A TMO-ZnO Heterojunction-Based Sensor for Transformer Defect Detection: A DFT Study
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. Gas Sensing Properties of the CuO-ZnO Heterojunction for H2, CO, and C2H4
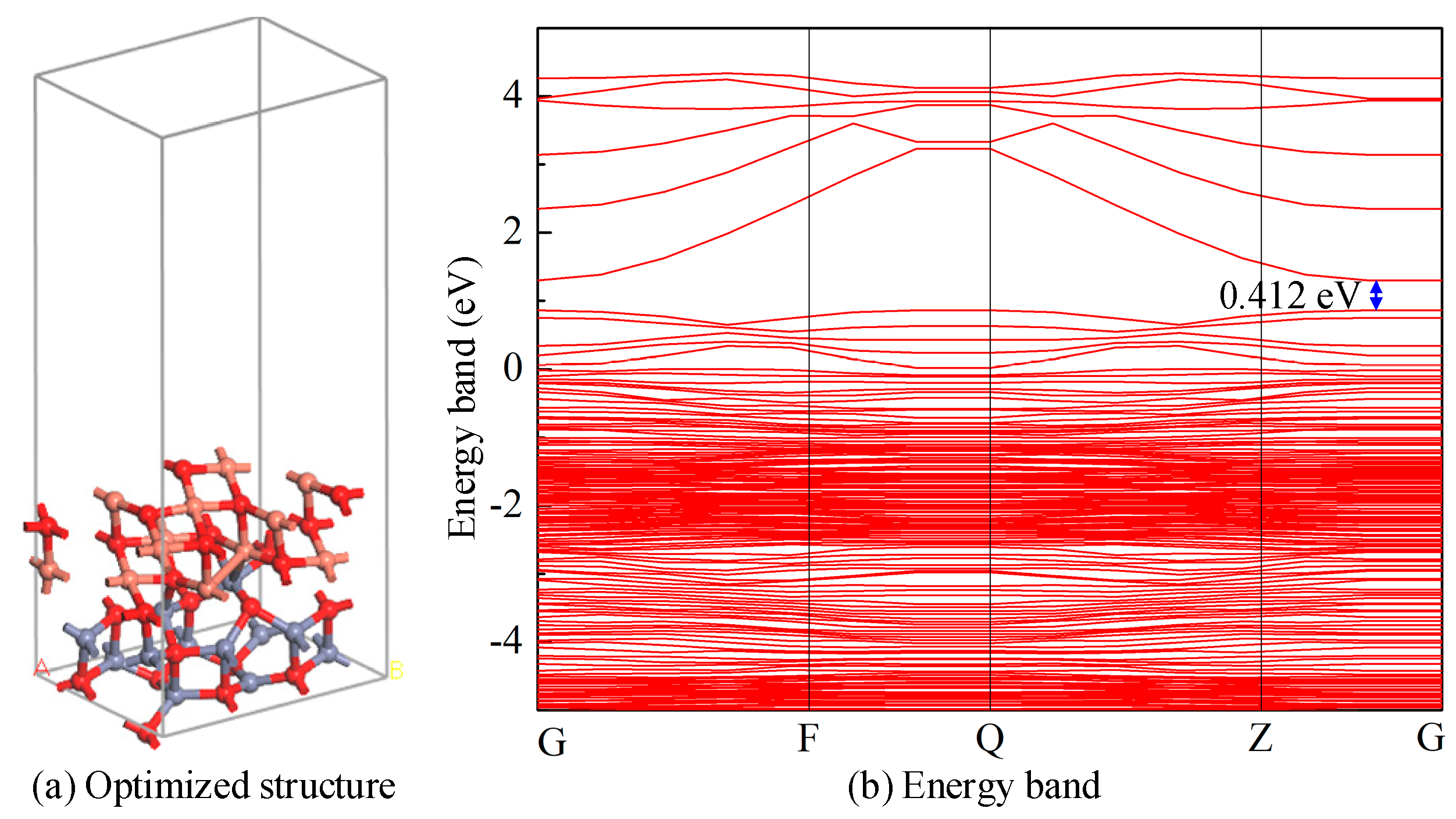
3.1.1. Structure and Electronic Analysis of the CuO-ZnO Heterojunction
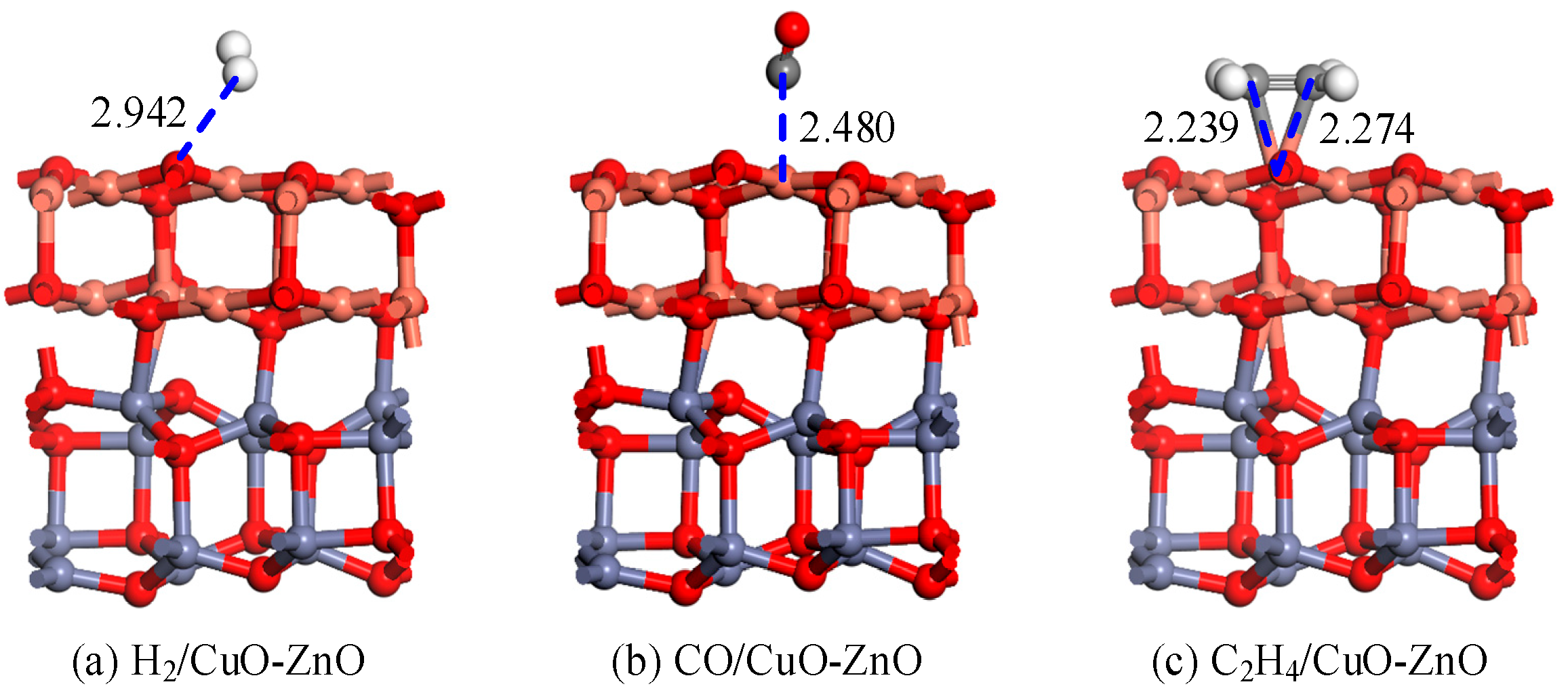
3.1.2. Structure and DCD Analysis of the Gas/CuO-ZnO Heterojunction System
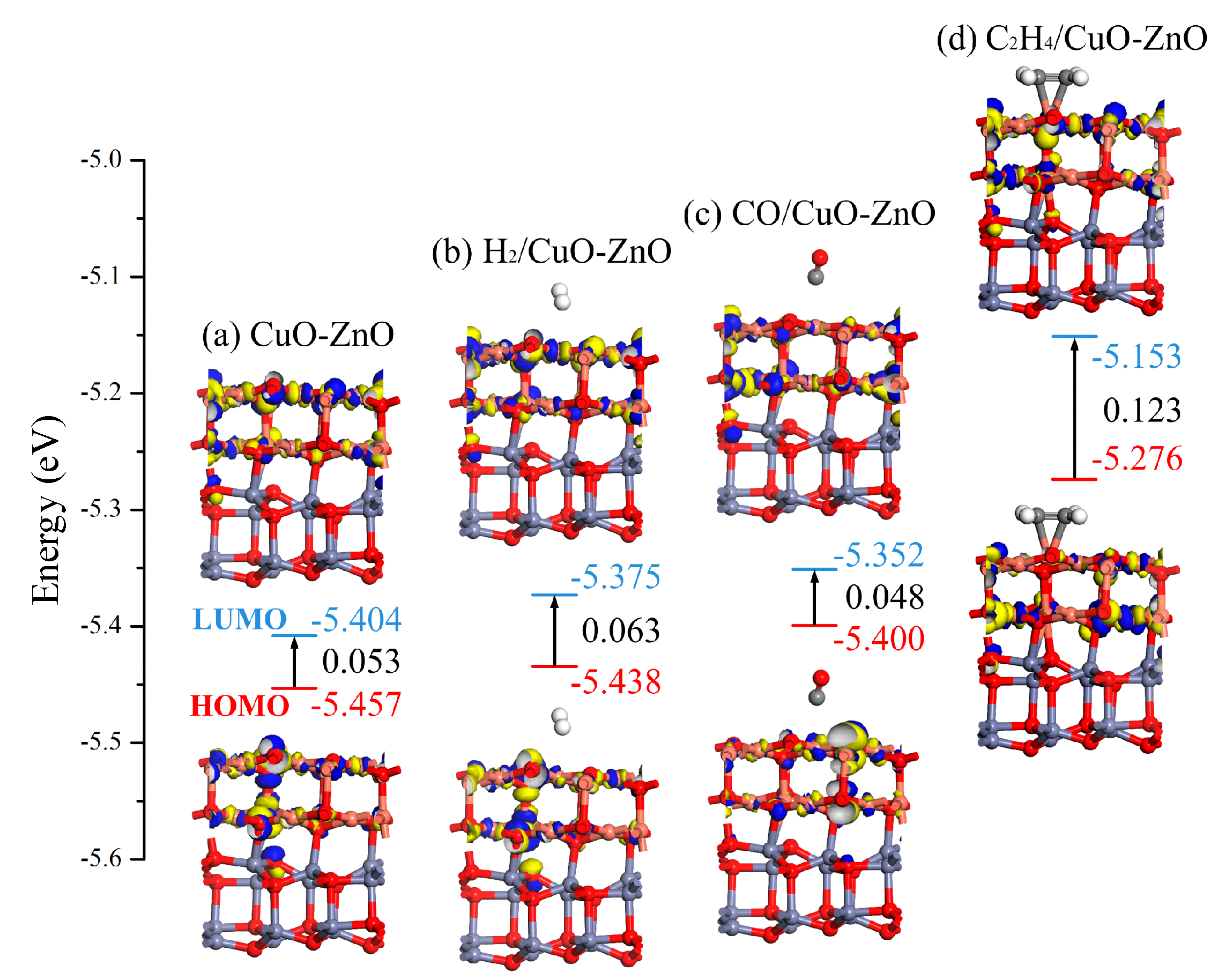
3.1.3. DOS and Molecular Orbital Analysis of the Gas/CuO-ZnO Heterojunction System
3.2. Gas Sensing Properties of the Ag2O-ZnO Heterojunction for H2, CO, and C2H4
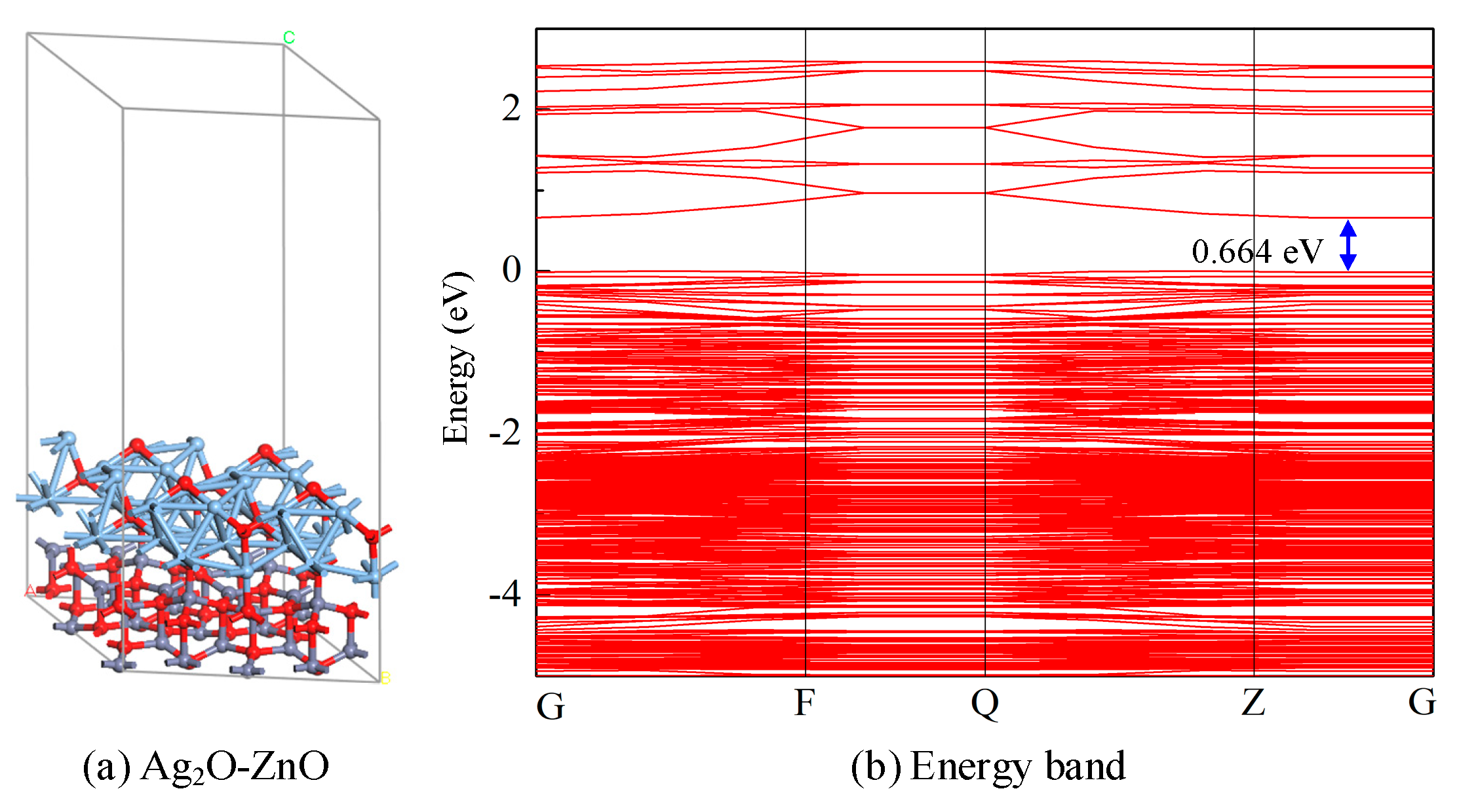
3.2.1. Structure and Electronic Analysis of Ag2O-ZnO Heterojunction
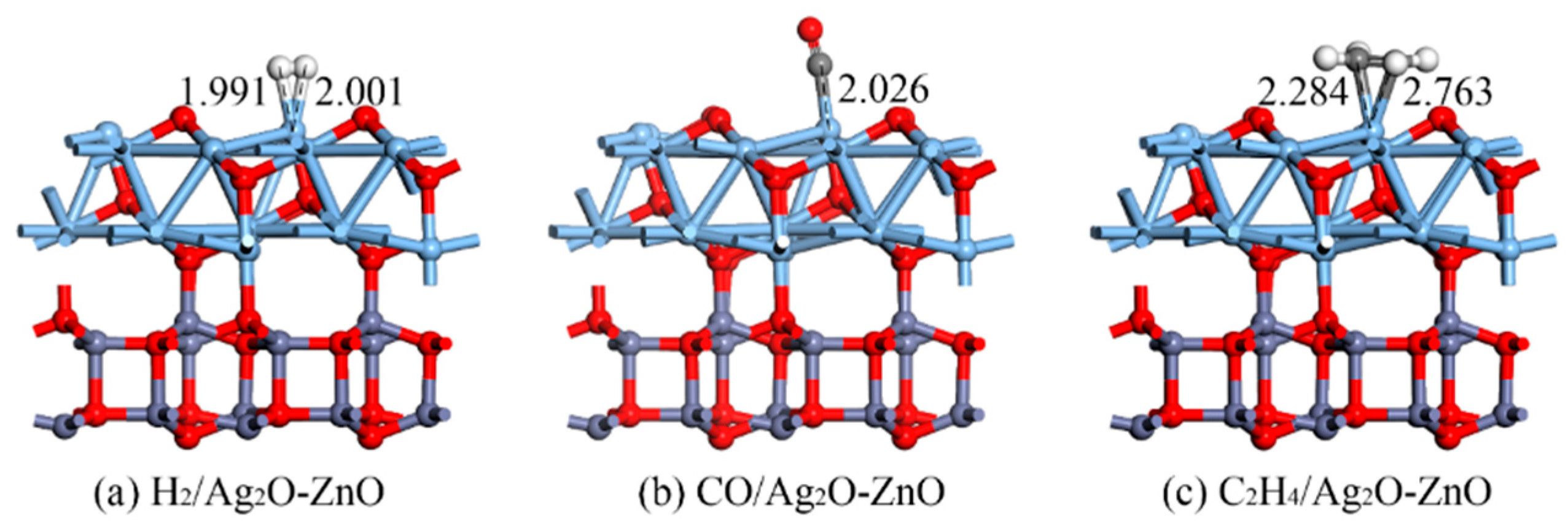
3.2.2. Structure and DCD Analysis of Gas/Ag2O-ZnO Heterojunction System
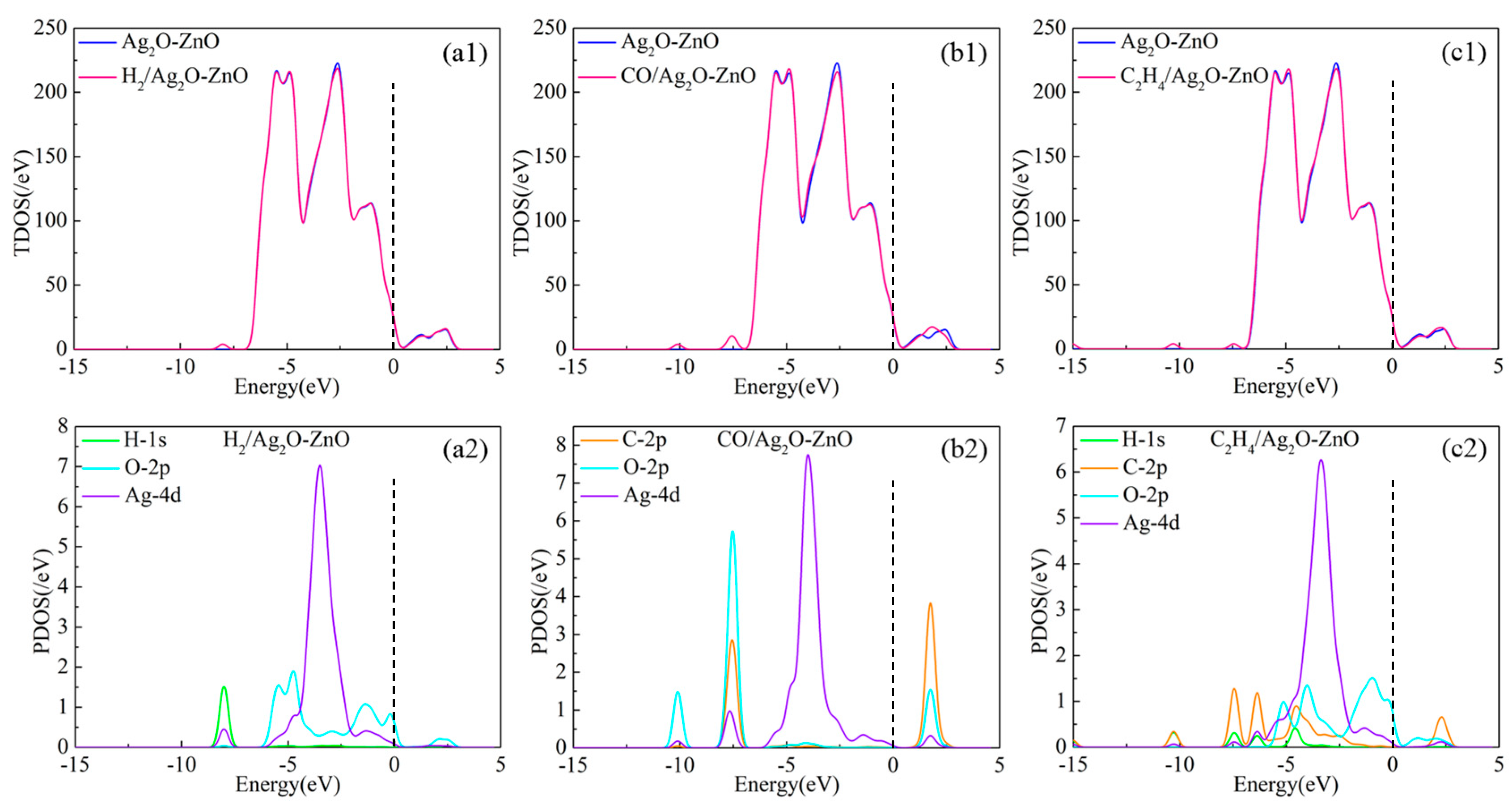
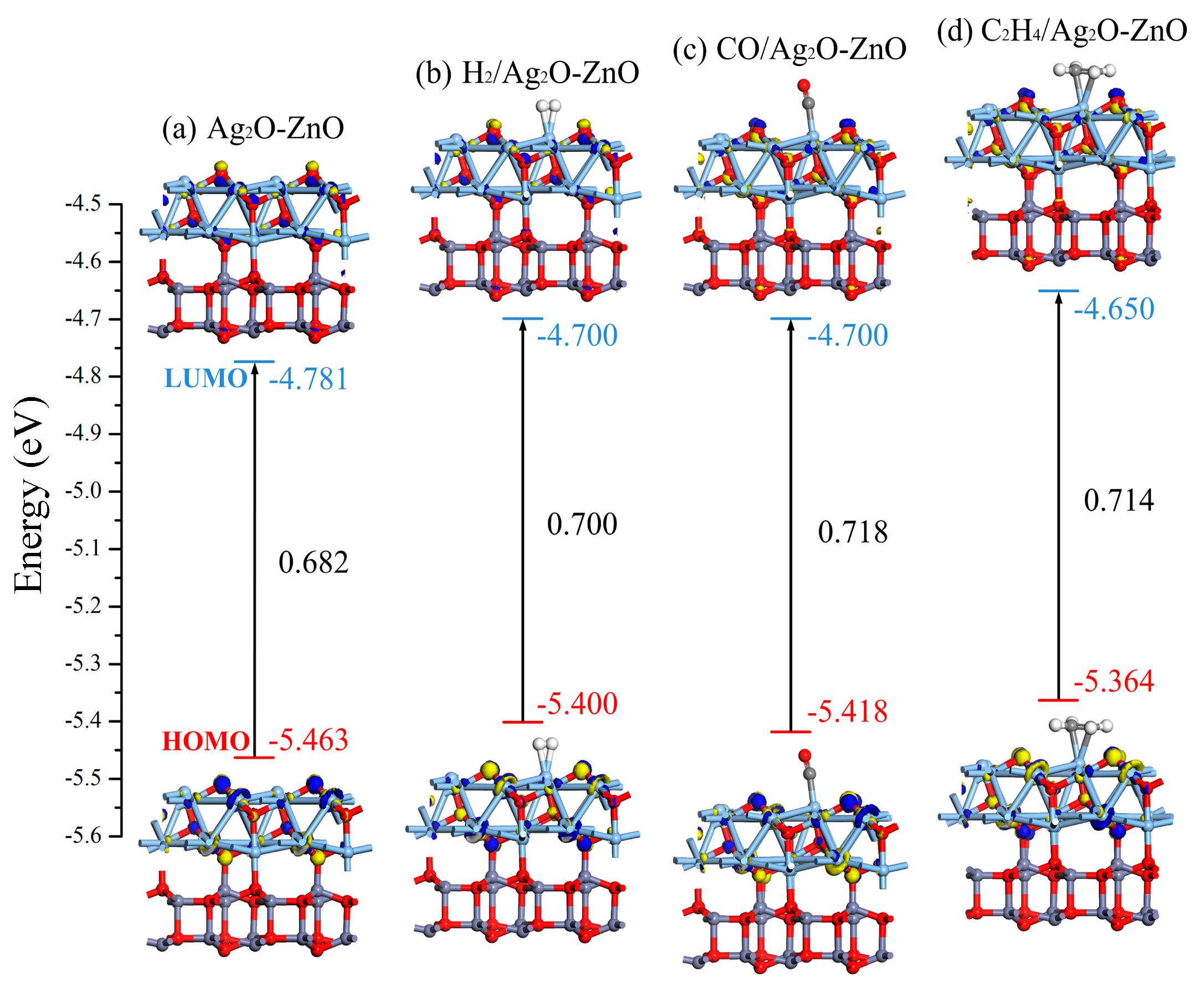
3.2.3. DOS and Molecular Orbital Analysis of Gas/Ag2O-ZnO Heterojunction System
3.3. Gas Sensing Properties of the Cu2O-ZnO Heterojunction for H2, CO, and C2H4
3.3.1. Structure and Electronic Analysis of the Cu2O-ZnO Heterojunction
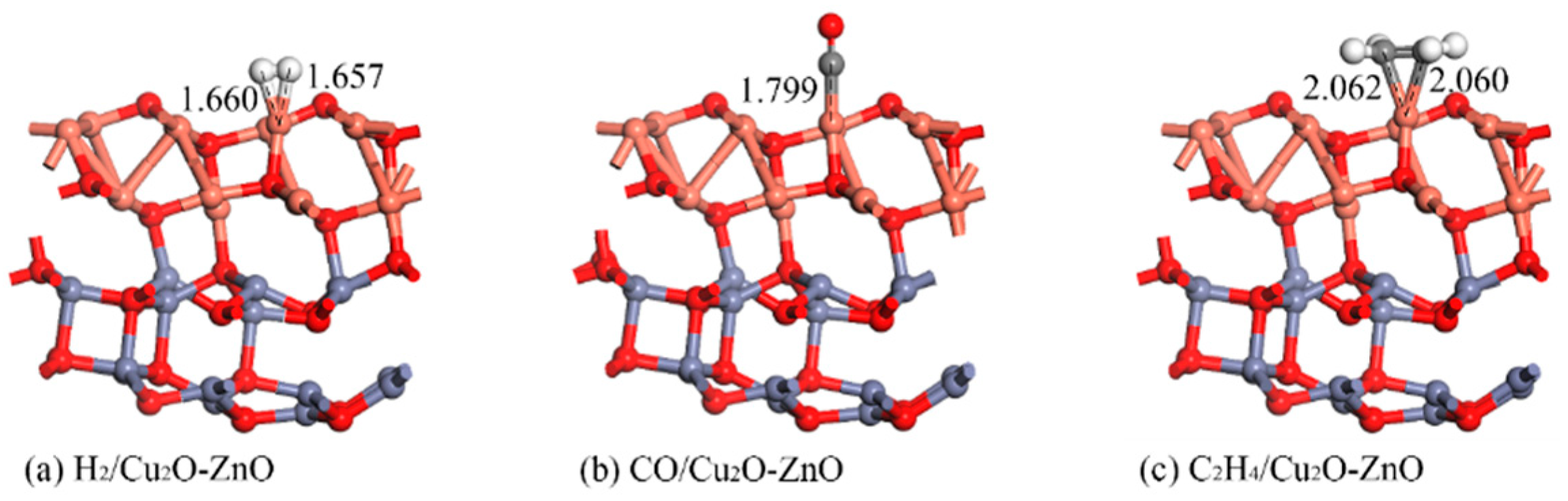
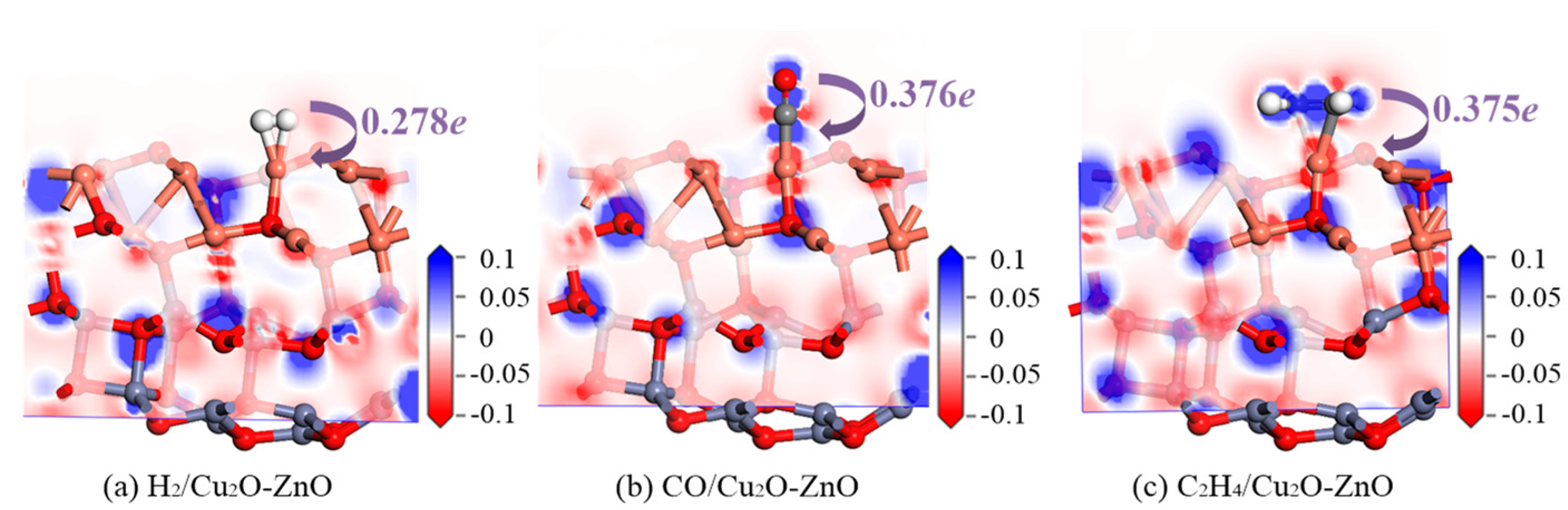
3.3.2. Structure and DCD Analysis of the Gas/Cu2O-ZnO Heterojunction System
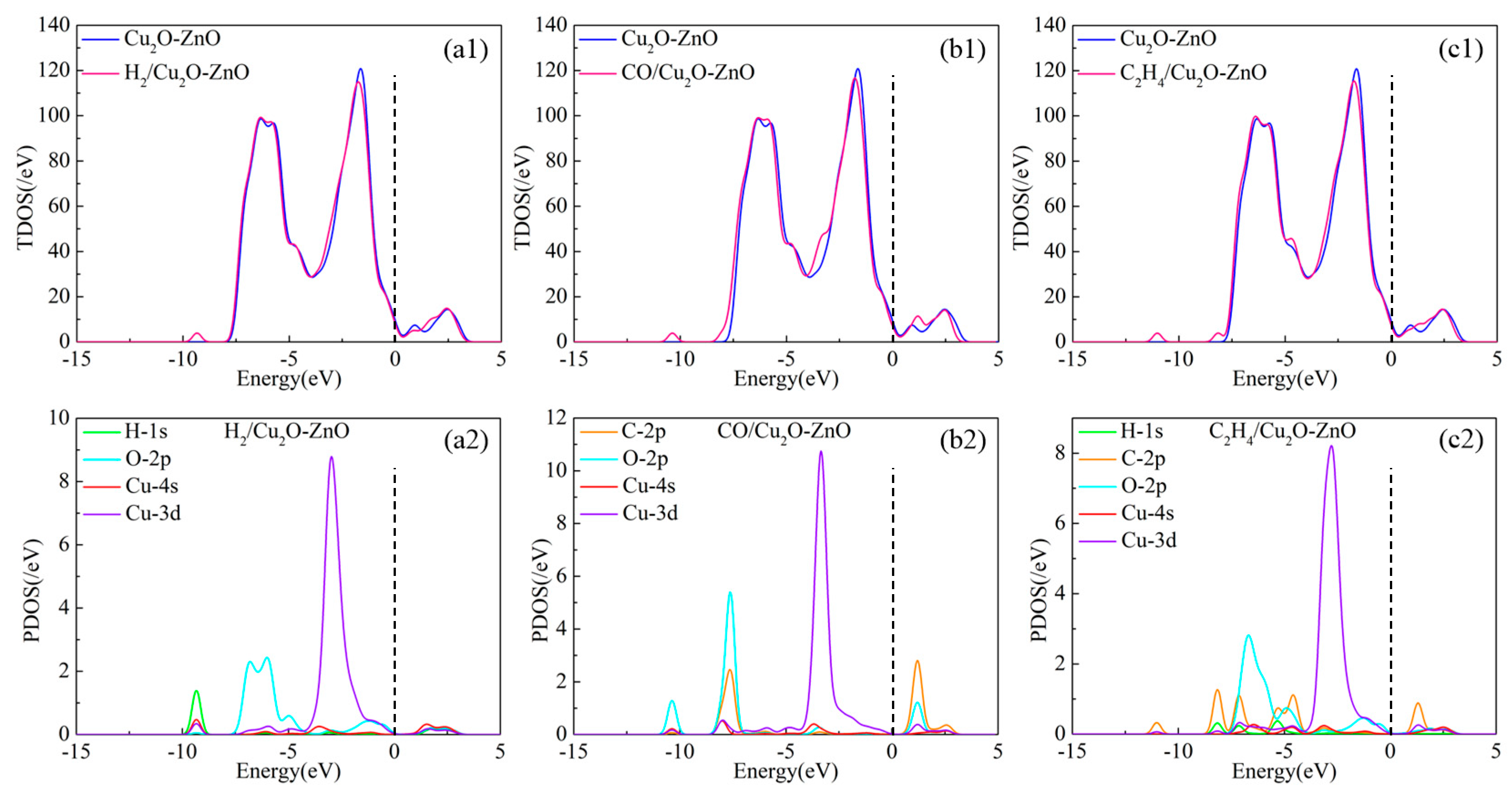
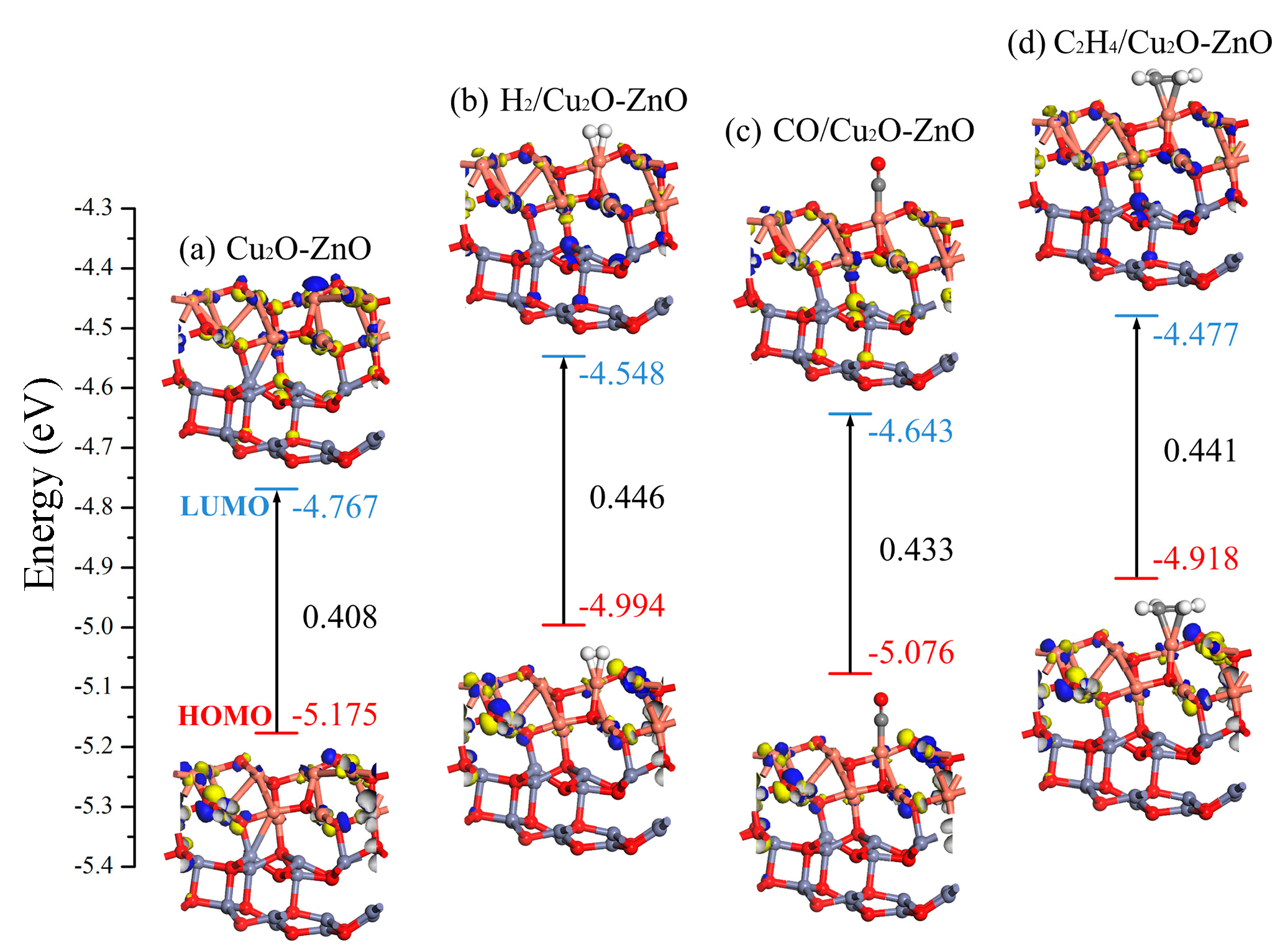
3.3.3. DOS and Molecular Orbital Analysis of the Gas/Cu2O-ZnO Heterojunction System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, L.; Kim, D.; Abu-Siada, A. State-of-the-art review on asset management methodologies for oil-immersed power transformers. Electr. Power Syst. Res. 2023, 218, 109194. [Google Scholar] [CrossRef]
- Jin, L.; Kim, D. Oil-Immersed Power Transformer Condition Monitoring Methodologies: A Review. Energies 2022, 15, 3379. [Google Scholar] [CrossRef]
- Fang, Q.; Ye, Z.; Chen, C. A Review of Power Transformer Vibration and Noise Caused by Silicon Steel Magnetostriction. Electronics 2024, 13, 968. [Google Scholar] [CrossRef]
- Battal, F.; Balci, S.; Sefa, I. Power electronic transformers: A review. Measurement 2021, 171, 108848. [Google Scholar] [CrossRef]
- Ruiz, F.; Perez, M.A.; Espinosa, J.R.; Gajowik, T.; Stynski, S.; Malinowski, M. Surveying Solid-State Transformer Structures and Controls: Providing Highly Efficient and Controllable Power Flow in Distribution Grids. IEEE Ind. Electron. Mag. 2020, 14, 56–70. [Google Scholar] [CrossRef]
- Wani, S.A.; Rana, A.S. Advances in DGA based condition monitoring of transformers: A review. Renew. Sustain. Energy Rev. 2021, 149, 111347. [Google Scholar] [CrossRef]
- Taha, I.B.M.; Ibrahim, S.; Mansour, D.E.A. Power Transformer Fault Diagnosis Based on DGA Using a Convolutional Neural Network with Noise in Measurements. IEEE Access 2021, 9, 111162–111170. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A.; Freudenberg, O.; Gspan, C.; Grogger, W.; Neuhold, A.; Resel, R. Gas sensing properties of novel CuO nanowire devices. Sens. Actuators B Chem. 2013, 187, 50–57. [Google Scholar] [CrossRef]
- Rizi, V.S.; Sharifianjazi, F.; Jafarikhorami, H.; Parvin, N.; Fard, L.S.; Irani, M. Esmaeilkhanian, A. Sol–gel derived SnO2/Ag2O ceramic nanocomposite for H2 gas sensing applications. Mater. Res. Express 2019, 6, 1150g2. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Zou, Y.-C.; Zhao, J.; Wang, P.-P.; Feng, L.-L.; Sun, L.-W.; Wang, D.-J.; Li, G.-D. Facile synthesis of highly stable and porous Cu2O/CuO cubes with enhanced gas sensing properties. Sens. Actuators B Chem. 2013, 188, 533–539. [Google Scholar] [CrossRef]
- Singh, A.; Sikarwar, S.; Verma, A.; Yadav, B.C. The recent development of metal oxide heterostructures based gas sensor, their future opportunities and challenges: A review. Sens. Actuators A Phys. 2021, 332, 113127. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe-Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Highly sensitive and selective triethylamine gas sensor based on hierarchical radial CeO2/ZnO n-n heterojunction. Sens. Actuators B Chem. 2022, 367, 132031. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, Z.; Nekouei, F.; Zhang, W.; Xu, J. Zeolitic imidazolate framework-derived n-ZnO/p-Co3O4 heterojunction by ion-etching method for superior CO toxic gas sensor. Sens. Actuators B Chem. 2023, 374, 132717. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.; Wang, G.; Shen, Y.; San, X.; Li, M.; Meng, F. Low-temperature formaldehyde gas sensors based on NiO-SnO2 hetero-junction microflowers assembled by thin porous nanosheets. Sens. Actuators B Chem. 2018, 273, 418–428. [Google Scholar] [CrossRef]
- Duoc, V.T.; Hung, C.M.; Nguyen, H.; Duy, N.V.; Hieu, N.V.; Hoa, N.D. Room temperature highly toxic NO2 gas sensors based on root-stock/scion nanowires of SnO2/ZnO, ZnO/SnO2, SnO2/SnO2 and, ZnO/ZnO. Sens. Actuators B Chem. 2021, 348, 130652. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Fang, T.-H.; Hsiao, Y.-J.; Li, Z.-J. Rapid detection of low concentrations of H2S using CuO-doped ZnO nanofibers. J. Alloys Compd. 2021, 852, 157014. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Weng, K.; Arramel; Jiang, J.; Ong, W.-J.; Zhang, P.; Zhao, X.; Li, N. Highly sensitive and selective gas sensor using het-eroatom doping graphdiyne: A DFT study. Adv. Electron. Mater. 2021, 7, 2001244. [Google Scholar] [CrossRef]
- Madsen, G.K.H. Functional form of the generalized gradient approximation for exchange: The PBE α functional. Phys. Rev. B 2007, 75, 195108. [Google Scholar] [CrossRef]
- Bučko, T.; Lebègue, S.; Ángyán, J.G.; Hafner, J. Extending the applicability of the Tkatchenko-Scheffler dispersion correction via iterative Hirshfeld partitioning. J. Chem. Phys. 2014, 141, 034114. [Google Scholar] [CrossRef]
- Peng, R.; Zeng, W.; Zhou, Q. Adsorption and gas sensing of dissolved gases in transformer oil onto Ru3-modified SnS2: A DFT study. Appl. Surf. Sci. 2023, 615, 156445. [Google Scholar] [CrossRef]
- Liu, H.; Tan, Z.; Niu, Y.; Wang, S.; Wang, Y. Ir-decorated MoS2 monolayer as a promising candidate to detect dissolved gas in transformer oil: A DFT study. Chem. Phys. Lett. 2023, 818, 140410. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, J.; Xu, L.; Ran, L.; Chen, X. Aun (n = 1–4) cluster doped MoSe2 nanosheet as a promising gas-sensing material for C2H4 gas in oil-immersed transformer. Appl. Surf. Sci. 2021, 541, 148356. [Google Scholar] [CrossRef]
- Choudhary, K.; Tavazza, F. Convergence and machine learning predictions of Monkhorst-Pack k-points and plane-wave cut-off in high-throughput DFT calculations. Comput. Mater. Sci. 2019, 161, 300–308. [Google Scholar] [CrossRef]
- Wisesa, P.; McDill, K.A.; Mueller, T. Efficient generation of generalized Monkhorst-Pack grids through the use of informatics. Phys. Rev. 2016, 93, 155109. [Google Scholar] [CrossRef]
- Delley, B. DMol3 DFT studies: From molecules and molecular environments to surfaces and solids. Comput. Mater. Sci. 2000, 17, 122–126. [Google Scholar] [CrossRef]
- Rahman, N.; Khan, A.; Ullah, R.; Ahmad, R.; Ahmad, I. Selective sensing of NH3 and CH2O molecules by novel 2D porous hexagonal boron oxide (B3O3) monolayer: A DFT approach. Surf. Interfaces 2022, 29, 101767. [Google Scholar] [CrossRef]
- Jiang, H. Band gaps from the Tran-Blaha modified Becke-Johnson approach: A systematic investigation. J. Chem. Phys. 2013, 138, 134115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lany, S.; Ghanbaja, J.; Fagot-Revurat, Y.; Chen, Y.; Soldera, F.; Horwat, D.; Mücklich, F.; Pierson, J.F. Electronic structures of Cu2O, Cu4O3, and CuO: A joint experimental and theoretical study. Phys. Rev. B 2016, 94, 245418. [Google Scholar] [CrossRef]




| System | Distance (Å) | Eads (eV) | Qt (e) |
|---|---|---|---|
| H2/CuO | 3.018 | −0.160 | 0.008 |
| CO/CuO | 2.543 | −0.280 | 0.070 |
| C2H4/CuO | 2.590 | −0.578 | 0.159 |
| H2/ZnO | 2.938 | −0.638 | 0.005 |
| CO/ZnO | 2.151 | −0.770 | 0.234 |
| C2H4/ZnO | 2.426 | −1.055 | 0.227 |
| H2/CuO-ZnO | 2.942 | −0.161 | 0.011 |
| CO/CuO-ZnO | 2.480 | −0.292 | 0.090 |
| C2H4/CuO-ZnO | 2.239 | −0.922 | 0.326 |
| System | Distance (Å) | Eads (eV) | Qt (e) |
|---|---|---|---|
| H2/Ag2O | 2.116 | −1.436 | 0.133 |
| CO/Ag2O | 2.042 | −2.133 | 0.306 |
| C2H4/Ag2O | 2.337 | −2.385 | 0.323 |
| H2/Ag2O-ZnO | 1.991 | −0.346 | 0.160 |
| CO/Ag2O-ZnO | 2.026 | −1.083 | 0.305 |
| C2H4/Ag2O-ZnO | 2.284 | −1.300 | 0.295 |
| System | Distance (Å) | Eads (eV) | Qt (e) |
|---|---|---|---|
| H2/Cu2O | 2.678 | −0.144 | 0.005 |
| CO/Cu2O | 1.809 | −1.490 | 0.380 |
| C2H4/Cu2O | 2.070 | −1.379 | 0.382 |
| H2/Cu2O-ZnO | 1.660 | −0.592 | 0.278 |
| CO/Cu2O-ZnO | 1.799 | −1.745 | 0.376 |
| C2H4/Cu2O-ZnO | 2.060 | −1.649 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Dai, W.; Zou, D.; Sun, H.; Tang, C.; Gui, Y. A TMO-ZnO Heterojunction-Based Sensor for Transformer Defect Detection: A DFT Study. Nanomaterials 2025, 15, 856. https://doi.org/10.3390/nano15110856
Yan J, Dai W, Zou D, Sun H, Tang C, Gui Y. A TMO-ZnO Heterojunction-Based Sensor for Transformer Defect Detection: A DFT Study. Nanomaterials. 2025; 15(11):856. https://doi.org/10.3390/nano15110856
Chicago/Turabian StyleYan, Jingyi, Weiju Dai, Dexu Zou, Haoruo Sun, Chao Tang, and Yingang Gui. 2025. "A TMO-ZnO Heterojunction-Based Sensor for Transformer Defect Detection: A DFT Study" Nanomaterials 15, no. 11: 856. https://doi.org/10.3390/nano15110856
APA StyleYan, J., Dai, W., Zou, D., Sun, H., Tang, C., & Gui, Y. (2025). A TMO-ZnO Heterojunction-Based Sensor for Transformer Defect Detection: A DFT Study. Nanomaterials, 15(11), 856. https://doi.org/10.3390/nano15110856








