaded PLGA Nanoparticles for the Chemotherapy of Glioblastoma: Towards the Pharmaceutical Development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Patel, T.; Jain, N. Multicellular Tumor Spheroids: A Convenient in Vitro Model for Translational Cancer Research. Life Sci. 2024, 358, 123184. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, 160, e60682. [Google Scholar] [CrossRef]
- Zambaux, M.F.; Bonneaux, F.; Gref, R.; Maincent, P.; Dellacherie, E.; Alonso, M.J.; Labrude, P.; Vigneron, C. Influence of Experimental Parameters on the Characteristics of Poly(Lactic Acid) Nanoparticles Prepared by a Double Emulsion Method. J. Control Release 1998, 50, 31–40. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- D’Intino, E.; Chirico, D.; Fabiano, M.G.; Buccini, L.; Passeri, D.; Marra, F.; Puglisi, R.; Rinaldi, F.; Mattia, G.; Carafa, M.; et al. Unveiling the Differences: A Comprehensive Multi-Technique Analysis of Hard and Soft Nanoparticles. Int. J. Pharm. 2025, 676, 125604. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.G.; Brown, K.; Moore, R.B.; McPhee, M.S.; Diwu, Z.J.; Liu, J.; Huang, L.; Lown, J.W.; Begg, D.A.; Chlumecky, V.; et al. Uptake Kinetics and Intracellular Localization of Hypocrellin Photosensitizers for photodynamic Therapy: A Confocal Microscopy Study. Photochem. Photobiol. 1995, 61, 632–638. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Devereux, C.J.; Keenan, S.N.; Lou, J.; Cho, E.; Nie, S.; De Souza, D.P.; Narayana, V.K.; Siddall, N.A.; Rodrigues, C.H.M.; et al. Proximity Proteomics Reveals a Mechanism of Fatty Acid Transfer at Lipid Droplet-Mitochondria- Endoplasmic Reticulum Contact Sites. Nat. Commun. 2025, 16, 2135. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Parmryd, I. Quantifying colocalization by correlation: The Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytom. Part A 2010, 77, 733–742. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Wang, B.; Wang, P.; Li, D.; Cao, T.; Zhang, D.; Han, H.; Bai, M.; Wang, X.; et al. Mitochondria-Targeted Photodynamic Therapy Triggers GSDME-Mediated Pyroptosis and Sensitizes Anti-PD-1 Therapy in Colorectal Cancer. J. Immunother. Cancer 2024, 12, e008054. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Molinari, M. ER-Phagy: Mechanisms, Regulation, and Diseases Connected to the Lysosomal Clearance of the Endoplasmic Reticulum. Physiol. Rev. 2022, 102, 1393–1448. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic Reticulum Stress Signals in the Tumour and Its Microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome Biogenesis and Lysosomal Membrane Proteins: Trafficking Meets Function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Romero, G.; Estrela-Lopis, I.; Zhou, J.; Rojas, E.; Franco, A.; Espinel, C.S.; Fernández, A.G.; Gao, C.; Donath, E.; Moya, S.E. Surface Engineered Poly(Lactide-Co-Glycolide) Nanoparticles for Intracellular Delivery: Uptake and Cytotoxicity—A Confocal Raman Microscopic Study. Biomacromolecules 2010, 11, 2993–2999. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual Polyvinyl Alcohol Associated with Poly (D,L-Lactide-Co-Glycolide) Nanoparticles Affects Their Physical Properties and Cellular Uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of Poly(DL-Lactide-Co-Glycolide) Nanoparticles by Modified Spontaneous Emulsification Solvent Diffusion Method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, S.; Zhang, M.; Shen, T. Spectroscopic Studies and Photodynamic Actions of Hypocrellin B in Liposomes. Photochem. Photobiol. 2001, 73, 482–488. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Xie, J.; Zhao, J.; Li, Z. Photogeneration of Free Radicals (*OH and HB*-) and Singlet Oxygen (1O2) by Hypocrellin B in TX-100 Micelles Microsurroundings. Free Radic. Res. 2003, 37, 1107–1112. [Google Scholar] [CrossRef]
- Zaleskis, G.; Garberyte, S.; Pavliukeviciene, B.; Krasko, J.A.; Skapas, M.; Talaikis, M.; Darinskas, A.; Zibutyte, L.; Pasukoniene, V. Comparative Evaluation of Cellular Uptake of Free and Liposomal Doxorubicin Following Short Term Exposure. Anticancer Res. 2021, 41, 2363–2370. [Google Scholar] [CrossRef]
- Cavalcanti, R.R.M.; Lira, R.B.; Riske, K.A. Membrane Fusion Biophysical Analysis of Fusogenic Liposomes. Langmuir 2022, 38, 10430–10441. [Google Scholar] [CrossRef]
- Andar, A.U.; Hood, R.R.; Vreeland, W.N.; Devoe, D.L.; Swaan, P.W. Microfluidic Preparation of Liposomes to Determine Particle Size Influence on Cellular Uptake Mechanisms. Pharm. Res. 2014, 31, 401–413. [Google Scholar] [CrossRef]
- Ashby, G.; Keng, K.E.; Hayden, C.C.; Gollapudi, S.; Houser, J.R.; Jamal, S.; Stachowiak, J.C. Selective Endocytic Uptake of Targeted Liposomes Occurs within a Narrow Range of Liposome Diameter. bioRxiv 2023. [Google Scholar] [CrossRef]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of Doxorubicin-Loaded PLGA Nanoparticles into U87 Human Glioblastoma Cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Song, K.; Li, J.; Li, L.; Zhang, P.; Geng, F.; Dong, R.; Yang, Q.; Qu, X.; Kong, B. Intracellular Metabolism, Subcellular Localization and Phototoxicity of HMME/HB in Ovarian Cancer Cells. Anticancer Res. 2011, 31, 3229–3235. [Google Scholar] [PubMed]
- Wang, X.; Luo, J.; Leung, A.W.; Li, Y.; Zhang, H.; Xu, C. Hypocrellin B in Hepatocellular Carcinoma Cells: Subcellular Localization and Sonodynamic Damage. Int. J. Radiat. Biol. 2015, 91, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ritz, R.; Roser, F.; Radomski, N.; Strauss, W.S.L.; Tatagiba, M.; Gharabaghi, A. Subcellular Colocalization of Hypericin with Respect to Endoplasmic Reticulum and Golgi Apparatus in Glioblastoma Cells. Anticancer Res. 2008, 28, 2033–2038. [Google Scholar] [PubMed]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-Based Photodynamic Action Induces Apoptosis in A549 Cells through ROS-Mediated Mitochondrial Signaling Pathway. Acta Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef]
- Du, J.; Cheng, L. The effect of hypocrellin A on the lipid bilayer of erythrocyte membrane. Shi Yan Sheng Wu Xue Bao 1991, 24, 153–158. [Google Scholar]
- Joyce, P.; Whitby, C.P.; Prestidge, C.A. Bioactive Hybrid Particles from Poly(D,L-Lactide-Co-Glycolide) Nanoparticle Stabilized Lipid Droplets. ACS Appl. Mater. Interfaces 2015, 7, 17460–17470. [Google Scholar] [CrossRef]
- Carrio, A.; Schwach, G.; Coudane, J.; Vert, M. Preparation and Degradation of Surfactant-Free PLAGA Microspheres. J. Control. Release 1995, 37, 113–121. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Indian J. Nephrol. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
| DPPC | Cholesterol | HB Conc. | Organic Solvent | Organic Solvent Removal | Particle Control |
|---|---|---|---|---|---|
| 12 mg | 3 mg | 5 μL of 40 mM HB in DMSO | chloroform | rotary evaporation for 2 h | extruder |
| No. | Inner Aqueous Phase | PLGA Conc. | EE, % | Average Diameter, nm | Dispersity (Đ) |
|---|---|---|---|---|---|
| 1 | 1% PVA | 10% | 0 | 455 ± 2.77 | 0.216 |
| 2 | 2% PVA | 10% | 0 | 341 ± 2.68 | 0.23 |
| 3 | 3% PVA | 10% | 0 | 579 ± 1.08 | 0.176 |
| 4 | 4% PVA | 10% | 73 | 281 ± 1.26 | 0.137 |
| 5 | 5% PVA | 10% | 89 | 390 ± 0.99 | 0.11 |
| No. | Inner Aqueous Phase | PLGA Conc. | EE, % | Average Diameter, nm | Dispersity (Đ) |
|---|---|---|---|---|---|
| 1 | 4% PVA | 10% | 73 | 281 ± 1.26 | 0.137 |
| 2 | 5% PVA | 10% | 89 | 390 ± 0.99 | 0.11 |
| 3 | 4% PVA | 15% | 92 | 748 ± 2.11 | 0.283 |
| 4 | 5% PVA | 15% | 67 | 445 ± 1.69 | 0.143 |
| 5 | 4% PVA | 20% | 89 | 669 ± 3.06 | 0.361 |
| 6 | 5% PVA | 20% | 91 | 701 ± 2.71 | 0.297 |
| HB Conc. | PLGA Conc. | Inner Aqueous Phase | External Aqueous Phase | Organic Solvent Removal | EE, % | Average Diameter, nm | Dispersity (Đ) |
|---|---|---|---|---|---|---|---|
| 20 μL of 40 mM HB in DMSO | 10% | 4% PVA | 1% PVA | Rotary evaporative for 1 h | 73 | 281 ± 1.26 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Zhao, F.; Cheng, J.; Feng, K.; Yan, L.; You, Y.; Li, J.; Meng, J. Comparative Study of Free and Encapsulated Hypocrellin B on Photophysical-Chemical Properties, Cellular Uptake, Subcellular Distribution, and Phototoxicity. Nanomaterials 2025, 15, 889. https://doi.org/10.3390/nano15120889
Kang W, Zhao F, Cheng J, Feng K, Yan L, You Y, Li J, Meng J. Comparative Study of Free and Encapsulated Hypocrellin B on Photophysical-Chemical Properties, Cellular Uptake, Subcellular Distribution, and Phototoxicity. Nanomaterials. 2025; 15(12):889. https://doi.org/10.3390/nano15120889
Chicago/Turabian StyleKang, Weiyan, Feng Zhao, Jixing Cheng, Kaijie Feng, Liang Yan, Yue You, Jinxia Li, and Jing Meng. 2025. "Comparative Study of Free and Encapsulated Hypocrellin B on Photophysical-Chemical Properties, Cellular Uptake, Subcellular Distribution, and Phototoxicity" Nanomaterials 15, no. 12: 889. https://doi.org/10.3390/nano15120889
APA StyleKang, W., Zhao, F., Cheng, J., Feng, K., Yan, L., You, Y., Li, J., & Meng, J. (2025). Comparative Study of Free and Encapsulated Hypocrellin B on Photophysical-Chemical Properties, Cellular Uptake, Subcellular Distribution, and Phototoxicity. Nanomaterials, 15(12), 889. https://doi.org/10.3390/nano15120889










