Boosting Photoresponse Performance and Stability of Photoelectrochemical Photodetectors by Chemical Bath Depositing Multilayer MoS2 on ZnO Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the ZnO Photoanode
2.2. Fabrication of the ZnO/MoS2 Photoanode
2.3. Characterization
2.4. Photoelectrochemical Measurements
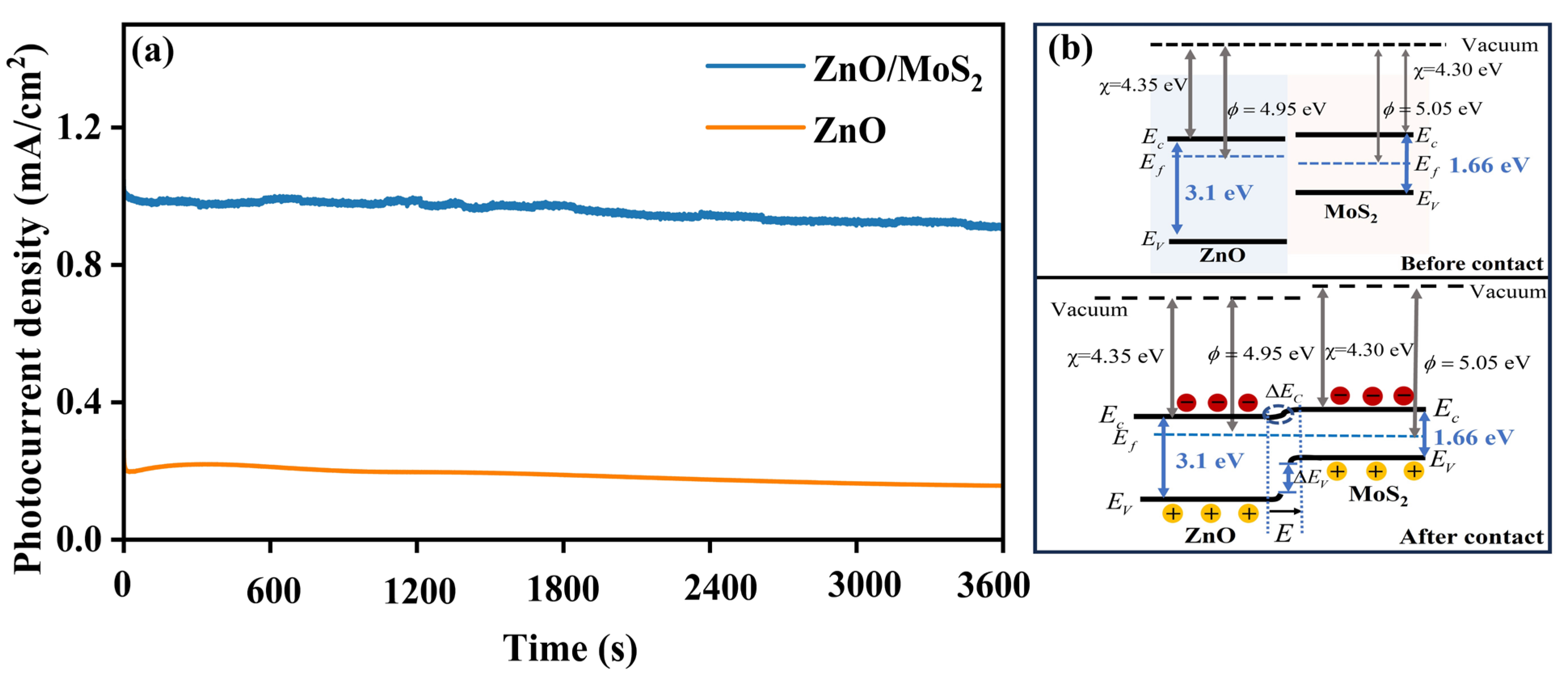
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Dong, M.; Li, Q.; Liu, Y.; Di, X.; Lu, X.; Meng, J.; Li, Z. High-performance UV photodetector via energy band engineering and LSPR-enhanced pyro-phototronic effect in Au decorated 2D-PbI2/1D-ZnO heterojunction. Adv. Opt. Mater. 2024, 12, 2303177. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Shi, Y.; Li, S.; Sun, F.; Lu, Q.; Shen, Y.; Feng, S.; Qin, S. Fast and high-performance self-powered photodetector based on the ZnO/metal-organic framework heterojunction. ACS Appl. Mater. Interfaces 2023, 15, 18236–18243. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, K.S.; Jayarathna, R.A.; Hwang, S.Y.; Thu, P.T.M.; Vidyasagar, D.; Shim, Y.-H.; Kim, E.-T.; Sohn, Y.; Kim, Y.H.; Kim, M.-D. NiO@GaN nanorods-based core-shell heterostructure for enhanced photoelectrochemical water splitting via efficient charge separation. J. Alloys Compd. 2024, 1009, 176882. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, Z.; Li, H.; Liu, S.; Lu, H.; Wen, X.; Yang, Y. ZnO quantum dot/MXene nanoflake hybrids for ultraviolet photodetectors. ACS Appl. Nano Mater. 2021, 4, 13674–13682. [Google Scholar] [CrossRef]
- Altaf, C.T.; Yaman, E.; Karagoz, E.; Colak, T.O.; Sankir, N.D.; Sankir, M. Self-powered photoelectrochemical photodetectors based on a CsPbBr3/S-g-C3N4 heterojunction-sensitized 3D ZnO nanostructured thin film. ACS Appl. Nano Mater. 2024, 7, 8186–8196. [Google Scholar] [CrossRef]
- Elahi, E.; Hassan, N.U.; Rehman, M.A.; Khalil, H.M.W.; Khan, M.A.; Rehman, S.; Hao, A.; Noh, H.; Khan, K.; Eom, J. Bipolar photoresponse of a graphene field-effect transistor induced by photochemical reactions. ACS Appl. Electron. Mater. 2023, 5, 5111–5119. [Google Scholar] [CrossRef]
- Yu, H.; Huang, L.; Zhou, L.; Peng, Y.; Li, X.; Yin, P.; Zhao, J.; Zhu, M.; Wang, S.; Liu, J.; et al. Wafer-scale epitaxial monolayer MoS2. Adv. Mater. 2024, 36, 2402855. [Google Scholar] [CrossRef]
- Lamouchi, A.; Ben Assaker, I.; Chtourou, R. Enhanced photoelectrochemical activity of MoS2-decorated ZnO nanowires electrodeposited onto stainless steel mesh for hydrogen production. Appl. Surf. Sci. 2019, 478, 937–945. [Google Scholar] [CrossRef]
- Gautam, C.; Verma, A.; Chaudhary, P.; Yadav, B. Development of 2D based ZnO-MoS2 nanocomposite for photodetector with light-induced current study. Opt. Mater. 2022, 123, 111860. [Google Scholar] [CrossRef]
- Ma, J.; Ge, Y.; Dai, P.; Lu, C.; Xu, X. Highly stable and sensitive photoelectrochemical photodetectors based on a ZnO nanorod/monolayer MoS2 nanosheets heterostructure. J. Alloys Compd. 2024, 976, 173315. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Niu, H.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. NiS2/MoS2 mixed phases with abundant active edge sites induced by sulfidation and graphene introduction towards high-rate supercapacitors. Chem. Eng. J. 2021, 406, 126713. [Google Scholar] [CrossRef]
- Mandavkar, R.; Lin, S.; Kulkarni, R.; Burse, S.; Habib, A.; Kunwar, S.; Lee, J. Dual-step photocarrier injection by mixture layer of ZnO QDs and MoS2 NPs on hybrid PdAu NPs. Mater. Res. Bull. 2022, 151, 111832. [Google Scholar] [CrossRef]
- Winata, S.M.; Zakaria, R.; Fauzia, V. Effect of UV-O3 treatment on the performance of UV detector based on zinc oxide/molybdenum disulfide nanosheet heterostructures. Opt. Mater. 2024, 147, 114722. [Google Scholar] [CrossRef]
- Bietti, S.; Somaschini, C.; Sanguinetti, S.; Koguchi, N.; Isella, G.; Chrastina, D. Chrastina. Fabrication of high efficiency III-V quantum nanostructures at low thermal budget on Si. Appl. Phys. Lett. 2009, 95, 3273860. [Google Scholar] [CrossRef]
- Bietti, S.; Somaschini, C.; Sanguinetti, S.; Koguchi, N.; Isella, G.; Chrastina, D. Thiol groups reutilization on chemical bath deposited tin oxide surface achieving interface anchoring and defects passivation for enhancing the performance and stability of perovskite solar cells. Small 2025, 21, 2408516. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Wang, Y.; Wang, H.; Li, J.; Lee, K. Sn-based metal oxides and sulfides anode materials for Na ion battery. Energy Storage Mater. 2021, 39, 21–44. [Google Scholar] [CrossRef]
- Tang, J.-F.; Yang, Y.-L.; Chen, L.-C.; Kang, C.-F.; Hsu, C.-L. Gas-sensing properties of p-type of nitrogen-doped ZnO nanorods prepared by deep cryogenic treatment. Appl. Surf. Sci. 2024, 658, 159871. [Google Scholar] [CrossRef]
- Momeni, M.M.; Aydisheh, H.M.; Lee, B.-K. Effectiveness of MnO2 and V2O5 deposition on light fostered supercapacitor performance of WTiO2 nanotube: Novel electrodes for photo-assisted supercapacitors. Chem. Eng. J. 2022, 450, 137941. [Google Scholar] [CrossRef]
- Li, Y.; Zuo, S.; Li, Q.-H.; Wu, X.; Zhang, J.; Zhang, H.; Zhang, J. Vertically aligned MoS2 with in-plane selectively cleaved Mo-S bond for hydrogen production. Nano Lett. 2021, 21, 1848–1855. [Google Scholar] [CrossRef]
- Hailili, R.; Ji, H.; Wang, K.; Dong, X.; Chen, C.; Sheng, H.; Bahnemann, D.W.; Zhao, J. ZnO with controllable oxygen vacancies for photocatalytic nitrogen oxide removal. ACS Catal. 2022, 12, 10004–10017. [Google Scholar] [CrossRef]
- Masar, M.; Ali, H.; Guler, A.C.; Suly, P.; Urbanek, P.; Antos, J.; Hanulikova, B.; Machovsky, M.; Kuritka, I. Unveiling significant impact of subtle atomic displacement from equilibrium position in crystal lattice on electronic properties and photocatalytic activity of ZnO. Mater. Today Chem. 2024, 38, 102136. [Google Scholar] [CrossRef]
- Singh, D.K.; Roul, B.; Pant, R.; Chowdhury, A.M.; Nanda, K.K.; Krupanidhi, S.B. Different types of band alignment at MoS2/(Al, Ga, In) N heterointerfaces. Appl. Phys. Lett. 2020, 116, 252102. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, S.; Peng, R.; Liang, J.; Cong, X.; Li, Y.; Li, C.; Wang, B.; Lin, M.-L.; Tan, P.-H.; et al. Abnormal out-of-plane vibrational Raman mode in electrochemically intercalated multilayer MoS2. Nano Lett. 2023, 23, 5342–5349. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.-H.; Liu, H.; Chen, L.; Liu, X.-K.; Min, L.; Li, Z.-W.; Zhu, H.; Sun, Q.-Q. Feasibility of large-scale MoS2 thin-film transistors on a GaN substrate. ACS Appl. Electron. Mater. 2019, 1, 1418–1423. [Google Scholar] [CrossRef]
- Vashishtha, P.; Prajapat, P.; Sharma, A.; Goswami, P.; Walia, S.; Gupta, G. Self-driven and thermally resilient highly responsive nano-fenced MoS2 based photodetector for near-infrared optical signal. Mater. Res. Bull. 2023, 164, 112260. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, Y.; Chen, Y.; Jiang, X.; Lin, M.; Lin, C.; Lin, T.; Gao, M.; Zhao, C.; Wu, X. Improved piezo-photocatalysis for aquatic multi-pollutant removal via BiOBr/BaTiO3 heterojunction construction. Chem. Eng. J. 2024, 488, 151002. [Google Scholar] [CrossRef]
- Ni, J.; Liu, D.; Wang, W.; Wang, A.; Jia, J.; Tian, J.; Xing, Z. Hierarchical defect-rich flower-like BiOBr/Ag nanoparticles/ultrathin g-C3N4 with transfer channels plasmonic Z-scheme heterojunction photocatalyst for accelerated visible-light-driven photothermal-photocatalytic oxytetracycline degradation. Chem. Eng. J. 2021, 419, 129969. [Google Scholar] [CrossRef]
- Velický, M.; Bissett, M.A.; Woods, C.R.; Toth, P.S.; Georgiou, T.; Kinloch, I.A.; Novoselov, K.S.; Dryfe, R.A.W. Photoelectrochemistry of pristine mono-and few-layer MoS2. Nano Lett. 2016, 16, 2023–2032. [Google Scholar] [CrossRef]
- Jian, W.; Cheng, X.; Huang, Y.; You, Y.; Zhou, R.; Sun, T.; Xu, J. Arrays of ZnO/MoS2 nanocables and MoS2 nanotubes with phase engineering for bifunctional photoelectrochemical and electrochemical water splitting. Chem. Eng. J. 2017, 328, 474–483. [Google Scholar] [CrossRef]
- Bonomo, M.; Zarate, A.S.; Fagiolari, L.; Damin, A.; Galliano, S.; Gerbaldi, C.; Bella, F.; Barolo, C. Unreported resistance in charge transport limits the photoconversion efficiency of aqueous dye-sensitised solar cells: An electrochemical impedance spectroscopy study. Mater. Today Sustain. 2023, 21, 100271. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Lan, H.; Liu, H.; Qu, J. Strongly coupled metal oxide/reassembled carbon nitride/Co–Pi heterostructures for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2018, 10, 6424–6432. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, H.; Huang, Q.; Li, X.; Hou, X.; Zhao, L.; Ma, R.; Chen, H.; Li, Y. Remarkable improvement of the turn–on characteristics of a Fe2O3 photoanode for photoelectrochemical water splitting with coating a FeCoW oxy–hydroxide gel. Appl. Catal. B. Environ. 2017, 212, 89–96. [Google Scholar] [CrossRef]
- Liang, B.-W.; Chang, W.-H.; Huang, C.-S.; Huang, Y.-J.; Chen, J.-H.; Li, K.-S.; Simbulan, K.B.; Kumar, H.; Su, C.-Y.; Kuan, C.-H.; et al. Self-powered broadband photodetection of MoS2/Sb2Se3 heterostructure. ACS Appl. Opt. Mater. 2023, 1, 1952–1962. [Google Scholar] [CrossRef]
- Khan, M.; Kumar, S.; Mishra, A.; Sulania, I.; Tripathi, M.N.A. Study of structural and electronic properties of few-layer MoS2 film. Mater. Today Proc. 2022, 57, 100–105. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Amézaga-Madrid, P.; Pizá-Ruiz, P.; Antúnez-Flores, W.; Miki-Yoshida, M. Optical band gap estimation of ZnO nanorods. Mater. Res. 2016, 19, 33–38. [Google Scholar] [CrossRef]
- Lu, C.; Dong, W.; Zou, Y.; Wang, Z.; Tan, J.; Bai, X.; Ma, N.; Ge, Y.; Zhao, Q.; Xu, X. Direct Z-scheme SnSe2/SnSe heterostructure passivated by Al2O3 for highly stable and sensitive photoelectrochemical photodetectors. ACS Appl. Mater. Interfaces 2023, 15, 6156–6168. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Hsieh, P.-Y.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Incorporating graphene quantum dots to enhance the photoactivity of CdSe-sensitized TiO2 nanorods for solar hydrogen production. J. Mater. Chem. A 2020, 8, 13971–13979. [Google Scholar] [CrossRef]
- Bhakhar, S.A.; Pataniya, P.M.; Tannarana, M.; Solanki, G.; Pathak, V. Electrophoretic deposition of MoS2 nanosheets for photoelectrochemical type photodetector. Opt. Mater. 2022, 125, 112097. [Google Scholar] [CrossRef]
- Lan, C.; Li, C.; Wang, S.; Yin, Y.; Guo, H.; Liu, N.; Liu, Y. ZnO-WS2 heterostructures for enhanced ultra-violet photodetectors. RSC Adv. 2016, 6, 67520–67524. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, J.; Liu, F.; Zhang, Y. Enhanced photoresponse performance of self-powered UV–visible photodetectors based on ZnO/Cu2O/electrolyte heterojunctions via graphene incorporation. J. Alloys Compd. 2017, 726, 803–809. [Google Scholar] [CrossRef]
- Zhan, Z.; Zheng, L.; Pan, Y.; Sun, G.; Li, L. Self-powered, visible-light photodetector based on thermally reduced graphene oxide-ZnO (rGO-ZnO) hybrid nanostructure. J. Mater. Chem. 2012, 22, 2589–2595. [Google Scholar] [CrossRef]
- Patel, M.; Pataniya, P.M.; Patel, V.; Sumesh, C.K. Flexible photodetector based on Graphite/ZnO-WS2 nanohybrids on paper. J. Mater. Sci. Mater. Electron. 2022, 33, 13771–13781. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Zhou, Y.; Zou, Z. Al-ZnO/CdS photoanode modified with a triple functions conformal TiO2 film for enhanced photoelectrochemical efficiency and stability, Applied Catalysis B: Environmental. Appl. Catal. B. Environ. 2019, 255, 117738. [Google Scholar] [CrossRef]
- Galán-González, A.; Sivan, A.K.; Hernández-Ferrer, J.; Bowen, L.; Di Mario, L.; Martelli, F.; Benito, A.M.; Maser, W.K.; Chaudhry, M.U.; Gallant, A.; et al. Cobalt-doped ZnO nanorods coated with nanoscale metal–organic framework shells for water-splitting photoanodes. ACS Appl. Nano Mater. 2020, 3, 7781–7788. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kalimuthu, K.R.; Kalimuthu, V. A type-II MoS2/ZnO heterostructure with enhanced photocatalytic activity. Mater. Lett. 2019, 243, 183–186. [Google Scholar] [CrossRef]
- Wei, J.; Lian, Z.; Liu, Y.; Xie, M.; Jia, Y.; Li, K.; Kudrawiec, R.; Dan, Y.; Yang, R. High-responsivity natural-electrolyte undersea photoelectrochemical photodetector with self-powered Cu@GaN nanowires network. Adv. Funct. Mater. 2023, 33, 2302872. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Z.; Qiu, H.; Tang, Y.; Yang, S.; Zhao, J.; Zhou, Q.; Wang, J.; Liu, G.; Zhao, Y.; et al. Fully suspended MoS2 photodetectors toward high response speed and stable responsivity. ACS Photonics 2025, 12, 2656–2663. [Google Scholar] [CrossRef]

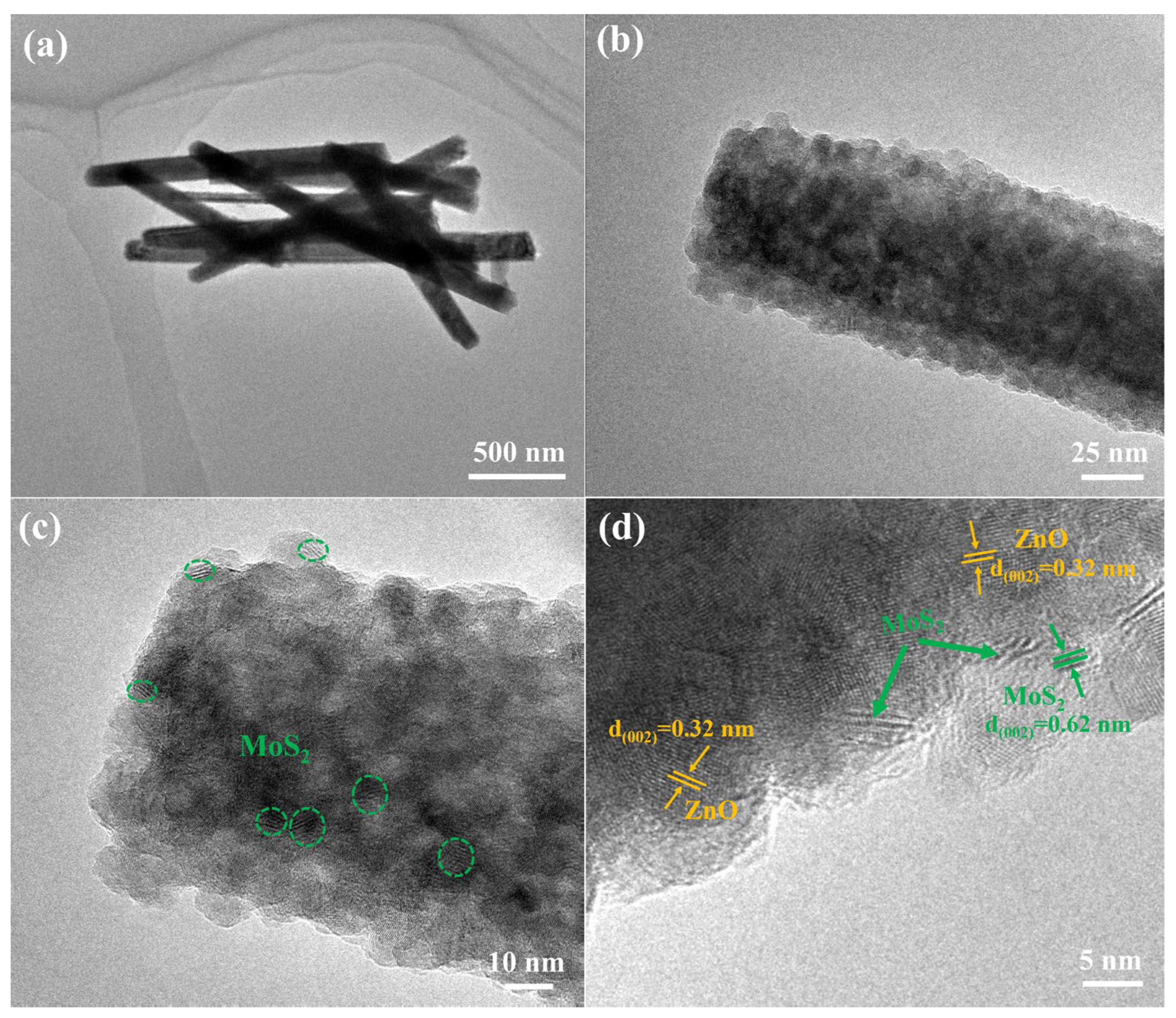
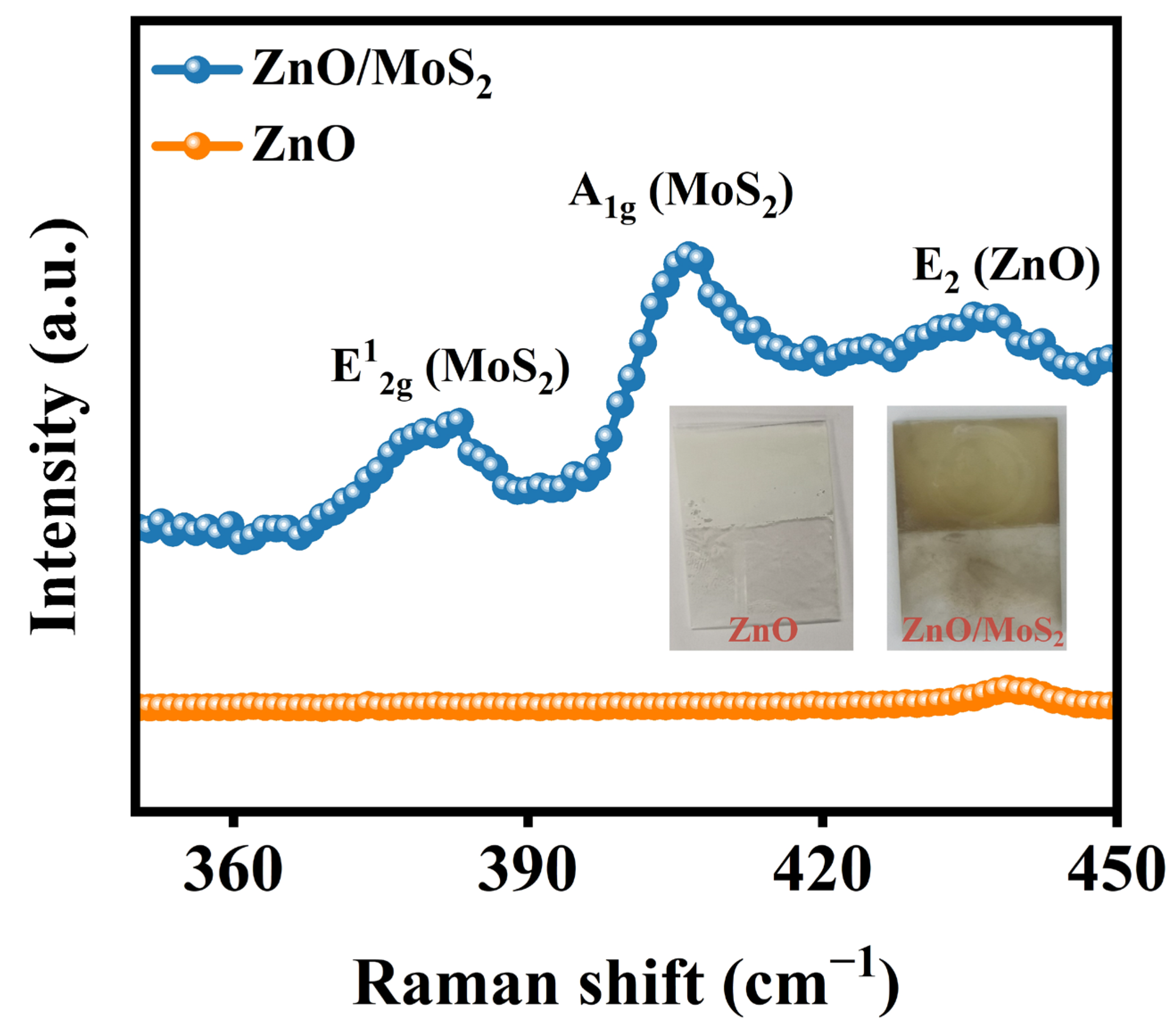
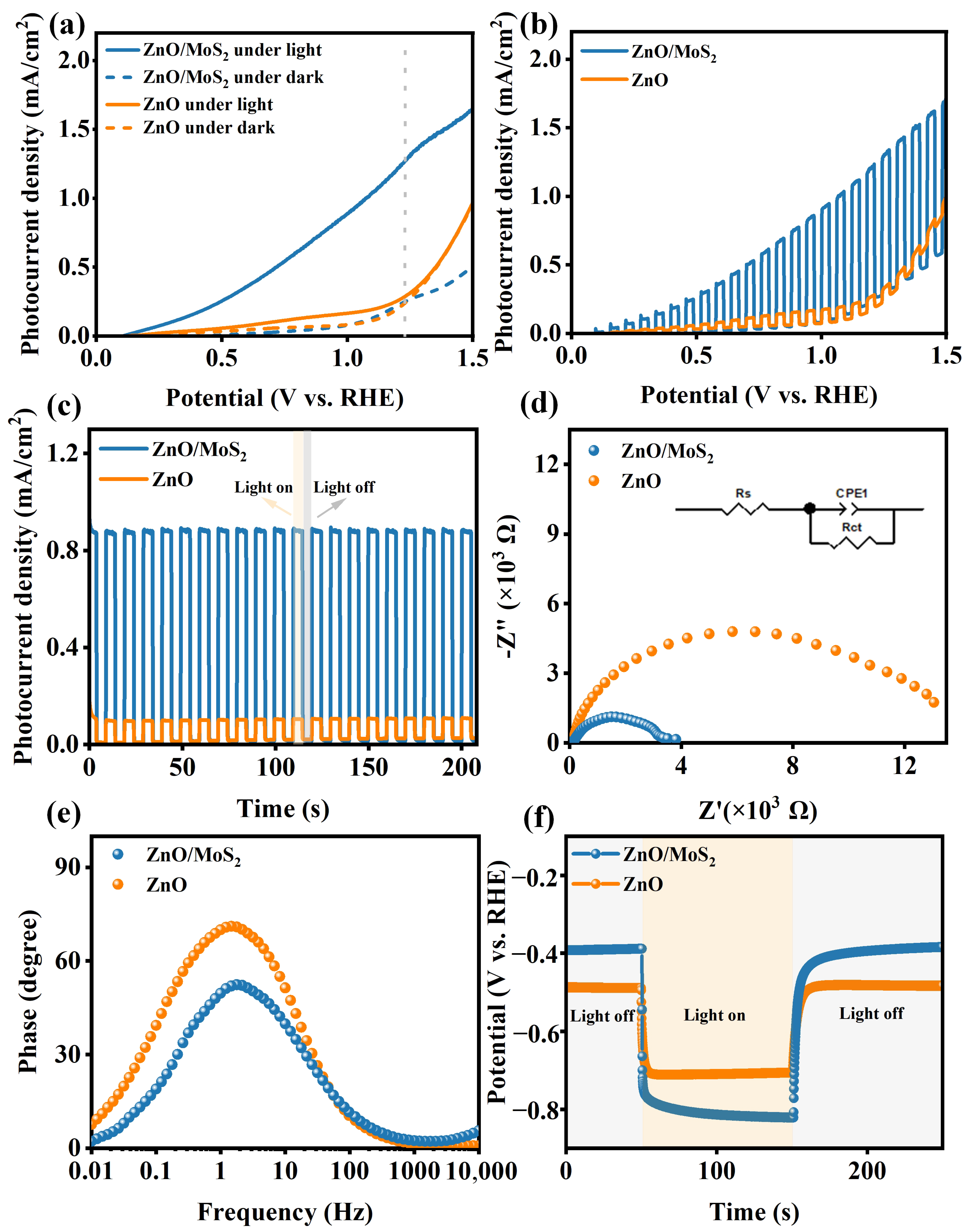
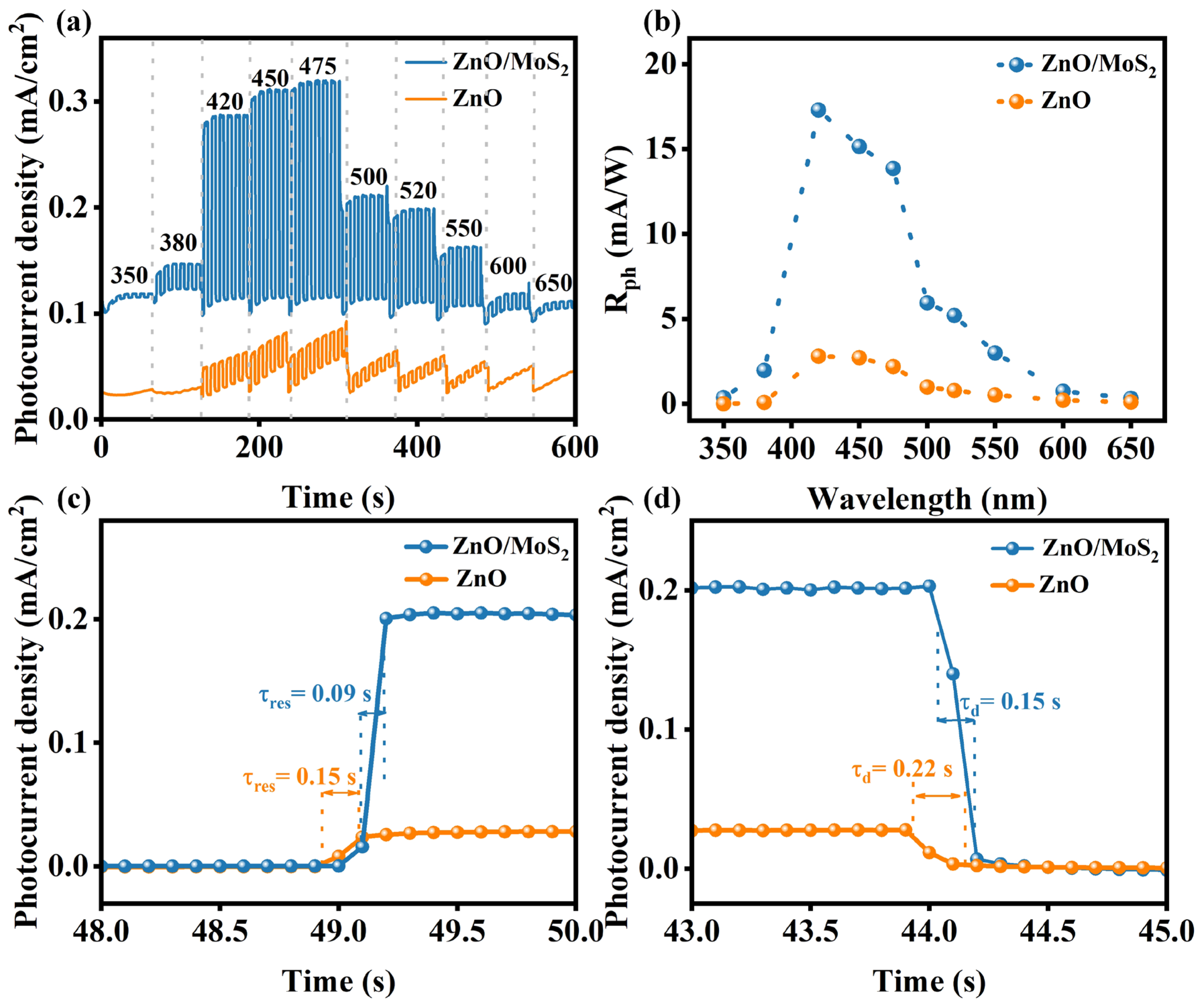
| Materials | Synthesis Method of Heterojunction | Rph (mA/W) | tres (s)/trec (s) | Stability | Refs. |
|---|---|---|---|---|---|
| MoS2 | Electrophoretic deposition method | 0.17 | 0.3/0.3 | / | [38] |
| ZnO-MoS2 | Solgel-assisted synthesis method | 34.50 | 2.46/4.82 | / | [9] |
| ZnO/MoS2/HNP | Dual-phase solid-state dewetting approach | 1430 | 3.37/0.35 | / | [12] |
| ZnO/MoS2 | Drop-casting method | 751 | 7/29.1 | / | [13] |
| nf-MoS2/Si3N4 | Radio frequency (RF) sputtering | 1358 | / | 1170 mAW−1 at 100 °C | [25] |
| MoS2 | Mechanically exfoliated | 140–138,400 | 68.6 × 10−6/100 × 10−6 | 80% retained | [47] |
| ZnO/monolayer MoS2 | Chemical vapor deposition methods | 4 | 0.15/0.17 | 21,600 s~98% retained | [10] |
| ZnO/MoS2 | Chemical bath deposition method | 17.29 | 0.09/0.15 | 3600 s~92% retained | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, J.; Shi, X.; Sun, T.; Dai, P. Boosting Photoresponse Performance and Stability of Photoelectrochemical Photodetectors by Chemical Bath Depositing Multilayer MoS2 on ZnO Electrode. Nanomaterials 2025, 15, 875. https://doi.org/10.3390/nano15120875
Ma J, Wang J, Shi X, Sun T, Dai P. Boosting Photoresponse Performance and Stability of Photoelectrochemical Photodetectors by Chemical Bath Depositing Multilayer MoS2 on ZnO Electrode. Nanomaterials. 2025; 15(12):875. https://doi.org/10.3390/nano15120875
Chicago/Turabian StyleMa, Jingyao, Jiawei Wang, Xin Shi, Tianqi Sun, and Pengpeng Dai. 2025. "Boosting Photoresponse Performance and Stability of Photoelectrochemical Photodetectors by Chemical Bath Depositing Multilayer MoS2 on ZnO Electrode" Nanomaterials 15, no. 12: 875. https://doi.org/10.3390/nano15120875
APA StyleMa, J., Wang, J., Shi, X., Sun, T., & Dai, P. (2025). Boosting Photoresponse Performance and Stability of Photoelectrochemical Photodetectors by Chemical Bath Depositing Multilayer MoS2 on ZnO Electrode. Nanomaterials, 15(12), 875. https://doi.org/10.3390/nano15120875





