Enhanced Nitrate Reduction Performance of Cu-Doped Nanoporous Co2P Electrocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Fabrication
2.2. Materials Characterization
2.3. Electrochemical Measurements
2.4. Determination of NH3
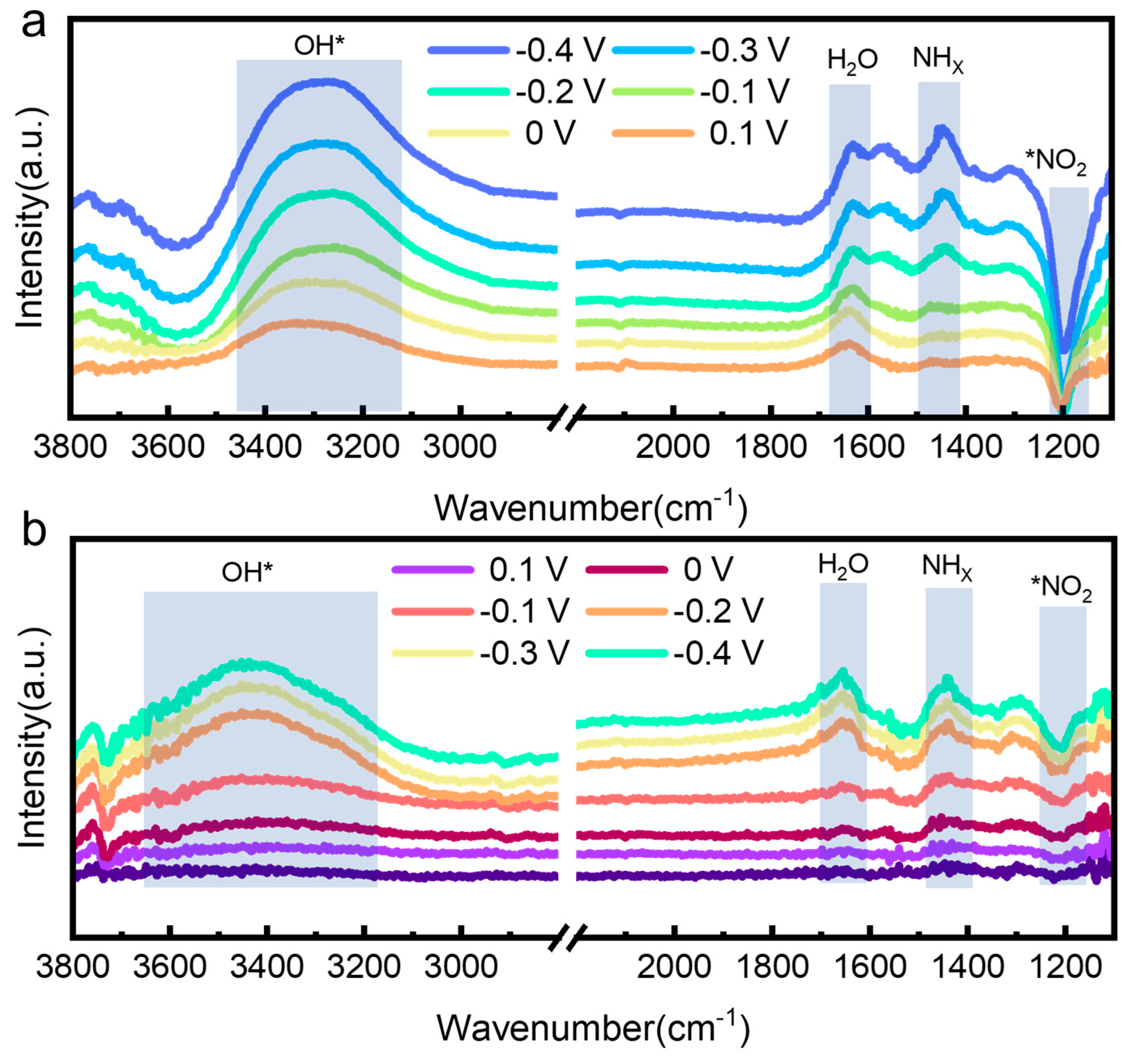
2.5. In-Situ ATR-SEIRAS Measurements
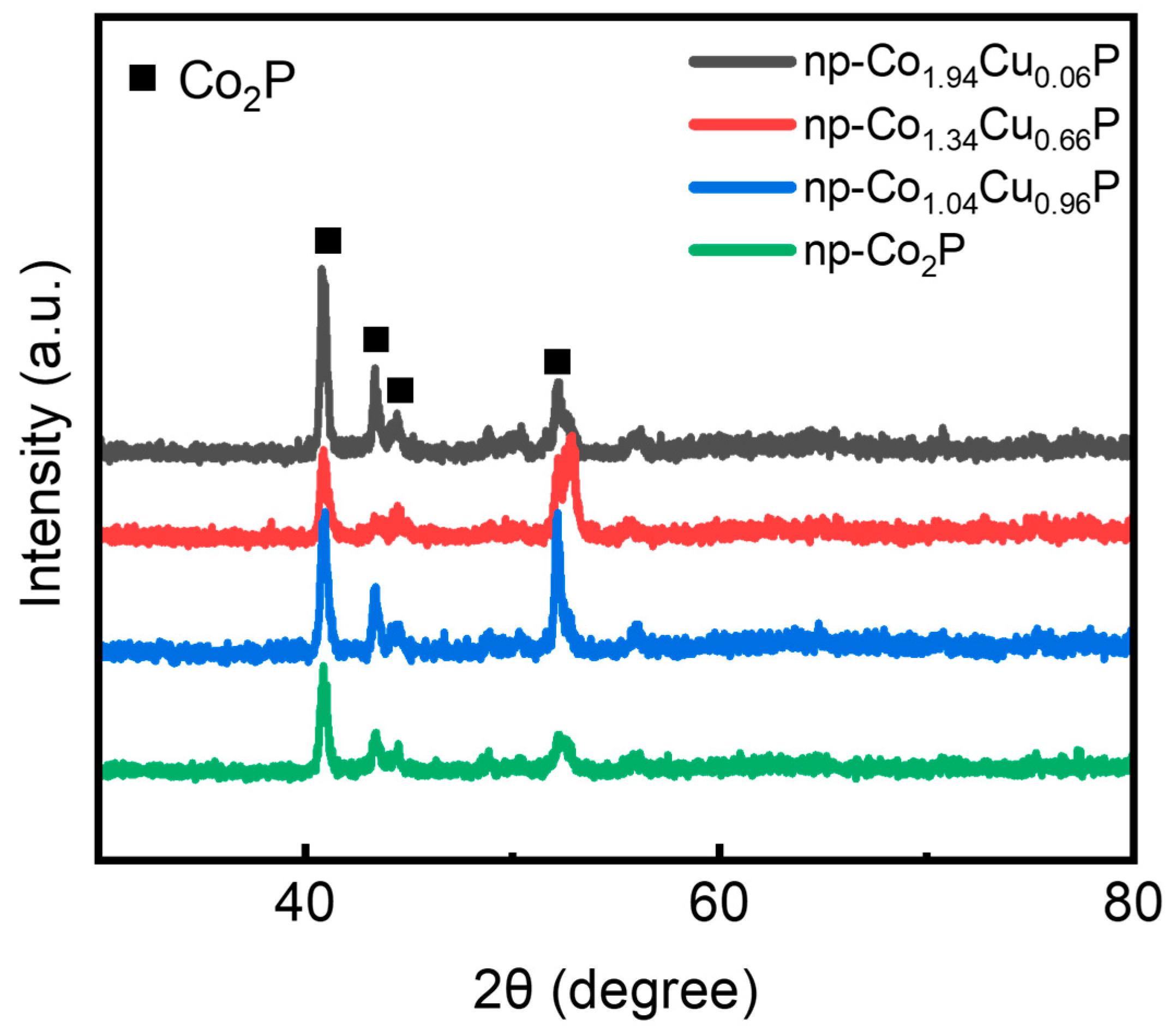
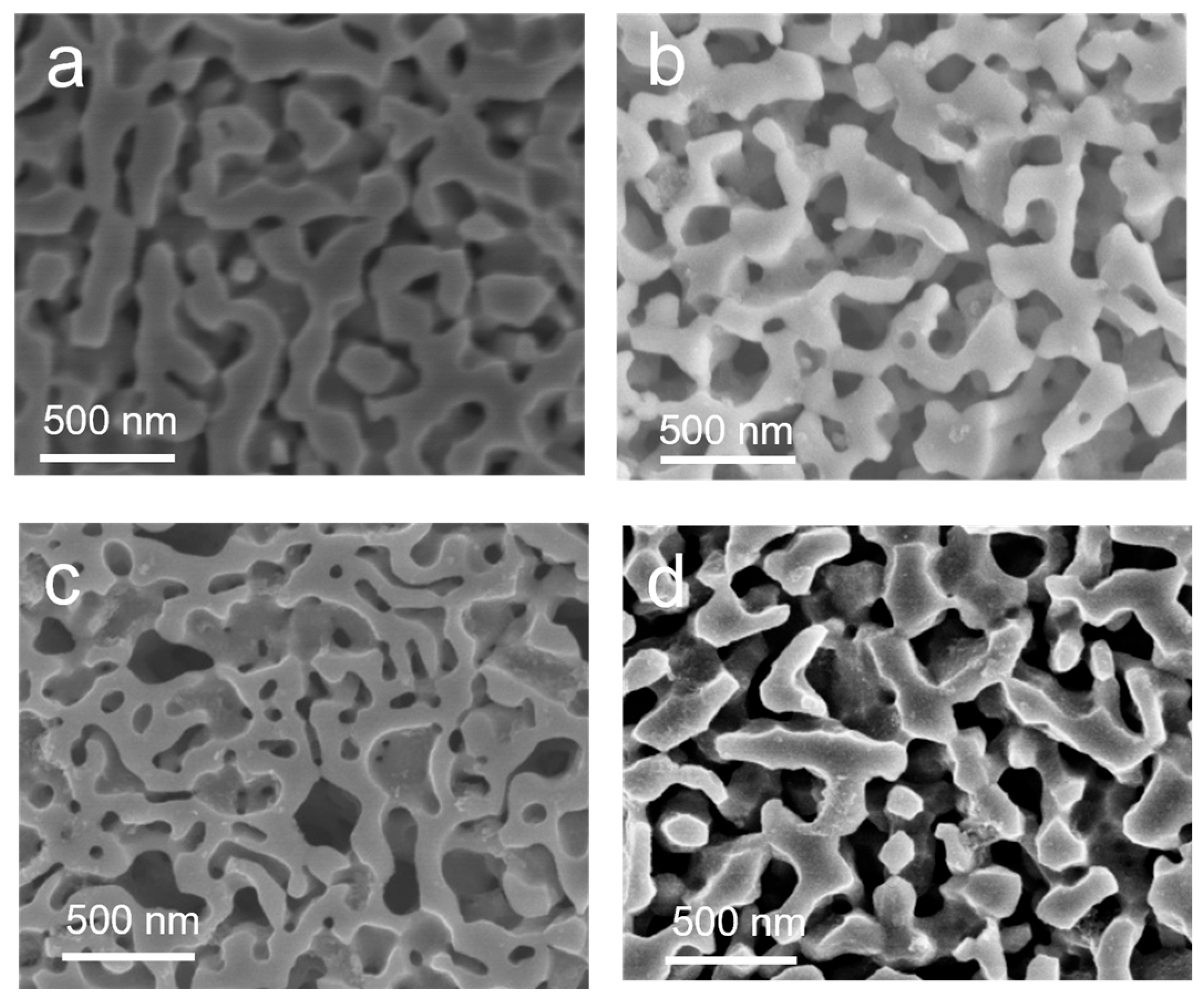
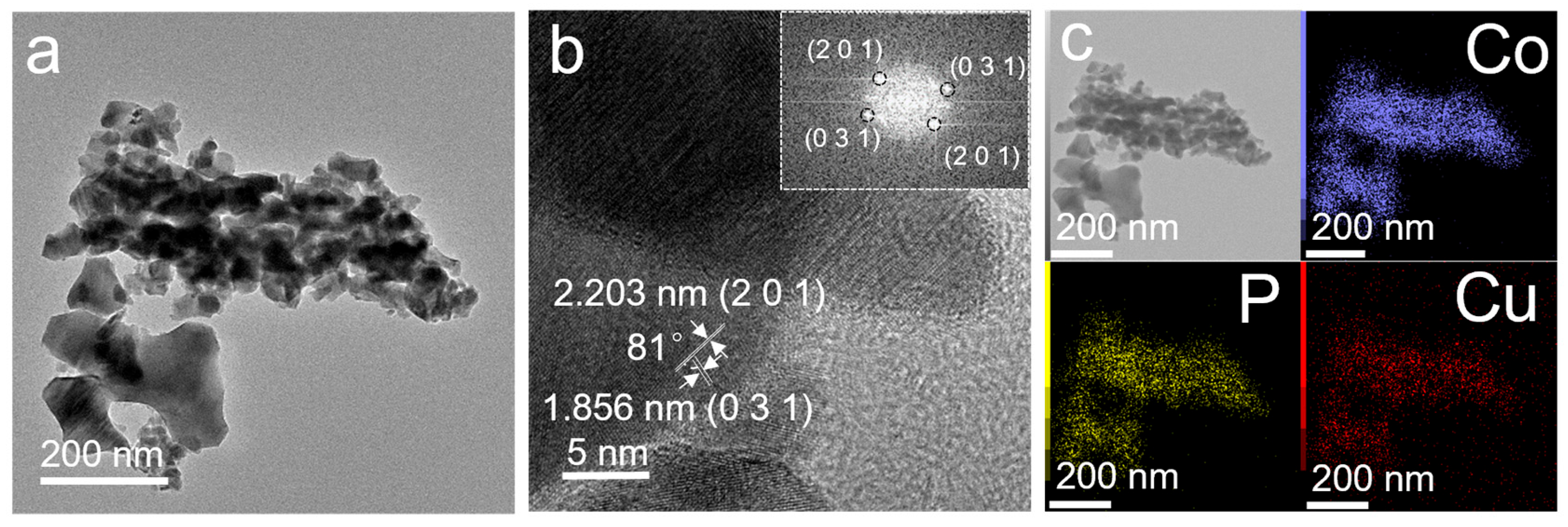
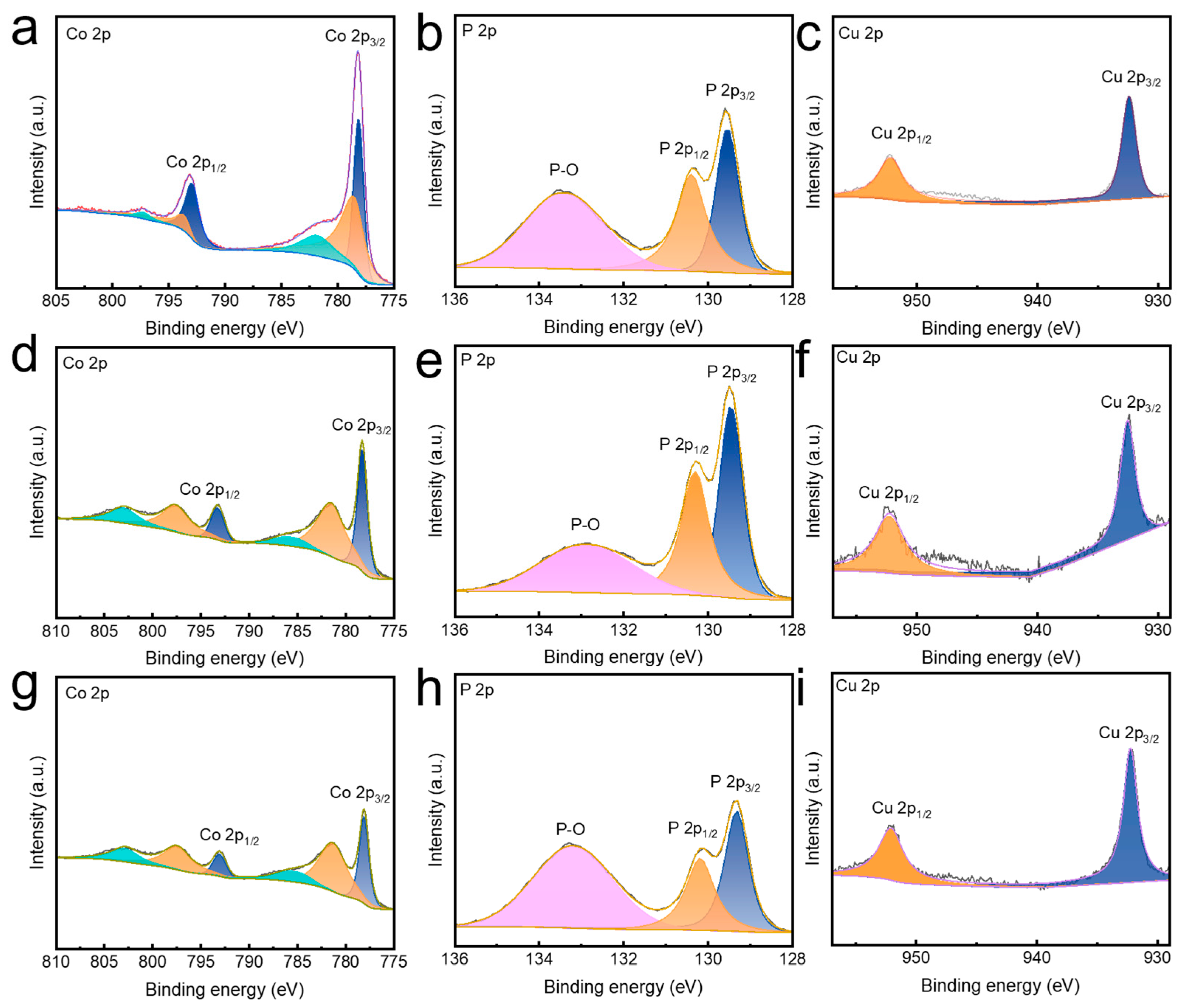
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gunbatar, S.A.; Kiran, B.E.; Boz, Y.; Oztay, E.S. A systematic review of green and sustainable chemistry training research with pedagogical content knowledge framework: Current trends and future directions. Chem. Educ. Res. Pract. 2025, 26, 34–52. [Google Scholar] [CrossRef]
- Cheng, Q.; Muhammad, A.; Kaario, O.; Ahmad, Z.; Martti, L. Ammonia as a sustainable fuel: Review and novel strategies. Renew. Sustain. Energy Rev. 2025, 207, 114995. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Mansourimarand, A.; Ajori, S.; Taghikhani, V.; Yoo, C. Waste-to-Ammonia: A sustainable pathway for energy transition. Renew. Sustain. Energy Rev. 2025, 208, 115012. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Lin, Y.; Liu, C.; Wang, X.; Xu, J. Optimal dispatching of integrated energy system with hydrogen-to-ammonia and ammonia-mixed/oxygen-enriched thermal power. Energy 2025, 316, 134514. [Google Scholar] [CrossRef]
- Fang, Y.; Li, M.; Gao, Y.; Wen, Y.; Shan, B. Static Organic p-n Junctions in Photoelectrodes for Solar Ammonia Production with 86 % Internal Quantum Efficiency. Angew. Chem. Int. Ed. Engl. 2025, 64, e202415729. [Google Scholar] [CrossRef]
- Ahsan, A.; Hussain, M.; Rashad, M.A.; Akhter, P.; Jamil, F.; Cho, K.; Park, Y.-K. Critical review on electrocatalytic reduction of nitrogen and nitrate to ammonia. J. Ind. Eng. Chem. 2025, 02, 29. [Google Scholar] [CrossRef]
- Jiang, H.; Li, T.; Gao, Y.; Fan, J.; Gan, D.; Yuan, S.; Hong, L.; Feng, Y.; Sun, J.; Song, Q.; et al. Sustainable ammonia synthesis: Opportunities for electrocatalytic nitrate reduction. J. Energy Chem. 2025, 105, 630–638. [Google Scholar] [CrossRef]
- Kang, B.; Xu, B.; Chen, Z.; Li, F.; Wang, Y. Promoting active hydrogen supply for kinetically matched tandem electrocatalytic nitrate reduction to ammonia. Appl. Catal. B-Environ. 2025, 360, 124528. [Google Scholar] [CrossRef]
- Bhatti, U.A.; Bhatti, M.A.; Tang, H.; Syam, M.S.; Awwad, E.M.; Sharaf, M.; Ghadi, Y.Y. Global production patterns: Understanding the relationship between greenhouse gas emissions, agriculture greening and climate variability. Environ. Res. 2024, 245, 118049. [Google Scholar] [CrossRef]
- Zhang, R.; Li, C.; Cui, H.; Wang, Y.; Zhang, S.; Li, P.; Hou, Y.; Guo, Y.; Liang, G.; Huang, Z.; et al. Electrochemical nitrate reduction in acid enables high-efficiency ammonia synthesis and high-voltage pollutes-based fuel cells. Nat. Commun. 2023, 14, 8036. [Google Scholar] [CrossRef]
- Wang, M.; Khan, M.A.; Mohsin, I.; Wicks, J.; Ip, A.H.; Sumon, K.Z.; Dinh, C.-T.; Sargent, E.H.; Gates, I.D.; Kibria, M.G. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 2021, 14, 2535–2548. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, K.; Yang, J.; Chen, H.; Ning, J.; Wang, H.; Hu, Y. Strategies and applications of electrocatalytic nitrate reduction towards ammonia. Coord. Chem. Rev. 2024, 506, 215723. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Li, T.; Chen, F.; Yang, R.; Yu, Y.; Zhang, B. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 2023, 6, 402–414. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Chen, J.; Zhang, W.X.; Yang, J. Electrocatalytic reduction of nitrate—a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 2022, 51, 2710–2758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Zhang, B.; Yu, Y. Self-template synthesis of hierarchically structured Co3O4@NiO bifunctional electrodes for selective nitrate reduction and tetrahydroisoquinolines semi-dehydrogenation. Sci. China Mater. 2020, 63, 2530–2538. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, K.; Peng, O.; Li, M.; Ma, L.; Huang, S.; Du, Y.; Xu, Z.X.; Wang, Q.; Chen, Z.; et al. Ammonia Electrosynthesis from Nitrate Using a Ruthenium-Copper Cocatalyst System: A Full Concentration Range Study. J. Am. Chem. Soc. 2024, 146, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ji, Y.G.; Xue, Q.; Li, F.M.; Zhao, G.T.; Jin, P.J.; Li, S.N.; Chen, Y. Efficient Nitrate-to-Ammonia Electroreduction at Cobalt Phosphide Nanoshuttles. ACS Appl. Mater. Interfaces 2021, 13, 45521–45527. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Z.; Zhang, G.; Chen, W.; Peng, C.; Yang, X.; Zheng, L.; Li, Y.; Ren, X.; Cao, H.; et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 2022, 15, 760–770. [Google Scholar] [CrossRef]
- Qi, R.; Wang, Z.; Zhong, M.; Wang, C.; Bai, F.; Lu, X. Synergistic Integration of Amorphous Cobalt Phosphide with a Conductive Channel for Highly Efficient Electrocatalytic Nitrate Reduction to Ammonia. Small 2024, 20, 2308311. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, H.; Liu, P.; Han, R.; Wang, Y.-Q. A Cu/Fe3O4@CN tandem catalyst for efficient ammonia electrosynthesis from nitrate reduction. J. Colloid Interface Sci. 2025, 682, 703–714. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, J.; Song, Q.; Liu, X. High-ammonia selective metal organic framework derived Co-doped Fe/Fe2O3 catalysts for electrochemical nitrate reduction. Proc. Natl. Acad. Sci. USA 2022, 119, e2115504119. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.; Zhang, Y.; Song, X.; Jiang, H. Precisely anchoring Ni-doped cobalt phosphide nanoparticles on phosphatized carbon nitride for efficient photocatalytic water splitting. Chem. Eng. J. 2023, 472, 144898. [Google Scholar] [CrossRef]
- Li, F.; Bu, Y.; Lv, Z.; Mahmood, J.; Han, G.-F.; Ahmad, I.; Kim, G.; Zhong, Q.; Baek, J.-B. Porous Cobalt Phosphide Polyhedrons with Iron Doping as an Efficient Bifunctional Electrocatalyst. Small 2017, 13, 1701167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zheng, L.; Yan, X.; Xie, J.; Yu, Z.; Zhang, S.; Jiang, F.; Chen, H. The synergistic catalysis effect on electrochemical nitrate reduction at the dual-function active sites of the heterostructure. Energy Environ. Sci. 2024, 17, 4582–4593. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Liu, P.; Cheng, C.; Zhu, F.; Hirata, A.; Chen, M. 3D Nanoporous Metal Phosphides toward High-Efficiency Electrochemical Hydrogen Production. Adv. Mater. 2016, 28, 2951–2955. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P Electrocatalyst for the Hydrogen Evolution Reaction and Direct Comparison with Morphologically Equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic Structure Modulation of Nanoporous Cobalt Phosphide by Carbon Doping for Alkaline Hydrogen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2107333. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Y.; Zhang, X.; Yang, H.; Hu, T. Synthesis of Co0·52Cu0·48/Cu@S–C arrays for the enhanced performance of hydrazine-assisted hydrogen production. Int. J. Hydrogen Energy 2024, 68, 472–480. [Google Scholar] [CrossRef]
- Sang, W.; Wang, C.; Zhang, X.; Yu, X.; Yu, C.; Zhao, J.; Wang, X.; Yang, X.; Li, L. Dendritic Co0.52Cu0.48 and Ni0.19Cu0.81 alloys as hydrogen generation catalysts via hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2017, 42, 30691–30703. [Google Scholar] [CrossRef]
- Liu, G.; Wang, M.; Xu, Y.; Wang, X.; Li, X.; Liu, J.; Cui, X.; Jiang, L. Porous CoP/Co2P heterostructure for efficient hydrogen evolution and application in magnesium/seawater battery. J. Power Sources 2021, 486, 229351. [Google Scholar] [CrossRef]
- Chen, L.; Song, X.-L.; Ren, J.-T.; Yuan, Z.-Y. Precisely modifying Co2P/black TiO2 S-scheme heterojunction by in situ formed P and C dopants for enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2022, 315, 121546. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.; Zhang, Z.; Zheng, Y.; Wu, Y.; Fu, X.; Su, J.; Gao, Y. CoP/Cu3P heterostructured nanoplates for high-rate supercapacitor electrodes. Chem. Eng. J. 2022, 437, 135352. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Xu, C.; Fang, H.; Yu, H.; Zhang, H.; Li, S.; Li, C.; Huang, F. Enhanced electrocatalytic nitrate reduction through phosphorus-vacancy-mediated kinetics in heterogeneous bimetallic phosphide hollow nanotube array. Appl. Catal. B-Environ. 2023, 330, 122627. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; An, N.; Zhang, S.; Song, Q.; Yang, Y.; Li, J.; Liu, X. Boosted ammonium production by single cobalt atom catalysts with high Faradic efficiencies. Proc. Natl. Acad. Sci. USA 2022, 119, e2123450119. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Z.; Zhang, Y.; Zheng, Y.; Liu, N.; Su, J.; Gao, Y. Interior and Exterior Decoration of Transition Metal Oxide Through Cu0/Cu+ Co-Doping Strategy for High-Performance Supercapacitor. Nano-Micro Lett. 2021, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lim, K.Y.; Chung, Y.D.; Whang, C.N.; Jeon, Y. XPS core-level shifts and XANES studies of Cu–Pt and Co–Pt alloys. Surf. Interface Anal. 2000, 30, 475–478. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. Engl. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Yang, M.; Li, B.; Li, S.; Dong, Q.; Huang, Z.; Zheng, S.; Fang, Y.; Zhou, G.; Chen, X.; Zhu, X.; et al. Highly Selective Electrochemical Nitrate to Ammonia Conversion by Dispersed Ru in a Multielement Alloy Catalyst. Nano Lett. 2023, 23, 7733–7742. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Wu, Z.; Wang, Z. Direct Electron Transfer Coordinated by Oxygen Vacancies Boosts Selective Nitrate Reduction to N2 on a Co-CuOx Electroactive Filter. Environ. Sci. Technol. 2022, 56, 8673–8681. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Yu, Y.; Wang, Y.; Zhang, B. Promoting selective electroreduction of nitrates to ammonia over electron-deficient Co modulated by rectifying Schottky contacts. Sci. China Chem. 2020, 63, 1469–1476. [Google Scholar] [CrossRef]
- Jiang, M.; Su, J.; Song, X.; Zhang, P.; Zhu, M.; Qin, L.; Tie, Z.; Zuo, J.-L.; Jin, Z. Interfacial Reduction Nucleation of Noble Metal Nanodots on Redox-Active Metal–Organic Frameworks for High-Efficiency Electrocatalytic Conversion of Nitrate to Ammonia. Nano Lett. 2022, 22, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wang, Y.; Wang, C.; Ling, Y.; Yu, Y.; Zhang, B. Boosting Selective Nitrate Electroreduction to Ammonium by Constructing Oxygen Vacancies in TiO2. ACS Catal. 2020, 10, 3533–3540. [Google Scholar] [CrossRef]
- Yang, J.; Qi, H.; Li, A.; Liu, X.; Yang, X.; Zhang, S.; Zhao, Q.; Jiang, Q.; Su, Y.; Zhang, L. Potential-driven restructuring of Cu single atoms to nanoparticles for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. [Google Scholar] [CrossRef]
- Lv, Y.; Ke, S.W.; Gu, Y.; Tian, B.; Tang, L.; Ran, P.; Zhao, Y.; Ma, J.; Zuo, J.L.; Ding, M. Highly Efficient Electrochemical Nitrate Reduction to Ammonia in Strong Acid Conditions with Fe2M-Trinuclear-Cluster Metal-Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305246. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, C.; Zhang, W.; Yang, X.; Ding, J.; Gao, G. Electrical pulse-driven periodic self-repair of Cu-Ni tandem catalyst for efficient ammonia synthesis from nitrate. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217337. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, Y.; Zhong, Y.; Guo, Q.; Zeng, J.; Geng, Z. Efficient electroreduction of nitrate into ammonia at ultralow concentrations via an enrichment effect. Adv. Mater. 2022, 34, 2204306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, e202003619. [Google Scholar] [CrossRef]
- Mao, X.; Bai, X.; Wu, G.; Qin, Q.; O’Mullane, A.P.; Jiao, Y.; Du, A. Electrochemical Reduction of N2 to Ammonia Promoted by Hydrated Cation Ions: Mechanistic Insights from a Combined Computational and Experimental Study. J. Am. Chem. Soc. 2024, 146, 18743–18752. [Google Scholar] [CrossRef]
- Yu, S.; Yamauchi, H.; Wang, S.; Aggarwal, A.; Kim, J.; Gordiz, K.; Huang, B.; Xu, H.; Zheng, D.J.; Wang, X.; et al. CO2-to-methanol electroconversion on a molecular cobalt catalyst facilitated by acidic cations. Nat. Catal. 2024, 7, 1000–1009. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zheng, S.; Yang, W.M.; Zhou, R.Y.; He, Q.F.; Radjenovic, P.; Dong, J.C.; Li, S.; Zheng, J.; Yang, Z.L.; et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 2021, 600, 81–85. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, X.; Yao, Y.; Shao, M. pH-Dependent Hydrogen and Water Binding Energies on Platinum Surfaces as Directly Probed through Surface-Enhanced Infrared Absorption Spectroscopy. J. Am. Chem. Soc. 2020, 142, 8748–8754. [Google Scholar] [CrossRef] [PubMed]
- Concha, B.M.; Chatenet, M.; Coutanceau, C.; Hahn, F. In situ infrared (FTIR) study of the borohydride oxidation reaction. Electrochem. Commun. 2009, 11, 223–226. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Zheng, Q.-Z.; Lou, Y.-Y.; Zhao, K.-M.; Hu, S.-N.; Li, G.; Akdim, O.; Huang, X.-Y.; Sun, S.-G. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef]
- Zhang, J.; Quast, T.; Eid, B.; Chen, Y.-T.; Zerdoumi, R.; Dieckhöfer, S.; Junqueira, J.R.C.; Seisel, S.; Schuhmann, W. In-situ electrochemical reconstruction and modulation of adsorbed hydrogen coverage in cobalt/ruthenium-based catalyst boost electroreduction of nitrate to ammonia. Nat. Commun. 2024, 15, 8583. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhang, X.; Liang, Y.; Jiang, H.; Wu, S.; Li, Z.; Cui, Z.; Zhu, S.; Gao, Z.; Xu, W. Enhanced Nitrate Reduction Performance of Cu-Doped Nanoporous Co2P Electrocatalyst. Nanomaterials 2025, 15, 753. https://doi.org/10.3390/nano15100753
Huang Y, Zhang X, Liang Y, Jiang H, Wu S, Li Z, Cui Z, Zhu S, Gao Z, Xu W. Enhanced Nitrate Reduction Performance of Cu-Doped Nanoporous Co2P Electrocatalyst. Nanomaterials. 2025; 15(10):753. https://doi.org/10.3390/nano15100753
Chicago/Turabian StyleHuang, Yunduo, Xiechen Zhang, Yanqin Liang, Hui Jiang, Shuilin Wu, Zhaoyang Li, Zhenduo Cui, Shengli Zhu, Zhonghui Gao, and Wence Xu. 2025. "Enhanced Nitrate Reduction Performance of Cu-Doped Nanoporous Co2P Electrocatalyst" Nanomaterials 15, no. 10: 753. https://doi.org/10.3390/nano15100753
APA StyleHuang, Y., Zhang, X., Liang, Y., Jiang, H., Wu, S., Li, Z., Cui, Z., Zhu, S., Gao, Z., & Xu, W. (2025). Enhanced Nitrate Reduction Performance of Cu-Doped Nanoporous Co2P Electrocatalyst. Nanomaterials, 15(10), 753. https://doi.org/10.3390/nano15100753







