Endophytic Bacterial Community Structure and Function Response of BLB Rice Leaves After Foliar Application of Cu-Ag Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis of Nanoparticles
2.3. The Characterization of Nanoparticles
2.4. Plant Growth
2.5. Plant Spraying with Nanoparticles
2.6. Phenylalanine Ammonia-Lyase (PAL) Activity Measurements
2.7. Determination of Rice Leaves Mineral Element and Metal Contents
2.8. Rice Leave Samples Harvest for Amplicon Sequencing
2.9. DNA Extraction and Amplicon Sequencing
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
3.1. Morphology Characterization of Nanoparticles
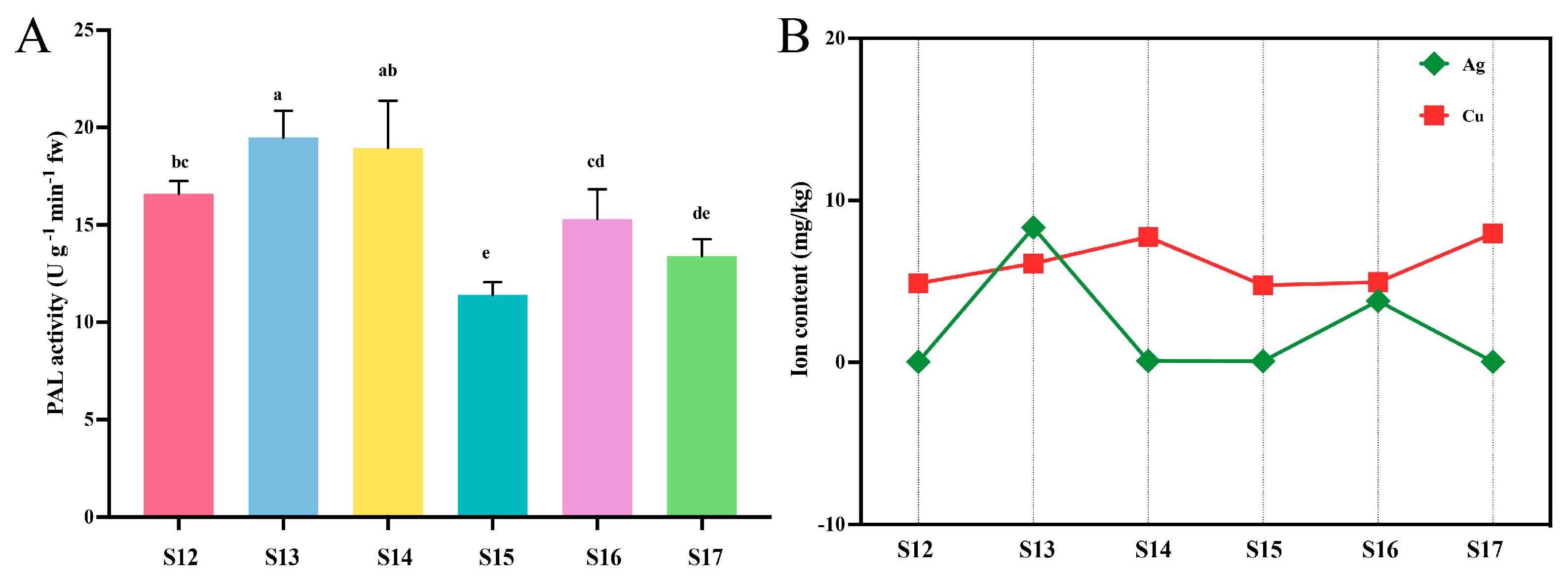
3.2. Phenylalanine Ammonia-Lyase (PAL) Activity
3.3. Mineral and Metal Content in Rice Leaves
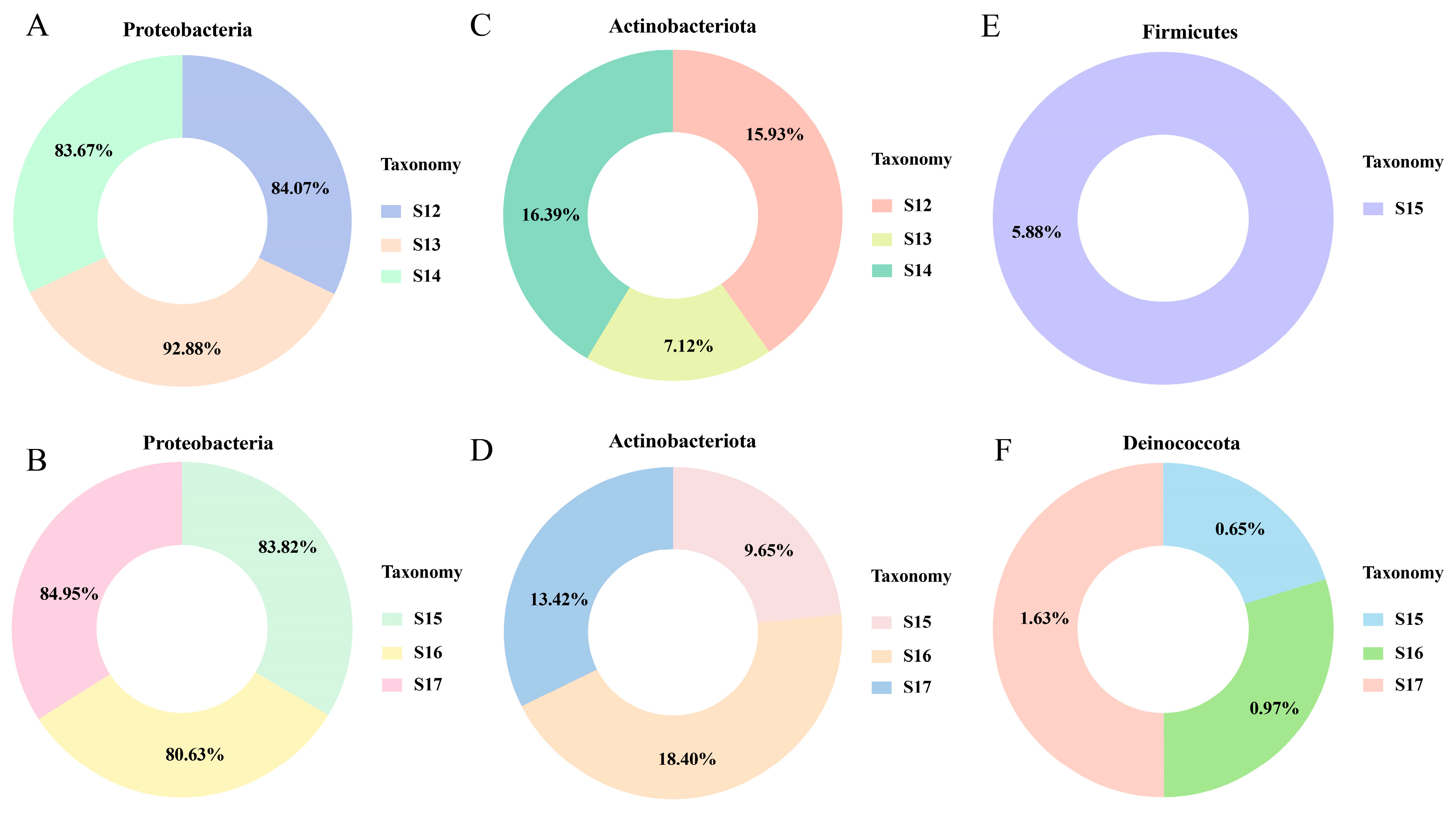
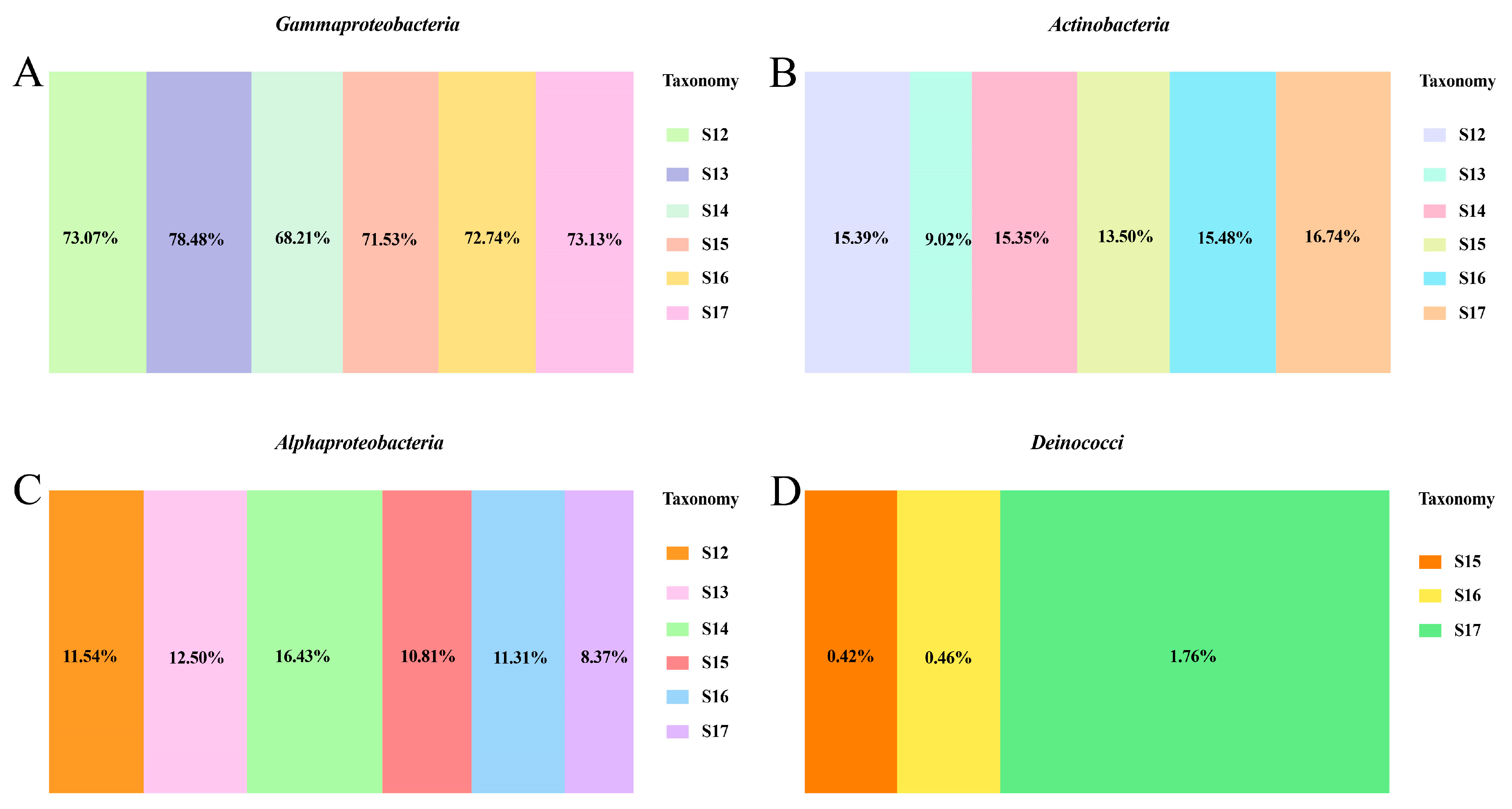
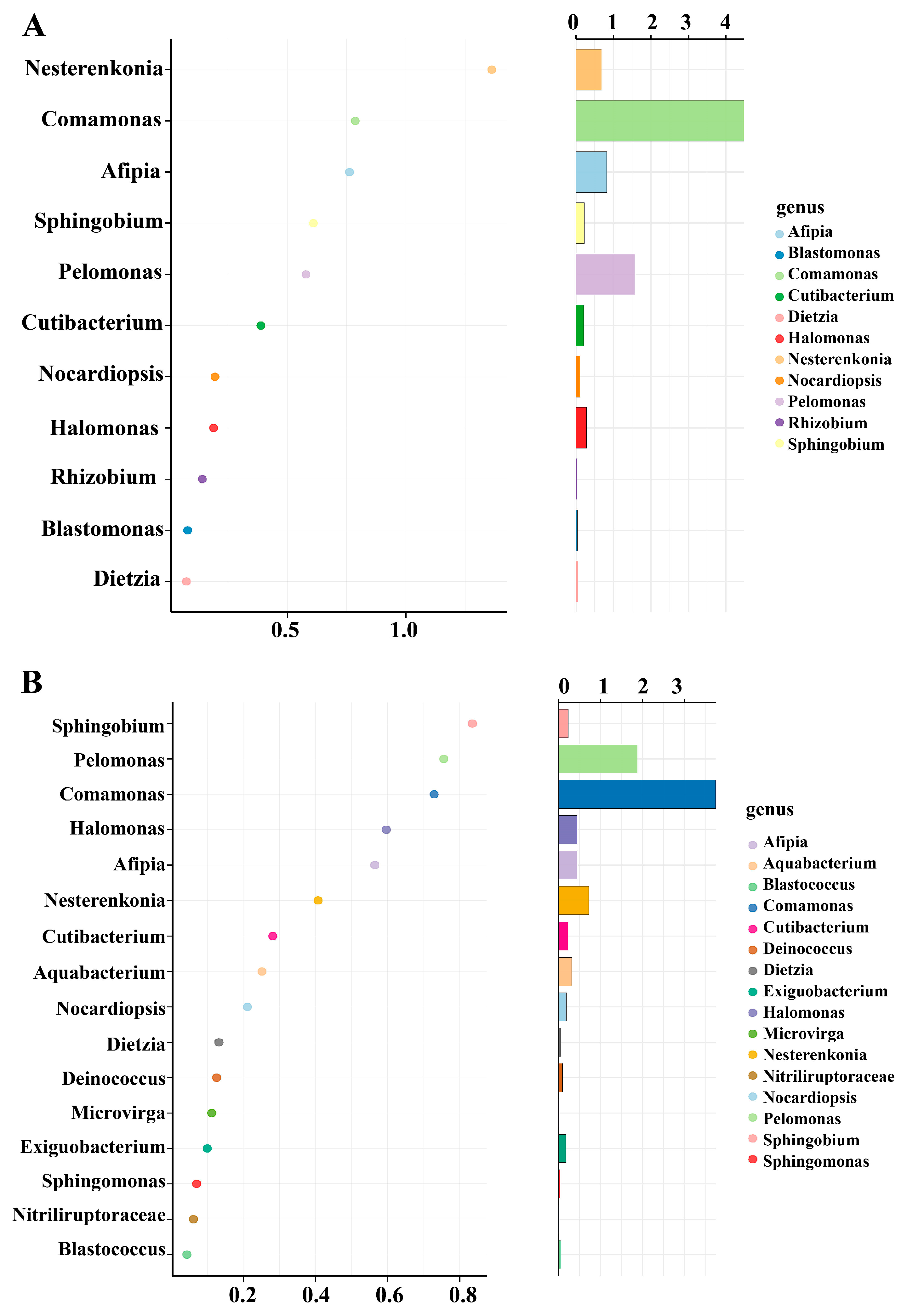
3.4. Changes in Endophytic Microbial Members
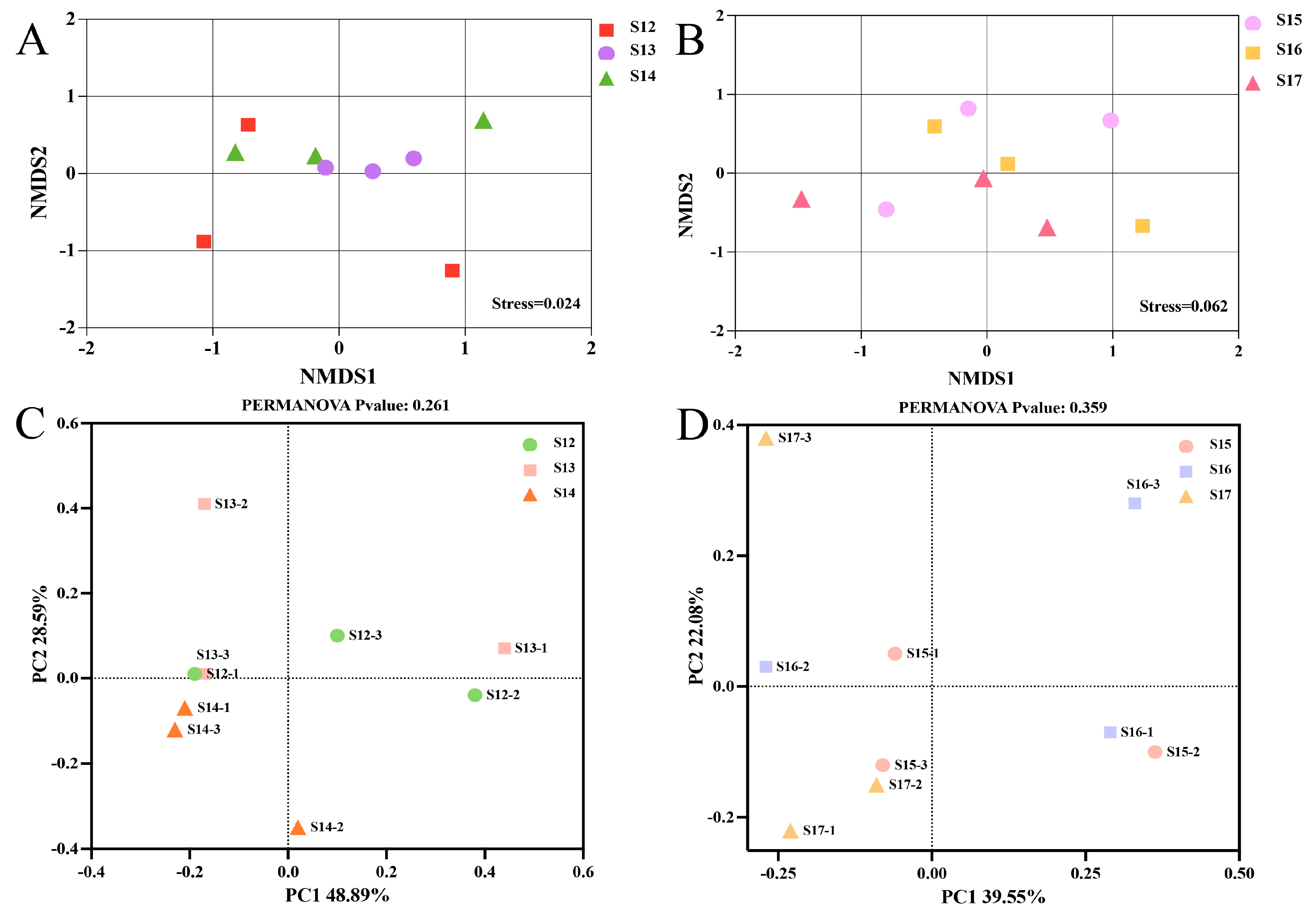
3.5. The Beta Diversity in Rice Endophytic Microbiota
3.6. Nanoparticles Charge the Leaf Endophytic Bacterial Microbiome
3.7. Unique Bacterial Species in the Rice Leaves
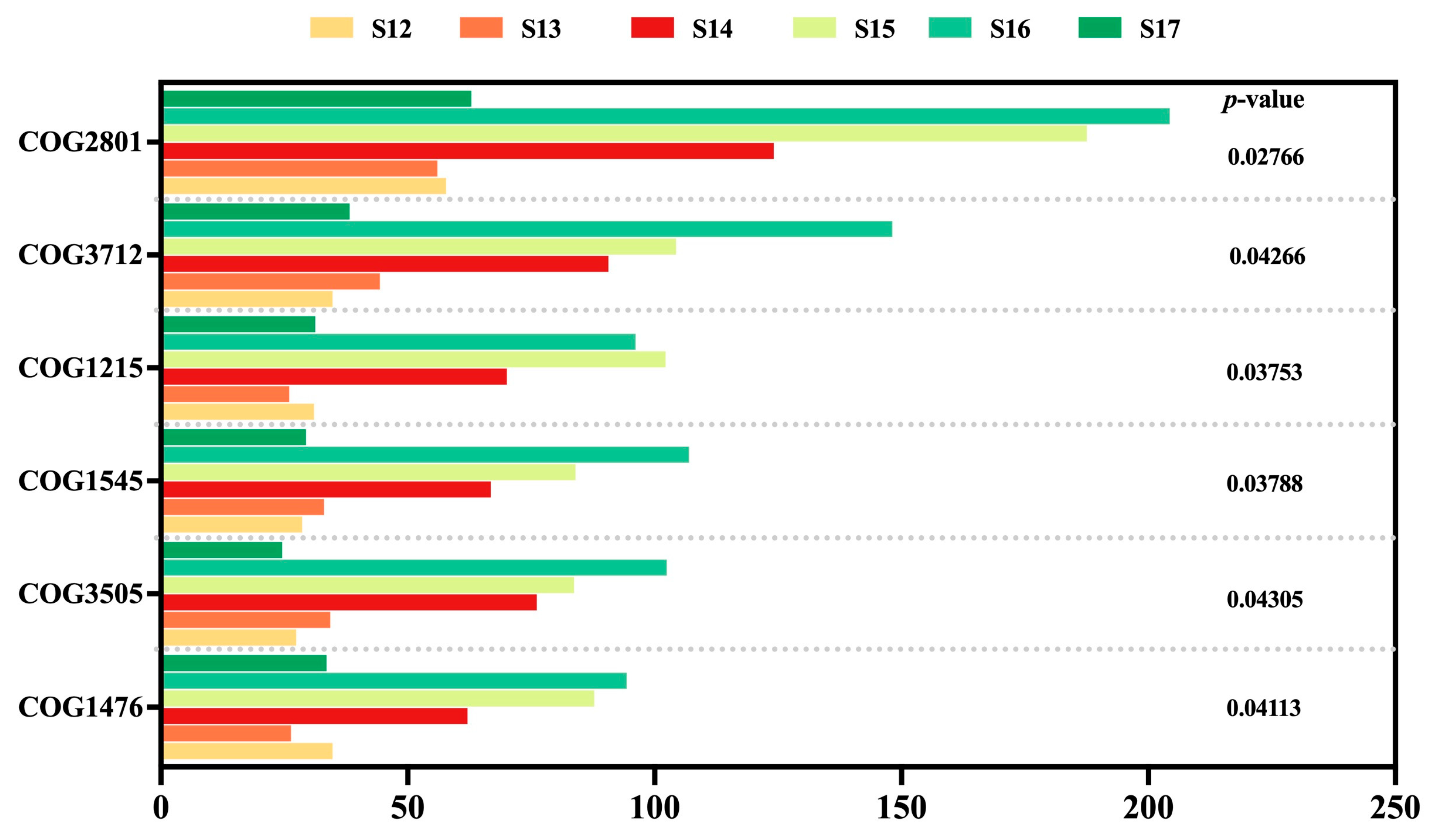
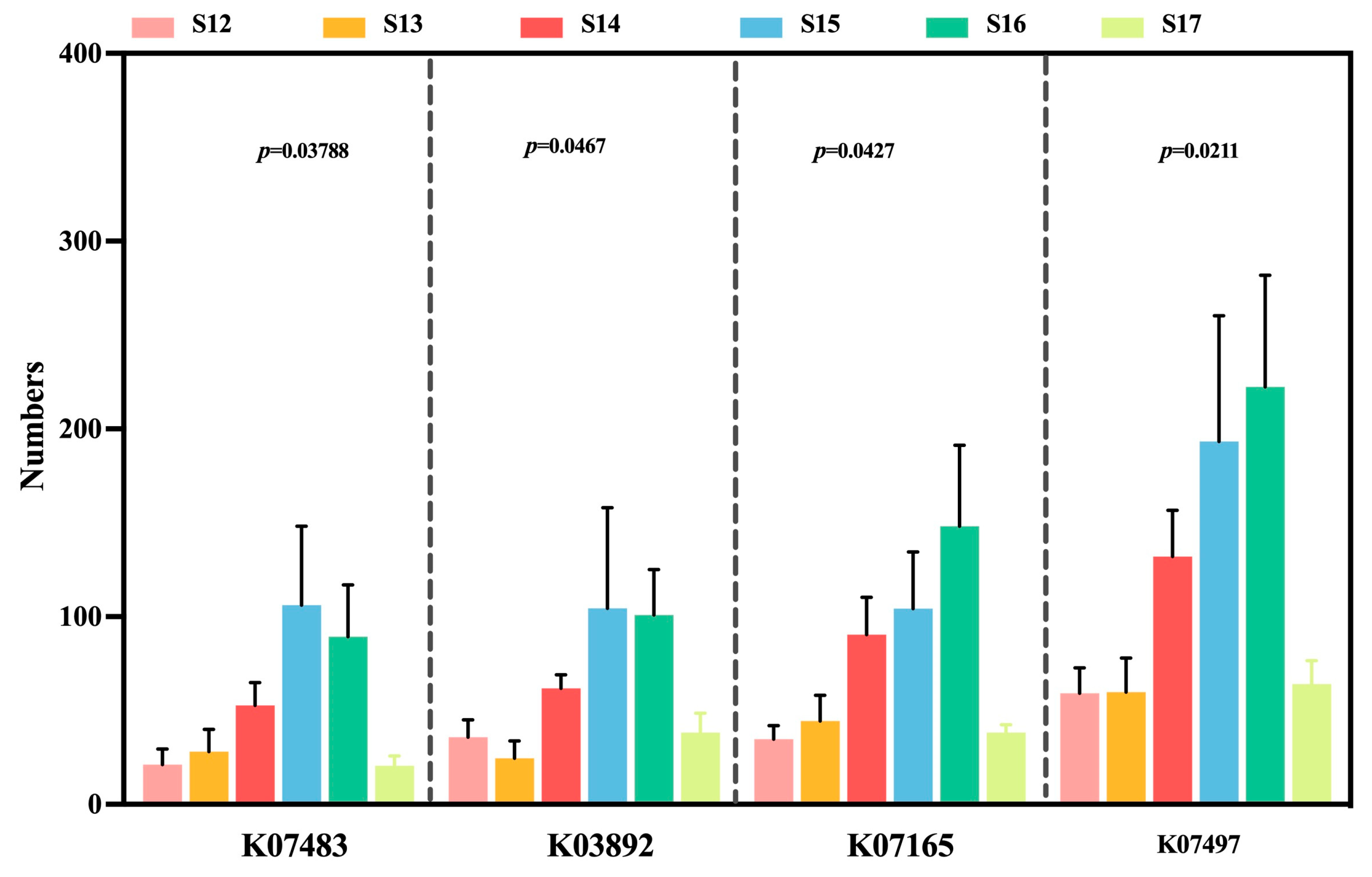
3.8. Changes in Endophytic Microbial Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alsakar, Y.M.; Sakr, N.A.; Elmogy, M. An enhanced classification system of various rice plant diseases based on multi-level handcrafted feature extraction technique. Sci. Rep. 2024, 14, 30601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance Genes and their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa L.)—An Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef]
- Fred, A.K.; Kiswara, G.; Yi, G.; Kim, K.M. Screening Rice Cultivars for Resistance to Bacterial Leaf Blight. J. Microbiol. Biotechnol. 2016, 26, 938–945. [Google Scholar] [CrossRef]
- Yazid, S.N.; Ahmad, K.; Razak, M.; Rahman, Z.A.; Ramachandran, K.; Mohamad, S.N.A.; Ghaffar, M.B.A. Introgression of bacterial leaf blight (BLB) resistant gene, Xa7 into MARDI elite variety, MR219 by marker assisted backcrossing (MABC) approach. Braz. J. Biol. 2021, 84, e248359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, H.; Wang, R.; He, H.; Song, B.; Song, R. Bactericidal bissulfone B(7) targets bacterial pyruvate kinase to impair bacterial biology and pathogenicity in plants. Sci. China Life Sci. 2024, 67, 391–402. [Google Scholar] [CrossRef]
- Joshi, T.; Joshi, T.; Sharma, P.; Chandra, S.; Pande, V. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by targeting peptide deformylase. J. Biomol. Struct. Dyn. 2021, 39, 823–840. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhang, S.; Sun, M.L.; Su, H.N.; Li, H.Y.; Kun, L.; Zhang, Y.Z.; Chen, X.L.; Cao, H.Y.; Song, X.Y. Antibacterial activity of peptaibols from Trichoderma longibrachiatum SMF2 against gram-negative Xanthomonas oryzae pv. oryzae, the causal agent of bacterial leaf blight on rice. Front. Microbiol. 2022, 13, 1034779. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Pandey, S.C.; Maiti, P.; Tripathi, M.; Paliwal, A.; Nand, M.; Sharma, P.; Samant, M.; Pande, V.; Chandra, S. Antimicrobial activity of methanolic extracts of Vernonia cinerea against Xanthomonas oryzae and identification of their compounds using in silico techniques. PLoS ONE 2021, 16, e0252759. [Google Scholar] [CrossRef]
- Singh, U.M.; Dixit, S.; Alam, S.; Yadav, S.; Prasanth, V.V.; Singh, A.K.; Venkateshwarlu, C.; Abbai, R.; Vipparla, A.K.; Badri, J.; et al. Marker-assisted forward breeding to develop a drought-, bacterial-leaf-blight-, and blast-resistant rice cultivar. Plant Genome 2022, 15, e20170. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, G.; Chen, W.; Tan, L.; Long, Q.; Cui, F.; Tan, L.; Zou, G.; Tan, Y. Current status of molecular rice breeding for durable and broad-spectrum resistance to major diseases and insect pests. Theor. Appl. Genet. 2024, 137, 219. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Teja, B.S.; Jamwal, G.; Gupta, V.; Verma, M.; Sharma, A.; Sharma, A.; Pandit, V. Biological control of bacterial leaf blight (BLB) in rice—A sustainable approach. Heliyon 2025, 11, e41769. [Google Scholar] [CrossRef] [PubMed]
- Ritbamrung, O.; Inthima, P.; Ratanasut, K.; Sujipuli, K.; Rungrat, T.; Buddhachat, K. Evaluating Xanthomonas oryzae pv. oryzae (Xoo) infection dynamics in rice for distribution routes and environmental reservoirs by molecular approaches. Sci. Rep. 2025, 15, 1408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; El-Sappah, A.H.; Khaskhoussi, A.; Huang, Q.; Atif, A.M.; Elhamid, M.A.A.; Ihtisham, M.; El-Maati, M.F.A.; Soaud, S.A.; Tahri, W. Nanoparticles: A promising tool against environmental stress in plants. Front. Plant Sci. 2024, 15, 1509047. [Google Scholar] [CrossRef]
- Abolarinwa, T.O.; Ajose, D.J.; Oluwarinde, B.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Plant-derived nanoparticles as alternative therapy against Diarrheal pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2022, 13, 1007115. [Google Scholar] [CrossRef]
- Javeedvali, S.; Gopalakrishnan, C.; Kannan, R.; Manonmani, S.; Prasanthrajan, M.; Varanavasiappan, S. Strategies for sustainable rice bacterial leaf blight management: A holistic approach through phage biocontrol and nanoparticle encapsulation. J. Plant Pathol. 2024, 1–12. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Zhang, M.; Abdallah, Y.; Ahmed, T.; Qiu, W.; Ali, M.A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. The Bio-Synthesis of Three Metal Oxide Nanoparticles (ZnO, MnO2, and MgO) and Their Antibacterial Activity Against the Bacterial Leaf Blight Pathogen. Front. Microbiol. 2020, 11, 588326. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.; Patel, M.; Patel, P.; Tiwari, S.; Jinal, H.N.; Amaresan, N. Assessment of copper (Cu) nanoparticle for their biocontrol activity against Xanthomonas oryzae pv. oryzae, growth promotion, and physiology of rice (Oryza sativa L.) plants. Lett. Appl. Microbiol. 2023, 76, ovac066. [Google Scholar] [CrossRef]
- Abdallah, Y.; Nehela, Y.; Ogunyemi, S.O.; Ijaz, M.; Ahmed, T.; Elashmony, R.; Alkhalifah, D.H.M.; Hozzein, W.N.; Xu, L.; Yan, C.; et al. Bio-functionalized nickel-silica nanoparticles suppress bacterial leaf blight disease in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1216782. [Google Scholar] [CrossRef]
- Shobha, B.; Lakshmeesha, T.R.; Ansari, M.A.; Almatroudi, A.; Alzohairy, M.A.; Basavaraju, S.; Alurappa, R.; Niranjana, S.R.; Chowdappa, S. Mycosynthesis of ZnO Nanoparticles Using Trichoderma spp. Isolated from Rhizosphere Soils and Its Synergistic Antibacterial Effect against Xanthomonas oryzae pv. Oryzae. J. Fungi 2020, 6, 181. [Google Scholar] [CrossRef]
- Namburi, K.R.; Kora, A.J.; Chetukuri, A.; Kota, V. Biogenic silver nanoparticles as an antibacterial agent against bacterial leaf blight causing rice phytopathogen Xanthomonas oryzae pv. Oryzae. Bioprocess Biosyst. Eng. 2021, 44, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Abdallah, Y.; Ibrahim, E.; Zhang, Y.; Bi, J.; Wang, F.; Ahmed, T.; Alkhalifah, D.H.M.; Hozzein, W.N.; Yan, C.; et al. Bacteriophage-mediated biosynthesis of MnO2NPs and MgONPs and their role in the protection of plants from bacterial pathogens. Front. Microbiol. 2023, 14, 1193206. [Google Scholar] [CrossRef] [PubMed]
- Vishakha, K.; Das, S.; Ganguli, A. The Facile Synthesis of Eco-Friendly Zinc Magnesium Bimetal Nanoparticles and its Application in the Eradication of Xanthomonas oryzae pv. oryzae that Causes Leaf Blight Disease of Rice. Curr. Microbiol. 2023, 80, 340. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yang, D.; Jin, Q.; Wu, C.; Cui, J. Multifunctional molybdenum disulfide-copper nanocomposite that enhances the antibacterial activity, promotes rice growth and induces rice resistance. J. Hazard. Mater. 2020, 394, 122551. [Google Scholar] [CrossRef]
- Unimke, A.A.; Okezie, O.; Mohammed, S.E.; Mmuoegbulam, A.O.; Abdullahi, S.; Ofon, U.A.; Olim, D.M.; Badamasi, H.; Galadima, A.I.; Fatunla, O.K.; et al. Microbe-plant-nanoparticle interactions: Role in bioremediation of petroleum hydrocarbons. Water Sci. Technol. 2024, 90, 2870–2893. [Google Scholar] [CrossRef]
- Adomako, M.O.; Yu, F.H. Potential effects of micro- and nanoplastics on phyllosphere microorganisms and their evolutionary and ecological responses. Sci. Total Environ. 2023, 884, 163760. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Rani, N.; Hooda, V. Unravelling the effects of nano SiO2, nano TiO2 and their nanocomposites on Zea mays L. growth and soil health. Sci. Rep. 2024, 14, 13996. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Rehman, H.M.; Lam, H.-M. Possible Roles of Rhizospheric and Endophytic Microbes to Provide a Safe and Affordable Means of Crop Biofortification. Agronomy 2019, 9, 764. [Google Scholar] [CrossRef]
- Jana, S.K.; Islam, M.M.; Mandal, S. Endophytic Microbiota of Rice and Their Collective Impact on Host Fitness. Curr. Microbiol. 2022, 79, 37. [Google Scholar] [CrossRef]
- Morales-Cedeno, L.R.; Orozco-Mosqueda, M.D.C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de Los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, J.; Zhang, H.; Ji, G.; Zeng, L.; Li, Y.; Yu, C.; Fernando, W.G.D.; Chen, W. Bacterial Blight Induced Shifts in Endophytic Microbiome of Rice Leaves and the Enrichment of Specific Bacterial Strains With Pathogen Antagonism. Front. Plant Sci. 2020, 11, 963. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, K.; Liang, S.; Liu, L.; Nian, H.; Lian, T. Investigating the synergistic effects of nano-zinc and biochar in mitigating aluminum toxicity in soybeans. Plant Physiol. Biochem. 2024, 217, 109275. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, R.; Lei, L.; Zhang, F. Green synthesis of silver nanoparticles from the endophytic fungus Panax notoginseng and their antioxidant and antimicrobial activities and effects on cherry tomato preservation. Int. J. Food Microbiol. 2025, 431, 111083. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Lv, L.; Ahmed, T.; Noman, M.; Manan, A.; Ijaz, R.; Hafeez, R.; Shahid, M.S.; Wang, D.; Ondrasek, G.; et al. Immunomodulating melatonin-decorated silica nanoparticles suppress bacterial wilt (Ralstonia solanacearum) in tomato (Solanum lycopersicum L.) through fine-tuning of oxidative signaling and rhizosphere bacterial community. J. Nanobiotechnol. 2024, 22, 617. [Google Scholar] [CrossRef]
- Ning, W.; Luo, X.; Zhang, Y.; Tian, P.; Xiao, Y.; Li, S.; Yang, X.; Li, F.; Zhang, D.; Zhang, S.; et al. Broad-spectrum nano-bactericide utilizing antimicrobial peptides and bimetallic Cu-Ag nanoparticles anchored onto multiwalled carbon nanotubes for sustained protection against persistent bacterial pathogens in crops. Int. J. Biol. Macromol. 2024, 265 Pt 2, 131042. [Google Scholar] [CrossRef]
- Barron, C.C.; Sponagle, B.J.; Arivalagan, P.; D’Cunha, G.B. Optimization of oligomeric enzyme activity in ionic liquids using Rhodotorula glutinis yeast phenylalanine ammonia lyase. Enzym. Microb. Technol. 2017, 96, 151–156. [Google Scholar] [CrossRef]
- Yenisoy-Karakas, S. Estimation of uncertainties of the method to determine the concentrations of Cd, Cu, Fe, Pb, Sn and Zn in tomato paste samples analysed by high resolution ICP-MS. Food Chem. 2012, 132, 1555–1561. [Google Scholar] [CrossRef]
- Rosa, A.C.G.; Melo, E.S.P.; Junior, A.S.A.; Gondim, J.M.S.; de Sousa, A.G.; Cardoso, C.A.L.; Viana, L.F.; Carvalho, A.M.A.; Machate, D.J.; do Nascimento, V.A. Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 660. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; Desantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Travanty, N.V.; Watson, D.W.; Seagle, S.W.; Boyce, R.M.; Reiskind, M.H. Microbiome of Invasive Tick Species Haemaphysalis longicornis in North Carolina, USA. Insects 2024, 15, 153. [Google Scholar] [CrossRef]
- Mohsen, A.; Chen, Y.A.; Allendes Osorio, R.S.; Higuchi, C.; Mizuguchi, K. Snaq: A Dynamic Snakemake Pipeline for Microbiome Data Analysis With QIIME2. Front. Bioinform. 2022, 2, 893933. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xian, X.; Gu, Y.; Castañé, C.; Arnó, J.; Wu, S.; Wan, F.; Liu, W.; Zhang, G.; Zhang, Y. Similar Bacterial Communities among Different Populations of a Newly Emerging Invasive Species, Tuta absoluta (Meyrick). Insects 2022, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Petermann, E.; Gnädinger, F.; Hartmann, P. Use of random forest algorithm for predictive modelling of transfer factor soil-plant for radiocaesium: A feasibility study. J. Environ. Radioact. 2023, 270, 107309. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Kristensen, D.M.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Microbial genome analysis: The COG approach. Brief. Bioinform. 2019, 20, 1063–1070. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Wang, N. KEGG orthology prediction of bacterial proteins using natural language processing. BMC Bioinform. 2024, 25, 146. [Google Scholar] [CrossRef]
- Naz, R.; Bano, A.; Nosheen, A.; Yasmin, H.; Keyani, R.; Shah, S.T.A.; Anwar, Z.; Roberts, T.H. Induction of defense-related enzymes and enhanced disease resistance in maize against Fusarium verticillioides by seed treatment with Jacaranda mimosifolia formulations. Sci. Rep. 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Bakhshi, B.; Malla, S.; Lokesh, S. Monitoring of Defense Enzymes (Phenylalanine Ammonia Lyase and Peroxidase) in Magnaporthe oryzae Infected Leaves after Treatment with Green Synthesized Silver Nanoparticles. Indian J. Pharm. Educ. Res. 2023, 57, 141–146. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, e14823. [Google Scholar] [CrossRef] [PubMed]
- Shams, A.H.M.; Helaly, A.A.; Algeblawi, A.M.; Awad-Allah, E.F.A. Efficacy of Seed-Biopriming with Trichoderma spp. and Foliar Spraying of ZnO-Nanoparticles Induce Cherry Tomato Growth and Resistance to Fusarium Wilt Disease. Plants 2023, 12, 3117. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.; Galazka, A.; Marzec-Grzadziel, A.; Frac, M. Microbial Community, Metabolic Potential and Seasonality of Endosphere Microbiota Associated with Leaves of the Bioenergy Tree Paulownia elongata × fortunei. Int. J. Mol. Sci. 2022, 23, 8978. [Google Scholar] [CrossRef]
- Ganie, S.A.; Bhat, J.A.; Devoto, A. The influence of endophytes on rice fitness under environmental stresses. Plant Mol. Biol. 2022, 109, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lv, L.; Ahmed, T.; Jin, S.; Shahid, M.; Noman, M.; Osman, H.H.; Wang, Y.; Sun, G.; Li, X.; et al. Effect of the Nanoparticle Exposures on the Tomato Bacterial Wilt Disease Control by Modulating the Rhizosphere Bacterial Community. Int. J. Mol. Sci. 2021, 23, 414. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Z.; Wan, X.; Munir, S.; Wang, X.; Wei, L.; Ji, G. Insights into the relevance between bacterial endophytic communities and resistance of rice cultivars infected by Xanthomonas oryzae pv. Oryzicola. 3 Biotech 2021, 11, 434. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, C.G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mehnaz, S.; Malik, K.A. Microbial diversity in the rhizosphere of plants growing under extreme environments and its impact on crop improvement. Environ. Sustain. 2019, 2, 329–338. [Google Scholar] [CrossRef]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287 Pt 2, 132107. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Chen, H.; Jing, X.; Zheng, W.; Li, R.; Sun, T.; Liu, J.; Fu, J.; Huo, L.; et al. Discovery of recombinases enables genome mining of cryptic biosynthetic gene clusters in Burkholderiales species. Proc. Natl. Acad. Sci. USA 2018, 115, e4255–e4263. [Google Scholar] [CrossRef]
- Liu, M.; Philp, J.; Wang, Y.; Hu, J.; Wei, Y.; Li, J.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Plant growth-promoting rhizobacteria Burkholderia vietnamiensis B418 inhibits root-knot nematode on watermelon by modifying the rhizosphere microbial community. Sci. Rep. 2022, 12, 8381. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Ye, M.; Wang, Y.; Foong, S.Y.; Wang, L.; Sun, F.; Cheng, H. Characteristics of Microbial Community and Function with the Succession of Mangroves. Front. Microbiol. 2021, 12, 764974. [Google Scholar] [CrossRef]
- Li, C.; Wei, Z.; Wang, X.; Ma, X.; Tang, Q.; Zhao, B.; Shan, J.; Yan, X. Biochar mitigates the stimulatory effects of straw incorporation on N2O emission and N2O/(N2O + N2) ratio in upland soil. J. Environ. Manag. 2024, 369, 122318. [Google Scholar] [CrossRef]
- Tian, H.; Kah, M.; Kariman, K. Are Nanoparticles a Threat to Mycorrhizal and Rhizobial Symbioses? A Critical Review. Front. Microbiol. 2019, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xing, Y.; Chen, C.; Wang, Z. Enhancing sugarcane’s drought resilience: The influence of Streptomycetales and Rhizobiales. Front. Plant Sci. 2024, 15, 1471044. [Google Scholar] [CrossRef]
- Rawle, R.; Saley, T.C.; Kang, Y.S.; Wang, Q.; Walk, S.; Bothner, B.; McDermott, T.R. Introducing the ArsR-Regulated Arsenic Stimulon. Front. Microbiol. 2021, 12, 630562. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Han, J.; Xi, L.; Li, M.; Li, Z.; Zhang, H. Functional insights into Brucella transcriptional regulator ArsR. Microb. Pathog. 2022, 168, 105557. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zong, G.; Chen, X.; Tan, M.; Gao, W.; Fu, J.; Zhang, P.; Wang, B.; Cao, G. Bifunctional protein ArsR(M) contributes to arsenite methylation and resistance in Brevundimonas sp. M20. BMC Microbiol. 2023, 23, 134. [Google Scholar] [CrossRef]
- Zhi, F.; Liu, K.; Geng, H.; Su, M.; Xu, J.; Fu, L.; Ma, K.; Gao, P.; Yuan, L.; Chu, Y. Copper sensing transcription factor ArsR2 regulates VjbR to sustain virulence in Brucella abortus. Emerg. Microbes Infect. 2024, 13, 2406274. [Google Scholar] [CrossRef]
- Braun, V.; Hantke, K. Ferric Citrate Regulator FecR Is Translocated across the Bacterial Inner Membrane via a Unique Twin-Arginine Transport-Dependent Mechanism. J. Bacteriol. 2020, 202, e00541-19. [Google Scholar] [CrossRef]
- Yokoyama, T.; Niinae, T.; Tsumagari, K.; Imami, K.; Ishihama, Y.; Hizukuri, Y.; Akiyama, Y. The Escherichia coli S2P intramembrane protease RseP regulates ferric citrate uptake by cleaving the sigma factor regulator FecR. J. Biol. Chem. 2021, 296, 100673. [Google Scholar] [CrossRef] [PubMed]
- D’Alvise, P.W.; Phippen, C.B.; Nielsen, K.F.; Gram, L. Influence of Iron on Production of the Antibacterial Compound Tropodithietic Acid and Its Noninhibitory Analog in Phaeobacter inhibens. Appl. Environ. Microbiol. 2016, 82, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.; Hernandez-Guerrero, R.; Mendez-Monroy, P.E.; Martinez-Nunez, M.A.; Ibarra, J.A.; Perez-Rueda, E. Evaluation of the Abundance of DNA-Binding Transcription Factors in Prokaryotes. Genes 2020, 11, 52. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Chen, W.; Ren, Z.H.; Gao, H.; Dai, J.; Luo, G.Z.; Wu, Z.; Ji, Q. A KDPG sensor RccR governs Pseudomonas aeruginosa carbon metabolism and aminoglycoside antibiotic tolerance. Nucleic Acids Res. 2024, 52, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, E.J.; Roe, J.H. uORF-mediated riboregulation controls transcription of whiB7/wblC antibiotic resistance gene. Mol. Microbiol. 2022, 117, 179–192. [Google Scholar] [CrossRef]
- Oka, G.U.; Souza, D.P.; Cenens, W.; Matsuyama, B.Y.; Cardoso, M.V.C.; Oliveira, L.C.; da Silva Lima, F.; Cuccovia, I.M.; Guzzo, C.R.; Salinas, R.K.; et al. Structural basis for effector recognition by an antibacterial type IV secretion system. Proc. Natl. Acad. Sci. USA 2022, 119, e2112529119. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Kim, E.Y.; Sang, P.B.; Chai, W. Roles of OB-Fold Proteins in Replication Stress. Front. Cell Dev. Biol. 2020, 8, 574466. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Lin, X.; Xu, H.; Li, Y.; Xue, R.; Wang, G.; Sun, S.; Li, J.; Lan, Z.; et al. Genetic characterization, mechanisms and dissemination risk of antibiotic resistance of multidrug-resistant Rothia nasimurium. Infect. Genet. Evol. 2021, 90, 104770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, W.; Li, M.; Jiang, L.; Yang, M.; Liu, M.; Liu, Y. Endophytic Bacterial Community Structure and Function Response of BLB Rice Leaves After Foliar Application of Cu-Ag Nanoparticles. Nanomaterials 2025, 15, 778. https://doi.org/10.3390/nano15110778
Ning W, Li M, Jiang L, Yang M, Liu M, Liu Y. Endophytic Bacterial Community Structure and Function Response of BLB Rice Leaves After Foliar Application of Cu-Ag Nanoparticles. Nanomaterials. 2025; 15(11):778. https://doi.org/10.3390/nano15110778
Chicago/Turabian StyleNing, Weimin, Mingxuan Li, Lei Jiang, Mei Yang, Maoyan Liu, and Yong Liu. 2025. "Endophytic Bacterial Community Structure and Function Response of BLB Rice Leaves After Foliar Application of Cu-Ag Nanoparticles" Nanomaterials 15, no. 11: 778. https://doi.org/10.3390/nano15110778
APA StyleNing, W., Li, M., Jiang, L., Yang, M., Liu, M., & Liu, Y. (2025). Endophytic Bacterial Community Structure and Function Response of BLB Rice Leaves After Foliar Application of Cu-Ag Nanoparticles. Nanomaterials, 15(11), 778. https://doi.org/10.3390/nano15110778






