Photoelectrochemical Biosensor Based on 1D In2O3 Tube Decorated with 2D ZnIn2S4 Nanosheets for Sensitive PSA Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of Hollow Tubular In2O3
2.3. Synthesis of Branched-Sheet Embedded Tubular In2O3-ZnIn2S4
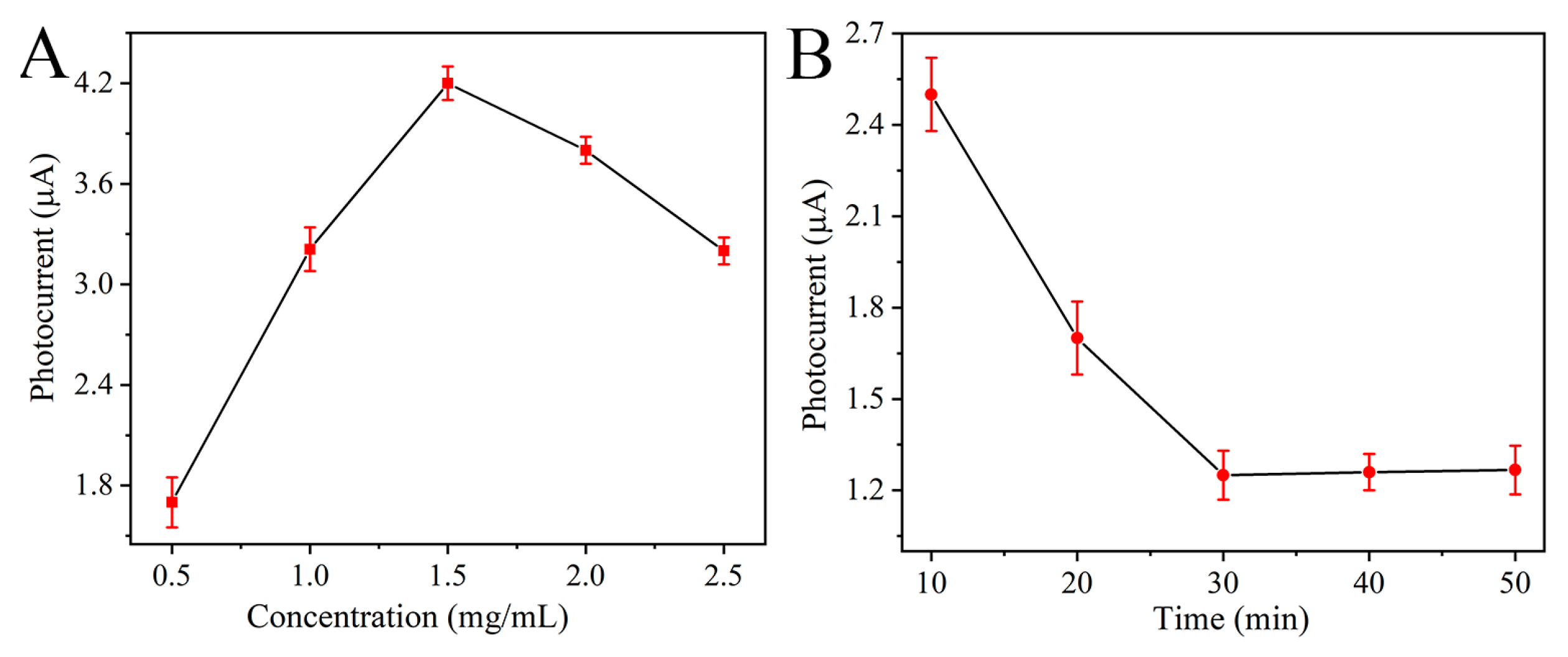
2.4. Fabrication of Sensing Platform and Analysis Protocol
2.5. Detection Limit Calculation
3. Results and Discussion
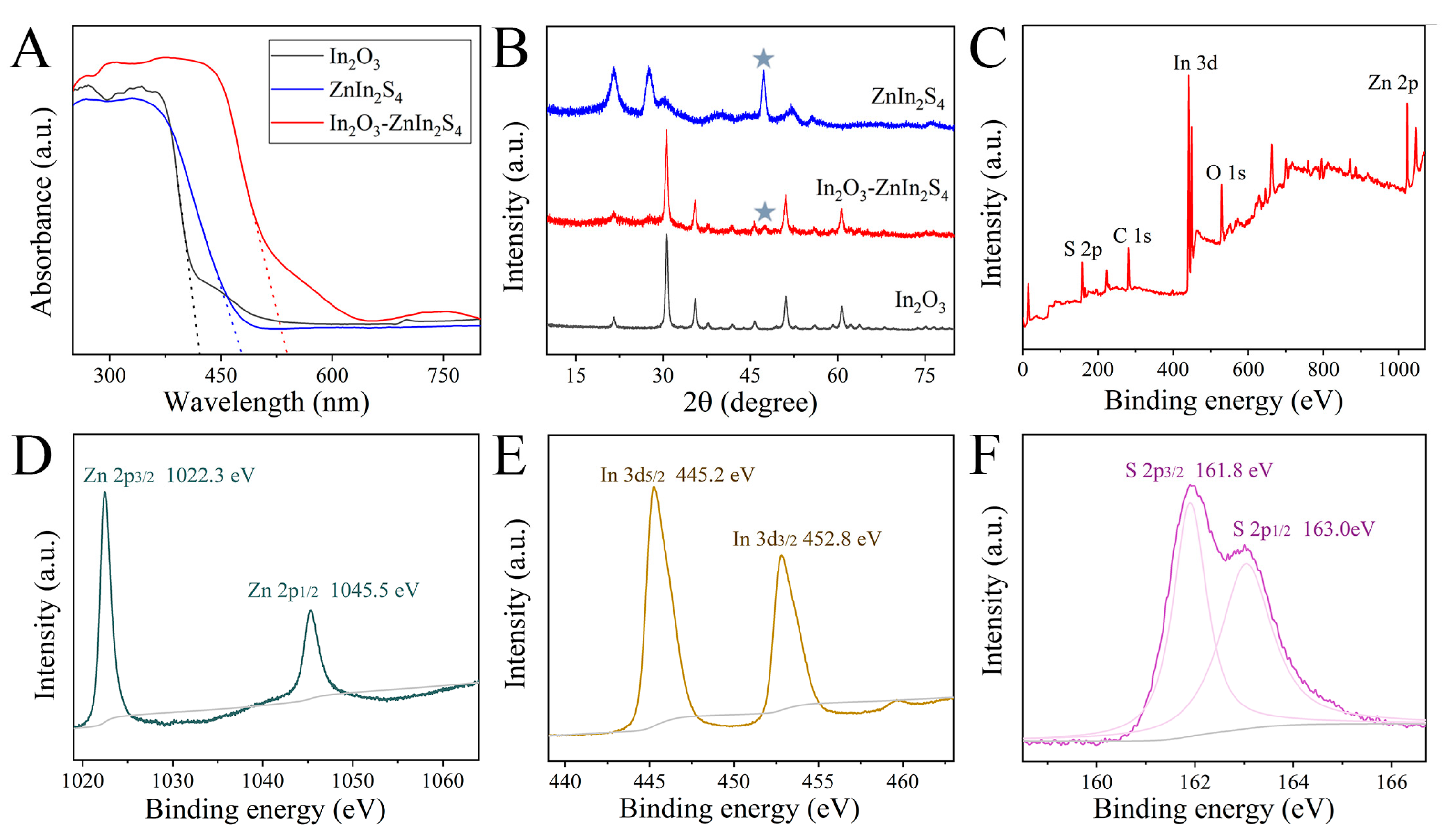
3.1. Morphology and Structure Characterization
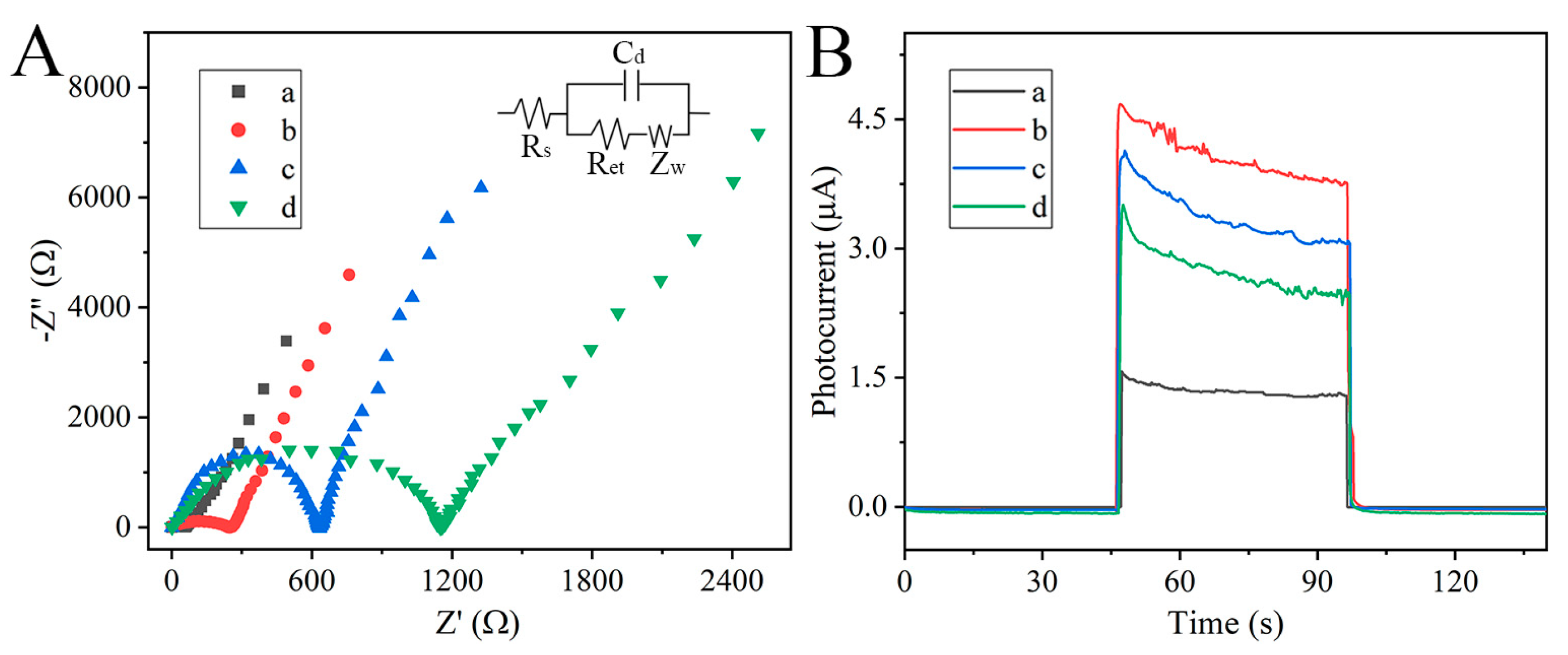
3.2. PEC and EIS Behaviors
3.3. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Homer, M.K.; Kuo, D.Y.; Dou, F.Y.; Cossairt, B.M. Photoinduced charge transfer from quantum dots measured by cyclic voltammetry. J. Am. Chem. Soc. 2022, 144, 14226–14234. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, J.; Tan, R.; Wu, Z.; Liu, M.; Li, J.; Xu, M.; Gu, W.; Zhu, C.; Hu, L. Small-molecule probe-induced in situ-sensitized photoelectrochemical biosensor for monitoring α-Glucosidase activity. ACS Sens. 2023, 8, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Feng, Y.; Xu, Y.; Xue, X.; Feng, R.; Yan, T.; Yan, L.; Wei, Q. Self-powered photoelectrochemical aptasensor based on MIL-68(In) derived In2O3 hollow nanotubes and Ag doped ZnIn2S4 quantum dots for oxytetracycline detection. Talanta 2022, 240, 123153. [Google Scholar] [CrossRef]
- Nam, B.; Ko, T.-K.; Hyun, S.-K.; Lee, C. NO2 sensing properties of WO3-decorated In2O3 nanorods and In2O3-decorated WO3 nanorods. Nano Converg. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cao, W.; Wang, P.-H.; Wang, J.; Yu, L.; Qiao, Z.; Ding, Z.-J. Fast and sensitive detection of CO by Bi-MOF-derived porous In2O3/Fe2O3 core-shell nanotubes. ACS Sens. 2023, 8, 4577–4586. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Huang, S.; Hu, Y.; Yang, Z.; Li, T.T.; Li, Q.; Qian, J. MOF-on-MOF-derived hollow Co3O4/In2O3 nanostructure for efficient photocatalytic CO2 reduction. Adv. Sci. 2023, 10, 2300797. [Google Scholar] [CrossRef]
- Shi, L.; Benetti, D.; Wei, Q.; Rosei, F. MOF-derived In2O3/CuO p-n heterojunction photoanode incorporating graphene nanoribbons for solar hydrogen generation. Small 2023, 19, 2300606. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, K.; Chen, Y.; Zheng, Q.; Huang, C.; Jia, N. In2O3/Bi2S3 S-scheme heterojunction-driven molecularly imprinted photoelectrochemical sensor for ultrasensitive detection of dlorfenicol. ACS Appl. Mater. Interfaces 2023, 15, 58397–58405. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Li, Y.; Xu, X.; Du, Y.; Jiang, Y.; Lin, K. A novel heterostructure coupling MOF-derived fluffy porous indium oxide with g-C3N4 for enhanced photocatalytic activity. Mater. Res. Bull. 2021, 133, 111078. [Google Scholar] [CrossRef]
- Ren, J.; Yuan, K.; Wu, K.; Zhou, L.; Zhang, Y. A robust CdS/In2O3 hierarchical heterostructure derived from a metal–organic framework for efficient visible-light photocatalytic hydrogen production. Inorg. Chem. Front. 2019, 6, 366–375. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; He, M.; Zhang, W.; Mo, Z.; Yuan, J.; She, Y.; Xu, H.; Li, H. Multidimensional In2O3/In2S3 heterojunction with lattice distortion for CO2 photoconversion. Chinese J. Catal. 2022, 43, 1286–1294. [Google Scholar] [CrossRef]
- Luo, D.; Peng, L.; Wang, Y.; Lu, X.; Yang, C.; Xu, X.; Huang, Y.; Ni, Y. Highly efficient photocatalytic water splitting utilizing a WO3-x/ZnIn2S4 ultrathin nanosheet Z-scheme catalyst. J. Mater. Chem. A 2021, 9, 908–914. [Google Scholar] [CrossRef]
- Ding, S.; Medic, I.; Steinfeldt, N.; Dong, T.; Voelzer, T.; Haida, S.; Rabeah, J.; Hu, J.; Strunk, J. Ultrathin defective nanosheet subunit ZnIn2S4 hollow nanoflowers for efficient photocatalytic hydrogen evolution. Small Struct. 2023, 4, 2300091. [Google Scholar] [CrossRef]
- Lu, P.; Liu, K.; Liu, Y.; Ji, Z.; Wang, X.; Hui, B.; Zhu, Y.; Yang, D.; Jiang, L. Heterostructure with tightly-bound interface between In2O3 hollow fiber and ZnIn2S4 nanosheet toward efficient visible light driven hydrogen evolution. Appl. Catal. B Environ. 2024, 345, 123697. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Zhou, R.; Li, Y.; He, Z.; Ding, H.; Chen, D.; Ao, W. A review: Synthesis, modification and photocatalytic applications of ZnIn2S4. J. Mater. Sci. Technol. 2021, 78, 1–19. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, W.; Xv, R.; Fu, L. TiO2 nanoparticles modified with ZnIn2S4 nanosheets and Co-Pi groups: Type II heterojunction and cocatalysts coexisted photoanode for efficient photoelectrochemical water splitting. Int. J. Hydrogen Energ. 2022, 47, 33361–33373. [Google Scholar] [CrossRef]
- Liu, M.; Xiong, J.; Kong, D.; Liu, Y.; Wu, H.; Li, F.; Hu, H.; Wang, D.; Guo, X.; Jiao, Y.; et al. Anchoring ZnIn2S4 nanosheets on oxygen-vacancy NiMoOx nanorods for efficient photocatalytic hydrogen evolution. Sep. Purif. Technol. 2025, 359, 130608. [Google Scholar] [CrossRef]
- Kong, D.; Hu, X.; Geng, J.; Zhao, Y.; Fan, D.; Lu, Y.; Geng, W.; Zhang, D.; Liu, J.; Li, H.; et al. Growing ZnIn2S4 nanosheets on FeWO4 flowers with pn heterojunction structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 2022, 591, 153256. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Guan, Z.; Yang, J.; Li, Q. Spatially separating redox centers and photothermal effect synergistically boosting the photocatalytic hydrogen evolution of ZnIn2S4 nanosheets. Small 2021, 17, 2006952. [Google Scholar] [CrossRef]
- Liu, T.; Shen, H.; Wang, M.; Feng, Q.; Chen, L.; Wang, W.; Zhang, J. Fabrication of ZnIn2S4 nanosheets decorated hollow CdS nanostructure for efficient photocatalytic H2-evolution and antibiotic removal performance. Sep. Purif. Technol. 2023, 315, 123698. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel and ultrasensitive sandwich-type electrochemical immunosensor based on delaminated MXene@ AuNPs as signal amplification for prostate specific antigen (PSA) detection and immunosensor validation. Talanta 2020, 220, 121403. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Ning, G.; Sun, W.; Wang, L.; Liang, H.; Xu, H.; He, C.; Zhao, H.; Li, C. P A novel affinity peptide–antibody sandwich electrochemical biosensor for PSA based on the signal amplification of MnO2-functionalized covalent organic framework. Talanta 2021, 233, 122520. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Bhattacharyya, I.M.; Prajapati, A.; Ron, I.; Shima-Edelstein, R.; Pikhay, E.; Greental, D.; Eisenberg-Lerner, A.; Rotfogel, Z.; Roizin, Y.; et al. Specific and label-free sensing of prostate-specific antigen (PSA) from an ultrasmall drop of diluted human serum with the meta-nano-channel Silicon field-effect biosensor. Adv. Mate. Technol. 2023, 8, 2202200. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Choi, M.; Baek, M.; Hsieh, P.-Y.; Yong, K.; Hsu, Y.-J. CdS/CdSe co-sensitized brookite H:TiO2 nanostructures: Charge carrier dynamics and photoelectrochemical hydrogen generation. Appl. Catal. B Environ. 2018, 225, 379–385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Xu, J.; Wang, Y. Photoelectrochemical Biosensor Based on 1D In2O3 Tube Decorated with 2D ZnIn2S4 Nanosheets for Sensitive PSA Detection. Nanomaterials 2025, 15, 855. https://doi.org/10.3390/nano15110855
Shi H, Xu J, Wang Y. Photoelectrochemical Biosensor Based on 1D In2O3 Tube Decorated with 2D ZnIn2S4 Nanosheets for Sensitive PSA Detection. Nanomaterials. 2025; 15(11):855. https://doi.org/10.3390/nano15110855
Chicago/Turabian StyleShi, Huihui, Jianjian Xu, and Yanhu Wang. 2025. "Photoelectrochemical Biosensor Based on 1D In2O3 Tube Decorated with 2D ZnIn2S4 Nanosheets for Sensitive PSA Detection" Nanomaterials 15, no. 11: 855. https://doi.org/10.3390/nano15110855
APA StyleShi, H., Xu, J., & Wang, Y. (2025). Photoelectrochemical Biosensor Based on 1D In2O3 Tube Decorated with 2D ZnIn2S4 Nanosheets for Sensitive PSA Detection. Nanomaterials, 15(11), 855. https://doi.org/10.3390/nano15110855







