Graphene Oxide-Doped CNT Membrane for Dye Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Buckypaper (GO-BP) Preparation
2.3. Thermodynamics and Kinetics of Dyes Adsorption
2.4. Characterizations
3. Results
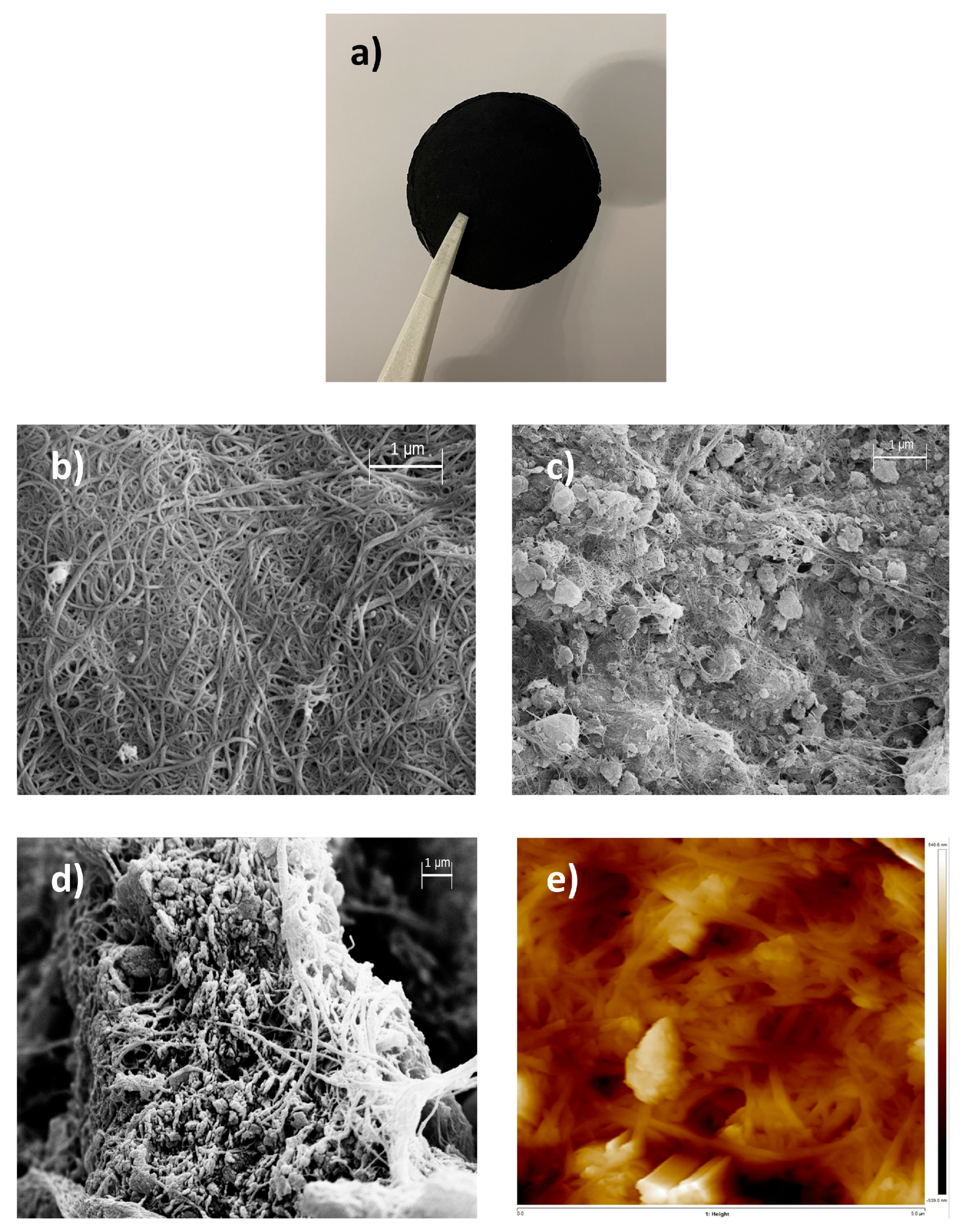
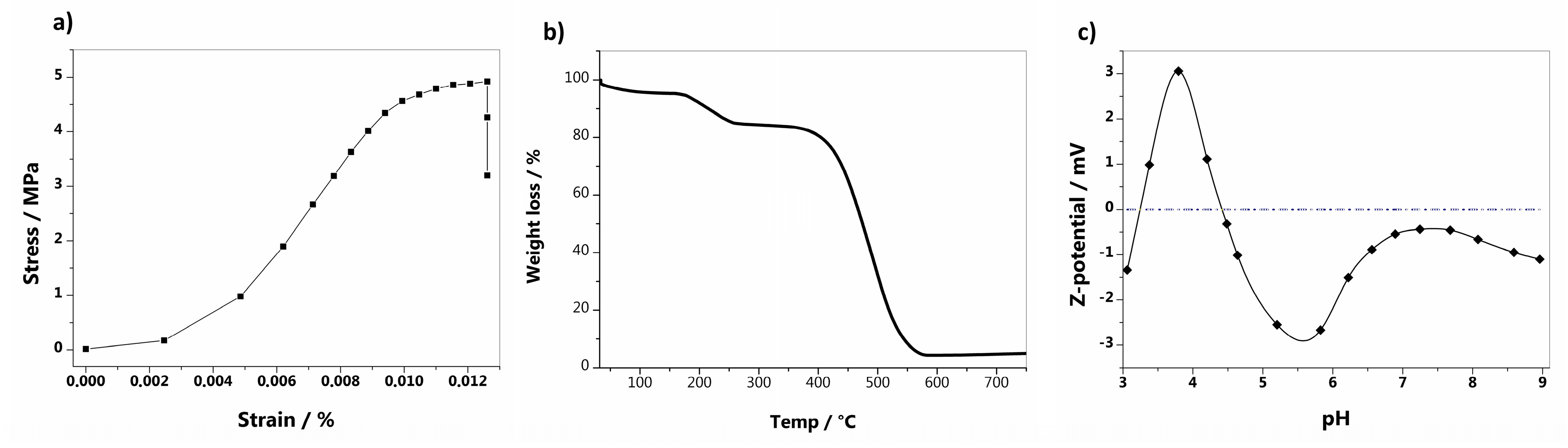
3.1. GO-BPs Characterization
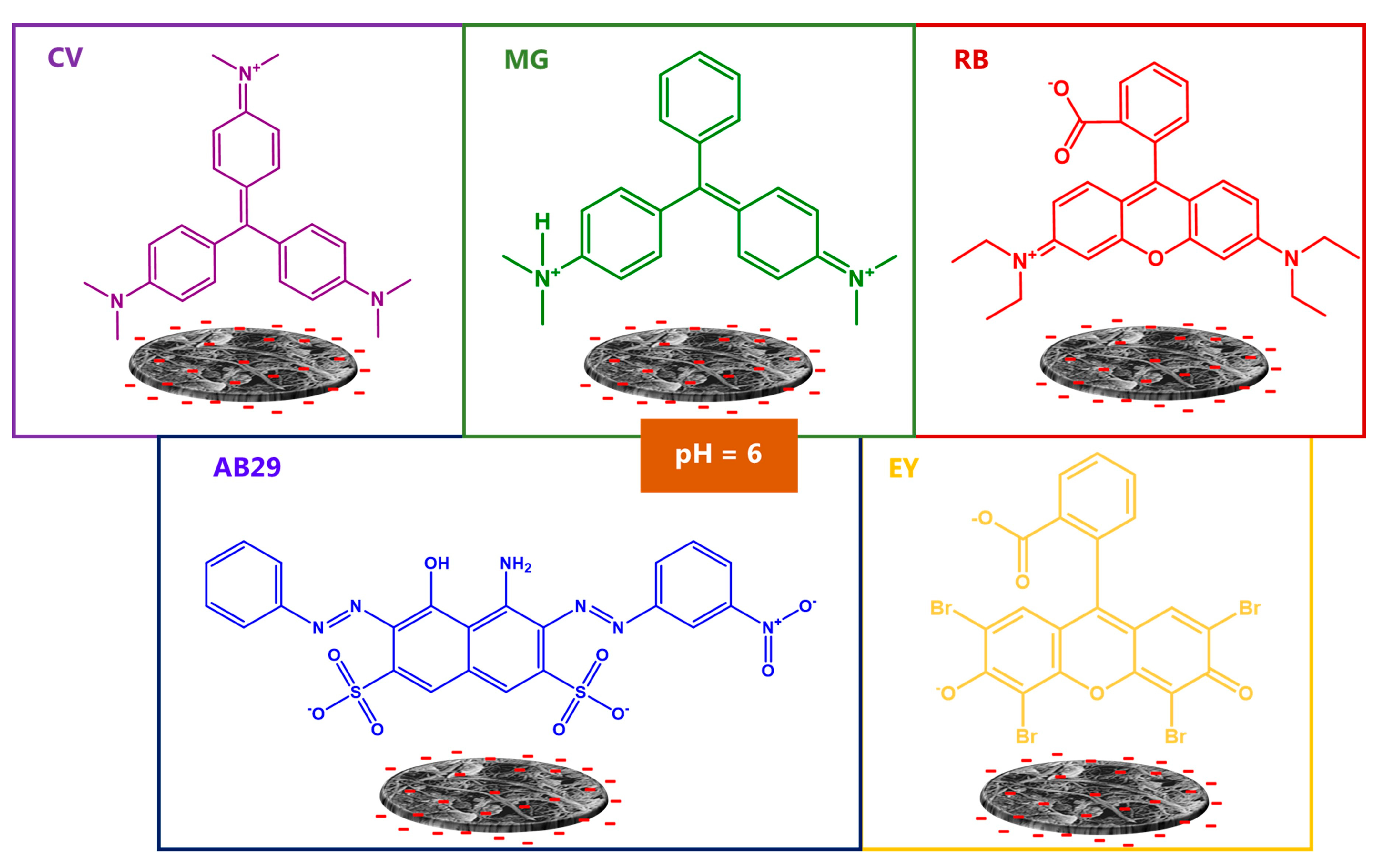
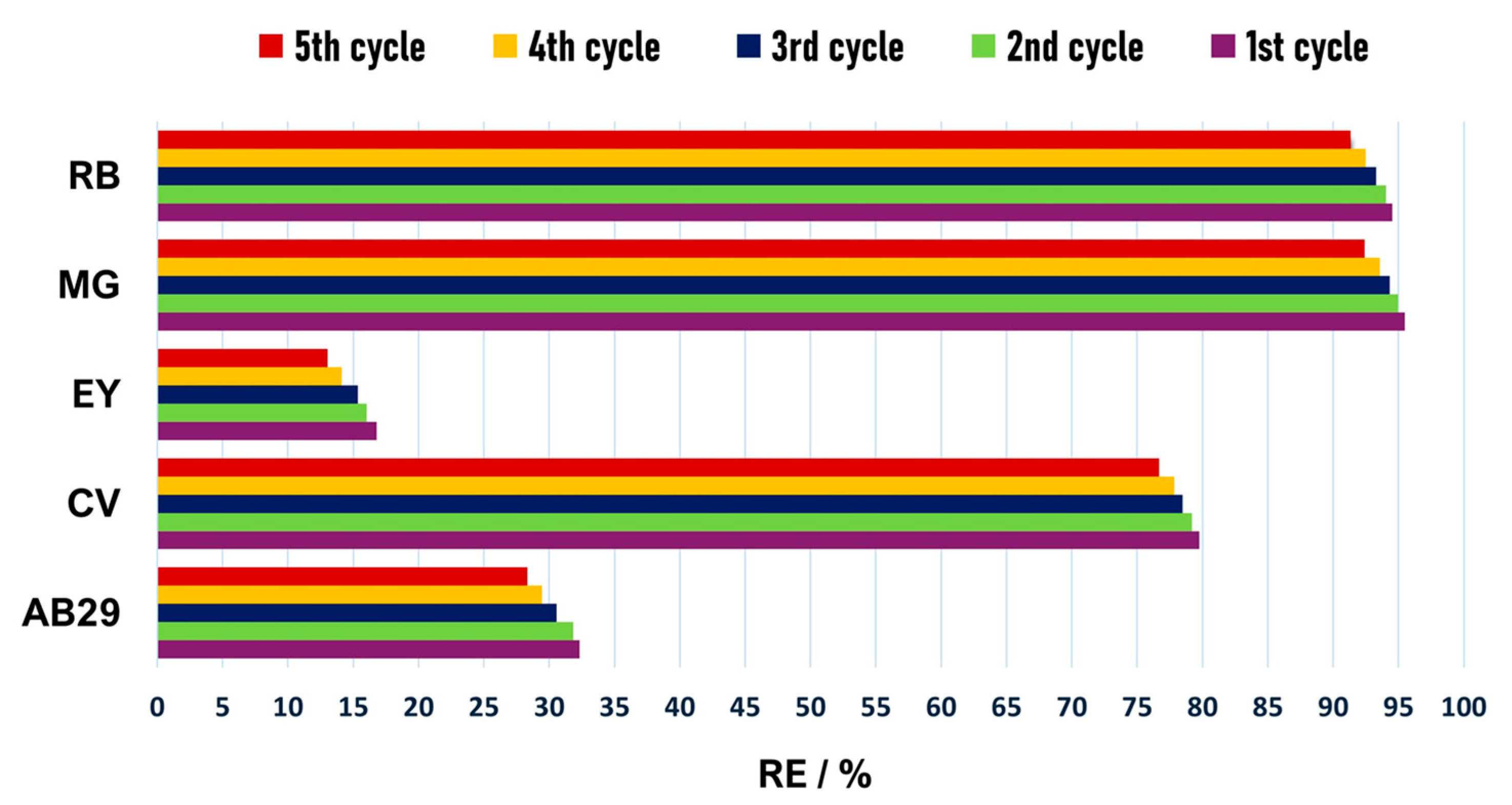
3.2. Dyes Adsorption
3.3. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SWCNT-BP | single-walled carbon nanotube buckypaper |
| MWCNTs | multi-walled carbon nanotubes |
| GO | graphene oxide |
| BP | buckypaper |
| AB29 | acid blue 29 |
| CV | crystal violet |
| EY | eosyn Y |
| MG | malachite green |
| RB | rhodamine B |
References
- United Nations Organization. The Sustainable Development Goals (SDGS); United Nations Organization: New York, NY, USA, 2024. [Google Scholar]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions; Springer: Berlin/Heidelberg, Germany, 2023; Volume 30, ISBN 0123456789. [Google Scholar]
- IMARC. Report Textile Market by Raw Material, Product, Application, and Region 2025–2033; IMARC: New York, NY, USA, 2025. [Google Scholar]
- Goswami, D.; Mukherjee, J.; Mondal, C.; Bhunia, B. Bioremediation of Azo Dye: A Review on Strategies, Toxicity Assessment, Mechanisms, Bottlenecks and Prospects. Sci. Total Environ. 2024, 954, 176426. [Google Scholar] [CrossRef] [PubMed]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Balakrishnan, V.K.; Shirin, S.; Aman, A.M.; de Solla, S.R.; Mathieu-Denoncourt, J.; Langlois, V.S. Genotoxic and Carcinogenic Products Arising from Reductive Transformations of the Azo Dye, Disperse Yellow 7. Chemosphere 2016, 146, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Chethana, M.; Sorokhaibam, L.G.; Bhandari, V.M.; Raja, S.; Ranade, V.V. Green Approach to Dye Wastewater Treatment Using Biocoagulants. ACS Sustain. Chem. Eng. 2016, 4, 2495–2507. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Joshi, H.; Mall, I.D.; Srivastava, V.C. Electrochemical Treatment of Acrylic Dye-Bearing Textile Wastewater: Optimization of Operating Parameters. Desalin. Water Treat. 2014, 52, 111–122. [Google Scholar] [CrossRef]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile Dye Wastewater Treatment by Direct Contact Membrane Distillation: Membrane Performance and Detailed Fouling Analysis. J. Memb. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.S.; Danquah, M.K. Potential and Challenges of Enzyme Incorporated Nanotechnology in Dye Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Chidichimo, F.; De Biase, M.; Tursi, A.; Maiolo, M.; Straface, S.; Baratta, M.; Olivito, F.; De Filpo, G. A Model for the Adsorption Process of Water Dissolved Elements Flowing into Reactive Porous Media: Characterization and Sizing of Water Mining/Filtering Systems. J. Hazard. Mater. 2023, 445, 130554. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wu, D.; Yu, J.; Gao, T.; Guo, L.; Li, F. Upgraded β-Cyclodextrin-Based Broad-Spectrum Adsorbents with Enhanced Antibacterial Property for High-Efficient Dyeing Wastewater Remediation. J. Hazard. Mater. 2024, 461, 132610. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Sun, Y.; Liu, W.; Pan, F.; Sun, P.; Fu, J. An Overview of Nanomaterials Applied for Removing Dyes from Wastewater. Environ. Sci. Pollut. Res. 2017, 24, 15882–15904. [Google Scholar] [CrossRef]
- Baratta, M.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Carbon Nanotubes Buckypapers: A New Frontier in Wastewater Treatment Technology. Sci. Total Environ. 2024, 924, 171578. [Google Scholar] [CrossRef]
- Umesh, A.S.; Puttaiahgowda, Y.M.; Thottathil, S. Enhanced Adsorption: Reviewing the Potential of Reinforcing Polymers and Hydrogels with Nanomaterials for Methylene Blue Dye Removal. Surf. Interfaces 2024, 51, 104670. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Singh, A. Adsorptive Removal of Heavy Metals, Dyes, and Pharmaceuticals: Carbon-Based Nanomaterials in Focus. Carbon 2024, 217, 118621. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers. Molecules 2022, 27, 7674. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Pantuso, E.; Mashin, A.I.; Baratta, M.; Nicoletta, F.P. WO3/Buckypaper Membranes for Advanced Oxidation Processes. Membranes 2020, 10, 157. [Google Scholar] [CrossRef]
- Baratta, M.; Nezhdanov, A.V.; Ershov, A.V.; Aiello, D.; Napoli, A.; Di Donna, L.; Mashin, A.I.; Nicoletta, F.P.; Filpo, G. De Improving the Catalytic Performance of TiO2 by Its Surface Deposition on CNT Buckypapers for Use in the Removal of Wastewater Pollutants. New Carbon Mater. 2025, 40, 438–455. [Google Scholar] [CrossRef]
- Tursi, A.; Mastropietro, T.F.; Bruno, R.; Baratta, M.; Ferrando-Soria, J.; Mashin, A.I.; Nicoletta, F.P.; Pardo, E.; De Filpo, G.; Armentano, D. Synthesis and Enhanced Capture Properties of a New BioMOF@SWCNT-BP: Recovery of the Endangered Rare-Earth Elements from Aqueous Systems. Adv. Mater. Interfaces 2021, 8, 2100730. [Google Scholar] [CrossRef]
- Baratta, M.; Mastropietro, T.F.; Bruno, R.; Tursi, A.; Negro, C.; Ferrando-Soria, J.; Mashin, A.I.; Nezhdanov, A.; Nicoletta, F.P.; De Filpo, G.; et al. Multivariate Metal-Organic Framework/Single-Walled Carbon Nanotube Buckypaper for Selective Lead Decontamination. ACS Appl. Nano Mater. 2022, 5, 5223–5233. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.; Mastropietro, T.F.; Escamilla, P.; Algieri, V.; Xu, F.; Nicoletta, F.P.; Ferrando-Soria, J.; Pardo, E.; De Filpo, G.; Armentano, D. Sulfur-Functionalized Single-Walled Carbon Nanotube Buckypaper/MTV-BioMetal-Organic Framework Nanocomposites for Gold Recovery. Inorg. Chem. 2024, 63, 18992–19001. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules 2022, 27, 4044. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Lima, E.C.; Juang, R.S.; Bollinger, J.C.; Chao, H.P. Thermodynamic Parameters of Liquid–Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. (Eds.) Adsorption Processes for Water Treatment and Purification; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 9783319581361. [Google Scholar]
- Chun, M.S.; Lee, S.Y.; Yang, S.M. Estimation of Zeta Potential by Electrokinetic Analysis of Ionic Fluid Flows through a Divergent Microchannel. J. Colloid Interface Sci. 2003, 266, 120–126. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Akrami, M.; Danesh, S.; Eftekhari, M. Comparative Study on the Removal of Cationic Dyes Using Different Graphene Oxide Forms. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1785–1797. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Rajendra Kumar, R.T. Adsorption Behaviour of Reduced Graphene Oxide towards Cationic and Anionic Dyes: Co-Action of Electrostatic and π–π Interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Mijowska, E. Equilibrium, Kinetic and Thermodynamic Studies on Adsorption of Cationic Dyes from Aqueous Solutions Using Graphene Oxide. Chem. Eng. Res. Des. 2017, 123, 35–49. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Alaabed, S.; Al-Akhras, M.A.; Obaidat, I.M. Molecular Simulation of Adsorption of Methylene Blue and Rhodamine B on Graphene and Graphene Oxide for Water Purification. Mater. Today Proc. 2019, 28, 1078–1083. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Vijaya Kumara, A.; Muralidhara, H.B.; Sampath, S. Graphene and Graphene Oxide as Effective Adsorbents toward Anionic and Cationic Dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.; Eugenia, L.; Mendoza-mart, E.; Joseph, A.; Soledad, S. Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals. Water 2019, 12, 29. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Bartczak, P.; Zdarta, J.; Jesionowski, T. Active MgO-SiO2 Hybrid Material for Organic Dye Removal: A Mechanism and Interaction Study of the Adsorption of C.I. Acid Blue 29 and C.I. Basic Blue 9. J. Environ. Manag. 2017, 204, 123–135. [Google Scholar] [CrossRef]
- Marrakchi, F.; Bouaziz, M.; Hameed, B.H. Adsorption of Acid Blue 29 and Methylene Blue on Mesoporous K2CO3-Activated Olive Pomace Boiler Ash. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 157–165. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Das, A.K.; Mondal, B.; Purkait, M.K. Kinetic and Equilibrium Studies on the Adsorption of Crystal Violet Dye Using Kaolin as an Adsorbent. Sep. Sci. Technol. 2008, 43, 1382–1403. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from Aqueous Solution onto NaOH-Modified Rice Husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of Crystal Violet onto Functionalised Multi-Walled Carbon Nanotubes: Equilibrium and Kinetic Studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Sumana, G. Highly Effective Adsorption of Crystal Violet Dye from Contaminated Water Using Graphene Oxide Intercalated Montmorillonite Nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Balogun, D.O.; Etemire, O.J.; Ezeh, P.N.; Tan, Y.H.; Mubarak, N.M. Rapid Adsorptive Removal of Eosin Yellow and Methyl Orange Using Zeolite Y. Sci. Rep. 2023, 13, 21373. [Google Scholar] [CrossRef]
- Yadav, S.K.; Dhakate, S.R.; Pratap Singh, B. Carbon Nanotube Incorporated Eucalyptus Derived Activated Carbon-Based Novel Adsorbent for Efficient Removal of Methylene Blue and Eosin Yellow Dyes. Bioresour. Technol. 2022, 344, 126231. [Google Scholar] [CrossRef]
- Veerakumar, P.; Tharini, J.; Ramakrishnan, M.; Panneer Muthuselvam, I.; Lin, K.C. Graphene Oxide Nanosheets as An Efficient and Reusable Sorbents for Eosin Yellow Dye Removal from Aqueous Solutions. ChemistrySelect 2017, 2, 3598–3607. [Google Scholar] [CrossRef]
- Ahmad, N.; Karim, S.; Hussain, D.; Mok, Y.S.; Siddiqui, G.U. Efficient Dual Adsorption of Eosin Y and Methylene Blue from Aqueous Solution Using Nanocomposite of Graphene Oxide Nanosheets and ZnO Nanospheres. Korean J. Chem. Eng. 2022, 39, 3155–3164. [Google Scholar] [CrossRef]
- Altintig, E.; Onaran, M.; Sarı, A.; Altundag, H.; Tuzen, M. Preparation, Characterization and Evaluation of Bio-Based Magnetic Activated Carbon for Effective Adsorption of Malachite Green from Aqueous Solution. Mater. Chem. Phys. 2018, 220, 313–321. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Meng, Y.; Cheng, Y.; Lu, J.; Wang, H. Novel Graphene Oxide/Aminated Lignin Aerogels for Enhanced Adsorption of Malachite Green in Wastewater. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 603, 125281. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene Oxide Decorated with Cellulose and Copper Nanoparticle as an Efficient Adsorbent for the Removal of Malachite Green. Int. J. Biol. Macromol. 2021, 167, 23–34. [Google Scholar] [CrossRef]
- Shirmardi, M.; Mahvi, A.H.; Hashemzadeh, B.; Naeimabadi, A.; Hassani, G.; Niri, M.V. The Adsorption of Malachite Green (MG) as a Cationic Dye onto Functionalized Multi Walled Carbon Nanotubes. Korean J. Chem. Eng. 2013, 30, 1603–1608. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Reduced Graphene Oxide as an Effective Adsorbent for Removal of Malachite Green Dye: Plausible Adsorption Pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Duan, X.; Srinivasakannan, C.; Liang, J. Preparation of Magnesium Silicate/Carbon Composite for Adsorption of Rhodamine B. RSC Adv. 2018, 8, 7873–7882. [Google Scholar] [CrossRef] [PubMed]
- Neelaveni, M.; Santhana Krishnan, P.; Ramya, R.; Sonia Theres, G.; Shanthi, K. Montmorillonite/Graphene Oxide Nanocomposite as Superior Adsorbent for the Adsorption of Rhodamine B and Nickel Ion in Binary System. Adv. Powder Technol. 2019, 30, 596–609. [Google Scholar] [CrossRef]
- Yu, Y.; Murthy, B.N.; Shapter, J.G.; Constantopoulos, K.T.; Voelcker, N.H.; Ellis, A.V. Benzene Carboxylic Acid Derivatized Graphene Oxide Nanosheets on Natural Zeolites as Effective Adsorbents for Cationic Dye Removal. J. Hazard. Mater. 2013, 260, 330–338. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Effectiveness of Carbon Nanotube-Cobalt Ferrite Nanocomposites for the Adsorption of Rhodamine B from Aqueous Solutions. RSC Adv. 2015, 5, 22724–22739. [Google Scholar] [CrossRef]

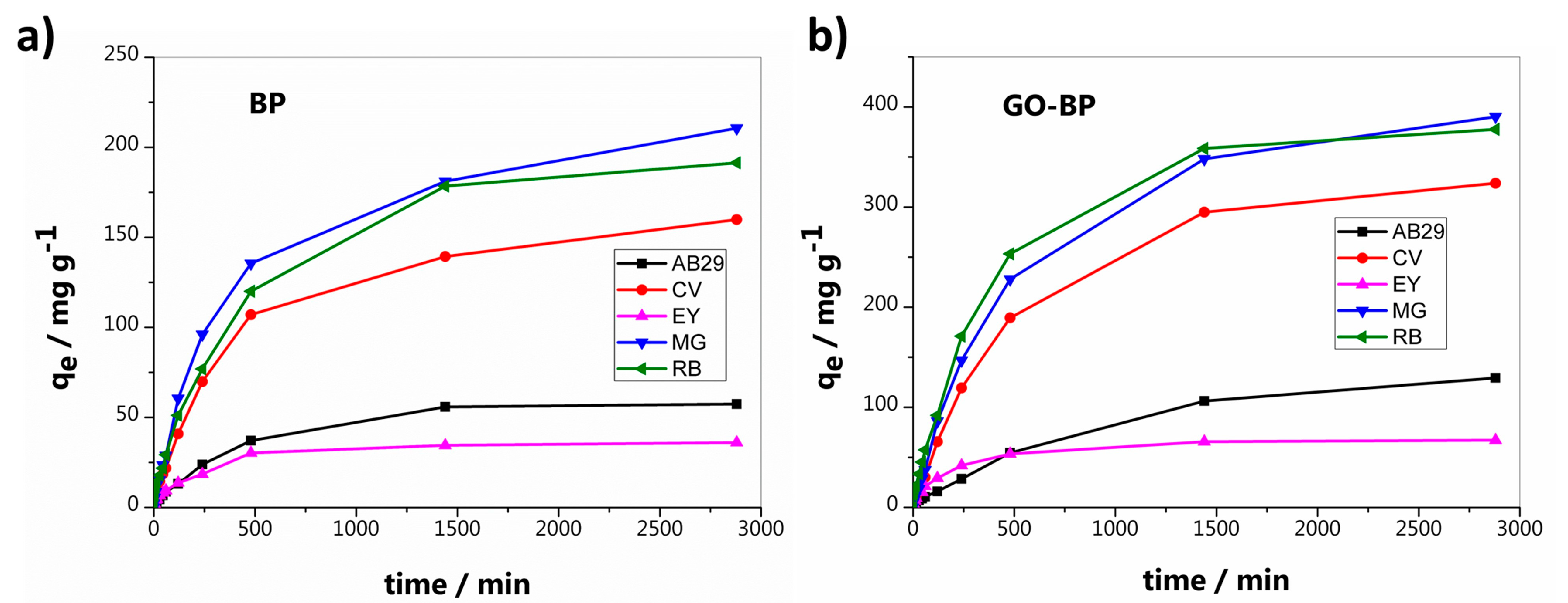
| Dye | LogKD | Maximun Adsorption Capacity (mg g−1) | 1st Order Kinetic Constant k1 × 104 (min−1) | R2 | 2nd Order Kinetic Constant k2 × 106 (g mg−1 min−1) | R2 |
|---|---|---|---|---|---|---|
| AB29 | 3.58 | 129.31 | 11.4 ± 0.2 | 0.9964 | 22.0 ± 1.0 | 0.9784 |
| CV | 4.50 | 323.74 | 16.9 ± 0.2 | 0.9988 | 21.3 ± 1.4 | 0.9532 |
| EY | 3.21 | 67.09 | 25.9 ± 1.0 | 0.9845 | 427.5 ± 35.3 | 0.9296 |
| MG | 5.23 | 390.17 | 15.6 ± 0.3 | 0.9968 | 14.3 ± 0.8 | 0.9672 |
| RB | 5.14 | 377.53 | 20.7 ± 0.3 | 0.9983 | 33.3 ± 2.5 | 0.9383 |
| Dye | Adsorbent Typology | Adsorbent Dose/(mg) | pH | Cdye/ (mg L−1) | qmax/ (mg g−1) | Ref. |
|---|---|---|---|---|---|---|
| AB29 | MgO-SiO2 | 500 | 10 | 100 | 44.9 | [41] |
| K2CO3-activated olive pomace boiler ash | 300 | 6 | 100 | 38.48 | [42] | |
| GO-BP | 50 | 6 | 100 | 162.65 | This work | |
| CV | Kaolin | 100 | 7 | 100 | 45.0 | [43] |
| NaOH-modified rice husk | 1000 | 7 | 50 | 44.87 | [44] | |
| MWCNT-COOH | 10 | 6 | 500 | 100.0 | [45] | |
| GO | 25 | 6 | 200 | 487.80 | [46] | |
| GO-BP | 50 | 6 | 100 | 374.53 | This work | |
| EY | Zeolite Y | 100 | 2.5 | 60 | 52.91 | [47] |
| CNTs-incorporated eucalyptus | 20 | 6 | 50 | 49.15 | [48] | |
| GO | 300 | 1 | 50 | 217.33 | [49] | |
| GO/ZnO nanocomposite | 230 | 2 | 100 | 555.55 | [50] | |
| GO-BP | 50 | 6 | 100 | 85.38 | This work | |
| MG | Fe3O4-AC | 100 | 6 | 300 | 217.68 | [51] |
| GO/aminated lignin aerogels | 20 | 8 | 50 | 113.5 | [52] | |
| GO-Cellulose-Cu | 1000 | 7 | 400 | 207.1 | [53] | |
| MWCNT-COOH | 40 | 7 | 100 | 142.85 | [54] | |
| rGO | 50 | 7 | 200 | 476.2 | [55] | |
| GO-BP | 50 | 6 | 100 | 493.44 | This work | |
| RB | Magnesium silicate/carbon composite | 100 | 6.5 | 600 | 244 | [56] |
| Montmorillonite/GO | 300 | 7 | 150 | 625.0 | [57] | |
| Benzene carboxylic acid/GO-zeolite | 10 | 3 | 500 | 67.56 | [58] | |
| MWCNT-COOH | 50 | 7 | 100 | 42.68 | [59] | |
| GO-BP | 50 | 6 | 100 | 467.35 | This work |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | qexp (mg/g) | qmax (mg/g) | KL (L/mg) | R2 | KF (L/mg) | nF | R2 | |
| AB29 | 298 | 162.6 | 227.3 | 0.034 | 0.9982 | 14.7 | 1.7 | 0.9660 |
| 313 | 177.3 | 222.2 | 0.051 | 0.9986 | 21.5 | 1.9 | 0.9606 | |
| CV | 298 | 374.5 | 476.2 | 0.140 | 0.9961 | 81.0 | 2.3 | 0.8543 |
| 313 | 407.6 | 454.5 | 0.338 | 0.9984 | 120.2 | 2.7 | 0.8468 | |
| EY | 298 | 85.4 | 200.0 | 0.009 | 0.9979 | 3.8 | 1.4 | 0.9678 |
| 313 | 101.5 | 227.3 | 0.012 | 0.9975 | 5.0 | 1.4 | 0.9629 | |
| MG | 298 | 493.4 | 526.3 | 0.731 | 0.9941 | 180.7 | 3.0 | 0.7881 |
| 313 | 504.1 | 500.0 | 1.667 | 0.9960 | 222.3 | 3.5 | 0.7764 | |
| RB | 298 | 467.3 | 526.3 | 0.576 | 0.9964 | 165.3 | 2.9 | 0.8224 |
| 313 | 478.1 | 500.0 | 1.428 | 0.9973 | 207.9 | 3.4 | 0.7750 | |
| T (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/(K·mol)) | |
|---|---|---|---|---|
| AB29 | 298 | −24.5 | 20.4 | 150.5 |
| 313 | −26.7 | 20.4 | 150.5 | |
| CV | 298 | −26.9 | 45.6 | 243.4 |
| 313 | −30.6 | 45.6 | 243.4 | |
| EY | 298 | −21.4 | 16.8 | 128.3 |
| 313 | −23.3 | 16.8 | 128.3 | |
| MG | 298 | −30.7 | 42.6 | 246.1 |
| 313 | −34.4 | 42.6 | 246.1 | |
| RB | 298 | −30.8 | 47.0 | 261.2 |
| 313 | −34.8 | 47.0 | 261.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, M.; Nicoletta, F.P.; De Filpo, G. Graphene Oxide-Doped CNT Membrane for Dye Adsorption. Nanomaterials 2025, 15, 782. https://doi.org/10.3390/nano15110782
Baratta M, Nicoletta FP, De Filpo G. Graphene Oxide-Doped CNT Membrane for Dye Adsorption. Nanomaterials. 2025; 15(11):782. https://doi.org/10.3390/nano15110782
Chicago/Turabian StyleBaratta, Mariafrancesca, Fiore Pasquale Nicoletta, and Giovanni De Filpo. 2025. "Graphene Oxide-Doped CNT Membrane for Dye Adsorption" Nanomaterials 15, no. 11: 782. https://doi.org/10.3390/nano15110782
APA StyleBaratta, M., Nicoletta, F. P., & De Filpo, G. (2025). Graphene Oxide-Doped CNT Membrane for Dye Adsorption. Nanomaterials, 15(11), 782. https://doi.org/10.3390/nano15110782







