Investigation of Annealing Temperature Effect of Tin Oxide on the Efficiency of Planar Structure Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
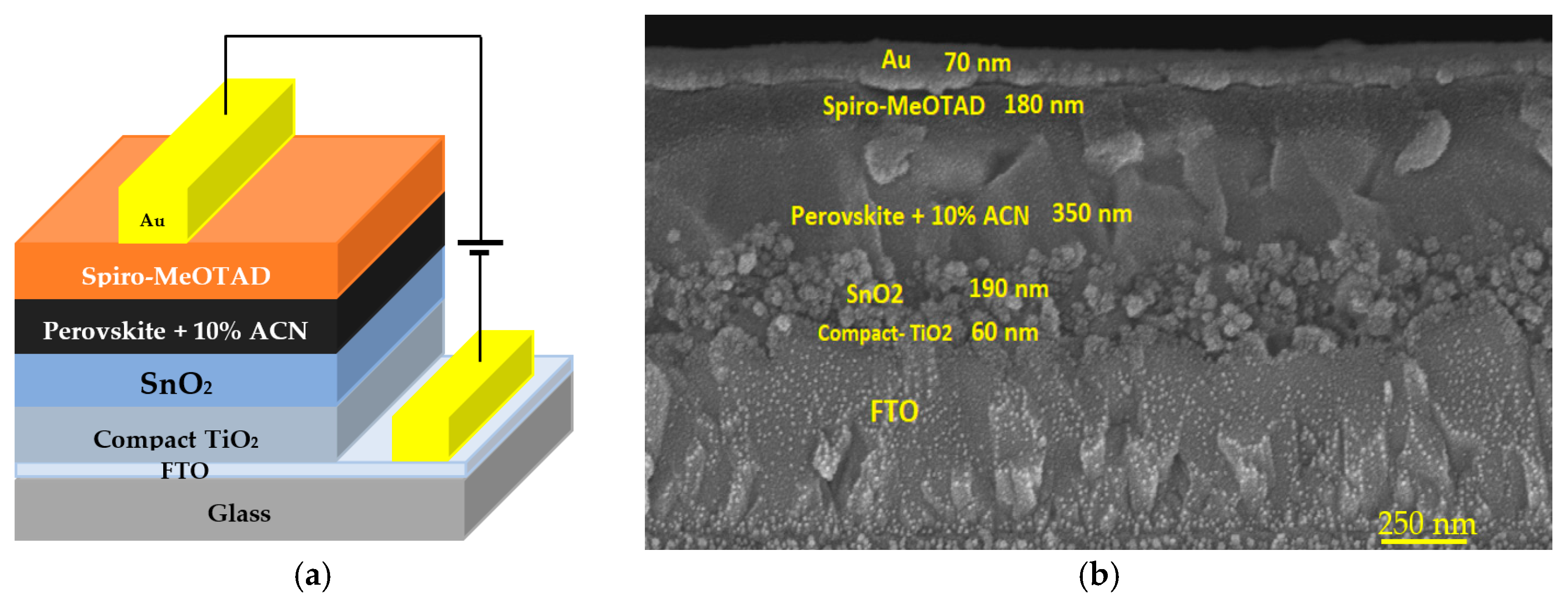
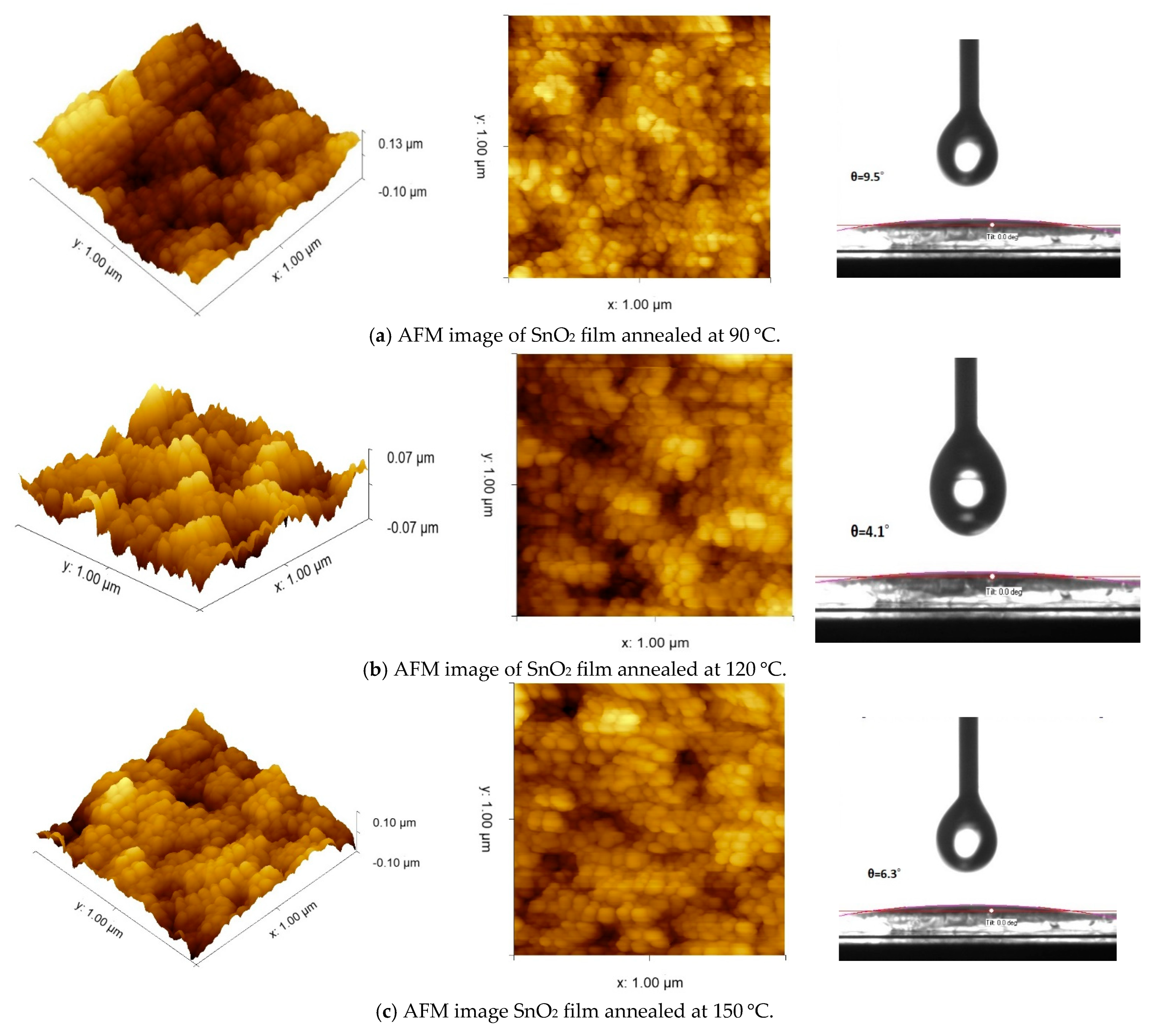
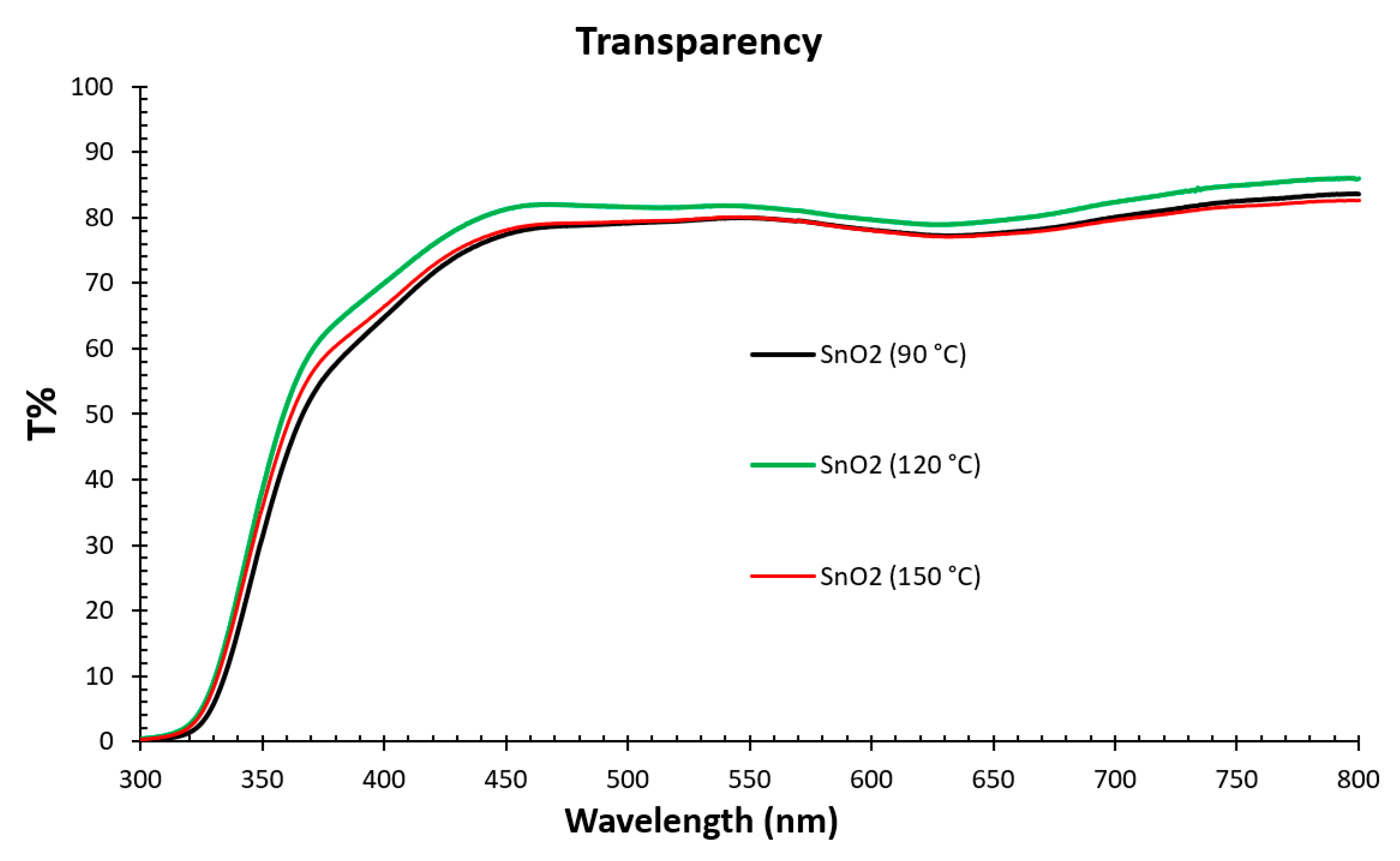
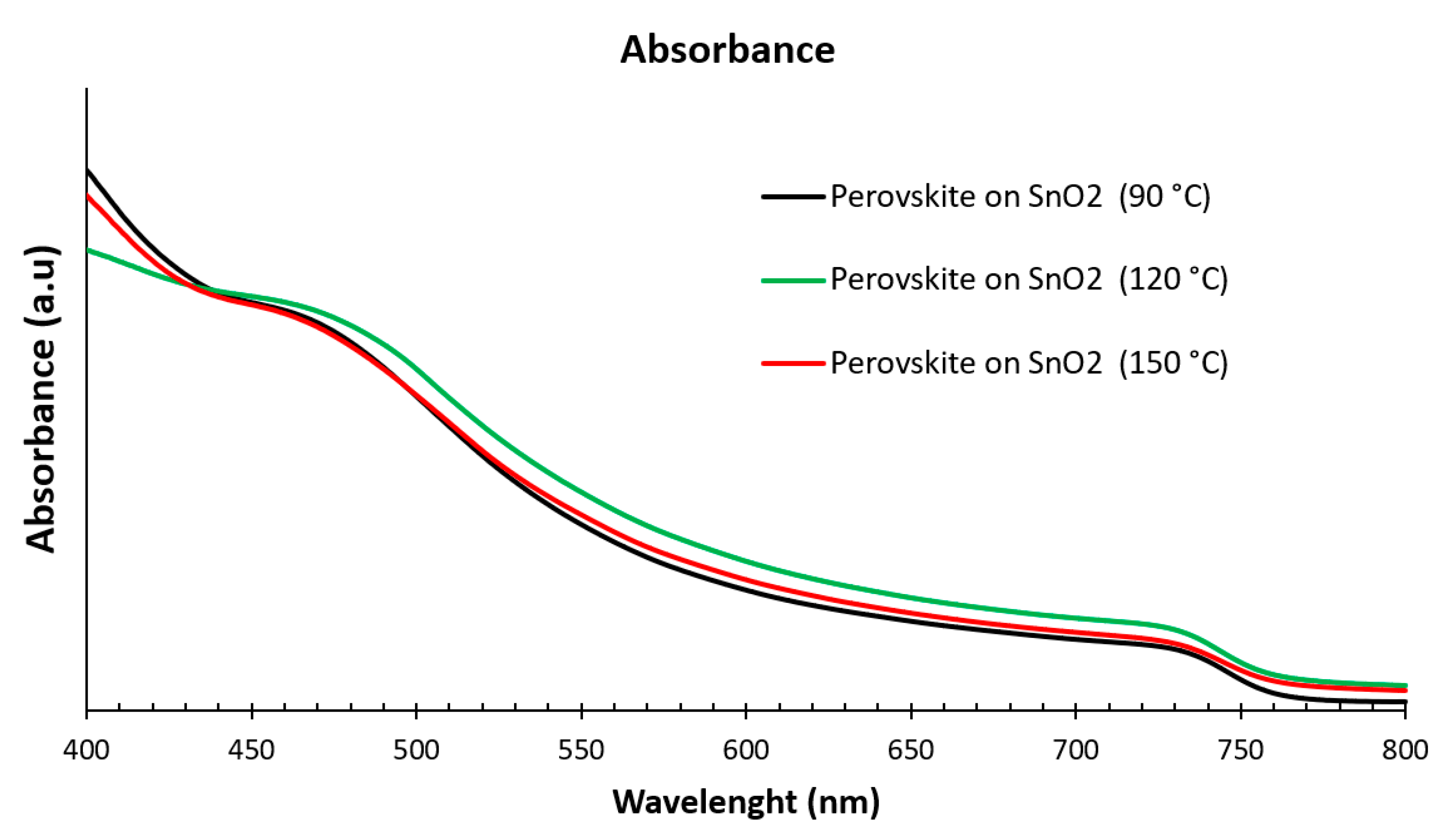
3.1. Structural and Optical Properties of the SnO2 and Perovskite Films
3.2. J-V Characteristics of Perovskite Solar Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Zhuang, R.; Wu, X.; Tian, C.; Sun, A.; Chen, C.; Guo, Y.; Hua, Y.; Meng, K.; et al. Towards 26% Efficiency in Inverted Perovskite Solar Cells via Interfacial Flipped Band Bending and Suppressed Deep-Level Traps. Energy Environ. Sci. 2023, 17, 1153–1162. [Google Scholar] [CrossRef]
- Leng, D.; Wu, L.; Jiang, H.; Zhao, Y.; Zhang, J.; Li, W.; Feng, L. Preparation and Properties of SnO2 Film Deposited by Magnetron Sputtering. Int. J. Photoenergy 2012, 2012, 235971. [Google Scholar] [CrossRef]
- Noh, M.F.M.; Arzaee, N.A.; Safaei, J.; Mohamed, N.A.; Kim, H.P.; Yusoff, A.R.M.; Jang, J.; Teridi, M.A.M. Eliminating oxygen vacancies in SnO2 films via aerosol-assisted chemical vapour deposition for perovskite solar cells and photoelectrochemical cells. J. Alloys Compd. 2019, 773, 997–1008. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Seo, G.; Paek, S.; Cho, K.T.; Huckaba, A.J.; Calizzi, M.; Choi, D.w.; Park, J.S.; Lee, D.; et al. Efficient Planar Perovskite Solar Cells Using Passivated Tin Oxide as an Electron Transport Layer. Adv. Sci. 2018, 5, 1800130. [Google Scholar] [CrossRef]
- Wu, W.Q.; Chen, D.; Cheng, Y.B.; Caruso, R.A. Thin Films of Tin Oxide Nanosheets Used as the Electron Transporting Layer for Improved Performance and Ambient Stability of Perovskite Photovoltaics. Sol. RRL 2017, 1, 1700117. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, Y.R.; Jang, H.; Lee, C.; Kang, M.G.; Jun, Y. Electrodeposition of SnO2 on FTO and Its Application in Planar Heterojunction Perovskite Solar Cells as an Electron Transport Layer. Nanoscale Res. Lett. 2017, 12, 10–16. [Google Scholar] [CrossRef]
- Kiruthiga, G.; Rajni, K.S.; Geethanjali, N.; Raguram, T.; Nandhakumar, E.; Senthilkumar, N. SnO2: Investigation of Optical, Structural, and Electrical Properties of Transparent Conductive Oxide Thin Films Prepared by Nebulized Spray Pyrolysis for Photovoltaic Applications. Inorg. Chem. Commun. 2022, 145, 109968. [Google Scholar] [CrossRef]
- Karmaoui, M.; Jorge, A.B.; McMillan, P.F.; Aliev, A.E.; Pullar, R.C.; Labrincha, J.A.; Tobaldi, D.M. One-Step Synthesis, Structure, and Band Gap Properties of SnO2 Nanoparticles Made by a Low Temperature Nonaqueous Sol-Gel Technique. ACS Omega 2018, 3, 13227–13238. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-Based Electron Transporting Layer Materials for Perovskite Solar Cells: A Review of Recent Progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Hayali, A.; Alkaisi, M.M. High Efficiency Perovskite Solar Cells Using DC Sputtered Compact TiO2 electron Transport Layer. EPJ Photovolt. 2021, 12, 8. [Google Scholar] [CrossRef]
- Hayali, A.; Reeves, R.J.; Alkaisi, M.M. Wavelength Selective Solar Cells Using Triple Cation Perovskite. Nanomaterials 2022, 12, 3299. [Google Scholar] [CrossRef] [PubMed]
- Hayali, A.; Alkaisi, M.M. Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique. Inorganics 2024, 12, 214. [Google Scholar] [CrossRef]
- Hayali, A.; Reeves, R.J.; Alkaisi, M.M. Influence of FK209 Cobalt Doped Electron Transport Layer in Cesium Based Perovskite Solar Cells. Appl. Sci. 2022, 12, 9382. [Google Scholar] [CrossRef]
- Sidhik, S.; Cerdan Pasarán, A.; Esparza, D.; López Luke, T.; Carriles, R.; De La Rosa, E. Improving the Optoelectronic Properties of Mesoporous TiO2 by Cobalt Doping for High-Performance Hysteresis-Free Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 3571–3580. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent Progress in Electron Transport Layers for Efficient Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, Z.; Chen, H.; Zhang, Q.; He, H.; Xiao, S.; Fan, Z.; Wong, K.S.; Yang, S. Designing Nanobowl Arrays of Mesoporous TiO2 as an Alternative Electron Transporting Layer for Carbon Cathode-Based Perovskite Solar Cells. Nanoscale 2016, 8, 6393–6402. [Google Scholar] [CrossRef]
- Singh, T.; Öz, S.; Sasinska, A.; Frohnhoven, R.; Mathur, S.; Miyasaka, T. Sulfate-Assisted Interfacial Engineering for High Yield and Efficiency of Triple Cation Perovskite Solar Cells with Alkali-Doped TiO2 Electron-Transporting Layers. Adv. Funct. Mater. 2018, 28, 1706287. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the Application of SnO2 in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Bai, G.; Wu, Z.; Li, J.; Bu, T.; Li, W.; Li, W.; Huang, F.; Zhang, Q.; Cheng, Y.B.; Zhong, J. High Performance Perovskite Sub-Module with Sputtered SnO2 Electron Transport Layer. Sol. Energy 2019, 183, 306–314. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Lowerature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhao, D.; Cimaroli, A.J.; Grice, C.R.; Qin, P.; Liu, Q.; Xiong, L.; Yan, Y.; Fang, G. Effects of Annealing Temperature of Tin Oxide Electron Selective Layers on the Performance of Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 24163–24168. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2PbI3-Based Perovskite Solar Cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Liu, H.; Yang, X.; Chen, W. Realization of p-type MA-based perovskite solar cells based on exposure of the (002) facet. Appl. Phys. Lett. 2025, 126, 021104. [Google Scholar] [CrossRef]
- Gao, C.; Jia, S.; Yin, X.; Li, Z.; Yang, G.; Chen, J.; Li, Z.; An, X.-T. Enhancing open-circuit voltage in FAPbI3 perovskite solar cells via self-formation of coherent buried interface FAPbIxCl3−x. Chem. Commun. 2025, 61, 2758–2761. [Google Scholar] [CrossRef]
- Bu, T.; Liu, X.; Zhou, Y.; Yi, J.; Huang, X.; Luo, L.; Xiao, J.; Ku, Z.; Peng, Y.; Huang, F.; et al. A Novel Quadruple-Cation Absorber for Universal Hysteresis Elimination for High Efficiency and Stable Perovskite Solar Cells. Energy Environ. Sci. 2017, 10, 2509–2515. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface Optimization to Eliminate Hysteresis for Record Efficiency Planar Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Yang, H.; Li, P.; Zhang, J.; Lin, Y. TiO2 Compact Layer for Dye-Sensitized SnO2 Nanocrystalline Thin Film. Electrochim. Acta 2014, 147, 366–370. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Shen, S.; Wang, F. TiO2/SnO2 Bilayer Electron Transport Layer for High Efficiency Perovskite Solar Cells. Nanomaterials 2023, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Z.; Liu, B.; Wu, W.; Zhu, Y.; Ma, R.; Wang, C. High-Performance Perovskite Solar Cells with Large Grain-Size Obtained by Using the Lewis Acid-Base Adduct of Thiourea. Sol. RRL 2018, 2, 1800034. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, M.; Wang, H.; Sun, Y.; Li, Z.; Ma, R.; Liu, H.; Dela Peña, T.A.; Chandran, H.T.; Li, M.; et al. Leveraging Compatible Iridium(III) Complexes to Boost Performance of Green Solvent-Processed Non-Fullerene Organic Solar Cells. Adv. Funct. Mater. 2024, 34, 2411058. [Google Scholar] [CrossRef]
- Wu, T.; Wu, J.; Tu, Y.; He, X.; Lan, Z.; Huang, M.; Lin, J. Solvent Engineering for High-Quality Perovskite Solar Cell with an Efficiency Approaching 20%. J. Power Sources 2017, 365, 1–6. [Google Scholar] [CrossRef]
- Ghobadi, N. Band Gap Determination Using Absorption Spectrum Fitting Procedure. Int. Nano Lett. 2013, 3, 2–5. [Google Scholar] [CrossRef]
- Kam, M.; Zhang, Q.; Zhang, D.; Fan, Z. Room-Temperature Sputtered SnO2 as Robust Electron Transport Layer for Air-Stable and Efficient Perovskite Solar Cells on Rigid and Flexible Substrates. Sci. Rep. 2019, 9, 6963. [Google Scholar] [CrossRef]
- Park, B.-W.; Kedem, N.; Kulbak, M.; Lee, D.Y.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; et al. Understanding how excess lead iodide precursor improves halide perovskite solar cell performance. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Kang, D.; Park, N. On the Current–Voltage Hysteresis in Perovskite Solar Cells: Dependence on Perovskite Composition and Methods to Remove Hysteresis. Adv. Mater. 2019, 31, e1805214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, Y.; Zhang, M.; Ni, Y.; Zhang, F.; Jeong, S.Y.; Zhang, F. Over 18.2% efficiency of layer–by–layer all–polymer solar cells enabled by homoleptic iridium (III) carbene complex as solid additive. Sci. Bull. 2024, 69, 2862–2869. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J-V Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef]
- Van Reenen, S.; Kemerink, M.; Snaith, H.J. Modeling Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, W.; Liu, S.; Yang, H.; Shao, Z. Fundamental Understanding of Photocurrent Hysteresis in Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803017. [Google Scholar] [CrossRef]

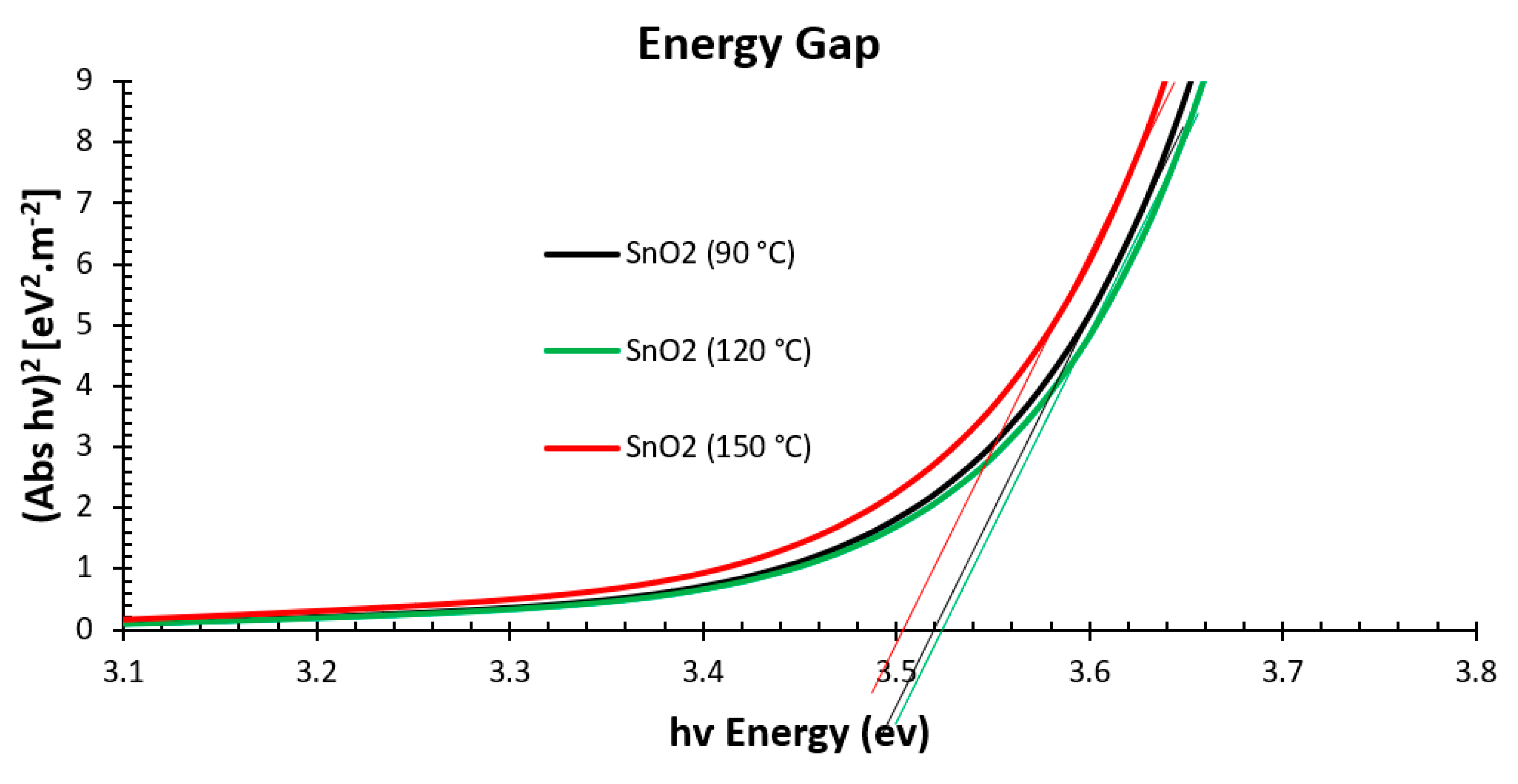
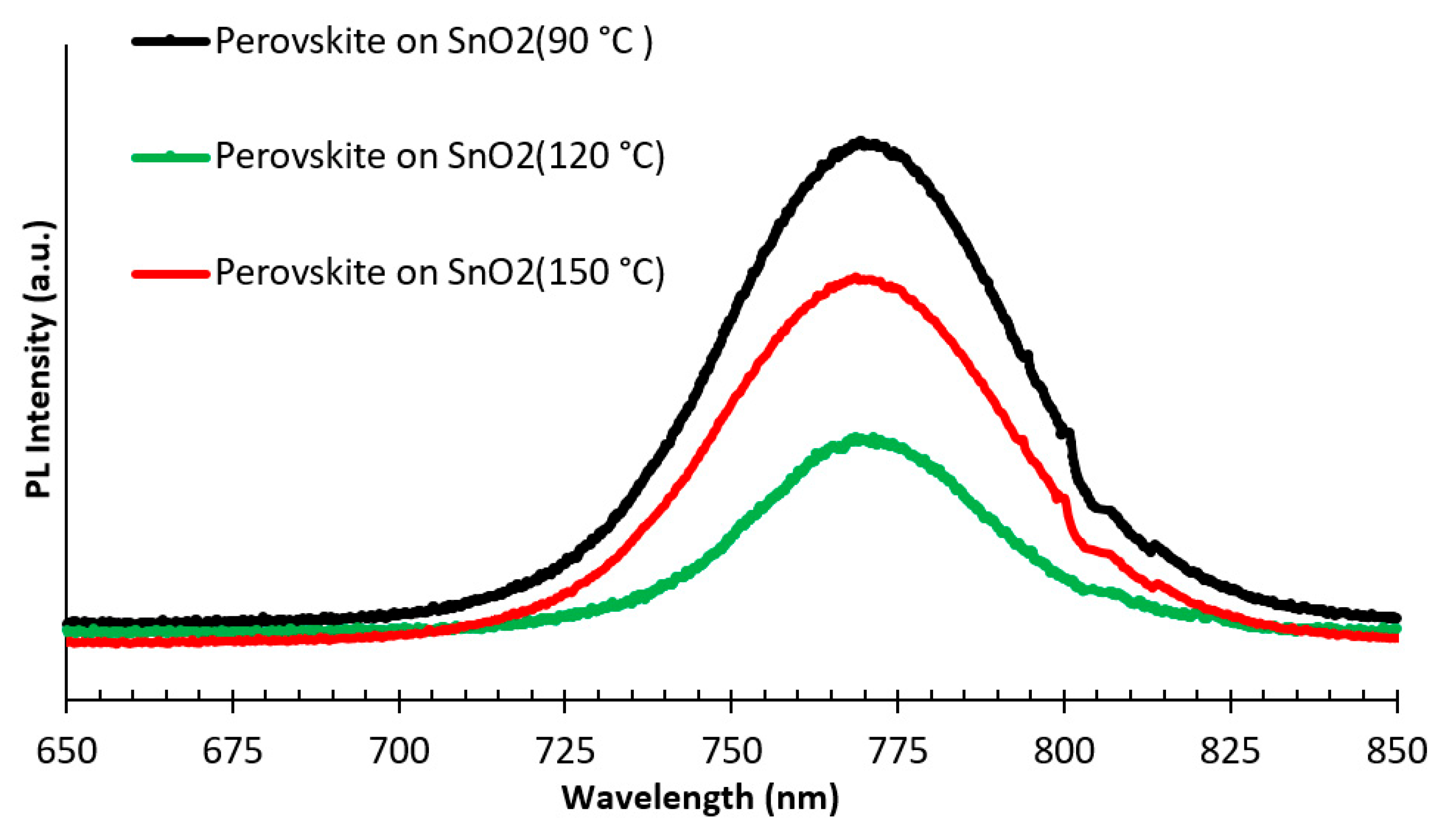
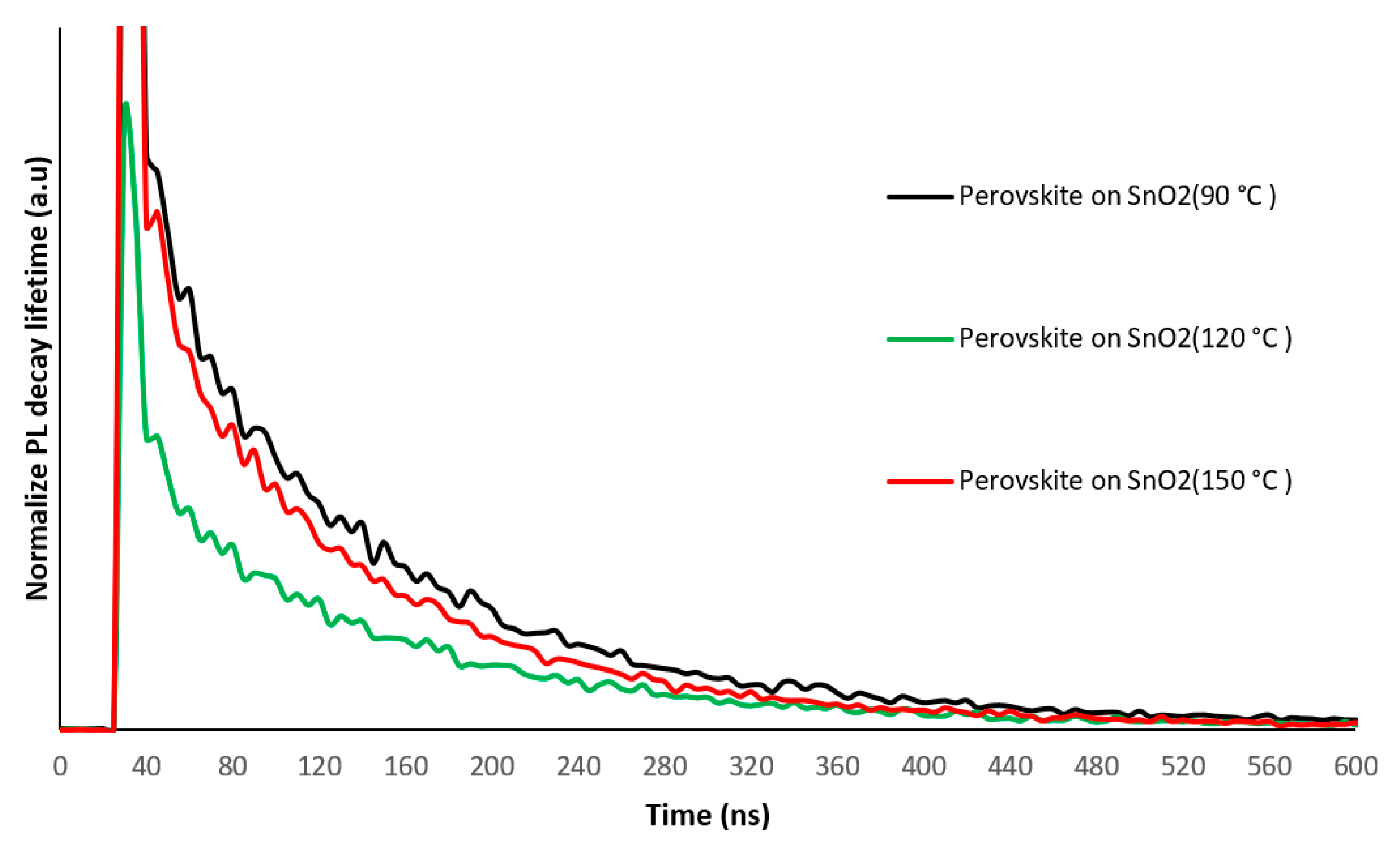


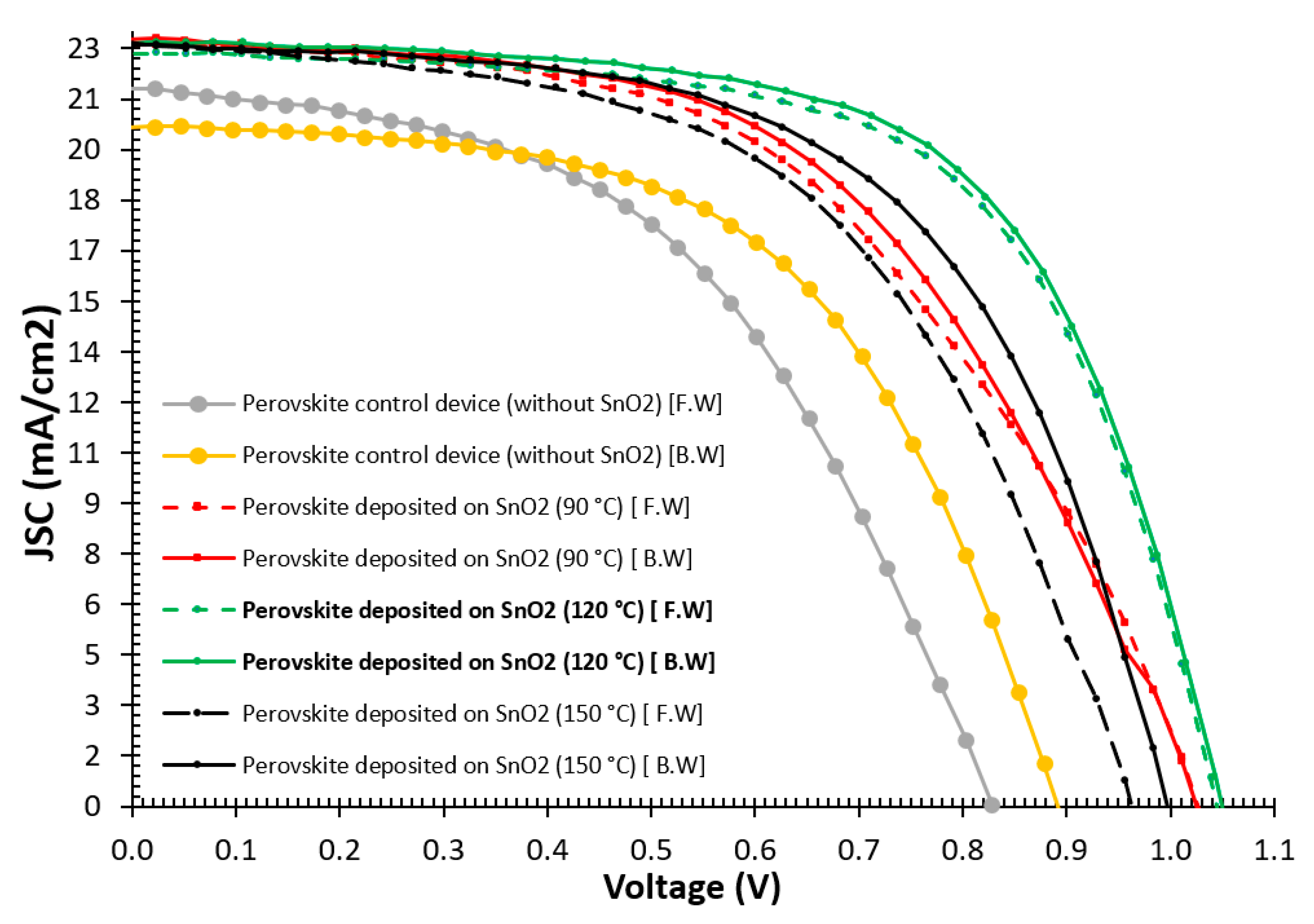
| Sample Description | Sweep Direction | EFF% | HI% | FF% | Voc [mV] | Jsc [mA/cm2] | Vmax [mV] | Jmax [mA/cm2] | Isc [mA] | Rshunt [Ω·cm2] | Rseries [Ω·cm2] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Perovskite control device without SnO2 | F.W | 8.72 | 44.5 | 829 | 21.3 | 526 | 16.6 | 7.6 | 865 | 15 | |
| B.W | 10.25 | 14.9 | 50.4 | 891 | 20.2 | 626 | 16.2 | 7.3 | 1250 | 13.4 | |
| Avg. | 9.4 | 47.4 | 860 | 20.7 | 576 | 16.4 | 7.4 | 1057 | 14.2 | ||
| Perovskite deposited on SnO2 (90 °C) | F.W | 12.2 | 51 | 1025 | 22.48 | 654 | 18.5 | 8.1 | 687 | 11.7 | |
| B.W | 12.8 | 4.6 | 52.7 | 1027 | 22.5 | 680 | 18.4 | 8.2 | 6863 | 10.8 | |
| Avg. | 12.5 | 51.85 | 1026 | 22.49 | 667 | 18.45 | 8.15 | 3775 | 11.25 | ||
| Perovskite deposited on SnO2 (120 °C) | F.W | 14.9 | 59.8 | 1023 | 22.7 | 749 | 19.16 | 7.9 | 7532 | 7.65 | |
| B.W | 15.1 | 1.3 | 61.8 | 1045 | 22.6 | 765 | 19.5 | 8.13 | 7698 | 7.7 | |
| Avg. | 15 | 60.8 | 1034 | 22.65 | 757 | 19.35 | 8.02 | 7615 | 7.7 | ||
| Perovskite deposited on SnO2 (150 °C) | F.W | 12.05 | 53 | 963 | 22.45 | 655 | 17.98 | 8.08 | 412 | 9.2 | |
| B.W | 13.4 | 7.9 | 57 | 1001 | 22.5 | 710 | 18.5 | 8.1 | 936 | 8.4 | |
| Avg. | 12.7 | 55 | 982 | 22.45 | 682.5 | 18.2 | 8.1 | 674 | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayali, A.; Alkaisi, M.M. Investigation of Annealing Temperature Effect of Tin Oxide on the Efficiency of Planar Structure Perovskite Solar Cells. Nanomaterials 2025, 15, 807. https://doi.org/10.3390/nano15110807
Hayali A, Alkaisi MM. Investigation of Annealing Temperature Effect of Tin Oxide on the Efficiency of Planar Structure Perovskite Solar Cells. Nanomaterials. 2025; 15(11):807. https://doi.org/10.3390/nano15110807
Chicago/Turabian StyleHayali, Ahmed, and Maan M. Alkaisi. 2025. "Investigation of Annealing Temperature Effect of Tin Oxide on the Efficiency of Planar Structure Perovskite Solar Cells" Nanomaterials 15, no. 11: 807. https://doi.org/10.3390/nano15110807
APA StyleHayali, A., & Alkaisi, M. M. (2025). Investigation of Annealing Temperature Effect of Tin Oxide on the Efficiency of Planar Structure Perovskite Solar Cells. Nanomaterials, 15(11), 807. https://doi.org/10.3390/nano15110807









